
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med. , 12 April 2024
Sec. Pediatric Cardiology
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1351530
This article is part of the Research Topic Neonatal Cardiology and Cardiac Surgery in Congenital Heart Disease. View all 8 articles
Published data estimate the prevalence of the vascular ring at approximately 7 per 10,000 live births. The association of a double aortic arch with a D-transposition of the great arteries has been rarely described in the literature. In this study, we report the prenatal diagnosis of a 28-year-old woman. A fetal echocardiography at a gestational age of 24 weeks + 6 days showed a D-transposition of the great arteries and a double aortic arch with a ventricular septal defect and pulmonary stenosis. On the first night after birth, the baby experienced an increase in lactate levels, with the rate of oxygen saturation consistently below 80%. A few hours after birth, the patient underwent a Rashkind procedure. An echocardiography, CT chest x-ray, and CT angiogram confirmed a diagnosis with a severe reduction of the tracheal lumen (>85%) and bronchomalacia. Then, the patient underwent posterior tracheopexy and aortopexy and later an arterial switch operation, ventricular septal defect closure, and resection of a part of the infundibular septum, accepting the risk of potential neoaortic obstruction. The literature has reported only two cases of patients with a fetal echocardiogram diagnosis. Therefore, our patient is only the third one with a fetal diagnosis and the second one with a complex intracardiac anatomy, characterized not only by a ventricular septal defect but also by two separate components of the obstruction (a bicuspid valve and a dysplastic valve with a posterior deviation of the infundibular septum). In conclusion, a D-transposition of the great arteries with a double aortic arch remains an extremely unusual association. The clinical outcome of these patients presents a high degree of variability and is entirely unpredictable in prenatal life. Our greatest aim as fetal and perinatal cardiologists is to improve the management and outcome of these patients through a fetal diagnosis, recognizing types of congenital heart disease in newborns who require early neonatal invasive procedures.
