- 1Virginia Heart, Falls Church, VA, United States
- 2Inova Schar Heart and Vascular Institute, Falls Church, VA, United States
- 3Willis Knighton Medical Center, Shreveport, LA, United States
- 4Wellspan Health, York, PA, United States
Coronary chronic total occlusions (CTO) are present in up to one-third of patients with coronary artery disease (CAD). It is thus essential for all clinical cardiologists to possess a basic awareness and understanding of CTOs, including optimal evaluation and management. While percutaneous coronary intervention (PCI) for CTO lesions has many similarities to non-CTO PCI, there are important considerations pertaining to pre-procedural evaluation, interventional techniques, procedural complications, and post-procedure management and follow-up unique to patients undergoing this highly specialized intervention. Distinct from other existing topical reviews, the current manuscript focuses on key knowledge relevant to non-interventional cardiologists.
Introduction
A chronic total occlusion (CTO) is defined as a 100% coronary artery occlusion that is non-acute and has been present for at least 3 months (1). Estimation of the occlusion duration is based upon first onset of classic anginal (or anginal equivalent) symptoms and/or history of myocardial infarction (MI) in the target vessel territory. Occluded coronary arteries discovered within 30 days of a MI are not considered CTOs, even though they may present technical revascularization challenges compared to acute lesions (2). There is an observed 15%–35% prevalence of CTOs in patients with CAD, increasing to 54%–89% for patients following coronary artery bypass grafting (CABG)—which has been noted to accelerate native vessel CAD and increase CTO prevalence post-operatively (3–8). The finding of a CTO during a diagnostic cardiac catheterization is also a common reason for referral to CABG, even though up to 30% of CTOs may still not be bypassed at surgery, as evidenced by data from the 2009 randomized SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) trial (9, 10).
Fortunately, there have been significant advances in both equipment and procedural techniques for performing CTO PCI over the last decade. Yet, PCI referrals and attempt rates remain low—often influenced by past decades’ technological limitations. Whereas previous historical CTO PCI success rates hovered around 60%, success rates have improved to 80%–90% in contemporary CTO PCI registries (11–14). CTO PCI is most commonly performed to ameliorate anginal symptoms and improve quality of life, based on current data and expert recommendations (15). Anginal symptoms may be both “classic” and “non-classic” and include exertional chest (or jaw, neck, shoulder, arm, or abdominal) discomfort, or shortness of breath, and/or decreased exercise tolerance (16). Many patients with less-typical symptoms may incorrectly attribute these adverse feelings to non-cardiac disorders or to the “normal aging process”—as may their physicians. Patients may often understandably (and unfortunately) reduce their daily physical activity (to include simple activities of daily living) progressively over time to prevent or attenuate anginal burden, at the expense of quality of life (QOL) (17, 18).
Indications: when to consider CTO PCI?
Current expert consensus indications to consider recanalization of a CTO include: (1) to alleviate lifestyle-limiting symptoms and/or to increase exercise capacity; (2) to reduce the extent of ischemia as detected by non-invasive testing; (3) to improve dyspnea related to reduced left ventricular (LV) dysfunction with demonstrable evidence of viable myocardium; and (4) to improve long-term prognosis in patients with high-risk and prognostically-significant multi-vessel CAD (19–27). A less certain clinical indication is the prevention of a “double jeopardy” acute coronary syndrome event—occurring with acute occlusion of a non-CTO coronary artery providing collateral flow to a CTO myocardial territory, resulting in acute multivessel MI with risk for complete circulatory collapse due to cardiogenic shock (Figure 1) (28, 29).
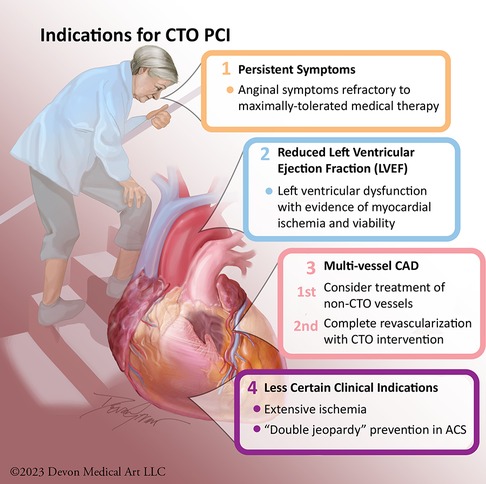
Figure 1. Indications for CTO PCI. Potential indications for CTO PCI primarily include: relief of angina, improvement in left ventricular systolic function, reduction of ischemia burden, prevention of “double jeopardy” in acute coronary syndrome, and complete revascularization.
The 2021 ACC/SCAI revascularization guidelines assign a Class IIb (treatment may be considered, but usefulness or efficacy is less well-established) recommendation for CTO PCI in patients with suitable anatomy and refractory angina despite medical therapy (30). The guidelines emphasize that the primary goal of CTO PCI should be to relieve symptoms, improve QOL, and increase exercise capacity (30). Based on the weight of existing randomized trial and observational data, anginal symptom improvement should remain the primary indication for consideration of CTO PCI.
Clinical evidence for CTO PCI
Randomized clinical trials demonstrate that CTO PCI is most beneficial for symptom relief (31–34). The 2018 Euro-CTO (Randomized Multicentre Trial to Evaluate the Utilization of Revascularization or Optimal Medical Therapy for the Treatment of Chronic Total Coronary Occlusions) trial assessed health status difference at 12-months between optimal medical therapy (OMT) alone or OMT combined with PCI (11). CTO PCI was associated with significantly improved health status at follow-up compared to OMT alone. Patients with successful CTO PCI were noted to have fewer physical limitations, less angina, better mobility, and increased physical activity after revascularization as compared with patients treated with OMT alone. Additionally, observed periprocedural risks were low, and 12-month MACE rates were comparable to the OMT group.
