
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cardiovasc. Med., 06 February 2024
Sec. General Cardiovascular Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1352675
This article is part of the Research TopicInsights in Cardiology from Caring for a Diverse Community: Perspectives from Inova Schar Heart and VascularView all 22 articles
Cardiovascular disease (CVD) is the leading cause of death worldwide and accounts for roughly 1 in 5 deaths in the United States. Women in particular face significant disparities in their cardiovascular care when compared to men, both in the diagnosis and treatment of CVD. Sex differences exist in the prevalence and effect of cardiovascular risk factors. For example, women with history of traditional cardiovascular risk factors including hypertension, tobacco use, and diabetes carry a higher risk of major cardiovascular events and mortality when compared to men. These discrepancies in terms of the relative risk of CVD when traditional risk factors are present appear to explain some, but not all, of the observed differences among men and women. Sex-specific cardiovascular disease research—from identification, risk stratification, and treatment—has received increasing recognition in recent years, highlighting the current underestimated association between CVD and a woman's obstetric and reproductive history. In this comprehensive review, sex-specific risk factors unique to women including adverse pregnancy outcomes (APO), such as hypertensive disorders of pregnancy (HDP), gestational diabetes mellitus, preterm delivery, and newborn size for gestational age, as well as premature menarche, menopause and vasomotor symptoms, polycystic ovarian syndrome (PCOS), and infertility will be discussed in full detail and their association with CVD risk. Additional entities including spontaneous coronary artery dissection (SCAD), coronary microvascular disease (CMD), systemic autoimmune disorders, and mental and behavioral health will also be discussed in terms of their prevalence among women and their association with CVD. In this comprehensive review, we will also provide clinicians with a guide to address current knowledge gaps including implementation of a sex-specific patient questionnaire to allow for appropriate risk assessment, stratification, and prevention of CVD in women.
Cardiovascular disease (CVD) is the leading cause of death in the United States among both men and women. Women in particular face significant disparities in their cardiovascular care when compared to men, both in the diagnosis and treatment of CVD (1–5). Even when traditional risk factors for CVD are present, clinicians are more likely to attribute a lower perceived risk in women leading to worse outcomes (1, 3, 5). For example, hypertension is more prevalent among women and carries a two-fold higher mortality risk compared to men (1, 6–9). Women with diabetes carry an excess risk of ischemic heart disease (IHD), and future risk of CVD by 3–7 fold vs. 2–3 fold compared to men (1, 10–14). Likewise, a recent meta-analysis demonstrated that tobacco use confers a 25% increased relative risk of major cardiovascular events in women when compared to men (1, 15). These discrepancies in the relative risk of CVD when conventional risk factors are present appear to explain some, but not all, of the observed differences among men and women.
Sex-specific risk factors and its association with CVD risk have become a highly researched field, stressing the importance of obtaining a thorough obstetric and reproductive history for cardiac risk stratification (1, 3, 5, 6, 8, 11, 16–18). Sex-specific risk factors including adverse pregnancy outcomes (e.g., hypertensive disorders of pregnancy, gestational diabetes, fetal growth restriction, preterm delivery, and placental abruption), premature menarche, premature menopause and vasomotor symptoms, polycystic ovarian syndrome, autoimmune disorders, infertility, and depression are all associated with increased future CVD risk. In fact, the American Heart Association (AHA)/American College of Cardiology (ACC) multi-society cholesterol guideline in 2018 and the AHA/ACC guideline on the primary prevention of CVD in 2019 identified “risk-enhancing factors” specific to women that are associated with increased incident atherosclerotic CVD risk (3). In this comprehensive review, we will cover each of these sex-specific risk factors in detail and their association with future CVD risk, heart failure (HF), and stroke. Additional entities including spontaneous coronary artery dissection (SCAD), coronary microvascular disease (CMD), systemic autoimmune disorders, and mental and behavioral health will be discussed in regards to their association with CVD. Additionally, we will provide strategies clinicians can utilize to incorporate a strong obstetric and reproductive history to better risk stratify for sex-specific CVD risk and directions for future research. Please note that we recognize patients have diverse gender identities and strive to use gender-inclusive language. In some instances throughout this review, we use the word “woman” (and the pronouns “she” and her”) to describe patients or individuals whose sex assigned at birth was female, whether they identify as female, male, or non-binary. When describing or referencing study populations used in prior research, we use the gender terminology reported by the study investigators.
Our comprehensive review used a structured systematic approach that included a methodical literature search of systematic peer-reviewed articles. We extracted data from landmark research between 1997 and 2023 from databases including PubMed, MEDLINE, EMBASE, Scopus, and the Cochrane Library. Keywords used in the selection of articles included terms referring to sex-specific risk factors in cardiovascular disease, adverse pregnancy outcomes, and hypertensive disorders of pregnancy. Our study has several limitations. First, our search was bound to certain inclusion criteria and a specific search strategy, which could have led to the non-inclusion of all relevant articles. Likewise, our search was limited to articles published in English; thus, perhaps not all relevant articles have been included. In addition, selection bias may have also affected our review. Lastly, the included articles are of different methodological quality, ranging from case reports to meta-analyses.
Pregnancy leads to metabolic, physiologic, and vascular changes in a mother which include insulin resistance, adipose deposition, hypercoagulability, cardiac remodeling, and decreased vascular resistance (19). Despite these necessary maternal adaptations to support fetal growth and development, the physiological stress of pregnancy can also cause adverse pregnancy outcomes (APOs) (19–24). APOs are common and occur in 17%–20% of all pregnancies in the US (16, 25–27), and are a constellation of interrelated maternal and fetal complications caused by incomplete placentation, oxidative stress, and/or vascular dysfunction (1, 19). The term encompasses disorders which will be discussed in detail under subparagraphs 3.1.1–3.1.5.
Hypertensive disorders of pregnancy (HDP) are common complications during pregnancy and the early postpartum period. Pre-pregnancy chronic hypertension, gestational hypertension, preeclampsia, and eclampsia encompass the most common forms of HDP. Research across retrospective and prospective cohort studies have identified HDPs as a significant sex-specific risk factor for both short- and long-term maternal CVD (Table 1) (28–44). Women with history of HDP have significantly increased odds of chronic hypertension later in life (28, 29, 41, 42), stroke (30, 33, 34, 36, 39–41, 44), MI (44), and cardiomyopathy (44) versus women without history of HDP. Women with history of HDPs also have earlier-onset CVD and valvular heart disease including aortic stenosis and mitral regurgitation, suggesting an association between HDPs and accelerated cardiovascular aging (32, 84–86). Furthermore, women with HDPs are at highest risk for morbidity and mortality in the years following pregnancy compared to women without HDPs, including the development of cardiovascular risk factors such as hypertension, diabetes, and hyperlipidemia (32, 38, 84).
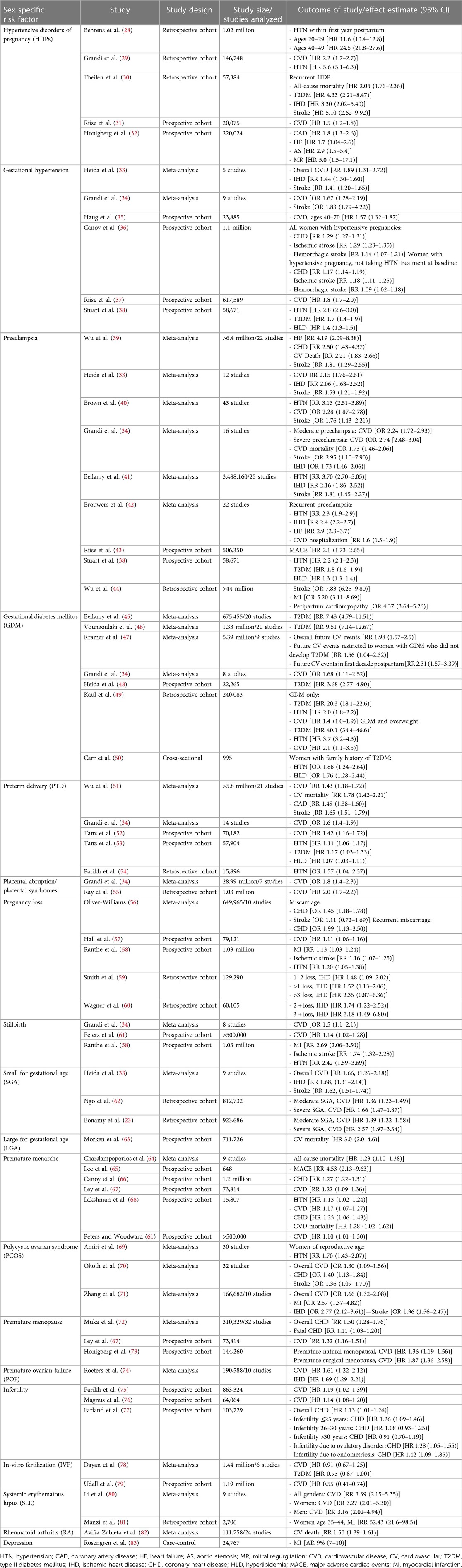
Table 1. A conglomerate of landmark studies describing statistically significant associations between various sex-specific risk factors and development of future cardiovascular risk factors, CVD, stroke, heart failure, and major adverse cardiac events.
Gestational hypertension is defined as pregnancy-induced hypertension (defined as SBP ≥ 140 mmHg or DBP ≥ 90 mmHg) after 20 weeks gestation without evidence of proteinuria or preeclampsia (3, 19). History of gestational hypertension has been consistently associated with increased CVD risk and increased odds of stroke across various studies (Table 1) (33–38).
Among the major types of HDPs, preeclampsia poses the greatest morbidity and mortality risk and affects 5%–10% of all pregnant women (16, 87–89). Preeclampsia is a condition in which preexisting or new-onset hypertension is complicated by proteinuria and/or other features of end-organ dysfunction after 20 weeks gestation (16). There is robust research to suggest that history of preeclampsia is independently associated with increased risk of CVD, IHD, stroke, and chronic hypertension later in life (Table 1) (33, 34, 38–44). For example, a meta-analysis by Wu et al. of 6.4 million women demonstrated a 4-fold increased risk of IHD and 2-fold increased risk of HF in women studied with preeclampsia compared to those without. Of note, women with recurrent preeclampsia compared to women with an isolated episode of preeclampsia are at significantly higher risk for future CVD (37, 84, 90), hypertension, and IHD (16, 30, 42). Despite the research demonstrating an independent association between preeclampsia and CVD, attempts to incorporate preeclampsia within risk scoring equations have led to only small improvements in discrimination and reclassification (91). This may be in part due to the population-based cohort studies including women well beyond their reproductive years rather than those of childbearing age (91, 92). Future studies should work to incorporate women closer to the target population intended for CVD screening and preventative intervention (6, 91).