In this study, we report the prenatal diagnosis of a combined D-transposition of the great arteries (D-TGA) with a ventricular septal defect (VSD), pulmonary stenosis, and a double aortic arch (DAA) in a 28-year-old woman at 24 weeks and 6 days of gestational age (GA). No family history of congenital heart disease or genetic disorders was found. A fetal echocardiography revealed a normal visceral situs, atrioventricular concordance, and a D-TGA, with the anterior aorta arising from the right ventricle and the posterior pulmonary trunk arising from the left ventricle. Two symmetrical aortic arches surrounded the trachea, each giving rise to separate arteries, respectively: the left carotid and left subclavian arteries, and the right carotid and right subclavian arteries (Figures 1, 2). Subpulmonary VSD and pulmonary valve (PV) dysplasia with a right outflow tract obstruction were also noted (Figure 3).
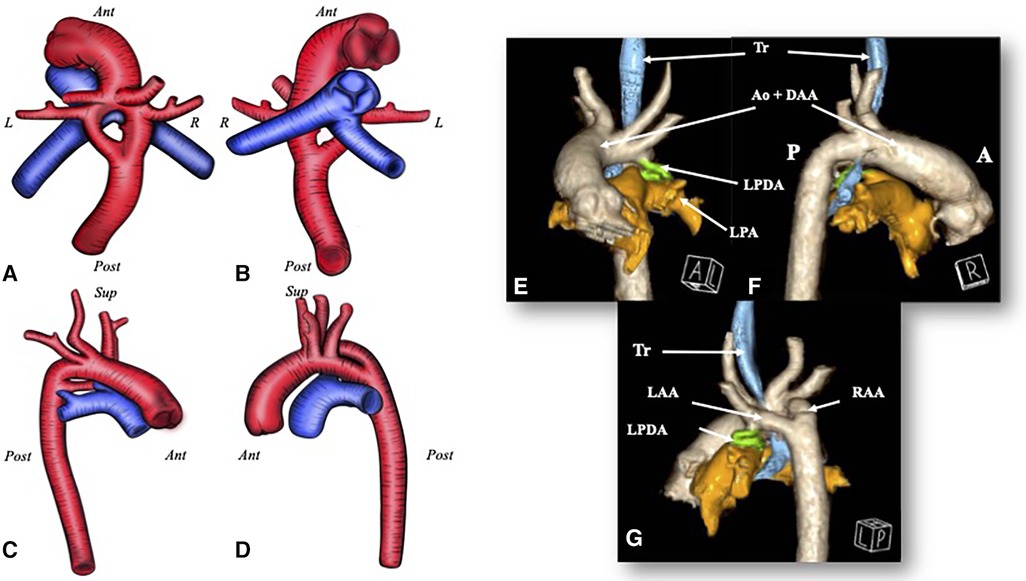
Figure 1. A D-TGA with a DAA. Image (A–D) The red blood vessel is the aorta with a double aortic arch; the blue one is the pulmonary artery. (A) A view from above; (B) a view from below; (C) a view from the right side; and (D) a view from the left side. (E–G) A CT scan 3D rendering of the double aortic arch in the transposition of the great arteries and the relationship with the trachea. (E) An anterior view; (F) a view from the right side; and (G) a posteroleft view. Ant, anterior; Post, posterior; R, right; L, left; Ao, aorta; LPA, left pulmonary artery; LPDA, left patent ductus arteriosus; Tr, trachea.
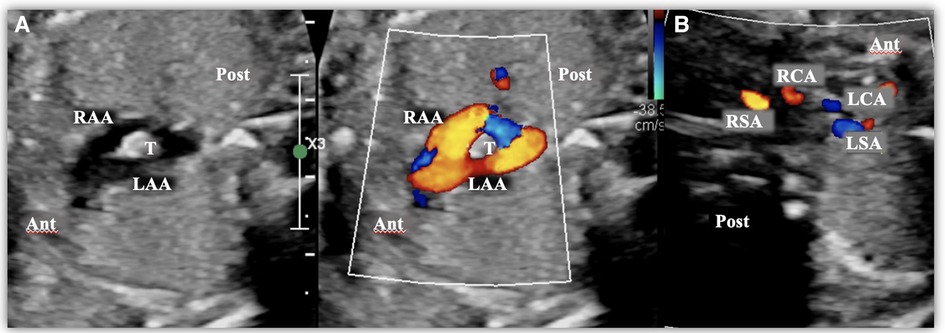
Figure 2. Images taken from a fetal transthoracic echocardiogram. (A) A cross-sectional view at the level of the aortic arch, with a color Doppler, where it is possible to observe the complete vascular ring surrounding the trachea, consisting of a double aortic arch. (B) A cross-sectional view at the level of the epiaortic vessels, with a color Doppler, showing the four epiaortic vessels originating separately (two on the right and two on the left). Ant, anterior; LAA, left aortic arch; LCA, Left carotid artery; LSA, left subclavian artery; Post, posterior; RCA, right carotid artery; RSA, right subclavian artery; T, trachea.
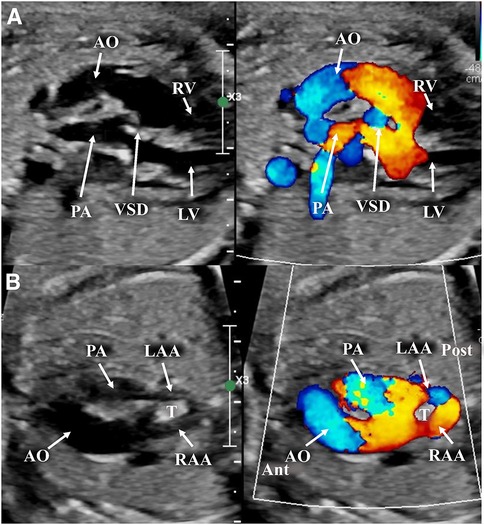
Figure 3. Images taken from a fetal transthoracic echocardiogram. (A) A long-axis view, with a color Doppler, where it is possible to observe the D-transposition with the anterior aorta and posterior pulmonary artery; the ventricular septal defect, with a dysplastic pulmonary valve. (B) An off-axis view at the level of the aortic arch, with a color Doppler, showing the anterior aorta with its complete vascular ring surrounding the trachea, consisting of a double aortic arch, and the posterior pulmonary artery. Ant, anterior; LAA, left aortic arch; LV, left ventricle; Post, posterior; RV, right ventricle; T, trachea.