The 2019 Decision-CTO (Drug-Eluting Stent Implantation Versus Optimal Medical Treatment in Patients With Chronic Total Occlusion) trial examined the outcomes of OMT alone compared to PCI coupled with OMT in patients with CTOs—and demonstrated low procedural complication rates and high procedural success but no difference in major adverse cardiovascular events (MACE) (35). Unfortunately, the study was limited by low power for clinical endpoints and was also terminated early due to slow enrollment and very high cross-over rates.
The 2016 EXPLORE (Evaluating Xience and left ventricular function in PCI on occlusiOns afteR STEMI) trial focused on LV function with concurrent CTO PCI for patients who presented with a ST-elevation MI and underwent primary PCI (36). While the trial had an overall low CTO PCI success rate of 73% and a high cross-over rate of 23%, a sub-study of patients with a left anterior descending coronary artery (LAD) CTO demonstrated benefit in LV ejection fraction (EF) improvement by cardiac MRI following successful CTO PCI—suggesting that CTO PCI to the LAD may improve not only clinical outcomes, but also LV geometry and function.
The 2017 OPEN-CTO (Outcomes, Patent Health Status, and Efficiency in Chronic Total Occlusion Hybrid Procedures) registry evaluated success rates, risks, and patient-reported benefits of contemporary CTO PCI (13). At one month following successful CTO PCI, significant improvements were seen in Seattle Angina Questionnaire (SAQ) QOL (49.4 ± 0.9–75.0 ± 0.7; p < 0.01), Rose Dyspnea Scale (2.0 ± 0.1–1.1 ± 0.1; p < 0.01), and Patient Health Questionnaire 8 (PHQ-8) (6.2 ± 0.2–3.5 ± 0.1; p < 0.01) parameters. Technical success rates in the registry were high, but complication rates were also higher than described for non-CTO PCI—highlighting the importance of careful evaluation of risks, benefits, and estimated technical success rates to most appropriately select optimal patients for CTO PCI and to best guide physician-patient shared decision-making conversations.
Overall, while there is abundant observational data suggesting that successful CTO PCI may be associated with improved clinical outcomes, prospective and randomized studies have been challenged by limitations in patient selection, trial design, and variable procedural success (37–40). Taken together, this data may be utilized to inform patient selection, education, pre-procedural counselling, and consent for CTO PCI—with evidence strongest at present for management of refractory anginal symptoms.
Preparation for CTO PCI: detective work and medication optimization
Once the decision has been made to proceed to CTO PCI, an in-depth review of patient coronary anatomy is essential. This involves a thorough examination of all recent and historical invasive coronary angiography (which should be acquired at low magnification and without panning to facilitate optimal evaluation of collateral routes and with administration of intraocoronary nitroglycerine to improve distal vessel filling), non-invasive coronary computed tomography angiography (CCTA), and prior percutaneous or surgical intervention records (41). CCTA can provide critical information regarding the vessel course within the CTO segment—to include the degree and extent of calcification—and may be superior to coronary angiography for analysing proximal cap morphology (42). In some facilities, integration of CCTA with invasive coronary angiography may also be possible during PCI—thereby delineating the course of the occluded segment and potential crossing obstacles. Overall, a more complete understanding of patient coronary anatomy via careful review of both CCTA and invasive angiography significantly aids technical decision-making regarding CTO crossing strategies (and their hierarchy) and risk assessment, and thus procedural consent.
Since the principal current indication for CTO PCI is symptom relief, we have adopted an algorithmic approach to optimal anti-anginal medication initiation in the outpatient setting prior to consideration of CTO PCI. These anti-anginal medications include a beta-blocker such as Metoprolol Succinate, a long-acting nitrate such as Imdur, a calcium channel blocker such as Amlodipine, and the metabolic modulator Ranolazine—all aimed at optimizing myocardial oxygen supply/demand. Only after patients are up-titrated over time to maximally tolerated doses of these medications (or prove intolerant to doses which adequately control symptoms due to adverse side effects), do we proceed with CTO PCI. Physicians who care for patients with CTOs should strongly consider referral to a CTO PCI specialist for further evaluation unless they are asymptomatic and with good exercise tolerance, normal EF, and minimal ischemic myocardium. When possible, it is advised that clinicians caring for patients undergoing evaluation for potential CTO PCI obtain prior invasive and non-invasive imaging studies in advance of CTO PCI specialty consultation and attempt medical optimization. Ultimately it is incumbent upon CTO PCI teams to complete any of this unfinished diagnostic or therapeutic work prior to pursuing invasive intervention (Figure 2).