Gestational diabetes mellitus (GDM) is a condition of impaired glucose tolerance during pregnancy that most commonly develops during the second and third trimester (16, 93). Paralleling the rise in prevalence of obesity, GDM has become increasingly prevalent, now estimated to affect 6%–9% of all pregnant women in the US (16, 94, 95). GDM results from inadequate response from pancreatic beta-cells to respond to the physiological and placental-mediated insulin resistance which occurs during pregnancy (84, 96). Several meta-analyses have shown that women with GDM are at increased risk of developing cardiovascular risk factors including type 2 diabetes mellitus (T2DM), hypertension, and hyperlipidemia leading to early-onset CVD, future cardiovascular events, and fatal IHD (Table 1) (34, 45–50). In fact, women with GDM have a 7- to 10-fold increased risk of developing T2DM (16, 45, 84) and nearly a 2-fold increased risk of developing hypertension and hyperlipidemia (Table 1) (16, 46, 49, 50, 97). This relative risk for future CVD remained statistically significant even after restricting the sensitivity analysis to women with GDM who did not subsequently develop T2DM (47). Proposed mechanisms to explain the association between GDM and early-onset CVD include epigenetics, elevated inflammatory markers including CRP and IL-6 associated with early atherosclerosis, and endothelial dysfunction leading to subsequent increased carotid artery thickness (16, 98). Some researchers suggest a dose-dependent relationship between the degree of glucose impairment during pregnancy with risk of subsequent CVD (84, 99). Nonetheless, documenting an obstetrical history of GDM in women is crucial given these associations with CVD which have been demonstrated consistently throughout studies (Table 1) (84, 99).
Spontaneous preterm delivery (sPTD), defined as a live birth before 37 weeks gestation, is a significant cause of neonatal mortality worldwide (16). Although our understanding of the underlying mechanism is limited, sPTD is associated with an increased development of cardiovascular risk factors and maternal CVD mortality (3, 52, 53, 84, 100, 101). For example, in the first decade after pregnancy, women with a history of sPTD are at increased risk of developing chronic hypertension, T2DM, hypercholesterolemia, and subclinical atherosclerosis (Table 1) (34, 51–53, 101). A meta-analysis by Wu et al. highlighted the association of sPTD with increased risk of future composite CVD, cardiovascular mortality, CAD, and stroke (Table 1) (51). Emerging research now suggests that the earlier sPTD occurs in pregnancy, the stronger its association with later development of hypertension and increased maternal CVD risk (3, 19, 52, 54, 84, 102).
Placental abruption is defined as the premature separation of a normally implanted placenta from the uterus before delivery most often occurring in the third trimester, and is strongly associated with cardiovascular risk factors and increased maternal CVD risk (3, 55, 84, 103). A meta-analysis by Grandi et al. demonstrated an increased risk of CVD in women with history of placental abruption (34), findings similarly documented in a large retrospective study by Ray et al., reporting a 1.7-fold risk of CVD in women with history of placental abruption or infarction (Table 1) (55). There is also a strong association of placental abruption with other concomitant APOs and cardiovascular risk factors such as higher BMI, hyperglycemia, and hyperlipidemia (84, 103).
Likewise, all forms of pregnancy loss (miscarriage, stillbirths, or combined) are associated with elevated risk of future cardiovascular risk factors and major cardiovascular events later in life (Table 1) (56–59, 61, 104). Recurrent pregnancy loss, defined as 3 or more losses, are associated with a particularly increased CVD risk (3). For example, a study by Wagner et al. demonstrated a higher risk of CVD for women who experienced two or three or more miscarriages as compared to those who did not experience miscarriage (Table 1) (60). Outcome data studying conventional CVD risk factors indicate that miscarriage is independently associated with future CVD and MI, highlighting its importance in obstetrical history for cardiovascular risk stratification in women (56, 59, 84).
The association between infant birth weight and future maternal CVD risk is well documented in current literature though studies are limited, thus warranting future investigation (Table 1) (23, 33, 62, 63). For example, in the Women’s Health Initiative, delivery of a small for gestational age (SGA) infant (defined as being ≤10th percentile in weight for their gestational age) was independently associated with increased maternal ASCVD risk after adjustment for conventional cardiovascular risk factors (19, 84, 105). A retrospective cohort study by Bonamy et al. observed similar findings, reporting a 3-fold maternal CVD risk in women with preterm or SGA infants even after accounting for pregnancy-related complications, socioeconomic factors, and tobacco use (23). This complex interplay between fetal growth restriction (FGR) and maternal CVD risk is hypothesized to be related to maternal vascular health (19). Many cases of FGR are thought to result from uteroplacental insufficiency due to poor implantation of the spiral arteries, or vascular insufficiency due to abnormal maternal uterine artery flow resulting in inadequate oxygen and nutrient supply to the fetus (19, 84). Thus, delivery of a SGA infant may unmask preexisting maternal vascular dysfunction which can result in a future increased predisposition for CVD including HF and stroke (19).
A need for further research is warranted in mothers who deliver infants large for gestational age (LGA), defined as an infant whose weight is ≥90th percentile for their gestational age, as emerging studies suggest that LGA delivery may be related to increased CVD risk—possibly mediated by its association with elevated BMI and diabetes (Table 1) (19, 63, 84, 106, 107).
Premature menarche, defined as menarche occurring before age 12, is strongly associated with an increased risk for developing future cardiovascular risk factors and CVD (3, 84). Though the mechanism linking early menarche to increased CVD risk is not entirely understood, it is postulated that given the strong association between childhood BMI and early menarche, premature menarche may reflect both genetic (e.g., elevated leptin levels associated with increased adiposity and higher BMI) and lifestyle risk factors (e.g., excess calorie consumption, lower birth weight, reduced physical activity) (84, 108, 109). One study estimated that premature menarche, independent of sociodemographic factors, is associated with a 15%–30% increased risk of future CVD (Table 1) (61, 64–66, 68). A meta-analysis by Charalampopoulos et al. reported a 3% reduction in the relative risk of all-cause mortality for every 1-year increase at menarche, and those women who experienced menarche at age <12 vs. ≥12 years were at an increased risk of all-cause mortality (Table 1) (64). The strong association intertwining premature menarche and increased CVD risk is likely due to women with history of early menarche being more susceptible to developing shared risk factors including hypertension, T2DM, hypercholesterolemia, and obesity later in life (3, 66, 68, 84).
Emerging data now suggest the relative risk for future CVD is elevated in both premature and delayed menarche, defined as menarche age ≥17 years, though further research is needed (1, 61, 65, 66, 84).
Polycystic ovarian syndrome (PCOS) is the most common cause of infertility in women and is often diagnosed in adolescence with key features including hyperandrogenism, ovulatory dysfunction, and polycystic kidneys on imaging (3, 84, 110). Women with PCOS are more likely to have traditional CVD risk factors including hypertension, insulin resistance, metabolic syndrome, elevated BMI, and dyslipidemia (1, 3, 69, 84, 110, 111). A meta-analysis by Zhang et al. demonstrated that the pooled risk of CVD events was higher in women with PCOS when compared to non-PCOS women, including increased risk of MI, IHD, and stroke (Table 1) (71). Likewise, a recent meta-analysis by Okoth et al. found that PCOS was associated with a 30% higher risk of overall CVD, including both in the risk of HF and stroke (Table 1) (70). These notable associations may be explained by the relationship between PCOS and carotid intima-media thickness (CIMT) and coronary artery calcium (CAC). Women with PCOS have greater CIMT and CAC even after adjusting for BMI when compared to non-PCOS women (3, 84, 112–115).
Premature menopause is commonly defined as the permanent cessation of menses before the age of 40 and is often attributed to premature ovarian failure (POF). POF, a condition characterized by hypergonadotropic hypogonadism, exhibits symptoms from hypoestrogenism including amenorrhea, hot flashes, and vaginal dryness. A shorter reproductive lifespan and an earlier age at menopause transition (MT) mediated by hypoestrogenism has been well-studied as an independent risk factor for CVD (3, 116). Estrogen assists in blood flow regulation and the relaxation of blood vessels, and in tandem with early loss of ovarian function can lead to long-term activation of the renin-angiotensin-aldosterone system, chronic inflammation, and vascular damage (3, 117). Hypoestrogenism also leads to dysfunction in cholesterol metabolism leading to atherosclerotic plaque formation and an elevated testosterone-to-estradiol ratio, factors which can increase subsequent risk of CVD and HF (3, 118).As such, a recent scientific statement by the AHA identified the MT as a particularly impactful period requiring an aggressive prevention-based approach for women to prevent accelerated CVD risk and future cardiovascular events (84, 119).
Vasomotor symptoms (VMS), including night sweats, hot flashes, and heat intolerance, are the hallmarks of the MT and can significantly impact quality of life (120–123). Emerging studies show evidence of an association between VMS with aortic calcification (124) and increased odds of elevated BMI, total cholesterol, and hypertension (125).
Premature menopause and POF have been consistently associated with greater maternal CVD and mortality risk across high-quality data studies cited in this review, as noted in Table 1 (67, 72–74, 84). For example, a meta-analysis by Muka et al. assessed the relationship between premature menopause and CVD among 190,588 women, demonstrating an increased risk of overall incident CVD and CVD mortality (72).
Women with a history of infertility, defined as the inability to achieve pregnancy after ≥12 months of unprotected intercourse, excluding causes of male infertility, have a higher prevalence of conventional CVD risk factors and a strong association with CVD (79, 84, 126, 127). The largest study to date using Swedish registry data analyzed 863,324 participants, reporting a 19% greater risk of CVD in women who experienced ≥5 years of infertility versus women who did not experience infertility (75). This significant association between infertility and CVD was consistent in both age-adjusted and multivariable adjusted models across other large prospective cohort studies (Table 1) (75–77). The risk of CVD appears to be the strongest among women with history of infertility at an earlier age and among women whose infertility is attributable to an ovulatory disorder or endometriosis (77). Further research is necessary, however, to identify infertility as an independent risk factor for CVD as there are many shared risk factors and comorbidities (84).
Emerging research has also shown that the use of assisted reproductive technology (ART), including in vitro fertilization (IVF) and intracytoplasmic sperm injection, are associated with increased CVD risk (Table 1) (79, 126). This may be due to a causal relationship between ART and APOs, as one systematic review reported an association between IVF and HDPs (79, 126), though further research regarding the long-term cardiovascular implications of ART is needed.