The baby was born by a scheduled cesarean section (GA 38 weeks + 5 days). At birth, the oxygen saturation value was 75%. Prostaglandin infusion was started after placing an umbilical catheter. Thereafter, the infant was quickly transferred from the birth center to our hospital.
A postnatal echocardiography confirmed a D-TGA with a DAA, with a dominant right-sided aortic arch, a hypoplastic left-sided aortic arch, and a small tortuous left ductus arteriosus (DA). The ductus arteriosus was non-hemodynamically significant. The interatrial communication was not a restricted foramen ovale. The PV was dysplastic and bicuspid with a posterior deviation of the infundibular septum. The degree of obstruction was classified as “moderate with a dynamic component.” The coronary arteries had a normal origin from a pathological perspective, but both were positioned in a paracommissural manner.
After a few hours, the patient developed severe acidosis, hypercapnia, dyspnea, and respiratory distress and required intubation. On the first night after birth, the baby experienced an increase in lactate levels, with the oxygen saturation rate consistently below 80%. Therefore, in order to allow an efficient mixing between the two circulations, atrial septostomy, as laid down by American cardiologist William Rashkind, was performed. After adequate mixing was achieved at the atrial level, we noticed an improvement in the oxygen saturation rate (>85%) and a gradual decrease in lactate levels, following which prostaglandin infusion was initiated.
A CT chest x-ray with angiography revealed severe tracheal narrowing with subocclusion (>85%) and tracheobronchomalacia caused by a vascular ring (VR), secondary to compression (Figure 1). Fibrobronchoscopy was performed the day before the surgery. The procedure, which was conducted with the patient awake under sedation but spontaneous breathing, revealed an approximately 70% reduction in the size of the respiratory tract at the transition between the middle and the distal third of the trachea.
The patient was admitted in the pediatric intensive care unit (ICU) but developed multiple respiratory infections caused by Klebsiella pneumoniae, Enterobacter cloacae, and Acinetobacter baumannii, which complicated the respiratory condition. Furthermore, multiple extubation attempts failed. The prolonged hospitalization, the tracheomalacia, and recurrent infections had certainly worsened the respiratory condition. For this reason, at seven days of life, the hypoplastic left arch and DA were resected to relieve the severe tracheobronchomalacia and its consequences. Despite this, extubation was unsuccessful, probably because of the unfavorable anatomy related to the TGA causing vascular compression.
Furthermore, at 2 months and 5 days of life, the patient underwent posterior tracheopexy and aortopexy with extracorporeal circulation. The cardiac surgeon secured a complete release of the esophagus, trachea, and left transverse arch by dissecting the surrounding tissue. Intraoperative endoscopic control and a 1-month follow-up bronchoscopy were performed, documenting a favorable caliber of the trachea and bronchi. Nevertheless, the patient remained dependent on invasive mechanical ventilation. Unfortunately, immediately after the procedure, a brain ultrasound revealed a slight and blurred subcortical hyperechogenicity in the left frontoparietal region, without an evident cavitation area. This was an element probably related to ischemic lesions. Consequently, a brain CT revealed two areas of cortico-subcortical hypodensity in the frontal and temporal regions on the right, indicative of new brain ischemic lesions. Given the timing of the onset, these lesions appeared to be correlated with extracorporeal circulation.
