
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 16 February 2024
Sec. Atherosclerosis and Vascular Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1345521
This article is part of the Research Topic Role of Colchicine in Atherosclerosis View all 5 articles
Background: Inflammation is pivotal to the progression of atherosclerosis. Cholesterol crystals (CCs) that grow and enlarge within the plaque core can cause plaque rupture and trigger inflammation as they deposit into the atherosclerotic bed. Thus, agents that affect CC formation, expansion, and morphology may reduce cardiovascular (CV) risk independent of lipid-lowering and anti-inflammatory therapy.
Objective: Because colchicine is highly concentrated in leukocytes that can enter the atherosclerotic plaque core, we tested its effect on the formation and growth of CCs in bench experiments to determine whether it may have direct effects on CCs, independent of its known anti-inflammatory actions.
Method: Different dosages of colchicine mixed with cholesterol (0.05–5 mg/ml/g of cholesterol) were used to influence the formation CCs and volume expansion in vitro. These were compared to control samples with cholesterol in ddH2O without colchicine. In an ex vivo study, fresh atherosclerotic human plaques were incubated with and without colchicine in a water bath at 37°C for 48 h to assess the impact of colchicine on CC morphology. Scanning electron microscopy (SEM) was utilized to analyze CC morphology in samples from the various treatment groups.
Results: The addition of colchicine to cholesterol caused a substantial dose-dependent reduction in volume (p < 0.05). Pairwise comparisons of volume reduction, showed a significant reduction in volume at 5 mg/ml/g when compared to control (p < 0.02) but the calculated Cohen's d effect size was large for five of the six pairwise comparisons. By SEM, CCs from both in vitro and ex vivo samples treated with colchicine had evidence of dissolution and changes in their morphology as evidenced by the loss of their sharp edges. In contrast, CCs in untreated specimens retained their typical geometric structure.
Conclusions: Colchicine can reduce CC formation and expansion and alter CC morphology. These previously unappreciated effects of colchicine may contribute to its clinical benefit in patients with CV disease independent of its anti-inflammatory effects.
Inflammation is fundamental to the development of atherosclerosis and plaque destabilization (1). Recent evidence suggests that cholesterol crystals (CCs) that form within the plaque core can cause plaque rupture as they enlarge and trigger inflammation when they are released into the atherosclerotic bed. Like uric acid crystals, CCs have been demonstrated to activate NLRP3 inflammasomes leading to IL-1β and then IL-6 and C-reactive protein (2–4). Furthermore, as CCs develop and enlarge, they develop sharp tips that can directly perforate the atherosclerotic plaque and trigger atherothrombosis independent of inflammation-induced disruption of the plaque structure (5, 6). Historically, colchicine has been used to treat gout and other inflammatory conditions (7, 8). However, recently it has been shown to significantly reduce recurrent cardiovascular events (CVEs) in high-risk patients (9). Given these findings, we proposed to test the effect of colchicine on CCs to evaluate a role other than anti-inflammation.
The actions of CCs can be interrupted by lowering the available free cholesterol within the plaque by a variety of lipid-lowering approaches but that requires long-term therapy (10). Of interest is the observation that statins and aspirin may directly dissolve CCs and alter their morphology (11–14). Colchicine has been shown to be highly concentrated within leukocytes that traverse the plaque bed and become entrapped in the plaque core. Thus, in contrast to statins and aspirin, it is likely that colchicine can accumulate within atherosclerotic plaque (15, 16). Thus, we evaluated the ability of colchicine to alter CC formation, expansion, and morphology in both in vitro and ex vivo experiments to determine whether it may have actions that could reduce the risk of atherosclerosis independent of its direct anti-inflammatory effects.
Purified cholesterol powder [5-cholesten-3β-ol; 3β-hydroxy-5-cholestene (C27H46O): molecular weight = 386.7; 95%–98% pure, Sigma, St. Louis, MO, USA] was melted in 10 ml graduated cylinders (Pyrex VISTA, Corning Inc., Corning, NY, USA) using a heating gun (HAG 1,400-U, GAR-TEC, Baden, Germany), and volume expansion was measured as previously described (13). Temperature achieved was at the melting point of cholesterol (147°C). The meniscus level of liquid cholesterol (V1) obtained upon melting was noted. The cylinder was then allowed to cool for 10 min at room temperature and maximal peak of CCs formed (V2) was noted. The peak volume expansion (ΔVE) was calculated by subtracting V1 from V2.