Spontaneous coronary artery dissection (SCAD) is an acute coronary event related to development of a hematoma within the tunica media causing separation of the intima or intima-media complex from the underlying vessel and compression of the true lumen, leading to ischemia and acute MI (128). Two hypotheses have been postulated to describe the pathophysiology of SCAD: the “inside-out” hypothesis and the “outside-in” hypothesis (128–130). The “inside-out” hypothesis suggests that blood enters the subintimal space from the true lumen after an endothelial-intimal disruption, while the “outside-in” hypothesis suggests that a hematoma arises de novo in the media perhaps from disruption of traversing microvessels (128–130). Current evidence favors the “outside-in” hypothesis for three reasons: (1) most SCAD cases demonstrate no communication between false and true lumens (128, 129, 131, 132); (2) serial angiograms following a SCAD event demonstrate that development of an intramural hematoma precedes intimal dissection (128, 129); and (3) optical coherence tomography (OCT) imaging suggests that observed fenestrations may arise from rupture of the false lumen into the true lumen, rather than vice versa (128, 132). Strikingly, women comprise 87%–95% of all SCAD events with literature describing SCAD as the underlying cause of up to 35% of all acute coronary syndrome cases in women ≤50 years of age and is the most common cause of pregnancy-associated MI (128, 133–138). The explanation for the astonishing over-representation of SCAD in women remains a hot topic for debate as many of the current leading theories have conflicting results and are not fully understood. Several postulated triggers for SCAD include but are not limited to: (1) genetic underpinnings; (2) regulation of autosomal susceptibility genes that exhibit sex-specific regulation (e.g., estrogen response element genes); (3) intrinsic, gene-independent differences in coronary biology in women; (4) endogenous and exogeneous sex hormones; and (5) extreme physical or emotional stress (128, 135, 139–141).
Aforementioned, pregnancy-associated SCAD (P-SCAD) is the most common cause of pregnancy-associated MI, estimated to affect 1.81 per 100,000 pregnancies and comprises 14.5%–43% of all pregnancy-associated MI events (128, 142–144). The majority of P-SCAD events occur in the third trimester or early postpartum, and when compared to non-P-SCAD women, these patients tend to be older at first childbirth with more severe clinical presentation (e.g., impaired left ventricular function, cardiogenic shock, left main disease, and multivessel dissections) (128, 145–148). The cause of P-SCAD is not fully understood, however hormonal changes during pregnancy leading to deleterious alterations in the architecture of the arterial wall has been hypothesized (138). Nonetheless, given the unpredictable and recurrent nature of SCAD, women are often advised to avoid subsequent pregnancy following an acute SCAD event (128). It should be highlighted that patients with SCAD experience a high frequency of major adverse cardiovascular events (MACE) driven primarily by recurrent SCAD, with rates of SCAD recurrence ranging from 10 to 30% by varying reports (128). Additionally, all patients diagnosed with SCAD should be assessed for other concomitant arterial abnormalities, given its high association with aneurysmal disease and fibromuscular dysplasia (138, 149–151).
There is now greater recognition and appreciation of the impact of structural and functional disorders that affect the entire coronary circulation, including microcirculation, termed coronary microvascular disease (CMD) (152, 153). Conceptually, the coronary arterial system can be divided into three compartments: (1) epicardial coronary arteries; (2) pre-arterioles; and (3) intramyocardial arterioles (152). Together, the pre-arterioles and intramyocardial arterioles directly interface with the capillary bed and comprise the microcirculation (152). In the absence of obstructive stenosis, the larger epicardial coronary arteries contribute only 10% of the coronary circulation volume, while the microcirculation contributes the remaining 90% and thus, is the site of the majority of coronary blood flow resistance and its regulation (152). The interconnected regulatory pathways which allow for dynamic regulation of microcirculatory resistance to match myocardial oxygen consumption is disrupted in CMD through a combination of structural (e.g., luminal narrowing, intramyocardial or perivascular fibrosis, decreased capillary density) and functional abnormalities (e.g., impaired endothelial dilation, microvascular spasm, enhanced constrictive reactivity), resulting in ischemia and a constellation of symptoms (152–154).
A proposed CMD classification scheme include the following subtypes: (1) primary CMD with evidence of ischemia with no obstructive CAD (INOCA); (2) CMD in MI with non-obstructive CAD (MINOCA) (3) CMD with obstructive CAD post-MI; (4) iatrogenic CMD associated with reperfusion injury and microvascular distal embolization following coronary revascularization; and (5) CMD unrelated to atherosclerosis (152–154). By far the most prevalent presentation of CMD occurs in patients with signs and symptoms of INOCA, seen most particularly in women (152). For example, in both the WISE (Women's Ischemia Syndrome Evaluation) and WISE-CVD (Women's Ischemia Syndrome Evaluation—Coronary Vascular Dysfunction) studies, nearly half of women with INOCA had CMD detected by invasive testing (152, 155, 156). Likewise, particularly in women, CMD is a major driver for adverse CV death and hospitalization for MI and HF (152, 157, 158). CMD is therefore an important and underrecognized entity to understand when observing similar or worse outcomes for women with INOCA despite a lower rate of obstructive epicardial CAD (152). Cardinal manifestations include angina, exertional dyspnea, and HF symptoms and when present without explanatory obstructive CAD, should prompt further diagnostic testing for CMD (152, 153). In the 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain, evaluation for CMD with invasive coronary function testing and non-invasive assessment of myocardial blood flow by positron emission tomography (PET), stress cardiac magnetic resonance (CMR) imaging, and stress echocardiography with coronary flow velocity reserve was provided a class 2a recommendation for patients with stable angina and evidence of non-obstructive CAD (152, 154, 159). Given the paucity of robust evidence from large-scale randomized trials, there are no existing management guidelines for CMD (152). Treatment is aimed at reducing risk of adverse CV events and treating symptoms targeted to the specific subtype of CMD (152). The emerging WARRIOR (Women's Ischemia Trial to Reduce Events in Non-Obstructive CAD) trial will provide important outcome data at 3-year follow-up on the impact of medical therapy MACE in women with symptoms of INOCA, a population with a high rate of CMD (152, 160).
Systemic inflammatory and autoimmune disorders, such as systemic erythematous lupus (SLE), rheumatoid arthritis (RA), and psoriasis are more prevalent in women and have shown clear association with increased MI and CVD mortality risk (Table 1) (1, 3, 7, 80–82, 161). For example, a meta-analysis by Li et al. demonstrated an elevated risk of CVD for both sexes with history of SLE, though this risk was disproportionately higher in women versus men (80). The Framingham Offspring study reported that young women with SLE were over 50 times more likely to suffer an MI versus those of similar age without history of SLE (13, 81, 162). Similarly, a meta-analysis by Aviña-Zubieta et al. reported a 50% increased risk of CVD mortality in women with RA when compared with the general population (82).
The link between systemic inflammatory disorders and CVD has been hypothesized to occur due to the pathological role that inflammation plays in the progression of atherosclerosis (1). Thus, these systemic rheumatologic conditions have been classified as risk-enhancing factors in the AHA/ACC 2018 Cholesterol Guidelines and should be considered for women during risk stratification and evaluation for statin initiation (3, 13, 163).
Many psychosocial, behavioral, and lifestyle factors have also been studied which disproportionally affect women and are strong risk factors for early-onset CVD (1, 13). Depression, for example, is 2-fold more common in women than men and is a recognized risk factor for incident MI and cardiac mortality, one study reporting a 9% attributable risk of acute MI from depression (Table 1) (13, 83, 164, 165). Current available research of other psychosocial factors which women have more exposure to including history of sexual and physical abuse, psychological stress, and post-traumatic stress disorder have also been postulated as strong risk factors for CVD (13, 166).
Unfortunately, the link between postpartum depression and anxiety for women during their childbearing years with future CVD risk has not been well studied and warrants future investigation (19). Likewise, additional research is needed to determine if addressing behavioral factors such as nutrition, stress, and exercise reduce a women's CVD risk, particularly women with history of APOs (16). Future clinical trials can investigate the efficacy of lifestyle interventions such as adopting a heart-healthy diet and regular physical activity in the prevention of future CVD (19).
The appropriate risk stratification and prevention of CVD in women remain a significant challenge and a principal issue given the considerable burden of CVD in women (2, 8, 84, 167, 168). It is reported that only 42% of cardiologists felt adequately prepared to assess CVD risk in their female patients, with only 22% reporting using guideline-directed sex-specific guidelines (169).
Although current prevention guidelines have mentioned the inclusion of pregnancy history in the assessment of CVD risk, limited studies have emphasized the incorporation of pregnancy risk factors into predictive CVD scoring (16, 85). In fact, current CVD risk assessment tools do not consider any female-specific risk factors including APOs (19, 163). Only a few published studies have thoroughly investigated the utility of incorporating APOs to conventional CVD risk stratification despite their strong association with increased maternal CVD risk (19, 85, 92, 104, 170). This may be due to uncertainty as to whether APOs provide a direct causal relationship to future maternal CVD or if they unmask shared risk factors (16). For example, it is unclear if the delivery of SGA infants is an association independent of other maternal placental syndromes given their many interrelated factors.
Thus, further research is required to elucidate the true pathophysiology between these important sex-specific CVD risk factors with future maternal CVD risk to improve screening strategies, refine risk assessment, and implement primordial and primary prevention for women beyond traditional risk scoring algorithms (84). Future clinical trials and female-specific risk prediction models should recognize the importance of including women of childbearing age as well as women transitioning through menopause to reflect the target subpopulations intended for screening (16, 34).
Improving patient and clinician education with regards to sex-specific CVD risk factors is vital. These risk factors can afflict women over a span of their lifetime, from young adulthood to childbearing age to their late adult and retirement years (Figure 1). Therefore, educating patients and clinicians, early and often, of these risk factors is essential to the identification and care of CVD in women. Most patients are not aware that having a pregnancy complication may increase their future CVD risk, with recent data showing that only 45% of women recognize that CVD is the leading cause of death (19, 169, 171). In particular, women with APOs should be informed that these disorders pose a higher lifetime risk of CVD and should undergo urgent risk assessment (19, 172, 173). Education and awareness of these risk factors have been shown to enhance the physician-patient relationship, improve engagement, and promote medication adherence (84, 174, 175). Likewise, educating clinicians and fellows-in-training regarding the importance of strong obstetrical and gynecological history-taking is fundamental and should be part of core and continuing medical education (84). Topics surrounding the identification of women with sex-specific risk factors should be featured at national and professional society conferences, such that all providers are better informed to provide comprehensive care for women at risk for CVD (84).