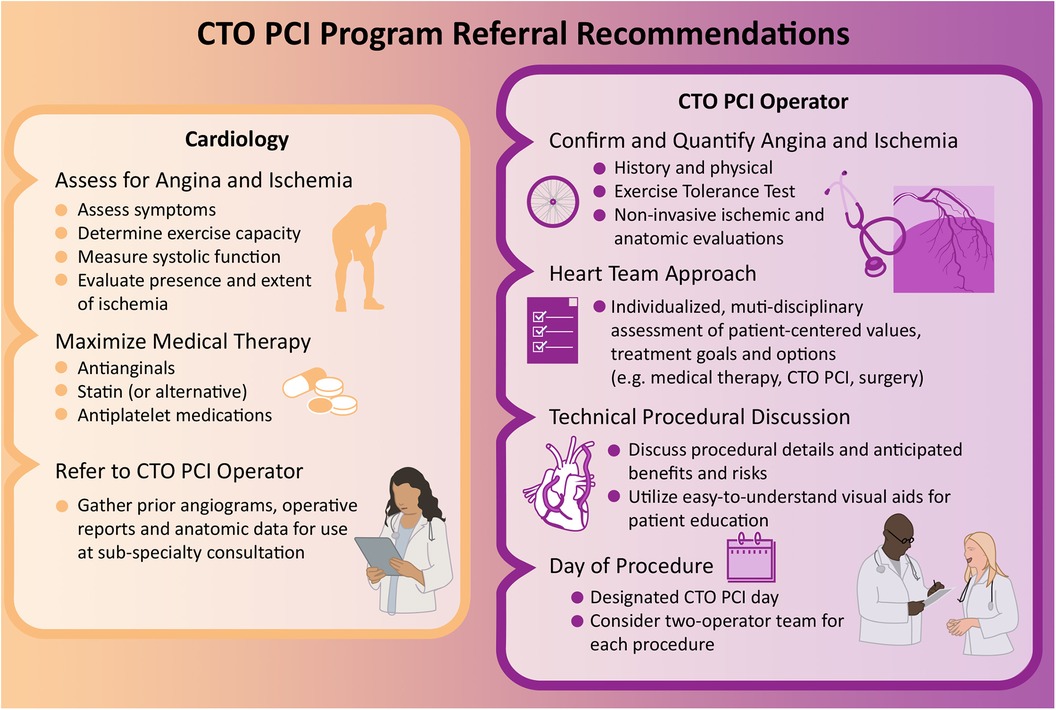
Figure 2. CTO PCI program referral recommendations. Evaluation and management of CTOs varies based on medical specialty and includes: assessment of angina and ischemia, review of patient comorbidities and values, maximization of medical therapy, referral to a CTO specialist, and consideration of PCI.
CTO crossing strategies and scoring systems
Given the complex technical strategies needed to perform CTO PCI safely and effectively and the enhanced procedural risks associated with these interventions, it is advised that patients undergoing evaluation for these procedures be referred to specialized and experienced interventional cardiologists at high-volume and high-complexity medical centers. Successful CTO PCI often requires multiple radial and/or femoral arterial access points to aid in vessel visualization and lesion crossing. There are essentially four techniques to traverse a CTO—antegrade wire escalation (AWE), antegrade dissection and re-entry (ADR), retrograde wire escalation (RWE), and retrograde dissection re-entry (RDR) (12, 15). In other words, CTO operators can cross CTO lesions four possible ways—from the antegrade direction straight through the blockage (“intraplaque” wire tracking within the occlusive intima-based plaque), from the antegrade direction “around” the blockage (“extraplaque” wire tracking outside the plaque but still contained within the adventitial layer), or from the retrograde direction via collateral vessels either straight through the blockage or “around” the blockage (31). Each individual crossing strategy and additional access point adds to procedural complexity and risk—advocating for both appropriate clinical indications and appropriate operator training and experience.
Multiple different CTO crossing expert consensus algorithms currently exist (e.g., Hybrid, Asia-Pacific, EuroCTO) and most recently a Global algorithm merging the best of each of these primary regional protocols has been proposed (43). Despite technical differences prioritizing one (initial) crossing strategy over another, all of the individual protocol share key guiding principles—to include a focus on the complementary nature of antegrade and retrograde wiring and reentry strategies, the importance of efficient switching between alternative crossing techniques to optimize success and shorten procedure time and radiation dose, and the critical importance of intracoronary imaging (15). Intracoronary imaging is vital for the optimal performance of all PCI, and CTO PCI in particular—for evaluation of intraplaque vs. extraplaque tracking, preintervention lesion assessment (to include assessment of plaque composition and follow-on optimal plaque modification technique), lesion preparation and stent deployment and optimization, and assessment of postprocedure endpoints and complications (44).
Various scoring systems have been developed to predict the technical success of CTO PCI and guide risk/benefit analysis and doctor/patient decision-making. Each scoring system considers multiple variables—to include both demographic and angiographic features. Two of the most common scoring systems to predict technical success are the J-CTO (Multicenter Chronic Total Occlusion Registry in Japan) and PROGRESS-CTO (Prospective Global Registry for the Study of Chronic Total Occlusion Intervention) scores (45, 46).
The J-CTO score predicts the likelihood of crossing the CTO lesion within 30 min. It includes five factors (each worth one point when present): occlusion length ≥20 mm, blunt stump appearance of the proximal cap of the occlusion, calcification within the lesion segment, presence of a >45-degree bend within the CTO, and prior failed PCI attempt. A J-CTO score of 0 is considered “easy”, 1 is intermediate, 2 is difficult, and ≥3 is very difficult, with the probability of a technically successful procedure described as 97.8%, 92.3%, 88.4%, and 73.3%, respectively (45). The PROGRESS-CTO score uses four independent variables to predict the likelihood of successful CTO recanalization: ambiguous proximal cap of the CTO, moderate or severe vessel tortuosity, circumflex artery as the target CTO vessel, and lack of “interventional collaterals” to support a retrograde procedural technique—with similarly graded success rates (which are also improving over time) as the J-CTO score (46, 47).
Complications: prevention, recognition, and management
CTO interventions are among the most complex and high-risk PCI procedures performed in the modern cardiac catheterization lab (48). Complications, while uncommon, can be catastrophic if not successfully anticipated, prevented, recognized, and managed (49). As presented in this document, meticulous procedural planning—to include detailed assessment of appropriate clinical indications, anticipated clinical benefit, anatomic complexity, and patient-specific procedural risk—is a critical aspect of CTO PCI (50). A detailed description of the technical aspects of management of specific CTO PCI complications has been well described elsewhere (51).
Many experienced CTO centers presently achieve high success rates (85%–90%) with low (2%–3%) risks of major periprocedural complications (52). By comparison, technical success rates >95% and complication rates <2% have been reported for non-complex non-CTO PCI—compared to success rates <60% (and >6% incidence of emergent CABG) at the dawn of PCI in the 1980s (53, 54). Still, despite lower technical success rates, equivalent MACE rates have been reported between the currently more routinely encountered complex (as opposed to non-complex) non-CTO PCI and CTO PCI (4.1% vs. 5.0% in a large recent single-center registry) (55, 56). Ultimately while there have been iterative advances in CTO PCI equipment and techniques and improvements in success and complication rates (in parallel with extension of procedures to increasingly more complex patient and lesion subsets), the benefit-to-risk ratio remains less favorable compared with non-CTO PCI and may be best limited to patients with refractory angina and dyspnea or high ischemic burden, and performed by high-volume, high-experience, CTO PCI teams and institutions (57).