Because of the restrictiveness of the foramen ovale and the poor clinical improvement, a second Rashkind procedure was performed with less improvement in clinical conditions. During hospitalization, the echocardiographic assessment of the VSD highlighted an evolution toward restrictiveness. After addressing the respiratory complications, the patient, who weighed 3.5 kg, underwent an arterial switch operation (ASO), VSD closure, and resection of a part of the infundibular septum, accepting the risk of potential neoaortic obstruction (Figure 4). During the intraoperative inspection, the neoaortic valve was bicuspid with normal leaflet excursion. The leaflets appeared slightly thickened, but the ring seemed to be of adequate size. The decision to perform an ASO instead of the Nikaidoh or Rastelli procedure was based on the patient's low weight. After the patient achieved clinical stability, he was discharged.
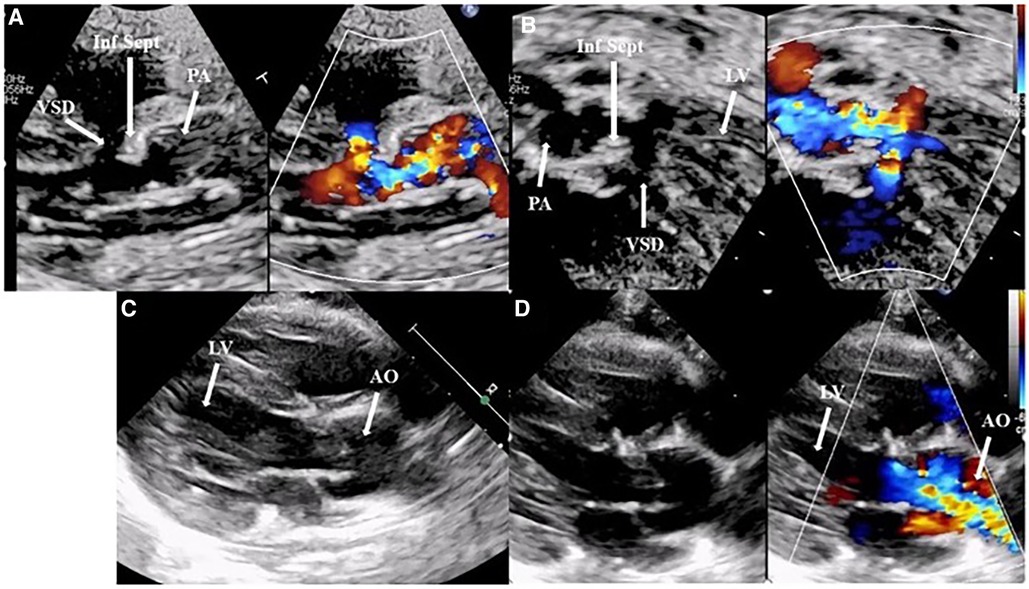
Figure 4. (A,B) A preoperative transthoracic echocardiogram: a parasternal long-axis view and five-chamber view with and without a color Doppler, highlighting the posterior deviation of the infundibular septum causing subvalvular and valvar pulmonary obstruction and the restrictive interventricular septal defect. Images (C,D), A postoperative transthoracic echocardiogram: a parasternal long-axis view without and with a color Doppler, showing an excellent surgical outcome after an arterial switch operation, closure of the interventricular defect, and resection of the infundibular septum. AO, aorta; Inf Sept, infundibular septum; LV, left ventricle; PA, pulmonary artery.
At the 1-year follow-up (6 months after corrective surgery), the patient was found to be stable, despite having a severe impairment of neurological function due to ischemic–hemorrhagic lesions; this was followed up with a brain MRI. He developed a mild neoaortic insufficiency with a mean pressure gradient of 10 mmHg. The patient is currently undergoing regular cardiological and neurological follow-ups.
Our patient represents a clinical case of a prenatal diagnosis of a D-TGA, VSD, and pulmonary and subpulmonary stenosis associated with a DAA, diagnosed in fetal life and confirmed during the postnatal period.
Published data estimate the prevalence of a VR at about 7 per 10,000 live births (1). The incidence of the DAA in the literature is 1.5 per 10,000. The most common type of vascular arch is the right aortic arch (RAA) with an aberrant left subclavian artery with an incidence of 5.4 per 10,000 (2).
The DAA is attributed to the absence of regression of the right fourth branchial arch during embryogenesis.