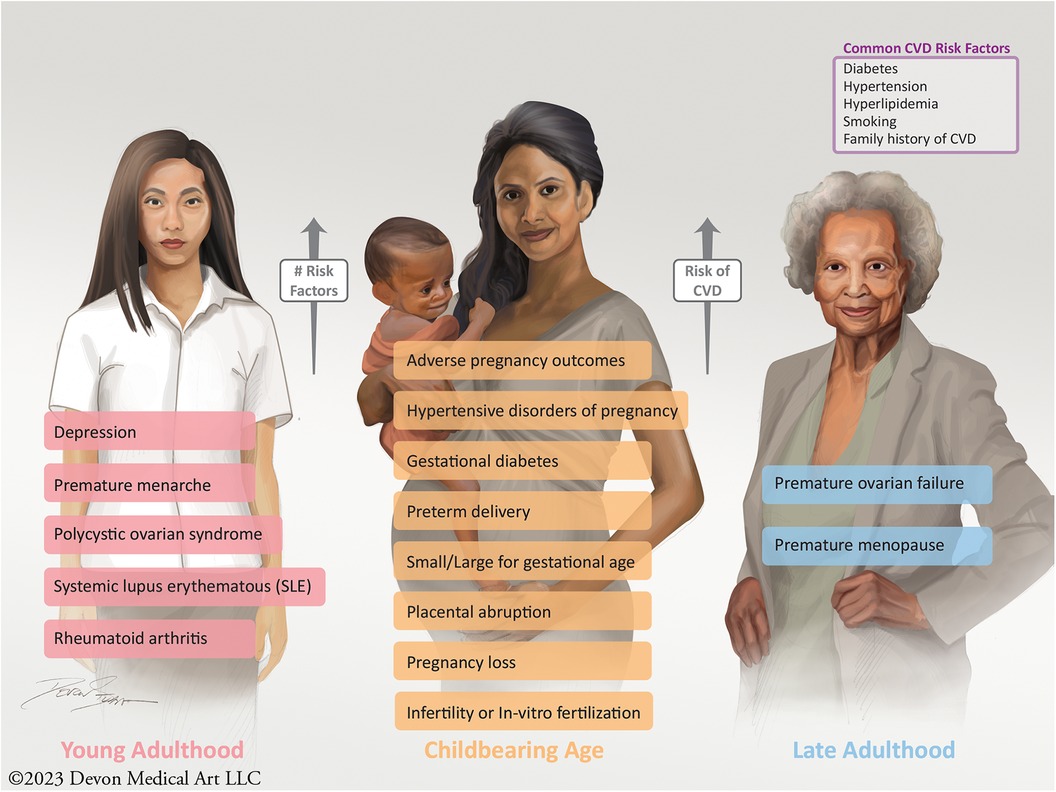
Figure 1. Sex-specific risk factors, which increase a women's future risk of CVD, can present over the span of a lifetime from young adulthood to childbearing age to late adulthood into retirement.
As evidenced by our discussion, a clinician's role in taking a strong obstetrical and reproductive history is an often neglected, though critical aspect, in the risk assessment and prevention of CVD in women. From preconception through pregnancy and into menopause, this continuum serves as an important opportunity for cardiovascular risk assessment. In fact, the American College of Obstetricians and Gynecologists (ACOG) recently formulated a concept called the “fourth trimester” of pregnancy, defined as a critical period for women after birth which warrant recurrent continuity of care beyond a traditional single postpartum visit (19, 171). With a multitude of elements of cardiovascular health to be discussed in a time-limited encounter, obtaining a strong sex-specific history poses a challenge (84).
To tackle this challenge, we developed a sex-specific screening questionnaire which can utilized and replicated throughout ambulatory clinics worldwide (Figure 2). This questionnaire highlights the many neglected sex-specific risk factors for women of reproductive age, which if recognized early, can assist in identifying high-risk individuals for close long-term follow-up and appropriate counseling regarding CVD prevention (Figure 2).
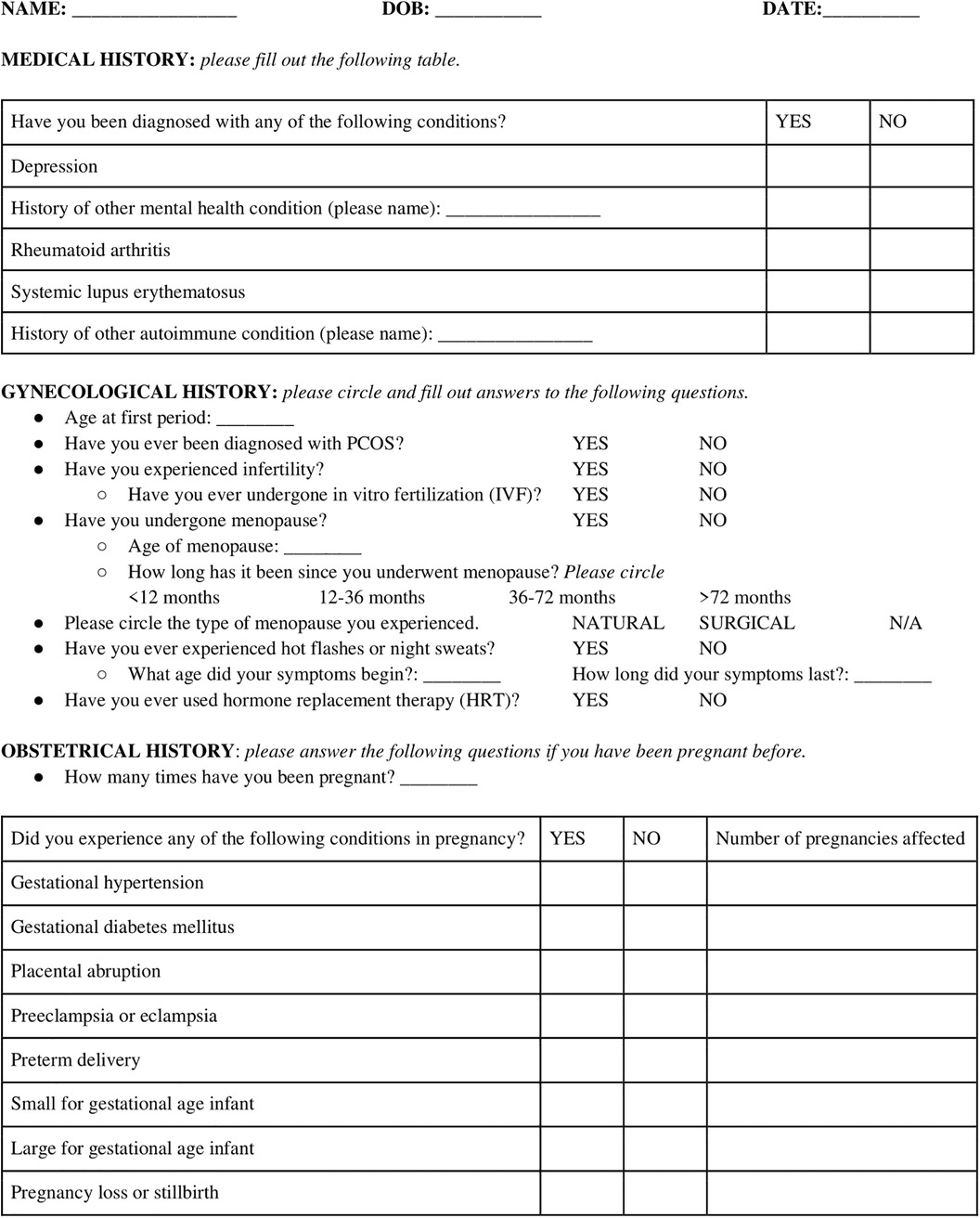
Figure 2. A screening questionnaire encapsulating pertinent medical, gynecologic, and obstetrical history to identify and document important sex-specific CVD risk factors.
Although beyond the scope of this review, the following prospective registries and cohort studies have been instrumental in understanding sex-specific risk factors and its association with CVD: NuMo2B, WISE, SCAPIS, SWAN, and CARPREG II. Active enrollment of eligible patients into current registries and cohort studies is a necessary element to propel the investigation of sex-specific risk factors forward.
Cardiovascular care for women in our current standard of practice is far from ideal. As outlined in this review, obtaining a thorough obstetrical history represents an opportunity to encourage sex-specific risk factor screening and refine risk prediction and stratification of CVD by recognizing important aspects of a women's reproductive and obstetrical history which affect long-term cardiometabolic health (84). Incorporation of sex-specific risk factors is one important step in shifting the paradigm of underdiagnosing and undertreating CVD in women which traditional risk models have done for years (3, 84, 176). Implementation of our patient questionnaire is an efficient, large-scale, standardized method of eliciting important medical history as it pertains to sex-specific risk factors, and can be utilized as a data analysis tool to develop a future prognostic model to improve the current inadequate care of CVD in women.