The decision to pursue CTO PCI—as with all medical interventions—depends on the balance of estimated risk and anticipated benefit. When indicated and successful, CTO PCI may offer relief of angina, improvement in QOL, and possible improvements in myocardial function, exercise capacity, prevention of arrhythmias, and long-term survival (58–60). In-hospital procedural complications are similar to non-CTO PCI and include death, MI, stroke, perforation, pericardial tamponade, side branch occlusion, coronary dissection, major bleeding and need for blood transfusion, contrast-induced nephropathy, vascular surgery repair, and urgent CABG (51). In the initial 2017 report of the multi-center OPEN-CTO Registry, in-hospital mortality occurred in 0.9% of patients, myocardial infarction in 2.6%, emergency CABG in 0.7%, and coronary perforation requiring treatment in 4.8% (13). Scoring systems such as the PROGRESS-CTO complication risk score can facilitate estimation of these periprocedural risks of death, MI, urgent target vessel revascularization, tamponade requiring intervention, and stroke in patients undergoing CTO PCI (overall 2.1% in PROGRESS-CTO) and thus inform physician-patient pre-procedure counseling and shared decision-making (61).
Is CTO PCI appropriate for every operator and every center?
While CTO PCI may not be appropriate for every operator or every institution, therapy awareness and the option to undergo this intervention should be available to all eligible patients as part of a multidisciplinary Heart Team management model (30, 62–64). Compared with complex non-CTO PCI, this intervention involves very unique techniques and equipment—to include specialized lesion crossing strategies, specialty wires and microcatheters, and re-entry devices (48). Dedicated CTO PCI operators need to be facile with all four of the main lesion crossing strategies (as well as additional sub-strategies) unique to CTO PCI (48, 65). Finally, in order to achieve high success rates, operators should possess the skillset to rapidly and fluidly transition between multiple alternative AWE, ADR, RWE, and RDR strategies—which often only occurs with dedicated individual and team training and accumulated experience. Additional optimal CTO PCI program requirements have been well described elsewhere (15, 41).
Karacsonyi et al. recently analyzed the association between operator volume and procedural outcomes of 7,035 CTO PCI procedures performed between 2012 and 2021 at 30 centers and observed that higher-volume operators (>60 CTO PCI cases/year) performed higher complexity procedures with higher rates of technical and procedural success (87.9%) (66). In another analysis by Zein et al. of 7,389 CTO PCIs performed between 2010 and 2018 at 46 sites in Michigan, combined operator and hospital CTO PCI experience was directly related to procedural success but not to major adverse cardiac events—although notably only 4 institutions performed >50 CTO PCIs per year (with 81% procedural success among this higher-volume center cohort) (67).
Presently more than two-thirds of practicing US interventional cardiologists perform fewer than 100 total (non-CTO) PCI procedures annually and nearly 50% perform fewer than 50 total (non-CTO) PCI procedures annually—with unsurprisingly lower mortality noted for high-volume vs. low-volume operators (1.53% vs. 1.86%) (68, 69). Additionally, while a volume-outcome relationship has been noted for a variety of complex PCI lesion subsets in multiple studies, non-CTO PCI volume, expertise, and outcomes do not directly translate to CTO PCI safety and success (70–72). Together, this data may advocate for the regionalization of CTO PCI care to teams of experienced high-volume CTO operators at experienced high-volume CTO centers (Figure 2).
Knowledge gaps and research opportunities
Multiple questions remain regarding the potential benefits of CTO PCI beyond angina relief and increase in exercise capacity. Ongoing randomized controlled trials (RCT), such as the NOBLE-CTO (Nordic-Baltic Randomized Registry Study for Evaluation of PCI in Chronic Total Coronary Occlusion; NCT03392415) and ISCHEMIA-CTO (Nordic and Spanish Randomized Trial on the Effect of Revascularization or Optimal Medical Therapy of Chronic Total Coronary Occlusions With Myocardial Ischemia; NCT03563417) trials, expected to complete enrollment in 2027 and 2028, respectively, may help address these unanswered questions and further inform future myocardial revascularization guidelines.
Conclusions
CTOs are common in daily practice and new and evolving treatment options now exist for lesions that were once considered “untreatable”. While many patients are asymptomatic with medical therapy alone, others suffer lifestyle-limiting angina which may significantly curtail their activities of daily living. Although there are multiple potential advantages to CTO intervention, the most proven current indication is for symptom relief and quality of life improvement rather than survival benefit. Due to higher procedural complexity and risk and a need for greater operator technical expertise, CTO PCI may be most appropriately performed by specially-trained and experienced teams at high-volume cardiac catheterization laboratories.
