- 1Department of Pharmacy, Wuzhou Red Cross Hospital, Wuzhou, Guangxi, China
- 2Office of Good Clinical Practice, Wuzhou Red Cross Hospital, Wuzhou, Guangxi, China
- 3Department of Pharmacy, Mianyang Central Hospital, Mianyang, Sichuan, China
- 4Department of Pharmacy, The Third Affiliated Hospital of Chongqing Medical University, Chongqing, China
Purpose: To identify the most commonly reported drugs associated with QT interval prolongation in the FDA Adverse Event Reporting System (FAERS) and evaluate their risk for QT interval prolongation.
Methods: We employed the preferred term (PT) “electrocardiogram QT prolonged” from the Medical Dictionary for Regulatory Activities (MedDRA) 26.0 to identify adverse drug events (ADEs) of QT interval prolongation in the FAERS database from the period 2004–2022. Reporting odds ratio (ROR) was performed to quantify the signals of ADEs.
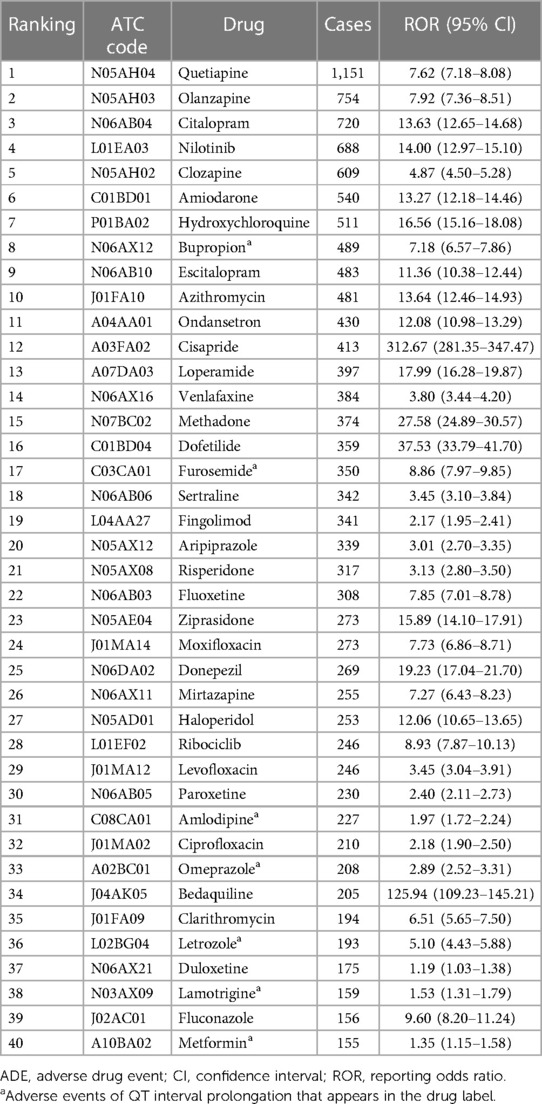
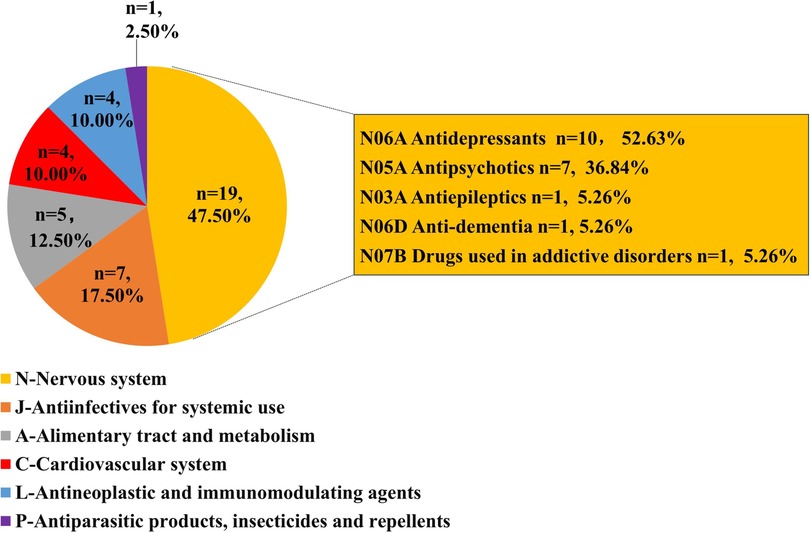
Results: We listed the top 40 drugs that caused QT interval prolongation. Among them, the 3 drugs with the highest number of cases were quetiapine (1,151 cases, ROR = 7.62), olanzapine (754 cases, ROR = 7.92), and citalopram (720 cases, ROR = 13.63). The two most frequently reported first-level Anatomical Therapeutic Chemical (ATC) groups were the drugs for the nervous system (n = 19, 47.50%) and antiinfectives for systemic use (n = 7, 17.50%). Patients with missing gender (n = 3,482, 23.68%) aside, there were more females (7,536, 51.24%) than males (5,158, 35.07%) were involved. 3,720 patients (25.29%) suffered serious clinical outcomes resulting in deaths or life-threatening conditions. Overall, most drugs that caused QT interval prolongation had early failure types according to the assessment of the Weibull's shape parameter (WSP) analysis.
Conclusions: Our study offered a list of drugs that frequently caused QT interval prolongation based on the FAERS system, along with a description of some risk profiles for QT interval prolongation brought on by these drugs. When prescribing these drugs in clinical practice, we should closely monitor the occurrence of ADE for QT interval prolongation.
1 Introduction
The QT interval, measured as the interval between the initiation of the Q wave and the termination of the T wave on an electrocardiogram, is a measure of ventricular depolarisation and repolarisation (1). As per the current diagnostic criteria, a corrected QT (QTc) interval longer than 450 milliseconds (ms) for men and 470 ms for women is considered a “prolonged QTc interval” (2). QT interval prolongation is correlated with the risk of cardiac events (3), and one of the most severe consequences of a prolonged QT interval is a ventricular arrhythmia known as torsade de pointes (TdP), which can lead to sudden death (4, 5).