AN: Conceptualization, Data curation, Project administration, Resources, Writing – original draft, Writing – review & editing. MH: Data curation, Resources, Writing – review & editing. SS: Writing – review & editing. AS: Writing – review & editing. JK: Writing – review & editing. GS: Writing – review & editing, Supervision.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
GS is supported by the American Heart Association (979462).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Garg K, Patel TR, Kanwal A, Villines TC, Aggarwal NR, Nasir K, et al. The evolving role of coronary computed tomography in understanding sex differences in coronary atherosclerosis. J Cardiovasc Comput Tomogr. (2022) 16(2):138–49. doi: 10.1016/j.jcct.2021.09.004
2. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics—2021 update: a report from the American heart association. Circulation. (2021) 143(8):e439–55. doi: 10.1161/CIR.0000000000000950
3. Agarwala A, Michos ED, Samad Z, Ballantyne CM, Virani SS. The use of sex-specific factors in the assessment of women’s cardiovascular risk. Circulation. (2020) 141(7):592–9. doi: 10.1161/CIRCULATIONAHA.119.043429
4. Mosca L, Linfante AH, Benjamin EJ, Berra K, Hayes SN, Walsh BW, et al. National study of physician awareness and adherence to cardiovascular disease prevention guidelines. Circulation. (2005) 111(4):499–510. doi: 10.1161/01.CIR.0000154568.43333.82
5. Garcia M, Mulvagh SL, Bairey Merz CN, Buring JE, Manson JE. Cardiovascular disease in women: clinical perspectives. Circ Res. (2016) 118(8):1273–93. doi: 10.1161/CIRCRESAHA.116.307547
6. Elder P, Sharma G, Gulati M, Michos ED. Identification of female-specific risk enhancers throughout the lifespan of women to improve cardiovascular disease prevention. Am J Prev Cardiol. (2020) 2:100028. doi: 10.1016/j.ajpc.2020.100028
7. Mehta LS, Beckie TM, Devon HA, Grines CL, Krumholz HM, Johnson MN, et al. Acute myocardial infarction in women: a scientific statement from the American heart association. Circulation. (2016) 133(9):916–47. doi: 10.1161/CIR.0000000000000351
8. Brown HL, Warner JJ, Gianos E, Gulati M, Hill AJ, Hollier LM, et al. Promoting risk identification and reduction of cardiovascular disease in women through collaboration with obstetricians and gynecologists: a presidential advisory from the American heart association and the American college of obstetricians and gynecologists. Circulation. (2018) 137(24):e843–52. doi: 10.1161/CIR.0000000000000582
9. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. (2004) 364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9
10. Peters SAE, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. (2014) 57(8):1542–51. doi: 10.1007/s00125-014-3260-6
11. Gnatiuc L, Herrington WG, Halsey J, Tuomilehto J, Fang X, Kim HC, et al. Sex-specific relevance of diabetes to occlusive vascular and other mortality: a collaborative meta-analysis of individual data from 980 793 adults from 68 prospective studies. Lancet Diabetes Endocrinol. (2018) 6(7):538–46. doi: 10.1016/S2213-8587(18)30079-2
12. Huxley RR, Peters SAE, Mishra GD, Woodward M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. (2015) 3(3):198–206. doi: 10.1016/S2213-8587(14)70248-7
13. Cho L, Davis M, Elgendy I, Epps K, Lindley KJ, Mehta PK, et al. Summary of updated recommendations for primary prevention of cardiovascular disease in women. J Am Coll Cardiol. (2020) 75(20):2602–18. doi: 10.1016/j.jacc.2020.03.060
14. Hemal K, Pagidipati NJ, Coles A, Dolor RJ, Mark DB, Pellikka PA, et al. Sex differences in demographics, risk factors. Presentation, and noninvasive testing in stable outpatients with suspected coronary artery disease. JACC Cardiovasc Imaging. (2016) 9(4):337–46. doi: 10.1016/j.jcmg.2016.02.001
15. Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. (2011) 378(9799):1297–305. doi: 10.1016/S0140-6736(11)60781-2
16. Minhas AS, Ying W, Ogunwole SM, Miller M, Zakaria S, Vaught AJ, et al. The association of adverse pregnancy outcomes and cardiovascular disease: current knowledge and future directions. Curr Treat Options Cardio Med. (2020) 22(12):61. doi: 10.1007/s11936-020-00862-6
17. Hollier LM, Martin JN, Connolly H, Turrentine M, Hameed A, Arendt KW, et al. ACOG practice bulletin no. 212 summary: pregnancy and heart disease. Obstet Gynecol. (2019) 133(5):1067–72. doi: 10.1097/AOG.0000000000003244
18. Mann S, Hollier LM, McKay K, Brown H. What we can do about maternal mortality—and how to do it quickly. N Engl J Med. (2018) 379(18):1689–91. doi: 10.1056/NEJMp1810649
19. Parikh NI, Gonzalez JM, Anderson CAM, Judd SE, Rexrode KM, Hlatky MA, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American heart association. Circulation. (2021) 143(18):e902–16. doi: 10.1161/CIR.0000000000000961
20. Zeng Z, Liu F, Li S. Metabolic adaptations in pregnancy: a review. Ann Nutr Metab. (2017) 70(1):59–65. doi: 10.1159/000459633
21. Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr. (2000) 71(5):1256S–61S. doi: 10.1093/ajcn/71.5.1256s
22. Gillespie SL, Porter K, Christian LM. Adaptation of the inflammatory immune response across pregnancy and postpartum in black and white women. J Reprod Immunol. (2016) 114:27–31. doi: 10.1016/j.jri.2016.02.001
23. Bonamy AKE, Parikh NI, Cnattingius S, Ludvigsson JF, Ingelsson E. Birth characteristics and subsequent risks of maternal cardiovascular disease: effects of gestational age and fetal growth. Circulation. (2011) 124(25):2839–46. doi: 10.1161/CIRCULATIONAHA.111.034884
24. Sattar N. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? Br Med J. (2002) 325(7356):157–60. doi: 10.1136/bmj.325.7356.157
25. Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: final data for 2018. Natl Vital Stat Rep. (2019) 68(13):1–47. PMID: 32501202.
26. Lane-Cordova AD, Khan SS, Grobman WA, Greenland P, Shah SJ. Long-term cardiovascular risks associated with adverse pregnancy outcomes: JACC review topic of the week. J Am Coll Cardiol. (2019) 73(16):2106–16. doi: 10.1016/j.jacc.2018.12.092
27. Correa A, Bardenheier B, Elixhauser A, Geiss LS, Gregg E. Trends in prevalence of diabetes among delivery hospitalizations, United States, 1993–2009. Matern Child Health J. (2015) 19(3):635–42. doi: 10.1007/s10995-014-1553-5
28. Behrens I, Basit S, Melbye M, Lykke JA, Wohlfahrt J, Bundgaard H, et al. Risk of post-pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. Br Med J. (2017) 358:j3078. doi: 10.1136/bmj.j3078
29. Grandi SM, Vallée-Pouliot K, Reynier P, Eberg M, Platt RW, Arel R, Basso O, et al. Hypertensive disorders in pregnancy and the risk of subsequent cardiovascular disease. Paediatr Perinat Epidemiol. (2017) 31(5):412–21. doi: 10.1111/ppe.12388
30. Theilen LH, Meeks H, Fraser A, Esplin MS, Smith KR, Varner MW. Long-term mortality risk and life expectancy following recurrent hypertensive disease of pregnancy. Am J Obstet Gynecol. (2018) 219(1):107.e1–e6. doi: 10.1016/j.ajog.2018.04.002
31. Riise HKR, Sulo G, Tell GS, Igland J, Egeland G, Nygard O, et al. Hypertensive pregnancy disorders increase the risk of maternal cardiovascular disease after adjustment for cardiovascular risk factors. Int J Cardiol. (2019) 282:81–7. doi: 10.1016/j.ijcard.2019.01.097
32. Honigberg MC, Zekavat SM, Aragam K, Klarin D, Bhatt DL, Scott NS, et al. Long-term cardiovascular risk in women with hypertension during pregnancy. J Am Coll Cardiol. (2019) 74(22):2743–54. doi: 10.1016/j.jacc.2019.09.052
33. Heida KY, Bots ML, de Groot CJ, van Dunné FM, Hammoud NM, Hoek A, et al. Cardiovascular risk management after reproductive and pregnancy-related disorders: a Dutch multidisciplinary evidence-based guideline. Eur J Prev Cardiolog. (2016) 23(17):1863–79. doi: 10.1177/2047487316659573
34. Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, et al. Cardiovascular disease-related morbidity and mortality in women with a history of pregnancy complications: systematic review and meta-analysis. Circulation. (2019) 139(8):1069–79. doi: 10.1161/CIRCULATIONAHA.118.036748
35. Haug EB, Horn J, Markovitz AR, Fraser A, Klykken B, Dalen H, et al. Association of conventional cardiovascular risk factors with cardiovascular disease after hypertensive disorders of pregnancy: analysis of the nord-trøndelag health study. JAMA Cardiol. (2019) 4(7):628. doi: 10.1001/jamacardio.2019.1746
36. Canoy D, Cairns BJ, Balkwill A, Wright FL, Khalil A, Beral V, et al. Hypertension in pregnancy and risk of coronary heart disease and stroke: a prospective study in a large UK cohort. Int J Cardiol. (2016) 222:1012–8. doi: 10.1016/j.ijcard.2016.07.170
37. Riise HKR, Sulo G, Tell GS, Igland J, Nygard O, Iversen A, et al. Association between gestational hypertension and risk of cardiovascular disease among 617,589 Norwegian women. JAHA. (2018) 7(10):e008337. doi: 10.1161/JAHA.117.008337
38. Stuart JJ, Tanz LJ, Missmer SA, Rimm EB, Spiegelman D, James-Todd TM, et al. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: an observational cohort study. Ann Intern Med. (2018) 169(4):224. doi: 10.7326/M17-2740
39. Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. (2017) 10(2):e003497. doi: 10.1161/CIRCOUTCOMES.116.003497
40. Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. (2013) 28(1):1–19. doi: 10.1007/s10654-013-9762-6
41. Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. Br Med J. (2007) 335(7627):974. doi: 10.1136/bmj.39335.385301.BE
42. Brouwers L, van der Meiden-van Roest AJ, Savelkoul C, Vogelvang TE, Lely AT, Franx A, et al. Recurrence of pre-eclampsia and the risk of future hypertension and cardiovascular disease: a systematic review and meta-analysis. BJOG. (2018) 125(13):1642–54. doi: 10.1111/1471-0528.15394
43. Riise HKR, Sulo G, Tell GS, Igland J, Nygard O, Vollset SE, et al. Incident coronary heart disease after preeclampsia: role of reduced fetal growth, preterm delivery, and parity. JAHA. (2017) 6(3):e004158. doi: 10.1161/JAHA.116.004158
44. Wu P, Chew-Graham CA, Maas AH, Chappell LC, Potts JE, Gulati M, et al. Temporal changes in hypertensive disorders of pregnancy and impact on cardiovascular and obstetric outcomes. Am J Cardiol. (2020) 125(10):1508–16. doi: 10.1016/j.amjcard.2020.02.029
45. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. (2009) 373(9677):1773–9. doi: 10.1016/S0140-6736(09)60731-5
46. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. (2020) 13:m1361. doi: 10.1136/bmj.m1361
47. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. (2019) 62(6):905–14. doi: 10.1007/s00125-019-4840-2
48. Heida KY, Franx A, van Rijn BB, Eijkemans MJC, Boer JMA, Verschuren MWM, et al. Earlier age of onset of chronic hypertension and type 2 diabetes mellitus after a hypertensive disorder of pregnancy or gestational diabetes mellitus. Hypertension. (2015) 66(6):1116–22. doi: 10.1161/HYPERTENSIONAHA.115.06005
49. Kaul P, Savu A, Nerenberg KA, Donovan LE, Chik CL, Ryan EA, et al. Impact of gestational diabetes mellitus and high maternal weight on the development of diabetes, hypertension and cardiovascular disease: a population-level analysis. Diabet Med. (2015) 32(2):164–73. doi: 10.1111/dme.12635
50. Carr DB, Utzschneider KM, Hull RL, Tong J, Wallace TM, Kodama K, et al. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care. (2006) 29(9):2078–83. doi: 10.2337/dc05-2482
51. Wu P, Gulati M, Kwok CS, Wong CW, Narain A, O'Brien S, et al. Preterm delivery and future risk of maternal cardiovascular disease: a systematic review and meta-analysis. JAHA. (2018) 7(2):e007809. doi: 10.1161/JAHA.117.007809
52. Tanz LJ, Stuart JJ, Williams PL, Rimm EB, Missmer SA, Rexrode KM, et al. Preterm delivery and maternal cardiovascular disease in young and middle-aged adult women. Circulation. (2017) 135(6):578–89. doi: 10.1161/CIRCULATIONAHA.116.025954
53. Tanz LJ, Stuart JJ, Williams PL, Rimm EB, James-Todd TM, Rich-Edwards JW, et al. Preterm delivery and maternal cardiovascular disease risk factors: the nurses’ health study II. J Womens Health. (2019) 28(5):677–85. doi: 10.1089/jwh.2018.7150
54. Parikh NI, Norberg M, Ingelsson E, Cnattingius S, Vasan RS, Domellöf M, et al. Association of pregnancy complications and characteristics with future risk of elevated blood pressure: the Västerbotten intervention program. Hypertension. (2017) 69(3):475–83. doi: 10.1161/HYPERTENSIONAHA.116.08121
55. Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. (2005) 366(9499):1797–803. doi: 10.1016/S0140-6736(05)67726-4
56. Oliver-Williams CT, Heydon EE, Smith GCS, Wood AM. Miscarriage and future maternal cardiovascular disease: a systematic review and meta-analysis. Heart. (2013) 99(22):1636–44. doi: 10.1136/heartjnl-2012-303237
57. Hall PS, Nah G, Vittinghoff E, Parker DR, Manson JE, Howard BV, et al. Relation of pregnancy loss to risk of cardiovascular disease in parous postmenopausal women (from the women’s health initiative). Am J Cardiol. (2019) 123(10):1620–5. doi: 10.1016/j.amjcard.2019.02.012
58. Ranthe MF, Andersen EAW, Wohlfahrt J, Bundgaard H, Melbye M, Boyd HA. Pregnancy loss and later risk of atherosclerotic disease. Circulation. (2013) 127(17):1775–82. doi: 10.1161/CIRCULATIONAHA.112.000285
59. Smith GCS. Spontaneous loss of early pregnancy and risk of ischaemic heart disease in later life: retrospective cohort study. Br Med J. (2003) 326(7386):423–4. doi: 10.1136/bmj.326.7386.423
60. Wagner MM, Bhattacharya S, Visser J, Hannaford PC, Bloemenkamp KW. Association between miscarriage and cardiovascular disease in a Scottish cohort. Heart. (2015) 101(24):1954–60. doi: 10.1136/heartjnl-2015-307563
61. Peters SA, Woodward M. Women’s reproductive factors and incident cardiovascular disease in the UK biobank. Heart. (2018) 104(13):1069–75. doi: 10.1136/heartjnl-2017-312289
62. Ngo AD, Roberts CL, Chen JS, Figtree G. Delivery of a small-for-gestational-age infant and risk of maternal cardiovascular disease—a population-based record linkage study. Heart Lung Circ. (2015) 24(7):696–704. doi: 10.1016/j.hlc.2015.01.004
63. Morken NH, Halland F, DeRoo L, Wilcox A, Skjaerven R. Offspring birthweight by gestational age and parental cardiovascular mortality: a population-based cohort study. BJOG. (2018) 125(3):336–41. doi: 10.1111/1471-0528.14522
64. Charalampopoulos D, McLoughlin A, Elks CE, Ong KK. Age at menarche and risks of all-cause and cardiovascular death: a systematic review and meta-analysis. Am J Epidemiol. (2014) 180(1):29–40. doi: 10.1093/aje/kwu113
65. Lee JJ, Cook-Wiens G, Johnson BD, Braunstein GD, Berga SL, Stanczyk FZ, et al. Age at menarche and risk of cardiovascular disease outcomes: findings from the national heart lung and blood institute-sponsored women’s ischemia syndrome evaluation. JAHA. 2019;8(12):e012406. doi: 10.1161/JAHA.119.012406
66. Canoy D, Beral V, Balkwill A, Wright FL, Kroll ME, Reeves G, et al. Age at menarche and risks of coronary heart and other vascular diseases in a large UK cohort. Circulation. (2015) 131(3):237–44. doi: 10.1161/CIRCULATIONAHA.114.010070
67. Ley SH, Li Y, Tobias DK, Manson JE, Rosner B, Hu FB, et al. Duration of reproductive life span, age at menarche, and age at menopause aare associated with risk of cardiovascular disease in women. JAHA. (2017) 6(11):e006713. doi: 10.1161/JAHA.117.006713
68. Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw K, et al. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. (2009) 94(12):4953–60. doi: 10.1210/jc.2009-1789
69. Amiri M, Ramezani Tehrani F, Behboudi-Gandevani S, Bidhendi-Yarandi R, Carmina E. Risk of hypertension in women with polycystic ovary syndrome: a systematic review, meta-analysis and meta-regression. Reprod Biol Endocrinol. (2020) 18(1):23. doi: 10.1186/s12958-020-00576-1
70. Okoth K, Chandan JS, Marshall T, Thangaratinam S, Thomas GN, Nirantharakumar K, et al. Association between the reproductive health of young women and cardiovascular disease in later life: umbrella review. BMJ. (2020) 7:m3502. doi: 10.1136/bmj.m3502
71. Zhang J, Xu JH, Qu QQ, Zhong GQ. Risk of cardiovascular and cerebrovascular events in polycystic ovarian syndrome women: a meta-analysis of cohort studies. Front Cardiovasc Med. (2020) 7:552421. doi: 10.3389/fcvm.2020.552421
72. Muka T, Oliver-Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. (2016) 1(7):767. doi: 10.1001/jamacardio.2016.2415
73. Honigberg MC, Zekavat SM, Aragam K, Finneran P, Klarin D, Bhatt DL, et al. Association of premature natural and surgical menopause with incident cardiovascular disease. JAMA. (2019) 322(24):2411. doi: 10.1001/jama.2019.19191
74. Roeters Van Lennep JE, Heida KY, Bots ML, Hoek A, on behalf of the collaborators of the Dutch Multidisciplinary Guideline Development Group on Cardiovascular Risk Management after Reproductive Disorders. Cardiovascular disease risk in women with premature ovarian insufficiency: a systematic review and meta-analysis. Eur J Prev Cardiolog. (2016) 23(2):178–86. doi: 10.1177/2047487314556004
75. Parikh NI, Cnattingius S, Mittleman MA, Ludvigsson JF, Ingelsson E. Subfertility and risk of later life maternal cardiovascular disease. Hum Reprod. (2012) 27(2):568–75. doi: 10.1093/humrep/der400
76. Magnus MC, Fraser A, Rich-Edwards JW, Magnus P, Lawlor DA, Håberg SE. Time-to-pregnancy and risk of cardiovascular disease among men and women. Eur J Epidemiol. (2021) 36(4):383–91. doi: 10.1007/s10654-021-00718-8
77. Farland LV, Wang Y-X, Gaskins AJ, Rich-Edwards JW, Wang S, Magnus MC, et al. Infertility and risk of cardiovascular disease: a prospective cohort study. JAHA. (2023) 12(5):e027755. doi: 10.1161/JAHA.122.027755
78. Dayan N, Filion KB, Okano M, Kilmartin C, Reinblatt S, Landry T, et al. Cardiovascular risk following fertility therapy. J Am Coll Cardiol. (2017) 70(10):1203–13. doi: 10.1016/j.jacc.2017.07.753
79. Udell JA, Lu H, Redelmeier DA. Long-term cardiovascular risk in women prescribed fertility therapy. J Am Coll Cardiol. (2013) 62(18):1704–12. doi: 10.1016/j.jacc.2013.05.085
80. Li H, Tong Q, Guo L, Yu S, Li Y, Cao Q, et al. Risk of coronary artery disease in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Am J Med Sci. (2018) 356(5):451–63. doi: 10.1016/j.amjms.2018.08.001
81. Conte CG, Medsger TA Jr, Jansen-McWilliams L, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the framingham study. Am J Epidemiol. (1997) 145(5):408–15. doi: 10.1093/oxfordjournals.aje.a009122
82. Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. (2008) 59(12):1690–7. doi: 10.1002/art.24092
83. Rosengren A, Hawken S, Ôunpuu S, Sliwa K, Zubaid M, Almahmeed WA, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11,119 cases and 13,648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. (2004) 364(9438):953–62. doi: 10.1016/S0140-6736(04)17019-0
84. O’Kelly AC, Michos ED, Shufelt CL, Vermunt JV, Minissian MB, Quesada O, et al. Pregnancy and reproductive risk factors for cardiovascular disease in women. Circ Res. (2022) 130(4):652–72. doi: 10.1161/CIRCRESAHA.121.319895
85. Stuart JJ, Tanz LJ, Cook NR, Spiegelman D, Missmer SA, Rimm EB, et al. Hypertensive disorders of pregnancy and 10-year cardiovascular risk prediction. J Am Coll Cardiol. (2018) 72(11):1252–63. doi: 10.1016/j.jacc.2018.05.077
86. Haug EB, Horn J, Markovitz AR, Fraser A, Vatten LJ, Macdonald-Wallis C, et al. Life course trajectories of cardiovascular risk factors in women with and without hypertensive disorders in first pregnancy: the HUNT study in Norway. JAHA. (2018) 7(15):e009250. doi: 10.1161/JAHA.118.009250
87. Grill S, Rusterholz C, Zanetti-Dällenbach R, Tercanli S, Holzgreve W, Hahn S, et al. Potential markers of preeclampsia–a review. Reprod Biol Endocrinol. (2009) 7:70. doi: 10.1186/1477-7827-7-70
88. Hogan MC, Foreman KJ, Naghavi M, Ahn SY, Wang M, Makela SM, et al. Maternal mortality for 181 countries, 1980–2008: a systematic analysis of progress towards millennium development goal 5. Lancet. (2010) 375(9726):1609–23. doi: 10.1016/S0140-6736(10)60518-1
89. Wanderer JP, Leffert LR, Mhyre JM, Kuklina EV, Callaghan WM, Bateman BT. Epidemiology of obstetric-related ICU admissions in Maryland: 1999–2008*. Crit Care Med. (2013) 41(8):1844–52. doi: 10.1097/CCM.0b013e31828a3e24
90. Sep SJS, Schreurs MPH, Bekkers SaM, Kruse AJ, Smits LJ, Peeters LLH. Early-pregnancy changes in cardiac diastolic function in women with recurrent pre-eclampsia and in previously pre-eclamptic women without recurrent disease. BJOG. (2011) 118(9):1112–9. doi: 10.1111/j.1471-0528.2011.02951.x