The VR can be isolated or associated with intracardiac and/or extracardiac anomalies. With regard to the DAA, an underlying cardiac diagnosis is prevalent in up to 12% of cases and includes a VSD, Tetralogy of Fallot, and other complex congenital cardiac conditions (3).
The association of a DAA with a D-TGA has been rarely described (Table 1) in the literature, with only nine cases of patients being described to date. Of these nine patients, six were detected by a postnatal diagnosis with echocardiography (4), angiocardiography (5, 6), direct intraoperative inspection (7), or necropsy (8). The literature has reported just two cases of patients with a fetal echocardiogram diagnosis (9, 10). Therefore, our patient is the third one with a fetal diagnosis and the second one with a complex intracardiac anatomy, characterized not only by a VSD but also by two separate components of the obstruction (a bicuspid valve and a dysplastic valve with a posterior deviation of the infundibular septum).
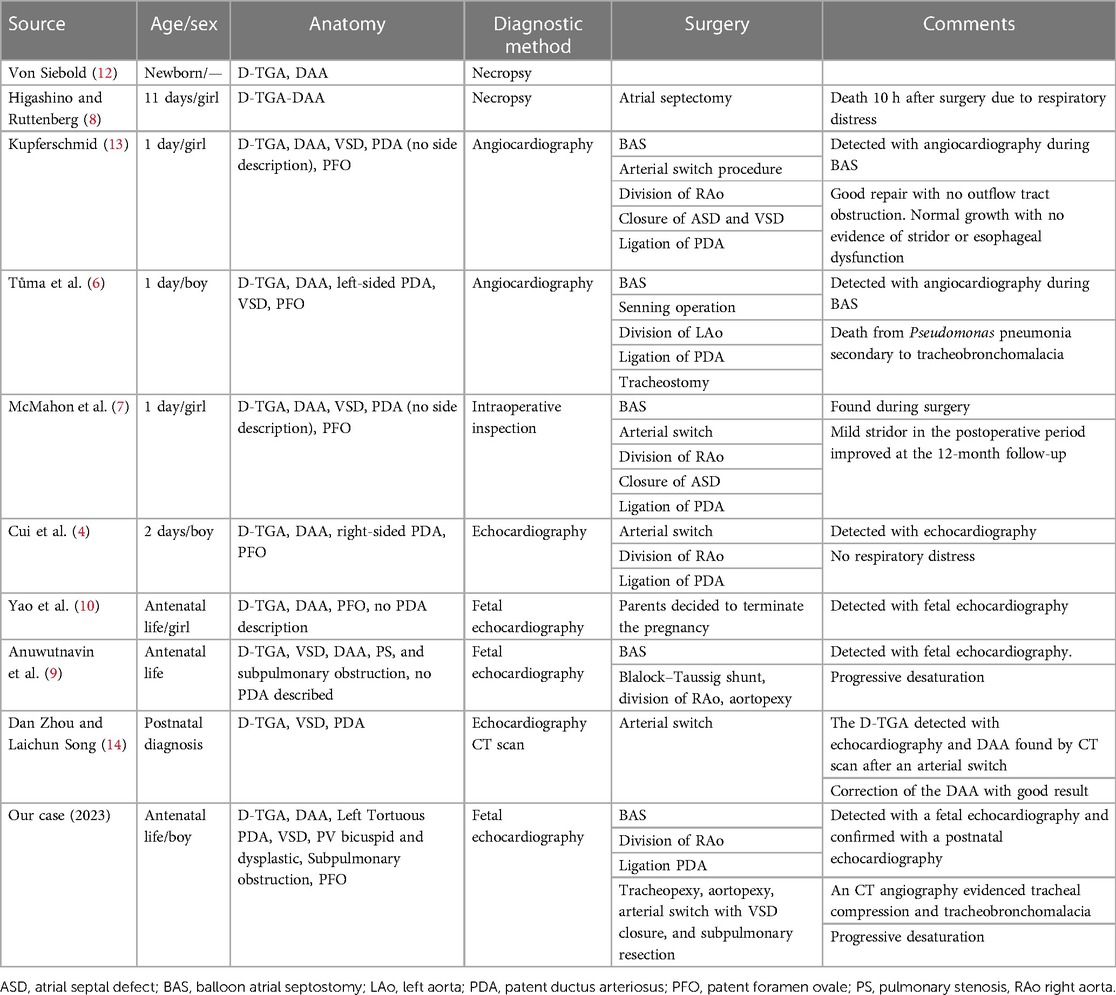
Table 1. Patients with a D-transposition of the great arteries with a double aortic arch described in the literature.