In general, hypocalcemia, hypokalemia, coronary artery disease, hypertension, diabetes mellitus, medications, and other factors result in QT interval prolongation (6–11). According to reports, antiarrhythmic medications, macrolides, fluoroquinolones, antifungal medications, antipsychotic medications, antihistamines, antiviral medications, anticancer medications, diuretics, and others might cause QT interval prolongation (12–14). Considering the medication's safety, drug-induced QT interval prolongation has been an important factor behind the removal or restricted use of medications during the past 20 years.
Because of the unpredictable and dangerous outcomes, drug-induced QT interval prolongation is still a concern that needs to be continuously monitored during treatment. Currently, there have been few studies that attempt to use big data mining in real-world pharmacovigilance to monitor drug-induced QT interval prolongation. Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) is a spontaneous reporting system that gathers a large number of adverse drug event (ADE) reports. The purpose of this database is to assist the FDA's post-marketing safety surveillance program for pharmaceutical and therapeutic biologic products. It can also be used to display adverse event profiles from actual clinical settings. Utilizing the FAERS database, we conducted data mining on ADEs with drug-induced QT interval prolongation in order to provide prescribers with reference information to ensure drug safety, as well as to give valuable information for pharmacoepidemiology.
2 Methods
2.1 Data source and mining
We obtained the American standard code for information interchange (ASCII) data file in the FAERS database (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html) from 2004 to 2022. The ASCII data file gathered seven subfiles individually, including DEMOyyQq (demographic information), DRUGyyQq (drug information), REACyyQq (adverse event), OUTCyyQq (outcome information), RPSRyyQq (report source), THERyyQq (treatment data), and INDIyyQq (indication for drug).
SAS (9.4) was applied in our study for data mining. We chose PRIMARYID, CASEID, and FDA_DT in the DEMO table and sorted them in the order of CASEID, FDA_DT, and PRIMARYID in accordance with the FDA's method for removing duplicate reports. We maintained the maximum value of FDA_DT for reports that had the same CASEID. Then, we reserved the report with the highest value of PRIMARYID for reports with the same CASEID and FDA_DT. The primary suspect drug (PS) was found by applying the preferred term (PT) “Electrocardiogram QT Prolonged” with the PT code 10014387 from the Medical Dictionary for Regulatory Activities (MedDRA) 26.0.
2.2 Discrimination of objective drugs
There was a lack of standardization in filling in the drug names in the FAERS database because of the wide range of reporting groups, including both healthcare professionals like physicians or pharmacists, and non-healthcare professionals like consumers and lawyers. For the same drug, we gathered all forms of drug names reported in FAERS, including generic name, brand name, drug code, active pharmaceutical ingredients, and their corresponding non-standard names in FAERS, in order to increase the data's accuracy. Then we categorized every drug using the World Health Organization's Anatomical Therapeutic Chemical (ATC) classification (https://www.whocc.no/atc_ddd_index/). Afterwards, we reviewed the package inserts of these drugs to see if there was an ADE for QT interval prolongation in their labels.
2.3 Disproportionality analysis
To find the ADE signal, we applied the reporting odds ratio (ROR) of the disproportionality method. This method compares the proportion of targeted events of the targeted drugs with the proportion of targeted events of all other drugs in order to mine potential risk signals of ADEs (15). The RORs for the drugs that caused QT interval prolongation were calculated by using a two-by-two contingency table, as shown in Supplementary Table S1. , and 95% confidence interval . When a ≥ 3 and the lower end of the 95% CI for the ROR value is higher than 1, the potential risk signal of ADE is satisfied. Respectively, we used the ASCII data file encompassing the time from the FDA approval time of each drug to the fourth quarter of 2022 in order to increase the accuracy of the computation for the ROR of each drug.
2.4 Time-to-onset analysis
Time-to-onset (TTO) was outlined as the period of time between the ADE occurrence date (EVENT_DT in the DEMO file) and the beginning date of drug use (START_DT in the THER file). In the meantime, we removed any inaccurate date inputs, missing specific data, or input errors (EVENT_DT comes before START_DT). Additionally, the TTO was assessed by using the medians, interquartile ranges (IQR), and Weibull's shape parameter (WSP) (16). The shape of the Weibull distribution was described by two parameters: scale (α) and shape (β). Early failure types are characterized by an ADE hazard that decreases with time (β <1% and 95% CI <1); random failure types are characterized by an ADE hazard that occurs continuously over time (β is equal to or close to 1 and its 95% CI contains the value 1); and wearout failure types are characterized by an increase in ADE hazard with time (β >1% and 95% CI >1) (17).
3 Results
3.1 Descriptive analysis
Following data mining, we discovered 1,579 different drugs responsible for 28,581 patients associated with “electrocardiogram QT prolongation”. The top 40 drugs-induced QT interval prolongation cumulatively involved 14,707 patients. The process of data mining is shown in Figure 1. Among 14,707 patients, there were 6,723 patients aged 18–65 years (45.71%). Patients with missing gender (n = 3,482, 23.68%) aside, there were more female patients (n = 7,536, 51.24%) than male patients (n = 5,158, 35.07%). In total, 3,721 patients (25.30%) experienced death or life-threatening clinical outcomes. Additionally, the United States reported the most number of cases (n = 4,646) out of all the reporting nations. When compared to non-healthcare professionals, healthcare professionals reported more cases. There has been an upward trend in the reporting of ADEs for QT interval prolongation since 2004, which should be considered of sufficient concern. More details on patients' characteristics and reporting information for the top 40 drugs are shown in Figure 2 and Supplementary Table S2.