91. Wong ND, Budoff MJ, Ferdinand K, Graham IM, Michos ED, Reddy T, et al. Atherosclerotic cardiovascular disease risk assessment: an American society for preventive cardiology clinical practice statement. Am J Prev Cardiol. (2022) 10:100335. doi: 10.1016/j.ajpc.2022.100335
92. Markovitz AR, Stuart JJ, Horn J, Williams PL, Rimm EB, Missmer SA, et al. Does pregnancy complication history improve cardiovascular disease risk prediction? Findings from the HUNT study in Norway. Eur Heart J. (2019) 40(14):1113–20. doi: 10.1093/eurheartj/ehy863
93. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. (2019) 42(Suppl 1):S13–28. doi: 10.2337/dc19-S002
94. Caughey AB, Turrentine M. ACOG practice bulletin no. 190: gestational diabetes mellitus. Obstet Gynecol. (2018) 131(2):e49–64. doi: 10.1097/AOG.0000000000002501
95. Garrison A. Screening, diagnosis, and management of gestational diabetes mellitus. Am Fam Physician. (2015) 91(7):460–7. PMID: 25884746.25884746
96. Buchanan TA. Pancreatic B-cell defects in gestational diabetes: implications for the pathogenesis and prevention of type 2 diabetes. J Clin Endocrinol Metab. (2001) 86(3):989–93. doi: 10.1210/jcem.86.3.7339
97. Retnakaran R, Qi Y, Connelly PW, Sermer M, Hanley AJ, Zinman B. The graded relationship between glucose tolerance status in pregnancy and postpartum levels of low-density-lipoprotein cholesterol and apolipoprotein B in young women: implications for future cardiovascular risk. J Clin Endocrinol Metab. (2010) 95(9):4345–53. doi: 10.1210/jc.2010-0361
98. Bo S, Valpreda S, Menato G, Bardelli C, Botto C, Gambino R, et al. Should we consider gestational diabetes a vascular risk factor? Atherosclerosis. (2007) 194(2):e72–79. doi: 10.1016/j.atherosclerosis.2006.09.017
99. Retnakaran R. Hyperglycemia in pregnancy and its implications for a woman’s future risk of cardiovascular disease. Diabetes Res Clin Pract. (2018) 145:193–9. doi: 10.1016/j.diabres.2018.04.008
100. Crump C, Sundquist J, Howell EA, McLaughlin MA, Stroustrup A, Sundquist K. Pre-term delivery and risk of ischemic heart disease in women. J Am Coll Cardiol. (2020) 76(1):57–67. doi: 10.1016/j.jacc.2020.04.072
101. Catov JM, Snyder GG, Bullen BL, Barinas-Mitchell EJM, Holzman C. Women with preterm birth have evidence of subclinical atherosclerosis a decade after delivery. J Womens Health. (2019) 28(5):621–7. doi: 10.1089/jwh.2018.7148
102. Minissian MB, Kilpatrick S, Eastwood JA, Robbins WA, Accortt EE, Wei J, et al. Association of spontaneous preterm delivery and future maternal cardiovascular disease. Circulation. (2018) 137(8):865–71. doi: 10.1161/CIRCULATIONAHA.117.031403
103. Veerbeek JHW, Smit JG, Koster MPH, Post Uiterweer ED, van Rijn BB, Koenen SV, et al. Maternal cardiovascular risk profile after placental abruption. Hypertension. (2013) 61(6):1297–301. doi: 10.1161/HYPERTENSIONAHA.111.00930
104. Parikh NI, Jeppson RP, Berger JS, Eaton CB, Kroenke CH, LeBlanc ES, et al. Reproductive risk factors and coronary heart disease in the women’s health initiative observational study. Circulation. (2016) 133(22):2149–58. doi: 10.1161/CIRCULATIONAHA.115.017854
105. Søndergaard MM, Hlatky MA, Stefanick M, Vittinghoff E, Nah G, Allison M, et al. Association of adverse pregnancy outcomes with risk of atherosclerotic cardiovascular disease in postmenopausal women. JAMA Cardiol. (2020) 5(12):1390. doi: 10.1001/jamacardio.2020.4097
106. Horn J, Haug EB, Markovitz AR, Fraser A, Vatten LJ, Romundstad PR, et al. Life course trajectories of maternal cardiovascular risk factors according to offspring birthweight: the HUNT study. Sci Rep. (2020) 10(1):10436. doi: 10.1038/s41598-020-66365-3
107. Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the avon longitudinal study of parents and children. Circulation. (2012) 125(11):1367–80. doi: 10.1161/CIRCULATIONAHA.111.044784
108. Matkovic V, Ilich JZ, Skugor M, Badenhop NE, Goel P, Clairmont A, et al. Leptin is inversely related to age at menarche in human females*. J Clin Endocrinol Metab. (1997) 82(10):3239–45. doi: 10.1210/jcem.82.10.4280
109. Juul F, Chang VW, Brar P, Parekh N. Birth weight, early life weight gain and age at menarche: a systematic review of longitudinal studies: birth weight early life weight gain and age at menarche. Obes Rev. (2017) 18(11):1272–88. doi: 10.1111/obr.12587
110. Osibogun O, Ogunmoroti O, Michos ED. Polycystic ovary syndrome and cardiometabolic risk: opportunities for cardiovascular disease prevention. Trends Cardiovasc Med. (2020) 30(7):399–404. doi: 10.1016/j.tcm.2019.08.010
111. Chen MJ, Yang WS, Yang JH, Chen CL, Ho HN, Yang YS. Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension. (2007) 49(6):1442–7. doi: 10.1161/HYPERTENSIONAHA.106.083972
112. Calderon-Margalit R, Siscovick D, Merkin SS, Wang E, Daviglus ML, Schreiner PJ, et al. Prospective association of polycystic ovary syndrome with coronary artery calcification and carotid-intima-Media thickness: the coronary artery risk development in young adults women’s study. ATVB. (2014) 34(12):2688–94. doi: 10.1161/ATVBAHA.114.304136
113. Christian RC, Dumesic DA, Behrenbeck T, Oberg AL, Sheedy PF, Fitzpatrick LA. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J Clin Endocrinol Metab. (2003) 88(6):2562–8. doi: 10.1210/jc.2003-030334
114. Meyer ML, Malek AM, Wild RA, Korytkowski MT, Talbott EO. Carotid artery intima-media thickness in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. (2012) 18(2):112–26. doi: 10.1093/humupd/dmr046
115. Guzick DS. Cardiovascular risk in PCOS. J Clin Endocrinol Metab. (2004) 89(8):3694–5. doi: 10.1210/jc.2004-1136
116. Torrealday S, Kodaman P, Pal L. Premature ovarian insufficiency—an update on recent advances in understanding and management. F1000Res. (2017) 6:2069. doi: 10.12688/f1000research.11948.1
117. Kalantaridou SN, Naka KK, Papanikolaou E, Kazakos N, Kravariti M, Calis KA, et al. Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy. J Clin Endocrinol Metab. (2004) 89(8):3907–13. doi: 10.1210/jc.2004-0015
118. Zhao D, Guallar E, Ouyang P, Subramanya V, Vaidya D, Ndumele CE, et al. Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J Am Coll Cardiol. (2018) 71(22):2555–66. doi: 10.1016/j.jacc.2018.01.083
119. El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American heart association. Circulation. (2020) 142(25):e506–32. doi: 10.1161/CIR.0000000000000912
120. Avis NE, Crawford SL, Greendale G, Bromberger JT, Everson-Rose SA, Gold EB, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. (2015) 175(4):531–9. doi: 10.1001/jamainternmed.2014.8063
121. Avis NE, Ory M, Matthews KA, Schocken M, Bromberger J, Colvin A. Health-related quality of life in a multiethnic sample of middle-aged women: study of women’s health across the nation (SWAN). Med Care. (2003) 41(11):1262–76. doi: 10.1097/01.MLR.0000093479.39115.AF
122. Blümel JE, Chedraui P, Baron G, Belzares E, Bencosme A, Calle A, et al. A large multinational study of vasomotor symptom prevalence, duration, and impact on quality of life in middle-aged women. Menopause. (2011) 18(7):778–85. doi: 10.1097/gme.0b013e318207851d
123. Williams RE, Levine KB, Kalilani L, Lewis J, Clark RV. Menopause-specific questionnaire assessment in US population-based study shows negative impact on health-related quality of life. Maturitas. (2009) 62(2):153–9. doi: 10.1016/j.maturitas.2008.12.006
124. Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease: findings from the study of women’s health across the nation heart study. Circulation. (2008) 118(12):1234–40. doi: 10.1161/CIRCULATIONAHA.108.776823
125. Franco OH, Muka T, Colpani V, Kunutsor S, Chowdhury S, Chowdhury R, et al. Vasomotor symptoms in women and cardiovascular risk markers: systematic review and meta-analysis. Maturitas. (2015) 81(3):353–61. doi: 10.1016/j.maturitas.2015.04.016
126. Luke B, Gopal D, Cabral H, Stern JE, Diop H. Adverse pregnancy, birth, and infant outcomes in twins: effects of maternal fertility status and infant gender combinations; the Massachusetts outcomes study of assisted reproductive technology. Am J Obstet Gynecol. (2017) 217(3):330.e1–330.e15. doi: 10.1016/j.ajog.2017.04.025
127. Mahalingaiah S, Sun F, Cheng JJ, Chow ET, Lunetta KL, Murabito JM. Cardiovascular risk factors among women with self-reported infertility. Fertil Res and Pract. (2017) 3(1):7. doi: 10.1186/s40738-017-0034-0
128. Hayes SN, Tweet MS, Adlam D, Kim ESH, Gulati R, Price JE, et al. Spontaneous coronary artery dissection. J Am Coll Cardiol. (2020) 76(8):961–84. doi: 10.1016/j.jacc.2020.05.084
129. Waterbury TM, Tweet MS, Hayes SN, Eleid MF, Bell MR, Lerman A, et al. Early natural history of spontaneous coronary artery dissection. Circ Cardiovasc Interv. (2018) 11(9):e006772. doi: 10.1161/CIRCINTERVENTIONS.118.006772
130. Waterbury TM, Tarantini G, Vogel B, Mehran R, Gersh BJ, Gulati R. Non-atherosclerotic causes of acute coronary syndromes. Nat Rev Cardiol. (2020) 17(4):229–41. doi: 10.1038/s41569-019-0273-3
131. Alfonso F, Paulo M, Lennie V, Dutary J, Bernardo E, Jiménez-Quevedo P, et al. Spontaneous coronary artery dissection: long-term follow-up of a large series of patients prospectively managed with a “conservative” therapeutic strategy. JACC Cardiovasc Interv. (2012) 5(10):1062–70. doi: 10.1016/j.jcin.2012.06.014
132. Jackson R, Al-Hussaini A, Joseph S, van Soest G, Wood A, Macaya F, et al. Spontaneous coronary artery dissection: pathophysiological insights from optical coherence tomography. JACC Cardiovasc Imaging. (2019) 12(12):2475–88. doi: 10.1016/j.jcmg.2019.01.015
133. Kok SN, Hayes SN, Cutrer FM, Raphael CE, Gulati R, Best PJM, et al. Prevalence and clinical factors of migraine in patients with spontaneous coronary artery dissection. J Am Heart Assoc. (2018) 7(24):e010140. doi: 10.1161/JAHA.118.010140
134. Lettieri C, Zavalloni D, Rossini R, Morici N, Ettori F, Leonzi O, et al. Management and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol. (2015) 116(1):66–73. doi: 10.1016/j.amjcard.2015.03.039