Author contributions
LC: Writing – original draft, Writing – review & editing. MM: Writing – review & editing. RD: Writing – review & editing. BT: Writing – original draft. WB: Writing – review & editing. AT: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Stone GW, Reifart NJ, Moussa I, Hoye A, Cox DA, Colombo A, et al. Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: part II. Circulation. (2005) 112:2530–7. doi: 10.1161/CIRCULATIONAHA.105.583716
2. Hochman JS, Lamas GA, Buller CE, Dzavik V, Reynolds HR, Abramsky SJ, et al. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. (2006) 355:2395–407. doi: 10.1056/NEJMoa066139
3. Jeroudi OM, Alomar ME, Michael TT, Sabbagh AE, Patel VG, Mogabgab O, et al. Prevalence and management of coronary chronic total occlusions in a tertiary veterans affairs hospital. Catheter Cardiovasc Interv. (2014) 84:637–43. doi: 10.1002/ccd.25264
4. Råmunddal T, Hoebers LP, Henriques JP, Dworeck C, Angerås O, Odenstedt J, et al. Prognostic impact of chronic total occlusions: a report from SCAAR (Swedish coronary angiography and angioplasty registry). JACC Cardiovasc Interv. (2016) 9:1535–44. doi: 10.1016/j.jcin.2016.04.031
5. Azzalini L, Jolicoeur EM, Pighi M, Millán X, Picard F, Tadros VX, et al. Epidemiology, management strategies, and outcomes of patients with chronic total coronary occlusion. Am J Cardiol. (2016) 118:1128–35. doi: 10.1016/j.amjcard.2016.07.023
6. Fefer P, Knudtson ML, Cheema AN, Galbraith PD, Osherov AB, Yalonetsky S, et al. Current perspectives on coronary chronic total occlusions: the Canadian multicenter chronic total occlusions registry. J Am Coll Cardiol. (2012) 59:991–7. doi: 10.1016/j.jacc.2011.12.007
7. Pereg D, Fefer P, Samuel M, Wolff R, Czarnecki A, Deb S, et al. Native coronary artery patency after coronary artery bypass surgery. JACC Cardiovasc Interv. (2014) 7:761–7. doi: 10.1016/j.jcin.2014.01.164
8. Pereg D, Fefer P, Samuel M, Shuvy M, Deb S, Sparkes JD, et al. Long-term follow-up of coronary artery bypass patients with preoperative and new postoperative native coronary artery chronic total occlusion. Can J Cardiol. (2016) 32:1326–31. doi: 10.1016/j.cjca.2016.01.015
9. Dautov R, Manh Nguyen C, Altisent O, Gibrat C, Rinfret S. Recanalization of chronic total occlusions in patients with previous coronary bypass surgery and consideration of retrograde access via saphenous vein grafts. Circ Cardiovasc Interv. (2016) 9:e003515. doi: 10.1161/CIRCINTERVENTIONS.115.003515
10. Farooq V, Serruys PW, Garcia-Garcia HM, Zhang Y, Bourantas CV, Holmes DR, et al. The negative impact of incomplete angiographic revascularization on clinical outcomes and its association with total occlusions: the SYNTAX (synergy between percutaneous coronary intervention with taxus and cardiac surgery) trial. J Am Coll Cardiol. (2013) 61:282–94. doi: 10.1016/j.jacc.2012.10.017
11. Werner GS, Martin-Yuste V, Hildick-Smith D, Boudou N, Sianos G, Gelev V, et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. (2018) 39:2484–93. doi: 10.1093/eurheartj/ehy220
12. Tajti P, Karmpaliotis D, Alaswad K, Jaffer FA, Yeh RW, Patel M, et al. The hybrid approach to chronic total occlusion percutaneous coronary intervention: update from the PROGRESS CTO registry. JACC Cardiovasc Interv. (2018) 11:1325–35. doi: 10.1016/j.jcin.2018.02.036
13. Sapontis J, Salisbury AC, Yeh RW, Cohen DJ, Hirai T, Lombardi W, et al. Early procedural and health Status outcomes after chronic total occlusion angioplasty: a report from the OPEN-CTO registry (outcomes, patient health status, and efficiency in chronic total occlusion hybrid procedures). JACC Cardiovasc Interv. (2017) 10:1523–34. doi: 10.1016/j.jcin.2017.05.065
14. Brilakis ES, Banerjee S, Karmpaliotis D, Lombardi WL, Tsai TT, Shunk KA, et al. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (national cardiovascular data registry). JACC Cardiovasc Interv. (2015) 8:245–53. doi: 10.1016/j.jcin.2014.08.014
15. Brilakis ES, Mashayekhi K, Tsuchikane E, Abi Rafeh N, Alaswad K, Araya M, et al. Guiding principles for chronic total occlusion percutaneous coronary intervention. Circulation. (2019) 140:420–33. doi: 10.1161/CIRCULATIONAHA.119.039797
16. Ford TJ, Colin B. Angina: contemporary diagnosis and management. Heart. (2020) 106:387. doi: 10.1136/heartjnl-2018-314661
17. Peri-Okonny PA, Spertus JA, Grantham JA, et al. Physical activity after percutaneous coronary intervention for chronic total occlusion and its association with health Status. J Am Heart Assoc. (2019) 8:e011629. doi: 10.1161/JAHA.118.011629
18. Yeh RW, Tamez H, Secemsky EA, Grantham JA, Sapontis J, Spertus JA, et al. Depression and angina among patients undergoing chronic total occlusion percutaneous coronary intervention. JACC Cardiovasc Interv. (2019) 12:651–8. doi: 10.1016/j.jcin.2018.12.029
19. Schumacher SP, Stuijfzand WJ, de Winter RW, van Diemen PA, Bom MJ, Everaars H, et al. Ischemic burden reduction and long-term clinical outcomes after chronic total occlusion percutaneous coronary intervention. JACC Cardiovasc Interv. (2021) 14:1407–18. doi: 10.1016/j.jcin.2021.04.044