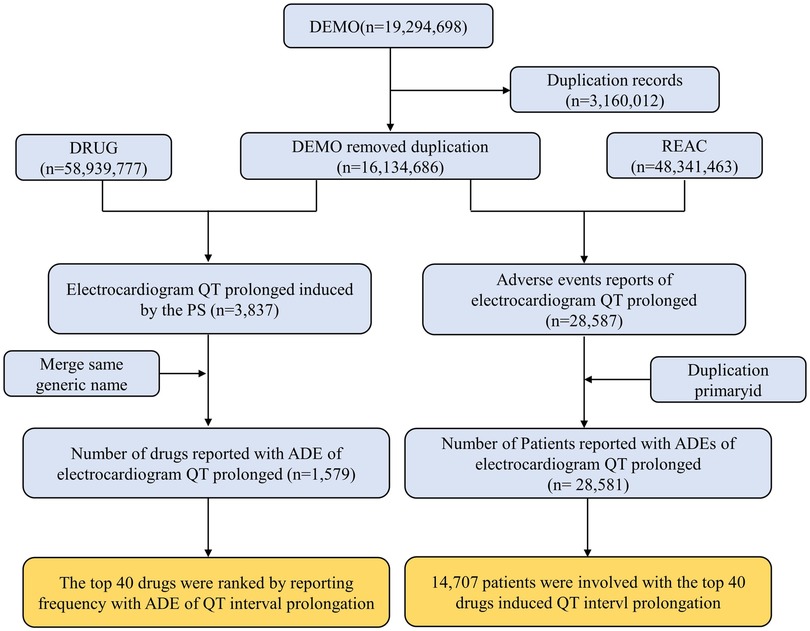
Figure 1. Flow chart for identification cases of QT interval prolongation in our study. PS, primary suspect drug; ADE, adverse drug event.
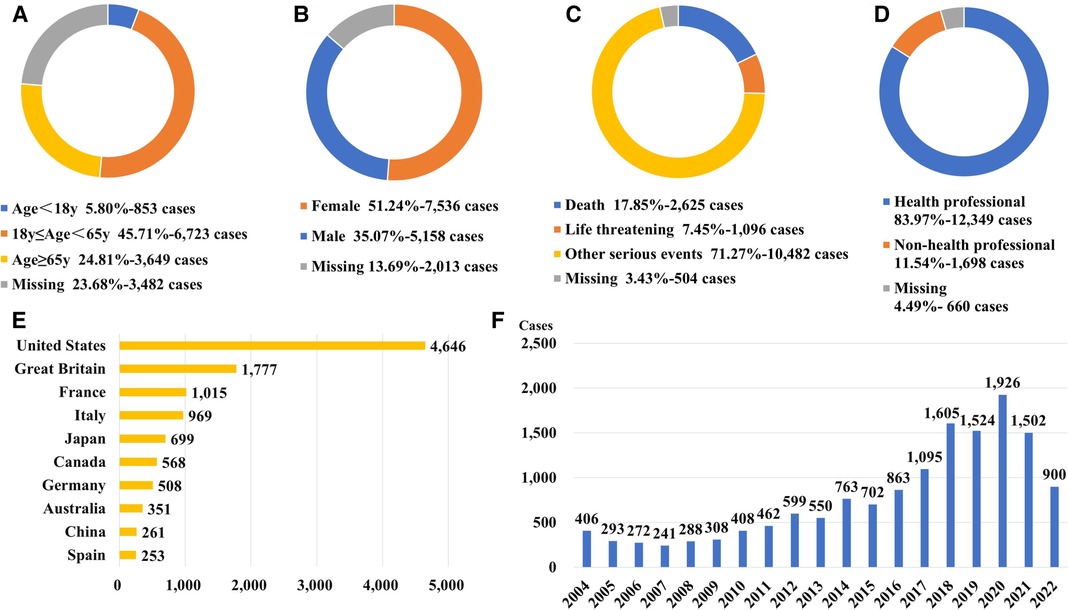
Figure 2. Patients’ characteristics and reporting information for the top 40 drugs with ADE of QT interval prolongation. (A) Distribution of patients’ age (B) Distribution of patients’ gender. (C) Distribution of patients’ outcome (D) Distribution of the reporters (E) Top 10 countries with the most sources of reports. (F) Distribution of the reporting year.
3.2 Culprit-drug list induced QT interval prolongation
The top 40 drugs (Table 1) by overall frequency of presence, with cases reported ranging from 155 to 1,151 and RORs ranging from 1.19 to 312.67, were taken into further analysis. The top 3 drugs with the highest number of reported cases were quetiapine (1,151 cases), olanzapine (754 cases), and citalopram (720 cases), and the 3 drugs with the highest ROR values were cisapride (ROR = 312.67, 95% CI: 281.35–347.47), bedaquiline (ROR = 125.94, 95% CI: 109.23–145.21), and dofetilide (ROR = 37.53, 95% CI: 33.79–41.70). According to the first level of the ATC classification, we divided the 40 drugs into categories. As shown in Figure 3, the most common drugs causing QT interval prolongation in the FAERS database were the drugs for the nervous system (ATC N, n = 19, 47.50%) and antiinfectives for systemic use (ATC J, n = 7, 17.50%). When we further classified the drugs for the nervous system at the third level of the ATC classification, we discovered that antidepressants (ATC N06A, n = 9, 47.37%) and antipsychotics (ATC N05A, n = 7, 36.84%) were the two primary drug classes.