The clinical presentation of patients with vascular rings is highly variable. Respiratory symptoms appear in 91% of presentations of the DAA, such as barky cough, wheezing, shortness of breath, and dysphagia, especially for solid food, with stridor being the most common presentation (77%) (11).
A multicenter study reported that respiratory signs were present in 21% of neonates on the day of birth: stridor was the most common clinical sign evaluated. Furthermore, 23% of the sample population were initially asymptomatic and not operated upon in the first year, and of these, 27% of patients developed respiratory symptoms/signs between 1 and 2 years of age (15).
Published data on the DAA indicate an approximately 5% association with array abnormalities. Postnatal series on the DAA are reported in the literature, and these describe an association of the DAA with 22q11 microdeletion. Other genetic anomalies that have been identified are trisomy 21, 15q24 microdeletion, and benign copy number variant (16). The majority of patients with a D-TGA have undefined genetics (17–19). The prevalence of genetic anomalies in patients with a D-TGA/VSD/PS and DAA has not been estimated; in the case of our patient with this combination, the results of the karyotype and array-CGH tests were negative. Screening test results for autoimmune and humoral immune function were also negative.
Fleenor et al. observed that patients with a VR who are symptomatic have a significantly altered tracheal geometry compared with non-symptomatic individuals in all dimensions measured, including the area and the longest and shortest diameters (20). In addition, tracheal compression impacts mucociliary clearance, leading to secretion stasis; symptoms become evident during expiration when the intrathoracic pressure (Ppl) overcomes the intratracheal pressure (Ptr), leading to a worsening of airway narrowing (20, 21). The worsening of symptoms is explained by Bernoulli's principle, according to which the increase in speed of a fluid occurs simultaneously with a decrease in pressure at the point where the channel narrows. This may result in inadequate removal of secretions (22). This, in turn, can lead to the recurrence and/or prolongation of respiratory infections.
Although a DAA carries a good prognosis, its association with tracheal anomalies could worsen the outcome (23). Reported that tracheal stenosis with pulmonary or cardiovascular malformations was associated with a 79% mortality rate.
The gold standard of treatment is surgical correction. Furthermore, an accurate estimation of the degree of tracheal compression during the prenatal period is a challenging task, yet it significantly impacts patient outcomes. In the case of our patient, it could be assumed that, although there is no evidence of tracheal compression given the rarity of the condition, the unpredictability of tracheal compression is also related to the different anatomical arrangements of the great vessels in the D-TGA. In fact, in addition to the compression and the tracheomalacia determined by the VR, the anterior position of the aorta and its course could contribute to a worsening of compression. Furthermore, the posterior pulmonary artery could cause a displacement of the trachea and its bifurcation, influencing respiratory dynamics. This may predispose to nosocomial infections along with a prolonged hospitalization in the ICU.
In conclusion, a D-TGA with a DAA remains an extremely unusual association. The outcome of patients with this condition presents a high degree of variability and is entirely unpredictable in prenatal life. Factors influencing this phenotypic range are related not only to intracardiac anatomy but also to the arrangement of the great vessels, tracheal compression, and tracheomalacia associated with the VR. The last two features represent negative prognostic factors, as they determine an increased risk of prolonged ICU stay and prolonged intubation.