20. Zhao S, Wang J, Chen Y, Wang W, Hu W, Zou Y, et al. Improvement of symptoms and quality of life after successful percutaneous coronary intervention for chronic total occlusion in elderly patients. J Am Heart Assoc. (2023) 12:e029034. doi: 10.1161/JAHA.123.029034
21. Kucukseymen S, Iannaccone M, Grantham JA, Sapontis J, Juricic S, Ciardetti N, et al. Association of successful percutaneous revascularization of chronic total occlusions with quality of life: a systematic review and meta-analysis. JAMA Netw Open. (2023) 6:e2324522. doi: 10.1001/jamanetworkopen.2023.24522
22. Megaly M, Brilakis ES, Abdelsalam M, Pershad A, Saad M, Garcia S, et al. Impact of chronic total occlusion revascularization on left ventricular function assessed by cardiac magnetic resonance. JACC Cardiovasc Imaging. (2021) 14:1076–8. doi: 10.1016/j.jcmg.2020.10.012
23. Galassi AR, Brilakis ES, Boukhris M, Tomasello SD, Sianos G, Karmpaliotis D, et al. Appropriateness of percutaneous revascularization of coronary chronic total occlusions: an overview. Eur Heart J. (2015) 37:2692–700. doi: 10.1093/eurheartj/ehv391
24. Stone GW, Ali ZA, O'Brien SM, Rhodes G, Genereux P, Bangalore S, et al. Impact of complete revascularization in the ISCHEMIA trial. J Am Coll Cardiol. (2023) 82:1175–88. doi: 10.1016/j.jacc.2023.06.015
25. Garcia S, Sandoval Y, Roukoz H, Adabag S, Canoniero M, Yannopoulos D, et al. Outcomes after complete versus incomplete revascularization of patients with multivessel coronary artery disease: a meta-analysis of 89,883 patients enrolled in randomized clinical trials and observational studies. J Am Coll Cardiol. (2013) 62:1421–31. doi: 10.1016/j.jacc.2013.05.033
26. Valenti R, Migliorini A, Signorini U, Vergara R, Parodi G, Carrabba N, et al. Impact of complete revascularization with percutaneous coronary intervention on survival in patients with at least one chronic total occlusion. Eur Heart J. (2008) 29:2336–42. doi: 10.1093/eurheartj/ehn357
27. Jang WJ, Yang JH, Song YB, Hahn JY, Choi JH, Chun WJ, et al. Clinical implications of residual SYNTAX score after percutaneous coronary intervention in patients with chronic total occlusion and multivessel coronary artery disease: a comparison with coronary artery bypass grafting. EuroIntervention. (2017) 13:97–105. doi: 10.4244/EIJ-D-16-00421
28. Watanabe H, Morimoto T, Shiomi H, Furukawa Y, Nakagawa Y, Ando K, et al. Chronic total occlusion in a non-infarct-related artery is closely associated with increased five-year mortality in patients with ST-segment elevation acute myocardial infarction undergoing primary percutaneous coronary intervention (from the CREDO-Kyoto AMI registry). EuroIntervention. (2017) 12:e1874–82. doi: 10.4244/EIJ-D-15-00421
29. Watanabe H, Morimoto T, Shiomi H, Kawaji T, Furukawa Y, Nakagawa Y, et al. Chronic total occlusion in non-infarct-related artery is associated with increased short-and long-term mortality in patients with ST-segment elevation acute myocardial infarction complicated by cardiogenic shock (from the CREDO-Kyoto AMI registry). Catheter Cardiovasc Interv. (2018) 92:455–63. doi: 10.1002/ccd.27330
30. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2022) 145:e18–114. doi: 10.1161/CIRCULATIONAHA.121.057538
31. Ybarra LF, Rinfret S, Brilakis ES, Karmpaliotis D, Azzalini L, Grantham JA, et al. Definitions and clinical trial design principles for coronary artery chronic total occlusion therapies: CTO-ARC consensus recommendations. Circulation. (2021) 143:479–500. doi: 10.1161/CIRCULATIONAHA.120.046754
32. Simsek B, Kostantinis S, Karacsonyi J, Alaswad K, Megaly M, Karmpaliotis D, et al. A systematic review and meta-analysis of clinical outcomes of patients undergoing chronic total occlusion percutaneous coronary intervention. J Invasive Cardiol. (2022) 34:E763–75.36227013
33. di Mario C, Mashayekhi AK, Garbo R, Pyxaras AS, Ciardetti N, Werner SG. Recanalisation of coronary chronic total occlusions. EuroIntervention. (2022) 18:535–61. doi: 10.4244/EIJ-D-21-01117
34. Stazi F. Dilemmas in cardiology: when to recanalize a chronic total occlusion. Eur Heart J Suppl. (2023) 25:B149–54. doi: 10.1093/eurheartjsupp/suad094
35. Lee SW, Lee PH, Ahn JM, Park DW, Yun SC, Han S, et al. Randomized trial evaluating percutaneous coronary intervention for the treatment of chronic total occlusion. Circulation. (2019) 139:1674–83. doi: 10.1161/CIRCULATIONAHA.118.031313
36. Henriques JP, Hoebers LP, Ramunddal T, Laanmets P, Eriksen E, Bax M, et al. Percutaneous intervention for concurrent chronic total occlusions in patients with STEMI: the EXPLORE trial. J Am Coll Cardiol. (2016) 68:1622–32. doi: 10.1016/j.jacc.2016.07.744
37. Kirschbaum SW, Baks T, van den Ent M, Sianos G, Krestin GP, Serruys PW, et al. Evaluation of left ventricular function three years after percutaneous recanalization of chronic total coronary occlusions. Am J Cardiol. (2008) 101:179–85. doi: 10.1016/j.amjcard.2007.07.060
38. George S, Cockburn J, Clayton TC, Ludman P, Cotton J, Spratt J, et al. Long-term follow-up of elective chronic total coronary occlusion angioplasty: analysis from the U. K. central cardiac audit database. J Am Coll Cardiol. (2014) 64:235–43. doi: 10.1016/j.jacc.2014.04.040