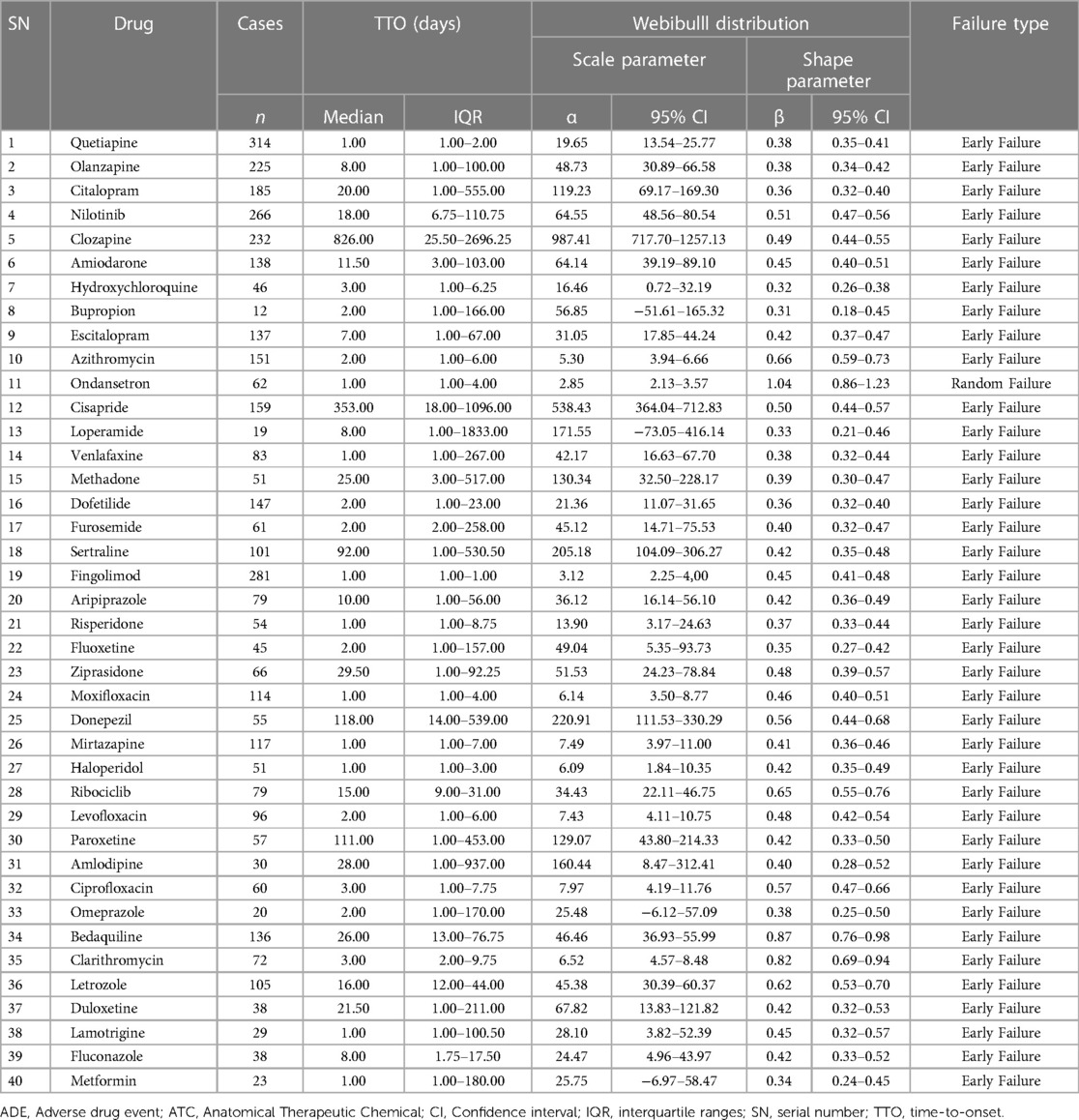
3.3 Time-to-onset analysis
In order to guarantee the accuracy of the TTO analysis, we excluded any data that was incorrect or missing. As a result, fewer cases were included for further analysis than were actually reported. With the exception of ondansetron, all shape parameters' 95% CI upper limits were smaller than 1, indicating that these medications have early failure types, according to the assessment of the WSP analysis. Due to its shape parameter of 1.04 (95% CI: 0.86–1.23), ondansetron had a random failure type. In Table 2, the results of TTO and WSP analyses for the top 40 drugs linked to reports of QT interval prolongation are presented.
4 Discussion
The standardized code of ADE terms is the key to identifying signals of drug safety in millions of ADEs. MedDRA is a clinically validated international medical terminology used to classify ADEs. As an important signal recognition tool, it has been increasingly used for drug safety analysis. The PT names associated with QT interval prolongation recorded in the FAERS are “electrocardiogram QT prolonged” and “long QT syndrome”. Long QT syndrome has been classified as being either congenital or acquired (18). However, congenital QT prolongation is caused by mendelian genetic disorders (19), and it is not associated with our current study. In order to improve the accuracy of our study, we only used the PT name “electrocardiogram QT prolonged” when we assessed the drug-induced prolongation of the QT interval in the FAERS database.
In our study, we found that drug-induced QT prolongation occurred in a higher percentage of females than males, which was consistent with previous studies (20, 21). The 20 ms longer baseline QTc intervals in women compared to men had been considered to be linked to the increased risks in women (22). It was reported that testosterone accelerates ventricular repolarization (23). Furthermore, the expression of several repolarizing ion-channels, including human ether-a-go-go-related gene (hERG), was found to be decreased in female hearts than in male hearts (24). Multiple factors make women more likely to be induced by drugs to prolong the QT interval. Rosen et al. (12) reported that age above 65 was a high-risk factor for QT interval prolongation. In contrast to previous studies, our study found that the proportion of QT interval prolongation in the 18–64 age group was higher than in the over-65 age group. This may be closely associated with the fact that the top 40 drugs for QT interval prolongation reported in the FAERS database included a higher percentage of patients (26.59%) who were 18–65 years old and took antidepressants and antipsychotics. Based on epidemiological investigations, mental disease tends to start at a younger age (25, 26).