Varying doses of colchicine (C22H25NO6, >95%, Sigma-Aldrich Co. St. Louis, MO, USA) mixed with cholesterol powder and water (0.05–5 mg/ml/g of cholesterol) were melted, and ΔVE was measured as mentioned above and compared to control samples without colchicine. The experiment was repeated six times each and the results were averaged. The crystals were then examined by scanning electron microscopy (SEM). The morphology of CCs formed with colchicine was compared to CC controls without colchicine.
The experimental doses of colchicine employed were designed to be analogous to the exposure levels commonly encountered by arterial plaques in humans. This equivalence was derived from the analysis of colchicine concentrations in the human serum following a single oral administration of 0.5 mg, achieving a maximum serum concentration (Cmax) of 2.1 ng/ml (17). Given that disrupted plaques contain an average of 30 mg/g of free cholesterol and considering a cardiac output of 5 L/min, it was determined that 0.01 mg of colchicine interfaces with free cholesterol at a rate of 30 mg/min. Higher colchicine doses were administered in the experiments, reflecting concentrations up to 600 times greater found in leukocytes that are equivalent to 6.3 mg in contact with 30 mg/min of free cholesterol (16–18). Although the serum levels of colchicine in humans are markedly lower than what was used in the in vitro test tube study, the doses used were designed to provide the visual measurements needed for the in vitro experiment. Moreover, another study showed that low doses of colchicine (0.1, 1, and 2 mg) all had a comparable effect on ΔVE (19).
Three fresh human carotid plaques were obtained from endarterectomy procedures and cut into equal halves and incubated in a water bath for 48 h at 37°C. One half was incubated in ddH2O with colchicine (5 mg/ml), and the other half was incubated in ddH2O alone. Plaque specimens were de-identified and were consistent with Sparrow Hospital and Michigan State University IRB approval (# 0518-exempt and no consent was required). Treated plaques were then prepared for SEM and crystal morphology analyzed in samples from the treatment groups of atherosclerotic human plaques incubated with and without colchicine. Two individuals concurred on the SEM findings and a third individual adjudicated if there was disagreement.
Atherosclerotic plaques were fixed overnight in buffered 4% glutaraldehyde and then cut into 5-mm segments and air dried as previously described (6). All samples were then mounted on stubs and gold coated in an EMSCOPE SC500 sputter coater (UK), and the surface was examined for CCs using a JEOL scanning electron microscope (Model JSM-6610LV, JEOL., Tokyo, Japan). Also, control and colchicine samples treated in vitro were collected and scanned for their morphology.
During SEM examination, crystals were evaluated for elemental composition using energy-dispersive x-ray spectroscopy (EDS) (AZtec system, Oxford Instruments, High Wycombe, Bucks, England). CCs analyzed with EDS were used to confirm the chemical composition corresponding to crystal shapes as previously reported (13).
All statistical analyses were performed using SAS version 9.4 (SAS Inc. Cary, NC, USA). Data were the difference between V2 and V1 (ΔVE). One-way ANOVA with Tukey–Kramer multiple-comparison post-tests and non-parametric equivalent, and the Kruskal–Wallis test followed by the Dwass–Steel–Critchlow–Fligner (DSCF) multiple-comparisons test were performed to compare peak volume expansions at various doses of colchicine. The results were the same for the two approaches; therefore, only the parametric comparisons are reported. We also calculated Cohen's d effect size (ES) for all pairwise comparisons because of the small sample size. In addition, simple linear regression of ΔVE on the colchicine dose was performed without and with control value (0 colchicine) in the model. Results are reported as means ± SD, and p < 0.05 is used to report statistical significance in all tests.