39. Hoebers LP, Vis MM, Claessen BE, van der Schaaf RJ, Kikkert WJ, Baan J Jr, et al. The impact of multivessel disease with and without a co-existing chronic total occlusion on short- and long-term mortality in ST-elevation myocardial infarction patients with and without cardiogenic shock. Eur J Heart Fail. (2013) 15:425–32. doi: 10.1093/eurjhf/hfs182
40. Nombela-Franco L, Mitroi CD, Fernández-Lozano I, García-Touchard A, Toquero J, Castro-Urda V, et al. Ventricular arrhythmias among implantable cardioverter-defibrillator recipients for primary prevention: impact of chronic total coronary occlusion (VACTO primary study). Circ Arrhythm Electrophysiol. (2012) 5:147–54. doi: 10.1161/CIRCEP.111.968008
41. Azzalini L, Karmpaliotis D, Santiago R, Mashayekhi K, Di Mario C, Rinfret S, et al. Contemporary issues in chronic total occlusion percutaneous coronary intervention. JACC Cardiovasc Interv. (2022) 15:1–21. doi: 10.1016/j.jcin.2021.09.027
42. Werner GS. Use of coronary computed tomographic angiography to facilitate percutaneous coronary intervention of chronic total occlusions. Circ Cardiovasc Interv. (2019) 12:e007387. doi: 10.1161/CIRCINTERVENTIONS.119.007387
43. Wu EB, Brilakis ES, Mashayekhi K, Tsuchikane E, Alaswad K, Araya M, et al. Global chronic total occlusion crossing algorithm: JACC state-of-the-art review. J Am Coll Cardiol. (2021) 78:840–53. doi: 10.1016/j.jacc.2021.05.055
44. Truesdell AG, Alasnag MA, Kaul P, Rab ST, Riley RF, Young MN, et al. Intravascular imaging during percutaneous coronary intervention: JACC state-of-the-art review. J Am Coll Cardiol. (2023) 81:590–605. doi: 10.1016/j.jacc.2022.11.045
45. Morino Y, Abe M, Morimoto T, Kimura T, Hayashi Y, Muramatsu T, et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30min: the J-CTO (multicenter CTO registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. (2011) 4:213–21. doi: 10.1016/j.jcin.2010.09.024
46. Christopoulos G, Kandzari DE, Yeh RW, Jaffer FA, Karmpaliotis D, Wyman MR, et al. Development and validation of a novel scoring system for predicting technical success of chronic total occlusion percutaneous coronary interventions: the PROGRESS CTO (prospective global registry for the study of chronic total occlusion intervention) score. JACC Cardiovasc Interv. (2016) 9:1–9. doi: 10.1016/j.jcin.2015.09.022
47. Karacsonyi J, Stanberry L, Alaswad K, Krestyaninov O, Choi JW, Rangan BV, et al. Predicting technical success of chronic total occlusion percutaneous coronary intervention. Circ Cardiovasc Interv. (2021) 14:e009860. doi: 10.1161/CIRCINTERVENTIONS.120.009860
48. Riley RF, Henry TD, Mahmud E, Kirtane AJ, Brilakis ES, Goyal A, et al. SCAI position statement on optimal percutaneous coronary interventional therapy for complex coronary artery disease. Catheter Cardiovasc Interv. (2020) 96:346–62. doi: 10.1002/ccd.28994
49. Riley RF, Sapontis J, Kirtane AJ, Karmpaliotis D, Kalra S, Jones PG, et al. Prevalence, predictors, and health status implications of periprocedural complications during coronary chronic total occlusion angioplasty. EuroIntervention. (2018) 14:e1199–206. doi: 10.4244/EIJ-D-17-00976
50. Naidu SS, Abbott JD, Bagai J, Blankenship J, Garcia S, Iqbal SN, et al. SCAI expert consensus update on best practices in the cardiac catheterization laboratory. Catheter Cardiovasc Interv. (2021) 98:255–76. doi: 10.1002/ccd.29744
51. Doll JA, Hira RS, Kearney KE, Kandzari DE, Riley RF, Marso SP, et al. Management of percutaneous coronary intervention complications. Circ Cardiovasc Interv. (2020) 13:e008962. doi: 10.1161/CIRCINTERVENTIONS.120.008962
52. Karacsonyi J, Vemmou E, Nikolakopoulos I, Ungi I, Abi Rafeh N, ElGuindy A, et al. Current challenges and prevention strategies for chronic total occlusion (CTO) complications. Expert Rev Cardiovasc Ther. (2021) 19:337–47. doi: 10.1080/14779072.2021.1905521
53. Waldo SW, Gokhale M, O'Donnell CI, Plomondon ME, Valle JA, Armstrong EJ, et al. Temporal trends in coronary angiography and percutaneous coronary intervention: insights from the VA clinical assessment, reporting, and tracking program. JACC Cardiovasc Interv. (2018) 11:879–88. doi: 10.1016/j.jcin.2018.02.035
54. Kent KM, Bentivoglio LG, Block PC, Cowley MJ, Dorros G, Gosselin AJ, et al. Percutaneous transluminal coronary angioplasty: report from the registry of the national heart, lung, and blood institute. Am J Cardiol. (1982) 49:2011–20. doi: 10.1016/0002-9149(82)90223-5
55. Azzalini L, Carlino M, Bellini B, Marini C, Pazzanese V, Toscano E, et al. Long-term outcomes of chronic total occlusion recanalization versus percutaneous coronary intervention for complex non-occlusive coronary artery disease. Am J Cardiol. (2020) 125:182–8. doi: 10.1016/j.amjcard.2019.10.034
56. Kheifets M, Vons SA, Bental T, Assa HV, Greenberg G, Samara A, et al. Temporal trends in complex PCI interventions. Eur Heart J. (2022) 43:ehac544.1243. doi: 10.1093/eurheartj/ehac544.1243
57. Mohamed OM, Polad J, Hildick-Smith D, Bizeau O, Baisebenov RK, Roffi M, et al. Impact of coronary lesion complexity in percutaneous coronary intervention: one-year outcomes from the large, multicentre e-ultimaster registry. EuroIntervention. (2020) 16:603–12. doi: 10.4244/EIJ-D-20-00361
58. Shah A. Chronic total occlusion coronary intervention: in search of a definitive benefit. Methodist Debakey Cardiovasc J. (2018) 14:50–9. doi: 10.14797/mdcj-14-1-50