Over the past decade, the effects on the QT interval and the associated risk of heart rhythm disorders of antidepressants had been raising concerns, while the risks of QT interval prolongation varied with different antidepressants. Among the most common antidepressants that caused QT interval prolongation in our study, citalopram (ROR = 13.63, 95% CI: 12.65–14.68) and escitalopram (ROR = 11.36, 95% CI: 10.38–12.44) had a higher risk of QT interval prolongation, and venlafaxine (ROR = 3.80, 95% CI: 3.44–4.20), sertraline (ROR = 3.45, 95% CI: 3.10–3.44), paroxetine (ROR = 2.40, 95% CI: 2.11–2.73), and duloxetine (ROR = 1.19, 95% CI: 1.03–1.38) had a relatively low risk of QT interval prolongation, similar to the findings of Funk et al. (27) and Jasiak et al. (28). Consequently, it is critical to appropriately evaluate the risk of medicine when treating depression, and we advise selecting antidepressants with a reduced risk for individuals who are at a high risk of developing QT interval prolongation.
In clinical practice, reports of QT interval prolongation caused by antipsychotics are more frequent. Currently, except for lurasidone, cariprazine, and brexpiprazole, most antipsychotic drugs can cause QT interval prolongation at therapeutic doses or in overdose by blocking the kalium current (IKr) ion channels (29). Ziprasidone, quetiapine, and risperidone showed the most significant inhibitory effects on IKr (30). Additionally, the cytochrome P450 (CYP) enzyme is responsible for the majority of antipsychotic drug metabolism, so combining them with other medications may increase the risk of QT interval prolongation. In our study, quetiapine (1,151 cases), olanzapine (754 cases), and clozapine (609 cases) were three of the top five drugs causing QT interval prolongation. These drugs belong to the second generation of antipsychotics, which gained popularity because they caused fewer side effects than those from the first generation. Since they were often utilized in clinics, more patients were observed to experience QT interval prolongation as a result.
Systemic anti-infective drugs, of which macrolides and fluoroquinolones were the most common, were another class of drugs that frequently resulted in QT interval prolongation in our study. The mechanism for the QT interval prolongation induced by macrolides is the inhibition of rectification of IKr ion channels and the suppression of auto-metabolism by CYP3A4 enzyme (31, 32). Fluoroquinolones, including ciprofloxacin, levofloxacin, and moxifloxacin, have been reported to cause QT interval prolongation and TdP (33–36). In addition, azole antifungal drugs are mostly potent inhibitors of the CYP3A4 enzyme, and when combined, they can increase the concentration of other drugs that prolong the QT interval. We are unable to avoid utilizing macrolides, fluoroquinolones, and azole antifungals since they are essential in the treatment of infectious illnesses. Therefore, we must be alert to the potential side effect of QT interval prolongation when using these drugs and avoid concomitantly using drugs that may interact with them.
In addition, our study discovered that the adverse effect of QT interval prolongation was not mentioned in the drug package inserts for seven medications, including bupropion, furosemide, amlodipine, omeprazole, letrozole, lamotrigine, and metformin. For these drugs, QT interval prolongation is their new risk signal for ADE. A list of drugs from CredibleMeds (available at www.crediblemeds.org) that increase the risk of QT interval prolongation and TdP includes furosemide and omeprazole in the conditional risk of TdP. This shows that omeprazole and furosemide might prolong the QT interval in specific situations, which may result in TdP. It is worth noting that on March 31, 2021, FDA released information that lamotrigine may increase the risk of arrhythmia in patients with heart disease. However, studies found that lamotrigine didn't prolong the QT interval in healthy subjects (37). Therefore, we need to pay attention to the risk of QT interval prolongation caused by lamotrigine in patients with heart disease in clinical practice. Previous studies had suggested that bupropion, which blocked IKr ion channels, can cause QT interval prolongation when used in excess (38, 39). According to an animal study, administering therapeutic doses of metformin for a lengthy period of time (7 weeks) could prolong the duration of QTc by delay cardiac repolarization (40). There were no studies on the QT interval prolongaton brought on by letrozole or amlodipine. Using data mining techniques, the current study rapidly identified cases of drug-induced QT interval prolongation and used ROR to quantify their risk signals. Larger studies may still be required to further confirm the ADE of QT interval prolongation for medications such as letrozole, amlodipine, metformin, bupropion, and others that are not indicated for QT interval prolongation in their inserts.
Among the top 40 drugs, cisapride which was removed from the market by FDA, had the highest ROR of 312.67 (95% CI: 281.35–347.47). It prolonged the QT interval and causes serious arrhythmias by blocking potassium ion channels regulated by hERG. It is known to all that the hERG has drawn a lot of interest over the past few decades. The estimated hERG blockade and the increase in QT interval for 5 Ikr-blocking medications (haloperidol, olanzapine, risperidone, thioridazine, and ziprasidone) were found to be significantly correlated in a study involving 14 antipsychotics (41). Furthermore, genetic variations in CYP and P-glycoprotein, may impact an individual's susceptibility of drug-induced QT interval prolongation. Methadone users who had weak CYP2B6 metabolizers had been found to have an increased risk of QT interval prolongation (42). A higher risk of QT interval prolongation caused by antimicrobial drugs, with the exception of quinolones, was associated with variations of CYP3A4 and CYP2C9 (43). Variants in the CYP2D6 gene may impact exposure and result in the development of AEs associated with aripiprazole, haloperidol, and risperidone, whereas variations in the CYP3A4 gene may impact quetiapine (44). Compared to homozygous C allele carriers, risperidone users with a T allele of the C3435T polymorphism had a significantly longer QT interval (45). Therefore, it's critical to understand specific genetic backgrounds in order to lower the incidence drug-induced QT interval prolongation.
According to reports, the Weibull parameter can be utilized to forecast the period before an ADE happens and can offer helpful information for patient pharmacological management in clinical practice (16). Even though we meticulously adhered to the FDA's suggested TTO calculation, we discovered some seemingly aberrant TTO information that calls for caution, such as high median TTO values for cisapride and clozapine. Overall, most drugs that caused QT interval prolongation had early failure types, indicating that the majority of ADEs related to drug-induced QT interval prolongation occurred early in the prescribing period and then decreased over time. Early in the course of treatment, the emergence of QT interval prolongation should be closely monitored. Once QT interval prolongation is identified in a patient, the drug regimen can be changed or supportive measures can be implemented to help manage symptoms and prevent the onset of serious adverse events.