Increasing concentration of colchicine resulted in a progressive decrease in ΔVE for the same amount of cholesterol (Figure 1). When dose response for the ΔVE with increasing dose of colchicine was evaluated with simple linear regression, the trend of decrease in ΔVE was statistically significant, p < 0.05 (Figure 2). The same estimate of reduction in volume expansion was observed when the linear regression model included ΔVE values for control samples in which colchicine’s value was set to 0. However, by one-way ANOVA, followed by multiple-comparisons test, only ΔVE for 5 mg/ml/g differed significantly from the control value (p < 0.02) (Figure 2). When Cohen's d effect size, which is independent of the sample size, was calculated for all pairwise comparisons of ΔVE, we observed a large effect size in all but one comparison (Table 1), indicating a significant reduction in ΔVE with colchicine. Furthermore, by SEM, cholesterol crystal morphology with colchicine modified the typical sharp tips and edges causing them to become blunted and rounded compared to control crystals (Figure 3).
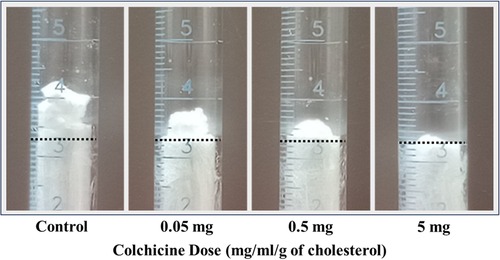
Figure 1. Example of dose-related effect of colchicine. The dotted line is the site of the original meniscus, which is almost equal for all the melted 3 g of cholesterol used.
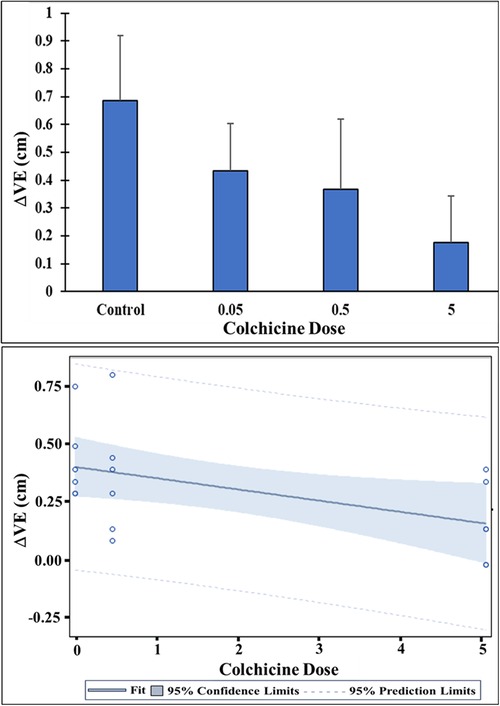
Figure 2. Change in cholesterol volume expansion (ΔVE) with and without colchicine. Control ΔVE differed significantly from ΔVE for the 5 mg/ml/g colchicine dose, p < 0.02. Colchicine treatment pairwise group comparisons were not significantly different possibly related to sample size. However, there is a dose-related decline in ΔVE with increasing levels of colchicine. Linear regression of ΔVE on the colchicine dose showed a significant decreasing trend in ΔVE with increasing dose of colchicine, at p < 0.05.