Our greatest aim as fetal and perinatal cardiologists is to improve the management and outcome of patients with the above condition through a fetal diagnosis, recognizing types of congenital heart disease in newborns who require early neonatal invasive procedures.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) and minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
MM: Writing – original draft, Conceptualization, Funding acquisition. AM: Writing – original draft, Conceptualization. CC: Writing – review & editing. PM: Writing – review & editing. MC: Writing – review & editing. LP: Writing – review & editing. AT: Writing – review & editing.
The authors declare that financial support was received for the research, authorship, and/or publication of this article.
This work was also supported by the Italian Ministry of Health with “Current Research funds”.
The authors thank the designer Antonio Tremante (antoniotremante1997@gmail.com) for providing original handmade drawings for Figure 1 in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor SA declared a shared affiliation with the authors MM, AM, CC, PM, MC, and AT at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1351530/full#supplementary-material.
ASO, arterial switch operation; D-TGA, D-transposition of the great arteries; DAA, double aortic arch; GA, gestational age; ICU, intensive care unit; PV, pulmonary valve; VSD, ventricular septal defect; VR, vascular ring.
1. Evans WN, Acherman RJ, Ciccolo ML, Lehoux J, Berthoty D, Montes A, et al. Isolated vascular rings are common cardiovascular malformations. World J Pediatr Congenit Heart Surg. (2023) 14(1):21–3. doi: 10.1177/21501351221122972
2. Vigneswaran TV, Jabak S, Syngelaki A, Charakida M, Simpson JM, Nicolaides KH, et al. Prenatal incidence of isolated right aortic arch and double aortic arch. J Matern Fetal Neonatal Med. (2021) 34(18):2985–90. doi: 10.1080/14767058.2019.1676413
3. Worhunsky DJ, Levy BE, Stephens EH, Backer CL. Vascular rings. Semin Pediatr Surg. (2021) 30(6):151128. doi: 10.1016/j.sempedsurg.2021.151128
4. Cui W, Patel D, Husayni TS, Roberson DA. Double aortic arch and D-transposition of the great arteries. Echocardiography. (2008) 25(1):91–5. doi: 10.1111/j.1540-8175.2007.00554.x
5. Shirali GS, Geva T, Ott DA, Bricker JT. Double aortic arch and bilateral patent ducti arteriosi associated with transposition of the great arteries: missing clinical link in an embryologic theory. Am Heart J. (1994) 127(2):451–3. doi: 10.1016/0002-8703(94)90143-0
6. Tůma S, Slavík Z, Tax P, Hučin B, Škovránek J. Double aortic arch in D-transposition of the great arteries complicated by tracheobronchomalacia. Cardiovasc Intervent Radiol. (1995) 18(2):115–7. doi: 10.1007/BF02807235
7. McMahon CJ, Bezold LI, Vick GW 3rd. Double aortic arch in D-transposition of the great arteries: confirmation of dominant arch by magnetic resonance imaging. Tex Heart Inst J. (2000) 27(4):398–400. PMID: 11198315. PMCID: PMC10111111198315
8. Higashino SM, Ruttenberg HD. Double aortic arch associated with complete transposition of the great vessels. Br Heart J. (1968) 30(4):579–81. doi: 10.1136/hrt.30.4.579
9. Anuwutnavin S, Satou G, Finn P, Lee L, Sklansky M. Fetal diagnosis of D-transposition of the great arteries associated with a double aortic arch. J Ultrasound Med. (2015) 34(9):1701–4. doi: 10.7863/ultra.15.14.10012
10. Yao WM, Wang JM, Huang HF, Ye YH. Prenatal diagnosis of double aortic arch and D-transposition of the great arteries: a case report. Prenat Diagn. (2010) 30(4):382–3. doi: 10.1002/pd.2472
11. Shah RK, Mora BN, Bacha E, Sena LM, Buonomo C, Del Nido P, et al. The presentation and management of vascular rings: an otolaryngology perspective. Int J Pediatr Otorhinolaryngol. (2007) 71(1):57–62. doi: 10.1016/j.ijporl.2006.08.025
12. Von Siebold C. Th. Aortenbogen, Ringformiger, bei einemneugenbornen, blausuchtigen Kinde. J Geburtschulfe Frauenzimmer Kinderkraukheiten. (1837) 16:294.