Mild QT interval prolongation is frequently asymptomatic, making it difficult for patients and physicians to recognize at first. However, when a QT interval is longer than 500 ms, there is an increased risk of cardiac events (46). Since our study, which was based on the FAERS database, was unable to gather information on whether or not patients had clinical symptoms when QT interval prolongation occurs, we simply went on to discuss the clinical outcomes which may cause by QT interval prolongation. According to the present study, 25.30% of the patients died or experienced life-threatening conditions, suggesting that the extent to which drug-induced QT interval prolongation jeopardizes patients should be of great concern. The greatest percentage of deaths or life-threatening conditions occurred in patients using metformin, which may have anything to do with the individuals' own diabetes. This was due to research suggesting that those with diabetes had a higher risk of sudden cardiac death compared to those without the disease (47).
5 Limitations
The real-world data mining strategy we used in our study based on the FAERS database has some advantages, several restrictions must be taken into account. These restrictions are as follows:
Firstly, FAERS is an open ADE reporting system that is widely used by the population, so it is difficult to avoid the situation of inaccurate, incomplete, and delayed reporting. Since voluntary reporting isn't restricted to healthcare professionals, ADEs may be reported voluntarily by non-healthcare professionals as well. Unfortunately, non-healthcare professionals are not very knowledgeable about medicine and are not likely to draw the right judgments. These could result in inevitable bias. Secondly, reports of patients using more than one prescription raise the possibility of erroneous correlations between target drugs and target events. The utilization of disproportionality analysis to establish a definitive causal relationship between target drugs and target events was limited, as it yielded only statistical associations. These can lead to possible false positive signals in the results of the study. However, these are intrinsic limitations of spontaneous reporting databases for pharmacovigilance studies. Thirdly, the FAERS database only contains cases of ADEs, but the total number of patients taking medication is unknown. Therefore, the incidence of drug-induced QT interval prolongation was not available. Furthermore, the number of reported cases of QT interval prolongation depends on both the tendency to prolong the QT interval and the number of patients treated. Consequently, the study's findings only provide a general overview of QT interval prolongation occurrences that have been recorded in the FAERS database. Fourthly, previous research indicates that the QT interval is significantly higher in the early morning compared to the evening (48), and that the dosage of the drug has an impact on this as well (39). Owing to the restricted data contained in the FAERS database, we were unable to conduct more in-depth analysis of these factors that influence the QT interval. So we need to be cautious in the interpretation of data mining results of our study and make comprehensive judgments in combination with evidence-based medical evidence.
6 Conclusion
Our study offered a list of drugs that frequently caused QT interval prolongation based on the FAERS system, along with a description of some risk profiles for QT interval prolongation brought on by these drugs. When prescribing these drugs in clinical practice, we should closely monitor the occurrence of ADE for QT interval prolongation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Ethical statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
HT: Writing – original draft, Writing – review & editing, Conceptualization, Methodology. XY: Data curation, Writing – review & editing. YC: Data curation, Writing – review & editing. GH: Data curation, Writing – review & editing. LL: Formal Analysis, Writing – review & editing. WL: Formal Analysis, Writing – review & editing. WL: Formal Analysis, Writing – review & editing. CC: Formal Analysis, Writing – review & editing. XX: Writing – original draft, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by the programs of key discipline within the Third Affiliated Hospital of Chongqing Medical University, China (No. Pharmacy ZK202002).
Acknowledgments
The FAERS database, which was made available by the FDA, was used to conduct this study. The FDA does not have any opinion about the data, findings, or interpretation of the current study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1363382/full#supplementary-material
References
1. Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics. (2013) 54(1):1–13. doi: 10.1016/j.psym.2012.11.001
2. Xiong GL, Pinkhasov A, Mangal JP, Huang H, Rado J, Gagliardi J, et al. QTc monitoring in adults with medical and psychiatric comorbidities: expert consensus from the association of medicine and psychiatry. J Psychosom Res. (2020) 135:110138. doi: 10.1016/j.jpsychores.2020.110138
3. Davies RA, Ladouceur VB, Green MS, Joza J, Juurlink DN, Krahn AD, et al. The 2023 Canadian cardiovascular society clinical practice update on management of the patient with a prolonged QT interval. Can J Cardiol. (2023) 39(10):1285–301. doi: 10.1016/j.cjca.2023.06.011