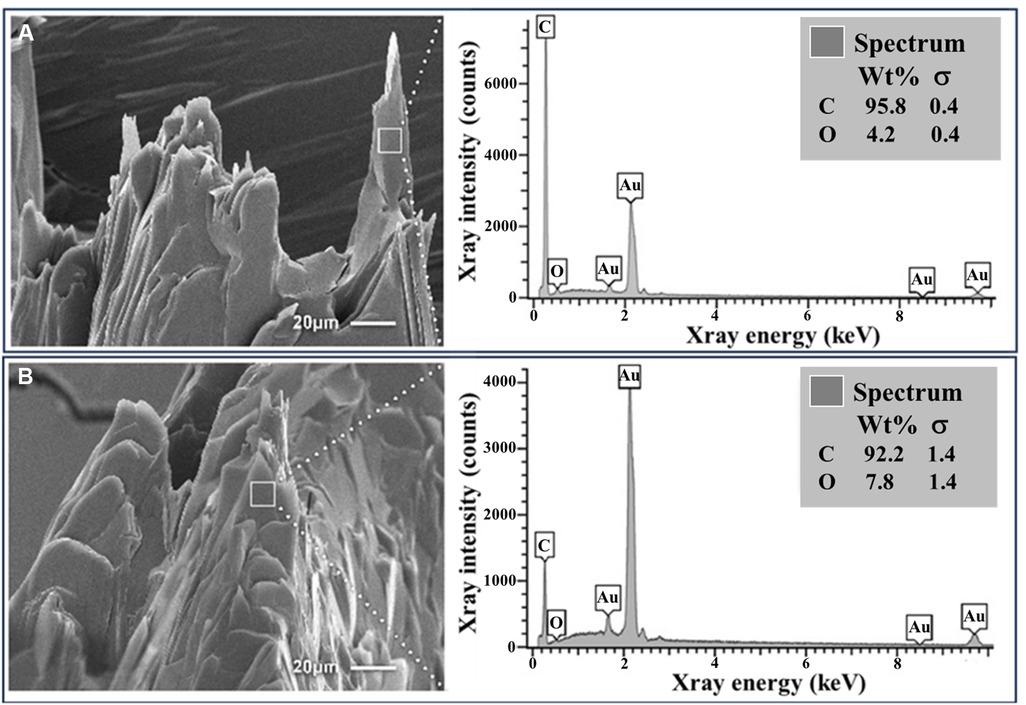
Figure 3. Cholesterol crystals with and without colchicine in vitro. (A) Basic cholesterol crystals with standard crystal morphology following crystallization. (B) Colchicine-exposed crystals with dissolving features and rounded edges. EDS demonstrates a predominance of carbon and oxygen as is consistent for cholesterol. Au, gold; C, carbon; O, oxygen.
SEM of the three carotid atherosclerotic plaques incubated in ddH2O had a similar crystal morphology as the in vitro controls with sharp edges but were mostly rhomboidal in shape as often seen in human plaques. However, all three colchicine-treated atherosclerotic plaque samples exhibited dissolving CCs with blunted edges and were fragmented as noted in the in vitro colchicine-treated samples (Figure 4). However, none of the ddH2O-treated samples exhibited dissolving crystals forms. Also, by visual observation, colchicine-treated samples appeared to have a lower crystal distribution compared to their matched halves incubated in ddH2O.
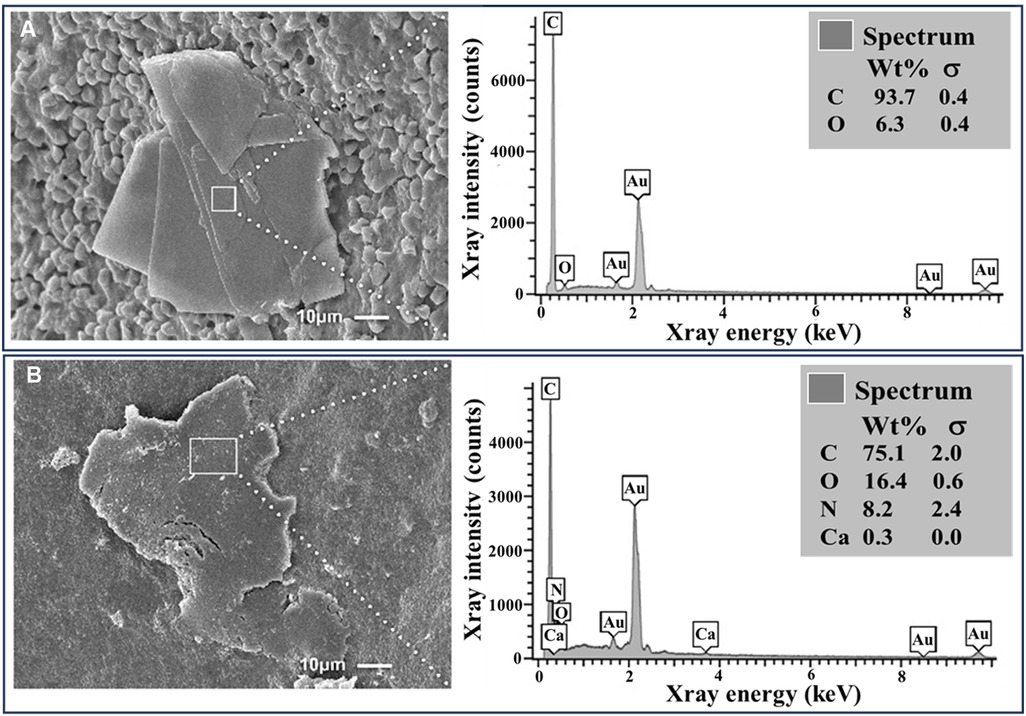
Figure 4. Cholesterol crystals with and without colchicine in ex vivo human plaques. (A) Intact cholesterol crystal in an atherosclerotic carotid plaque with surrounding RBCs. (B) Dissolving and fragmented cholesterol crystal from a plaque treated with colchicine (5 mg/ml). EDS demonstrates a predominance of carbon and oxygen with a small amount of nitrogen and calcium in the dissolving crystal (B) that is likely a contamination detected by the EDS from underlying tissues. Au, gold; Ca, calcium; C, carbon; N, nitrogen; O, oxygen.