135. Saw J, Starovoytov A, Humphries K, Sheth T, So D, Minhas K, et al. Canadian Spontaneous coronary artery dissection cohort study: in-hospital and 30-day outcomes. Eur Heart J. (2019) 40(15):1188–97. doi: 10.1093/eurheartj/ehz007
136. Nakashima T, Noguchi T, Haruta S, Yamamoto Y, Oshima S, Nakao K, et al. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: a report from the angina pectoris-myocardial infarction multicenter investigators in Japan. Int J Cardiol. (2016) 207:341–8. doi: 10.1016/j.ijcard.2016.01.188
137. Meng PN, Xu C, You W, Wu ZM, Xie DJ, Zhang H, et al. Spontaneous coronary artery dissection as a cause of acute myocardial infarction in young female population: a single-center study. Chin Med J (Engl). (2017) 130(13):1534–9. doi: 10.4103/0366-6999.208245
138. Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, et al. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American heart association. Circulation. (2018) 137(19):e523–57. doi: 10.1161/CIR.0000000000000564
139. Goel K, Tweet M, Olson TM, Maleszewski JJ, Gulati R, Hayes SN. Familial spontaneous coronary artery dissection: evidence for genetic susceptibility. JAMA Intern Med. (2015) 175(5):821–6. doi: 10.1001/jamainternmed.2014.8307
140. Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. (2012) 126(5):579–88. doi: 10.1161/CIRCULATIONAHA.112.105718
141. Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. (2014) 7(5):645–55. doi: 10.1161/CIRCINTERVENTIONS.114.001760
142. Elkayam U, Jalnapurkar S, Barakkat MN, Khatri N, Kealey AJ, Mehra A, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation. (2014) 129(16):1695–702. doi: 10.1161/CIRCULATIONAHA.113.002054
143. Smilowitz NR, Gupta N, Guo Y, Zhong J, Weinberg CR, Reynolds HR, et al. Acute myocardial infarction during pregnancy and the puerperium in the United States. Mayo Clin Proc. (2018) 93(10):1404–14. doi: 10.1016/j.mayocp.2018.04.019
144. Faden MS, Bottega N, Benjamin A, Brown RN. A nationwide evaluation of spontaneous coronary artery dissection in pregnancy and the puerperium. Heart. (2016) 102(24):1974–9. doi: 10.1136/heartjnl-2016-309403
145. Tweet MS, Hayes SN, Codsi E, Gulati R, Rose CH, Best PJM. Spontaneous coronary artery dissection associated with pregnancy. J Am Coll Cardiol. (2017) 70(4):426–35. doi: 10.1016/j.jacc.2017.05.055
146. Koul AK, Hollander G, Moskovits N, Frankel R, Herrera L, Shani J. Coronary artery dissection during pregnancy and the postpartum period: two case reports and review of literature. Catheter Cardiovasc Interv. (2001) 52(1):88–94. doi: 10.1002/1522-726x(200101)52:1%3C88::aid-ccd1022%3E3.0.co;2-p
147. Havakuk O, Goland S, Mehra A, Elkayam U. Pregnancy and the risk of spontaneous coronary artery dissection: an analysis of 120 contemporary cases. Circ Cardiovasc Interv. (2017) 10(3):e004941. doi: 10.1161/CIRCINTERVENTIONS.117.004941
148. Al-Hussaini A, Abdelaty AMSEK, Gulsin GS, Arnold JR, Garcia-Guimaraes M, Premawardhana D, et al. Chronic infarct size after spontaneous coronary artery dissection: implications for pathophysiology and clinical management. Eur Heart J. (2020) 41(23):2197–205. doi: 10.1093/eurheartj/ehz895
149. Eleid MF, Tweet MS, Young PM, Williamson E, Hayes SN, Gulati R. Spontaneous coronary artery dissection: challenges of coronary computed tomography angiography. Eur Heart J Acute Cardiovasc Care. (2018) 7(7):609–13. doi: 10.1177/2048872616687098
150. Pate GE, Lowe R, Buller CE. Fibromuscular dysplasia of the coronary and renal arteries? Catheter Cardiovasc Interv. (2005) 64(2):138–45. doi: 10.1002/ccd.20246
151. Saw J, Poulter R, Fung A, Wood D, Hamburger J, Buller CE. Spontaneous coronary artery dissection in patients with fibromuscular dysplasia: a case series. Circ Cardiovasc Interv. (2012) 5(1):134–7. doi: 10.1161/CIRCINTERVENTIONS.111.966630
152. Marano P, Wei J, Merz CNB. Coronary microvascular dysfunction: what clinicians and investigators should know. Curr Atheroscler Rep. (2023) 25(8):435–46. doi: 10.1007/s11883-023-01116-z
153. Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J Am Coll Cardiol. (2018) 72(21):2625–41. doi: 10.1016/j.jacc.2018.09.042
154. Smilowitz NR, Toleva O, Chieffo A, Perera D, Berry C. Coronary microvascular disease in contemporary clinical practice. Circ Cardiovasc Interv. (2023) 16(6):e012568. doi: 10.1161/CIRCINTERVENTIONS.122.012568
155. Bairey Merz CN, Kelsey SF, Pepine CJ, Reichek N, Reis SE, Rogers WJ, et al. The women’s ischemia syndrome evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol. (1999) 33(6):1453–61. doi: 10.1016/S0735-1097(99)00082-0
156. Jalnapurkar S, Landes S, Wei J, Mehta PK, Shufelt C, Minissian M, et al. Coronary endothelial dysfunction appears to be a manifestation of a systemic process: a report from the women’s ischemia syndrome evaluation—coronary vascular dysfunction (WISE-CVD) study. PLoS One. (2021) 16(9):e0257184. doi: 10.1371/journal.pone.0257184
157. Taqueti VR, Shaw LJ, Cook NR, Murthy VL, Shah NR, Foster CR, et al. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation. (2017) 135(6):566–77. doi: 10.1161/CIRCULATIONAHA.116.023266
158. Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the national heart, lung and blood institute WISE (women’s ischemia syndrome evaluation) study. J Am Coll Cardiol. (2010) 55(25):2825–32. doi: 10.1016/j.jacc.2010.01.054
159. Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2021) 144(22):e406–20. doi: 10.1161/CIR.0000000000001029
160. Handberg EM, Merz CNB, Cooper-Dehoff RM, Wei J, Conlon M, Lo MC, et al. Rationale and design of the women’s ischemia trial to reduce events in nonobstructive CAD (WARRIOR) trial. Am Heart J. (2021) 237:90–103. doi: 10.1016/j.ahj.2021.03.011
161. Westerweel PE, Luyten RKMAC, Koomans HA, Derksen RHWM, Verhaar MC. Premature atherosclerotic cardiovascular disease in systemic lupus erythematosus. Arthritis Rheum. (2007) 56(5):1384–96. doi: 10.1002/art.22568
162. Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcón GS, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with medicaid coverage, 2000–2004. Arthritis Rheum. (2013) 65(3):753–63. doi: 10.1002/art.37795
163. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. (2019) 139(25):e1091–122. doi: 10.1161/CIR.0000000000000625
164. Center for Behavioral Health Statistics and Quality. Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. (2015). Available online at: http://www.samhsa.gov/data (Accessed June 15, 2023)
165. Carney RM, Freedland KE. Depression and coronary heart disease. Nat Rev Cardiol. (2017) 14(3):145–55. doi: 10.1038/nrcardio.2016.181
166. Suglia SF, Koenen KC, Boynton-Jarrett R, Chan PS, Clark CJ, Danese A, et al. Childhood and adolescent adversity and cardiometabolic outcomes: a scientific statement from the American heart association. Circulation. (2018) 137(5):e15–28. doi: 10.1161/CIR.0000000000000536
167. Shaw LJ, Pepine CJ, Xie J, Mehta PK, Morris AA, Dickert NW, et al. Quality and equitable health care gaps for women. J Am Coll Cardiol. (2017) 70(3):373–88. doi: 10.1016/j.jacc.2017.05.051
168. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2019 update: a report from the American heart association. Circulation. (2019) 139(10):e257–80. doi: 10.1161/CIR.0000000000000659
169. Bairey Merz CN, Andersen H, Sprague E, Burns A, Keida M, Walsh MN, et al. Knowledge, attitudes, and beliefs regarding cardiovascular disease in women. J Am Coll Cardiol. (2017) 70(2):123–32. doi: 10.1016/j.jacc.2017.05.024
170. Timpka S, Fraser A, Schyman T, Stuart JJ, Åsvold BO, Mogren I, et al. The value of pregnancy complication history for 10-year cardiovascular disease risk prediction in middle-aged women. Eur J Epidemiol. (2018) 33(10):1003–10. doi: 10.1007/s10654-018-0429-1
171. McKinney J, Keyser L, Clinton S, Pagliano C. ACOG committee opinion no. 736: optimizing postpartum care. Obstet Gynecol. (2018) 132(3):784–5. doi: 10.1097/AOG.0000000000002849
172. Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American heart association. Circulation. (2011) 123(11):1243–62. doi: 10.1161/CIR.0b013e31820faaf8
173. Rich-Edwards JW, Klungsoyr K, Wilcox AJ, Skjaerven R. Duration of pregnancy, even at term, predicts long-term risk of coronary heart disease and stroke mortality in women: a population-based study. Am J Obstet Gynecol. (2015) 213(4):518.e1–e8. doi: 10.1016/j.ajog.2015.06.001
174. Valle JA, Ho PM. Medication adherence in secondary prevention post-myocardial infarction. Curr Treat Options Cardio Med. (2014) 16(12):349. doi: 10.1007/s11936-014-0349-7
175. Spoletini I, Ferrari R, Rosano GMC. Living with stable angina: patients’ pathway and needs in angina. J Cardiovasc Med. (2020) 21(5):377–82. doi: 10.2459/JCM.0000000000000954
Keywords: women's cardiovascular health, obstetrics and gynecology, reproductive health, pregnancy, cardiovascular disease, sex-specific risk factors, adverse pregnancy outcomes, women's health
Citation: Nguyen AH, Hurwitz M, Sullivan SA, Saad A, Kennedy JLW and Sharma G (2024) Update on sex specific risk factors in cardiovascular disease. Front. Cardiovasc. Med. 11:1352675. doi: 10.3389/fcvm.2024.1352675
Received: 8 December 2023; Accepted: 17 January 2024;
Published: 6 February 2024.
Edited by:
Maurice Enriquez-Sarano, Minneapolis Heart Institute Foundation (MHIF), United StatesReviewed by:
Sawan Jalnapurkar, Gadsden Regional Medical Center, United States© 2024 Nguyen, Hurwitz, Sullivan, Saad, Kennedy and Sharma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Garima Sharma Z2FyaW1hLnNoYXJtYUBpbm92YS5vcmc=
Abbreviations CVD, cardiovascular disease; IHD, ischemic heart disease; HF, heart failure; APO, adverse pregnancy outcome; HDP, hypertensive disorders of pregnancy; GDM, gestational diabetes mellitus; T2DM, type II diabetes Mellitus; sPTD, spontaneous preterm delivery; MI, myocardial infarction; SGA, small for gestational age; LGA, large for gestational age; FGR, fetal growth restriction; PCOS, polycystic ovarian syndrome; CIMT, carotid intima-media thickness; CAC, coronary artery calcium; POF, premature ovarian failure; MT, menopause transition; VMS, vasomotor symptoms; ART, assisted reproductive technology; IVF, in vitro fertilization; SLE, systemic erythematous lupus; RA, rheumatoid arthritis.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.