4. Ponte ML, Keller GA, Di Girolamo G. Mechanisms of drug induced QT interval prolongation. Curr Drug Saf. (2010) 5(1):44–53. doi: 10.2174/157488610789869247
5. Locati ET, Bagliani G, Padeletti L. Normal ventricular repolarization and QT interval: ionic background, modifiers, and measurements. Card Electrophysiol Clin. (2017) 9(3):487–513. doi: 10.1016/j.ccep.2017.05.007
6. Al-Khatib SM, LaPointe NM, Kramer JM, Califf RM. What clinicians should know about the QT interval [published correction appears in JAMA. 2003 Sep 10;290(10):1318]. JAMA. (2003) 289(16):2120–7. doi: 10.1001/jama.289.16.2120
7. Brown DW, Giles WH, Greenlund KJ, Valdez R, Croft JB. Impaired fasting glucose, diabetes mellitus, and cardiovascular disease risk factors are associated with prolonged QTc duration. Results from the third national health and nutrition examination survey. J Cardiovasc Risk. (2001) 8(4):227–33. doi: 10.1177/174182670100800407
8. Passino C, Franzoni F, Gabutti A, Poletti R, Galetta F, Emdin M. Abnormal ventricular repolarization in hypertensive patients: role of sympatho-vagal imbalance and left ventricular hypertrophy. Int J Cardiol. (2004) 97(1):57–62. doi: 10.1016/j.ijcard.2003.07.029
9. Tang JKK, Rabkin SW. Hypocalcemia-induced QT interval prolongation. Cardiology. (2022) 147(2):191–5. doi: 10.1159/000515985
10. Heemskerk CPM, Pereboom M, van Stralen K, Berger FA, van den Bemt PMLA, Kuijper AFM, et al. Risk factors for QTc interval prolongation. Eur J Clin Pharmacol. (2018) 74(2):183–91. doi: 10.1007/s00228-017-2381-5
11. Roden DM. A current understanding of drug-induced QT prolongation and its implications for anticancer therapy. Cardiovasc Res. (2019) 115(5):895–903. doi: 10.1093/cvr/cvz013
12. Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. (2004) 350(10):1013–22. doi: 10.1056/NEJMra032426
13. Schwartz PJ, Woosley RL. Predicting the unpredictable: drug-induced QT prolongation and torsades de pointes. J Am Coll Cardiol. (2016) 67(13):1639–50. doi: 10.1016/j.jacc.2015.12.063
14. Khatib R, Sabir FRN, Omari C, Pepper C, Tayebjee MH. Managing drug-induced QT prolongation in clinical practice. Postgrad Med J. (2021) 97(1149):452–8. doi: 10.1136/postgradmedj-2020-138661
15. Huang L, Guo T, Zalkikar JN, Tiwari RC. A review of statistical methods for safety surveillance. Ther Innov Regul Sci. (2014) 48(1):98–108. doi: 10.1177/2168479013514236
16. Mazhar F, Battini V, Gringeri M, Pozzi M, Mosini G, Marran AMN, et al. The impact of anti-TNFα agents on weight-related changes: new insights from a real-world pharmacovigilance study using the FDA adverse event reporting system (FAERS) database. Expert Opin Biol Ther. (2021) 21(9):1281–90. doi: 10.1080/14712598.2021.1948529
17. Nakamura M, Umetsu R, Abe J, Matsui T, Ueda N, Kato Y, et al. Analysis of the time-to-onset of osteonecrosis of jaw with bisphosphonate treatment using the data from a spontaneous reporting system of adverse drug events. J Pharm Health Care Sci. (2015) 1:34. doi: 10.1186/s40780-015-0035-2
18. Mahida S, Hogarth AJ, Cowan C, Tayebjee MH, Graham LN, Pepper CB. Genetics of congenital and drug-induced long QT syndromes: current evidence and future research perspectives. J Interv Card Electrophysiol. (2013) 37(1):9–19. doi: 10.1007/s10840-013-9779-5
19. Niemeijer MN, van den Berg ME, Eijgelsheim M, Rijnbeek PR, Stricker BH. Pharmacogenetics of drug-induced QT interval prolongation: an update. Drug Saf. (2015) 38(10):855–67. doi: 10.1007/s40264-015-0316-6
20. Uchikawa M, Hashiguchi M, Shiga T. Drug-Induced QT prolongation and torsade de pointes in spontaneous adverse event reporting: a retrospective analysis using the Japanese adverse drug event report database (2004–2021). Drugs Real World Outcomes. (2022) 9(4):551–9. doi: 10.1007/s40801-022-00328-0
21. Sarganas G, Garbe E, Klimpel A, Hering RC, Bronder E, Haverkamp W. Epidemiology of symptomatic drug-induced long QT syndrome and torsade de pointes in Germany. Europace. (2014) 16(1):101–8. doi: 10.1093/europace/eut214
22. Kurokawa J, Kodama M, Clancy CE, Furukawa T. Sex hormonal regulation of cardiac ion channels in drug-induced QT syndromes. Pharmacol Ther. (2016) 168:23–8. doi: 10.1016/j.pharmthera.2016.09.004
23. Charbit B, Christin-Maître S, Démolis JL, Soustre E, Young J, Funck-Brentano C. Effects of testosterone on ventricular repolarization in hypogonadic men. Am J Cardiol. (2009) 103(6):887–90. doi: 10.1016/j.amjcard.2008.11.041
24. Gaborit N, Varro A, Le Bouter S, Szuts V, Escande D, Nattel S, et al. Gender-related differences in ion-channel and transporter subunit expression in non-diseased human hearts. J Mol Cell Cardiol. (2010) 49(4):639–46. doi: 10.1016/j.yjmcc.2010.06.005
25. Herrman H, Patel V, Kieling C, Berk M, Buchweitz C, Cuijpers P, et al. Time for united action on depression: a lancet-world psychiatric association commission. Lancet. (2022) 399(10328):957–1022. doi: 10.1016/S0140-6736(21)02141-3
26. McGrath JJ, Al-Hamzawi A, Alonso J, Altwaijri Y, Andrade LH, Bromet EJ, et al. Age of onset and cumulative risk of mental disorders: a cross-national analysis of population surveys from 29 countries. Lancet Psychiatry. (2023) 10(9):668–81. doi: 10.1016/S2215-0366(23)00193-1
27. Funk KA, Bostwick JR. A comparison of the risk of QT prolongation among SSRIs. Ann Pharmacother. (2013) 47(10):1330–41. doi: 10.1177/1060028013501994