59. Megaly M, Buda K, Mashayekhi K, Werner GS, Grantham JA, Rinfret S, et al. Comparative analysis of patient characteristics in chronic total occlusion revascularization studies: trials vs real-world registries. JACC Cardiovasc Interv. (2022) 15:1441–9. doi: 10.1016/j.jcin.2022.05.023
60. Leone PP, Calò L, Donahue M, Gasparini G. Acute coronary complications in chronic total occlusion interventions. Eur Heart J Suppl. (2023) 25:C96–105. doi: 10.1093/eurheartjsupp/suad041
61. Simsek B, Kostantinis S, Karacsonyi J, Alaswad K, Krestyaninov O, Khelimskii D, et al. Predicting periprocedural complications in chronic total occlusion percutaneous coronary intervention: the PROGRESS-CTO complication scores. JACC Cardiovasc Interv. (2022) 15:1413–22. doi: 10.1016/j.jcin.2022.06.007
62. Grines CL, Box LC, Mamas MA, Abbott JD, Blankenship JC, Carr JG, et al. SCAI expert consensus statement on percutaneous coronary intervention without on-site surgical backup. JACC Cardiovasc Interv. (2023) 16:847–60. doi: 10.1016/j.jcin.2022.12.016
63. Kaier TE, Hurrell H, Patterson T, Fisk G, Stewart J, Baig K, et al. The impact of a dedicated chronic total occlusion PCI program on heart team decision making. J Invasive Cardiol. (2022) 34:E660–664. PMID: 35916923.35916923
64. Young MN, Kolte D, Cadigan ME, Laikhter E, Sinclair K, Pomerantsev E, et al. Multidisciplinary heart team approach for complex coronary artery disease: single center clinical presentation. J Am Heart Assoc. (2020) 9:e014738. doi: 10.1161/JAHA.119.014738
65. Riley RF, Walsh SJ, Kirtane AJ, Michael Wyman R, Nicholson WJ, Azzalini L, et al. Algorithmic solutions to common problems encountered during chronic total occlusion angioplasty: the algorithms within the algorithm. Catheter Cardiovasc Interv. (2019) 93:286–97. doi: 10.1002/ccd.27987
66. Karacsonyi J, Tsiafoutis I, Alaswad K, Karmpaliotis D, Choi JW, Khatri J, et al. Association of annual operator volume with the outcomes of chronic total occlusion percutaneous coronary intervention. J Invasive Cardiol. (2022) 34:E645–652. PMID: 35969838.35969838
67. Zein R, Seth M, Othman H, Rosman HS, Lalonde T, Alaswad K, et al. Association of operator and hospital experience with procedural success rates and outcomes in patients undergoing percutaneous coronary interventions for chronic total occlusions: insights from the blue cross blue shield of Michigan cardiovascular consortium. Circ Cardiovasc Interv. (2020) 13:e008863. doi: 10.1161/CIRCINTERVENTIONS.119.008863
68. Harold JG, Bass TA, Bashore TM, Brindis RG, Brush JE Jr, Burke JA, et al. ACCF/AHA/SCAI 2013 update of the clinical competence statement on coronary artery interventional procedures: a report of the American college of cardiology foundation/American heart association/American college of physicians task force on clinical competence and training (writing committee to revise the 2007 clinical competence statement on cardiac interventional procedures). J Am Coll Cardiol. (2013) 62:357–96. doi: 10.1016/j.jacc.2013.05.002
69. Fanaroff AC, Zakroysky P, Dai D, Wojdyla D, Sherwood MW, Roe MT, et al. Outcomes of PCI in relation to procedural characteristics and operator volumes in the United States. J Am Coll Cardiol. (2017) 69:2913–24. doi: 10.1016/j.jacc.2017.04.032
70. Kaul P, Kandzari DE. Chronic total occlusions. Circ Cardiovasc Interv. (2020) 13:e009724. doi: 10.1161/CIRCINTERVENTIONS.120.009724
71. Kinnaird T, Gallagher S, Anderson R, Sharp A, Farooq V, Ludman P, et al. Are higher operator volumes for unprotected left main stem percutaneous coronary intervention associated with improved patient outcomes? Circ Cardiovasc Interv. (2020) 13:e008782. doi: 10.1161/CIRCINTERVENTIONS.119.008782
Keywords: total coronary occlusion, coronary artery disease, chronic total occlusions, percutaneous coronary intervention, CABG, revascularization
Citation: Cilia L, Megaly M, Davies R, Tehrani BN, Batchelor WB and Truesdell AG (2024) A non-interventional cardiologist’s guide to coronary chronic total occlusions. Front. Cardiovasc. Med. 11:1350549. doi: 10.3389/fcvm.2024.1350549
Received: 5 December 2023; Accepted: 17 January 2024;
Published: 6 February 2024.
Edited by:
Maurice Enriquez-Sarano, Minneapolis Heart Institute Foundation (MHIF), United StatesReviewed by:
Yasushi Ueki, Shinshu University Hospital, Japan© 2024 Cilia, Megaly, Davies, Tehrani, Batchelor and Truesdell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lindsey Cilia bGNpbGlhQHZpcmdpbmlhaGVhcnQuY29t
Abbreviations ADR, antegrade dissection re-entry; AWE, antegrade wire escalation; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCTA, coronary computer tomography angiography; CTO, chronic total occlusion; EF, ejection fraction; LAD, left anterior descending coronary artery; LV, left ventricular; MACE, major adverse cardiovascular events; MI, myocardial infarction; OMT, optimal medical therapy; PCI, percutaneous coronary intervention; QOL, quality of life; RCT, randomized controlled trial; RDR, retrograde dissection re-entry; RWE, retrograde wire escalation.
 Lindsey Cilia
Lindsey Cilia Michael Megaly3
Michael Megaly3 Behnam N. Tehrani
Behnam N. Tehrani Wayne B. Batchelor
Wayne B. Batchelor