EDS showed that synthetic CCs were composed exclusively of carbon and oxygen. The CCs from the ex vivo carotid plaques also predominantly contained carbon and oxygen.
This investigation evaluated the impact of colchicine on CC formation, volume expansion, and morphology in a series of bench studies that included the assessment of CCs in human atherosclerotic plaques. In the in vitro study, we noted a trend showing a decrease in the volume of CCs as the colchicine dose increased. The effect size was large, suggesting a practical change in volume with colchicine. The lack of statistical significance in pairwise comparisons is likely attributed to the small sample size. By SEM, CC morphology from both in vitro and ex vivo samples treated with colchicine appeared to be dissolving with the loss of sharp edges.
This research was motivated by the recognition of the pivotal role that CCs play in the development and progression of atherosclerosis and CVEs and by evidence that both statins and aspirin may also affect CC development, expansion, and morphology (11–14). By focusing on CC formation and morphology, we sought to understand if the effects of colchicine in atherosclerosis may also relate to factors other than its anti-inflammatory effects. Since colchicine is highly concentrated in leukocytes by up to 600× than serum levels, once leukocytes traverse the atherosclerotic bed, it is likely that colchicine becomes highly concentrated as senescent leukocytes become trapped in the plaque core (15, 16).
Cholesterol crystals have been shown to trigger the innate immune system via NLRP3 inflammasomes similar to uric acid crystals (2–4). Moreover, other types of crystals have been shown to cause DNA damage as in the case of silicates and asbestos that have a mutagenic potential (20). Recently, we demonstrated that CCs are also present and actively engaged in neovascularization and enhancing cancer cell tumorigenesis (21). Thus, the disruption of crystalloids that act as an irritant within the biological system could potentially inhibit the acute inflammatory response as well as the chronic irritation that is persistent causing long-term tissue injury.
Previous studies have demonstrated that statins, aspirin, and metformin, which are known to reduce cardiovascular events, attenuate CCs expansion, dissolve them, and alter their morphology while at the same time attenuating inflammation, which may reduce disease progression and the risk of CVEs (12, 14, 22–26). In contrast to colchicine, it is not known if these agents can become concentrated within the atherosclerotic bed. Cecconi et al. have demonstrated that colchicine may stabilize atherosclerotic plaque by reducing inflammatory activity and plaque burden without affecting macrophage infiltration (27). This suggests that its actions are beyond having an effect on macrophages and may be related to its effects on CC development.
Although the control test tubes demonstrate an expansion of CCs, it is also important to mention that other agents (norepinephrine and steroids) that may trigger or worsen CVE also enhanced volume expansion emphasizing that the process of inhibition is not a non-specific effect (28).
In the LoDoCo2 trial of patients with chronic coronary disease, colchicine (0.5 mg/day) was found to provide significant protection from CVE in addition to standard treatment with statins, aspirin, and beta blockers (9). Following these results, the Food and Drug Administration has approved the use of colchicine for prevention in patients who are at a high risk for CVE (17). Our study provides physiologic and morphologic effects of colchicine on CCs beyond anti-inflammation as a benefit for CVE.
Colchicine does affect CC formation, expansion, and morphology. This suggests that some of its clinical benefits may be due to actions beyond its well-known anti-inflammatory effects. Importantly, these results suggest that agents that specifically reduce CC formation and expansion and affect their morphology may offer a therapeutic benefit beyond that obtained with lipid-lowering and anti-inflammatory therapy.
This study was conducted in vitro and ex vivo. An in vivo study would be needed to convey similar findings. However, we have conducted such a study in humans regarding statins and have shown similar CC breakdown in plaques removed from those who were on statins compared to intact CCs in those who were not taking statins (12).