28. Jasiak NM, Bostwick JR. Risk of QT/QTc prolongation among newer non-SSRI antidepressants. Ann Pharmacother. (2014) 48(12):1620–8. doi: 10.1177/1060028014550645
29. Lambiase PD, de Bono JP, Schilling RJ, Lowe M, Turley A, Slade A, et al. British Heart rhythm society clinical practice guidelines on the management of patients developing QT prolongation on antipsychotic medication. Arrhythm Electrophysiol Rev. (2019) 8(3):161–5. doi: 10.15420/aer.2019.8.3.G1
30. Beach SR, Celano CM, Sugrue AM, Adams C, Ackerman MJ, Noseworthy PA, et al. QT Prolongation, torsades de pointes, and psychotropic medications: a 5-year update. Psychosomatics. (2018) 59(2):105–22. doi: 10.1016/j.psym.2017.10.009
31. Ray WA, Murray KT, Meredith S, Narasimhulu SS, Hall K, Stein CM. Oral erythromycin and the risk of sudden death from cardiac causes. N Engl J Med. (2004) 351(11):1089–96. doi: 10.1056/NEJMoa040582
32. Stanat SJ, Carlton CG, Crumb WJ Jr, Agrawal KC, Clarkson CW. Characterization of the inhibitory effects of erythromycin and clarithromycin on the HERG potassium channel. Mol Cell Biochem. (2003) 254(1–2):1–7. doi: 10.1023/a:1027309703313
33. Haring B, Bauer W. Ciprofloxacin and the risk for cardiac arrhythmias: culprit delicti or watching bystander? Acta Cardiol. (2012) 67(3):351–4. doi: 10.1080/ac.67.3.2160727
34. Briasoulis A, Agarwal V, Pierce WJ. QT Prolongation and torsade de pointes induced by fluoroquinolones: infrequent side effects from commonly used medications. Cardiology. (2011) 120(2):103–10. doi: 10.1159/000334441
35. Khan F, Ismail M, Khan Q, Ali Z. Moxifloxacin-induced QT interval prolongation and torsades de pointes: a narrative review. Expert Opin Drug Saf. (2018) 17(10):1029–39. doi: 10.1080/14740338.2018.1520837
36. Falagas ME, Rafailidis PI, Rosmarakis ES. Arrhythmias associated with fluoroquinolone therapy. Int J Antimicrob Agents. (2007) 29(4):374–9. doi: 10.1016/j.ijantimicag.2006.11.011
37. Dixon R, Job S, Oliver R, Tompson D, Wright JG, Maltby K, et al. Lamotrigine does not prolong QTc in a thorough QT/QTc study in healthy subjects. Br J Clin Pharmacol. (2008) 66(3):396–404. doi: 10.1111/j.1365-2125.2008.03250.x
38. Caillier B, Pilote S, Castonguay A, Patoine D, Ménard-Desrosiers V, Vigneault P, et al. QRS widening and QT prolongation under bupropion: a unique cardiac electrophysiological profile. Fundam Clin Pharmacol. (2012) 26(5):599–608. doi: 10.1111/j.1472-8206.2011.00953.x
39. Campleman SL, Brent J, Pizon AF, Shulman J, Wax P, Manini AF. Drug-specific risk of severe QT prolongation following acute drug overdose. Clin Toxicol (Phila). (2020) 58(12):1326–34. doi: 10.1080/15563650.2020.1746330
40. Malagueta-Vieira L, Fernández-Ruocco J, Hortigón-Vinagre MP, Zamora V, Zayas-Arrabal J, Echeazarra L, et al. Metformin reduces potassium currents and prolongs repolarization in non-diabetic heart. Int J Mol Sci. (2022) 23(11):6021. doi: 10.3390/ijms23116021
41. Crumb WJ Jr, Ekins S, Sarazan RD, Wikel JH, Wrighton SA, Carlson C, et al. Effects of antipsychotic drugs on I(to), I (Na), I (sus), I (K1), and hERG: qT prolongation, structure activity relationship, and network analysis. Pharm Res. (2006) 23(6):1133–43. doi: 10.1007/s11095-006-0070-7
42. Eap CB, Crettol S, Rougier JS, Schläpfer J, Sintra Grilo L, Déglon JJ, et al. Stereoselective block of hERG channel by (S)-methadone and QT interval prolongation in CYP2B6 slow metabolizers. Clin Pharmacol Ther. (2007) 81(5):719–28. doi: 10.1038/sj.clpt.6100120
43. Owens RC Jr. QT prolongation with antimicrobial agents: understanding the significance. Drugs. (2004) 64(10):1091–124. doi: 10.2165/00003495-200464100-00005
44. Hulshof EC, Deenen MJ, Nijenhuis M, Soree B, de Boer-Veger NJ, Buunk AM, et al. Dutch pharmacogenetics working group (DPWG) guideline for the gene-drug interaction between UGT1A1 and irinotecan. Eur J Hum Genet. (2023) 31(9):1088–9. doi: 10.1038/s41431-023-01315-x
45. Suzuki Y, Tsuneyama N, Fukui N, Sugai T, Watanabe J, Ono S, et al. Effect of risperidone metabolism and P-glycoprotein gene polymorphism on QT interval in patients with schizophrenia. Pharmacogenomics J. (2014) 14(5):452–6. doi: 10.1038/tpj.2014.6
46. Li M, Ramos LG. Drug-induced QT prolongation and torsades de pointes. P T. (2017) 42(7):473–7. 33935476.28674475
47. Svane J, Pedersen-Bjergaard U, Tfelt-Hansen J. Diabetes and the risk of sudden cardiac death. Curr Cardiol Rep. (2020) 22(10):112. doi: 10.1007/s11886-020-01366-2
Keywords: QT interval prolongation, pharmacovigilance, FAERS, data mining, reporting odds ratio
Citation: Tan H, Yan X, Chen Y, Huang G, Luo L, Li W, Lan W, Chen C and Xi X (2024) A real-world pharmacovigilance study of drug-induced QT interval prolongation: analysis of spontaneous reports submitted to FAERS. Front. Cardiovasc. Med. 11:1363382. doi: 10.3389/fcvm.2024.1363382
Received: 30 December 2023; Accepted: 29 April 2024;
Published: 13 May 2024.
Edited by:
Xiaofeng Yang, Temple University, United StatesReviewed by:
Emanuela Teresina Locati, IRCCS San Donato Polyclinic, ItalyLei Zhao, Cincinnati Children's Hospital Medical Center, United States
© 2024 Tan, Yan, Chen, Huang, Luo, Li, Lan, Chen and Xi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Xi, NjUwMTAxQGhvc3BpdGFsLmNxbXUuZWR1LmNu
 Haowen Tan
Haowen Tan Xida Yan
Xida Yan Ying Chen2
Ying Chen2 Guili Huang
Guili Huang Wenjun Li
Wenjun Li Xin Xi
Xin Xi