13. Kupferschmid JP, Burns SA, Jonas RA, Keane JF, Spevak PJ, Wernovsky G. Repair of double aortic arch associated with D-transposition of the great arteries. Ann Thorac Surg. (1993) 56(3):570–2. doi: 10.1016/0003-4975(93)90905-w
14. Zhou D, Song L, Tao L, Zhou H. D-TGA combined with left arch atresia of a double aortic arch. Heart Lung Circ. (2017) 26(11):e76–8. doi: 10.1016/j.hlc.2017.03.155
15. Vigneswaran TV, Van Poppel MP, Griffiths B, James P, Jogeesvaran H, Rahim Z, et al. Postnatal impact of a prenatally diagnosed double aortic arch. Arch Dis Child. (2021) 106(6):564–9. doi: 10.1136/archdischild-2020-318946
16. McElhinney DB, Clark BJ, Weinberg PM, Kenton ML, McDonald-McGinn D, Driscoll DA, et al. Association of chromosome 22q11 deletion with isolated anomalies of aortic arch laterality and branching. J Am Coll Cardiol. (2001) 37(8):2114–9. doi: 10.1016/S0735-1097(01)01286-4
17. Goldmuntz E, Bamford R, Karkera JD, dela Cruz J, Roessler E, Muenke M. CFC1 mutations in patients with transposition of the great arteries and double-outlet right ventricle. Am J Hum Genet. (2002) 70(3):776–80. doi: 10.1086/339079
18. D’Alessandro LCA, Latney BC, Paluru PC, Goldmuntz E. The phenotypic spectrum of ZIC3 mutations includes isolated d-transposition of the great arteries and double outlet right ventricle. Am J Med Genet A. (2013) 161(4):792–802. doi: 10.1002/ajmg.a.35849
19. Chhin B, Hatayama M, Bozon D, Ogawa M, Schön P, Tohmonda T, et al. Elucidation of penetrance variability of a ZIC3 mutation in a family with complex heart defects and functional analysis of ZIC3 mutations in the first zinc finger domain. Hum Mutat. (2007) 28(6):563–70. doi: 10.1002/humu.20480
20. Fleenor JT, Weinberg PM, Kramer SS, Fogel M. Vascular rings and their effect on tracheal geometry. Pediatr Cardiol. (2003) 24(5):430–5. doi: 10.1007/s00246-002-0385-z
21. Porcaro F, Ciliberti P, Petreschi F, Secinaro A, Allegorico A, Coretti A, et al. Long term respiratory morbidity in patients with vascular rings: a review. Ital J Pediatr. (2023) 49(1):24. doi: 10.1186/s13052-023-01430-x
22. Wallis C, Alexopoulou E, Antón-Pacheco JL, Bhatt JM, Bush A, Chang AB, et al. ERS statement on tracheomalacia and bronchomalacia in children. Eur Respir J. (2019) 54(3):1900382. doi: 10.1183/13993003.00382-2019
Keywords: fetal diagnosis, transposition of the great arteries, double aortic arch, vascular ring, fetal echocardiography
Citation: Masci M, Missineo A, Campanale CM, Moras P, Colucci MC, Pasquini L and Toscano A (2024) Case Report: An unusual case of a transposition of the great arteries with a double aortic arch: a highly complex fetal diagnosis with an unpredictable outcome. Front. Cardiovasc. Med. 11:1351530. doi: 10.3389/fcvm.2024.1351530
Received: 6 December 2023; Accepted: 25 March 2024;
Published: 12 April 2024.
Edited by:
Salvatore Agati, Bambino Gesù Children's Hospital (IRCCS), ItalyReviewed by:
Maruti Haranal, U N Mehta Institute of Cardiology and Research, India© 2024 Masci, Missineo, Campanale, Moras, Colucci, Pasquini and Toscano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: A. Missineo YW5uYS5taXNzaW5lbzExQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.