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Michigan State University IRB # 0518. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated samples as part of a previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
ZA: Conceptualization, Data curation, Formal Analysis, Investigation, Project administration, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. DP: Investigation, Methodology, Validation, Visualization, Writing – review & editing. RS: Investigation, Methodology, Validation, Visualization, Writing – review & editing. MM: Investigation, Methodology, Validation, Visualization, Writing – review & editing. GA: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article.
Support was provided in part from Michigan State University, The Jean P. Schultz Biomedical Research Endowment, and Edward W. Sparrow Hospital, Lansing, MI; and the National Institutes of Health (2 R01 EY025383-05A1), Bethesda, MD.
The authors thank Abigail Vanderberg, BSc, Center for help in advanced microscopy for scanning. The authors thank Dr. Ara Pridjan and Mark Schnepp for atherosclerotic plaque sample collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1345521/full#supplementary-material
1. Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. (2012) 32:2045–51. doi: 10.1161/ATVBAHA.108.179705
2. Düewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind F, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. (2010) 464:1357–61. doi: 10.1038/nature08938
3. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. (2006) 440:237–41. doi: 10.1038/nature04516
4. Rajamäki K, Lappalainen J, Oörni K, Välimäki E, Matikainen S, Kovanen PT, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. (2010) 5(7):e11765. doi: 10.1371/journal.pone.0011765
5. Abela GS, Aziz K. Cholesterol crystals rupture biological membranes and human plaques during acute cardiovascular events: a novel insight into plaque rupture by scanning electron microscopy. Scanning. (2006) 28:1–10. doi: 10.1002/sca.4950280101
6. Abela GS, Aziz K, Vedre A, Pathak D, Talbott JD, DeJong J. Effect of cholesterol crystals on plaques and intima in arteries of patients with acute coronary and cerebrovascular syndromes. Am J Cardiol. (2009) 103:959–68. doi: 10.1016/j.amjcard.2008.12.019
7. Nuki G. Colchicine: its mechanism of action and efficacy in crystal-induced inflammation. Curr Rheumatol Rep. (2008) 10(3):218–27. doi: 10.1007/s11926-008-0036-3
8. Lazaros G, Imazio M, Brucato A, Vlachopoulos C, Lazarou E, Vassilopoulos D, et al. The role of colchicine in pericardial syndromes. Curr Pharm Des. (2018) 24:702–9. doi: 10.2174/1381612824666180116101823
9. Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. (2020) 383:1838–47. doi: 10.1056/NEJMoa2021372
10. Patel R, Janoudi A, Vedre A, Aziz K, Tamhane U, Rubinstein J, et al. Plaque rupture and thrombosis is reduced by lowering cholesterol levels and crystallization with ezetimibe and is correlated with FDG-PET. Arterioscler Thromb Vasc Biol. (2011) 31:2007–14. doi: 10.1161/A
11. Nasiri M, Janoudi A, Vanderberg A FM, Flegler C, Flegler S, Abela GS. Role of cholesterol crystals in atherosclerosis is unmasked by altering tissue preparation methods. Microsc Res Tech. (2015) 78:969–74. doi: 10.1002/jemt.22560
12. Abela GS, Vedre A, Janoudi A, Huang R, Durga S, Tamhane U. Effect of statins on cholesterol crystallization and atherosclerotic plaque stabilization. Am J Cardiol. (2011) 107:1710–17. doi: 10.1016/j.amjcard.2011.02.336
13. Vedre A, Pathak DR, Crimp M, Lum C, Koochesfahani M, Abela GS. Physical factors that trigger cholesterol crystallization leading to plaque rupture. Atherosclerosis. (2008) 203:89–96. doi: 10.1016/j.atherosclerosis.2008.06.027
14. Fry L, Lee A, Khan S, Aziz K, Vedre A, Abela GS. Effect of aspirin on cholesterol crystallization: a potential mechanism for plaque stabilization. Am Heart J Plus. (2022) 13. doi: 10.1016/j.ahjo.2021.100083
15. Chappey ON, Niel E, Wautier JL, Hung PP, Dervichian M, Cattan D, et al. Colchicine disposition in human leukocytes after single and multiple oral administration. Clin Pharmacol Ther. (1993) 54:360–7. doi: 10.1038/clpt.1993.161
16. Ben-Chetrit E, Bergmann S, Sood R. Mechanism of the anti-inflammatory effect of colchicine in rheumatic diseases: a possible new outlook through microarray analysis. Rheumatology. (2006) 4:274–82. doi: 10.1093/rheumatology/kei140
17. Food and Drug Administration. (2023). FDA Reference ID: 5192662. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/215727s000lbl.pdf (accessed July 15, 2023).
18. Felton CV, Crook D, Davies MJ, Oliver MF. Relation of plaque lipid composition and morphology to the stability of human aortic plaques. Arterioscler Thromb Vasc Biol. (1997) 17(7):1337–45. doi: 10.1161/01.atv.17.7.1337
19. Abela GS, Hammer S, Huang X, Busik JV, Nidorf SM. Agents that affect cholesterol crystallization and modify the risk of crystal induced traumatic and inflammatory injury. In: Abela GS, Nidorf SM, editors. Cholesterol crystals in atherosclerosis and other related diseases. Cham, Switzerland: Springer Nature (2023). p. 467–89.
20. Msiska Z, Pacurari M, Mishra A, Leonard SS, Castranova V, Vallyathan V. DNA double-strand breaks by asbestos, silica, and titanium dioxide: possible biomarker of carcinogenic potential? Am J Respir Cell Mol Biol. (2010) 43:210–9. doi: 10.1165/rcmb.2009-0062OC
21. Abela GS, Katkoori VR, Pathak DR, Bumpers HL, Leja M, Abideen ZU, et al. Cholesterol crystals induce mechanical trauma, inflammation, and neo-vascularization in solid cancers as in atherosclerosis. Am Heart J Plus. (2023) 35:100317. doi: 10.1016/j.ahjo.2023.100317
22. Abela GS, Kalavakunta JK, Janoudi A, Leffler D, Dhar G, Salehi N, et al. Frequency of cholesterol crystals in culprit coronary artery aspirate during acute myocardial infarction and their relation to inflammation and myocardial injury. Am J Cardiol. (2017) 120:1699–707. doi: 10.1016/j.amjcard.2017.07.075
23. Boumegouas M, Grondahl B, Fry L, Feijter-Rupp HD, Janoudi A, Abela GS. Metformin inhibition of volume expansion with cholesterol crystallization may contribute to reducing plaque rupture and improved cardiac outcomes. J Clin Lipidol. (2020) 14:579–80. doi: 10.1016/j.jacl.2020.05.058
24. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. (2009) 373(9678):1849–60. doi: 10.1016/S0140-6736(09)60503-1
25. Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US preventive services task force [published correction appears in JAMA. 2020 Feb 18;323(7):669]. JAMA. (2016) 316(19):2008–24. doi: 10.1001/jama.2015.15629
26. Zhang K, Yang W, Dai H, Deng Z. Cardiovascular risk following metformin treatment in patients with type 2 diabetes mellitus: results from meta-analysis. Diabetes Res Clin Pract. (2020) 160:108001. doi: 10.1016/j.diabres.2020.108001
27. Cecconi A, Vilchez-Tschischke JP, Mateo J, Sanchez-Gonzalez J, España S, Fernandez-Jimenez R, et al. Effects of colchicine on atherosclerotic plaque stabilization: a multimodality imaging study in an animal model. J Cardiovasc Transl Res. (2021) 14:150–60. doi: 10.1007/s12265-020
Keywords: cholesterol crystals, atherosclerosis, colchicine, plaque rupture, cardiovascular events
Citation: Abideen ZU, Pathak DR, Sabanci R, Manu M and Abela GS (2024) The effect of colchicine on cholesterol crystal formation, expansion and morphology: a potential mechanism in atherosclerosis. Front. Cardiovasc. Med. 11:1345521. doi: 10.3389/fcvm.2024.1345521
Received: 28 November 2023; Accepted: 22 January 2024;
Published: 16 February 2024.
Edited by:
Ashish Misra, Heart Research Institute, AustraliaReviewed by:
Solomon Arko Mensah, Worcester Polytechnic Institute, United States© 2024 Abideen, Pathak, Sabanci, Manu and Abela. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: George S. Abela YWJlbGFAbXN1LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.