- Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, United States
A 73-year-old woman presented to the emergency department with a syncopal episode and a history of dizzy spells. A transthoracic echocardiogram demonstrated a large left atrial mass extending into the right upper pulmonary veins. Subsequently, cardiac magnetic resonance imaging and coronary computed tomography angiography with three-dimensional reconstruction and printing of the heart and mass were performed, which demonstrated a high index of suspicion for an atypical left atrial myxoma. The mass was excised robotically, and the pathology report confirmed a diagnosis of myxoma.
Introduction
Cardiac myxoma is one of the most common primary cardiac neoplasms in adults, second only to papillary fibroelastomas (1). Patients with cardiac myxomas can present with a variety of symptoms such as chest pain, shortness of breath, palpitations, and embolic phenomena. Malaise or syncope was reported in 14% of patients in a case series describing 112 cases of left atrial myxoma (2).
Case report
History of presentation
A 73-year-old woman presented to an outside clinic complaining of dizzy spells leading to a single syncopal episode. Following the syncopal episode, she was taken to the emergency department (ED). She was noted to have supraventricular tachycardia (SVT). An echocardiogram demonstrated a left atrial mass. However, she did not report any symptoms such as chest pain, dyspnea, orthopnea, paroxysmal nocturnal dyspnea, palpitations, or irregular heart rhythm. She was referred to our institution for further evaluation and treatment of the mass.
Past medical history
The patient had a history of type 2 diabetes mellitus (on oral agents), well-controlled systemic hypertension, hyperlipidemia, and bronchial asthma.
Investigations
Her physical examination was unremarkable, as exemplified by the fact that her heart sounds were normal. A brain magnetic resonance imaging (MRI) did not show signs of ischemia or prior infarction. An echocardiogram in the ED demonstrated a left atrial mass with a broad base attachment to the atrial septum (Figure 1) that extended from the opening of the pulmonary veins to the mitral annulus. Subsequent investigation with coronary computed tomography angiography (CCTA) revealed a 41 mm × 27 mm × 33 mm broad-based lobulated mass in the left atrium that extended onto the mitral annulus (Figure 2). The mass was isointense on T1-weighted images and hyperintense on T2-weighted images and showed restricted diffusion (Supplementary Figure S1). There was heterogeneous enhancement on both early and late postcontrast images. The findings were compatible with a cardiac myxoma (Supplementary Video S1).
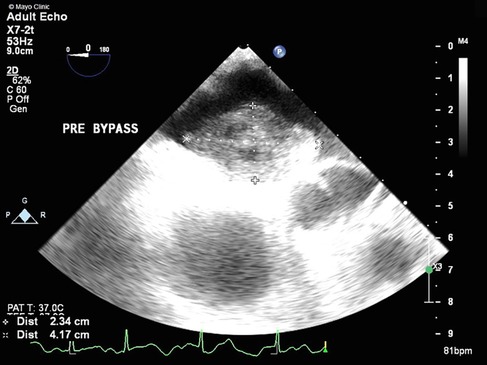
Figure 1. TEE showing a large mass (2.3 cm × 4.2 cm) with attachment to the left atrial side of the atrial septum.
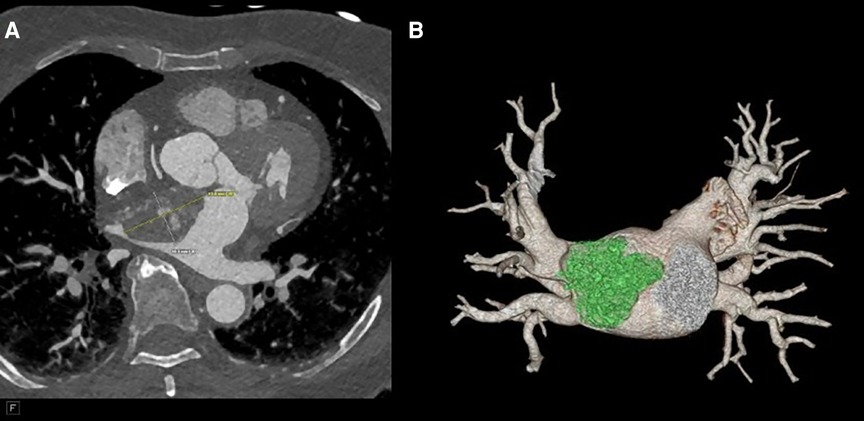
Figure 2. (A) CTA revealing a 41 mm × 27 mm × 33 mm broad-based lobulated mass in the left atrium that extends into the mitral annulus. (B) CTA reconstruction to show the mass extending to the right upper pulmonary vein (RUPV).
Differential diagnosis
Cardiac myxoma; metastases from unknown primary; undifferentiated high-grade pleomorphic sarcoma; thrombus; leiomyosarcoma; lipoma.
Management
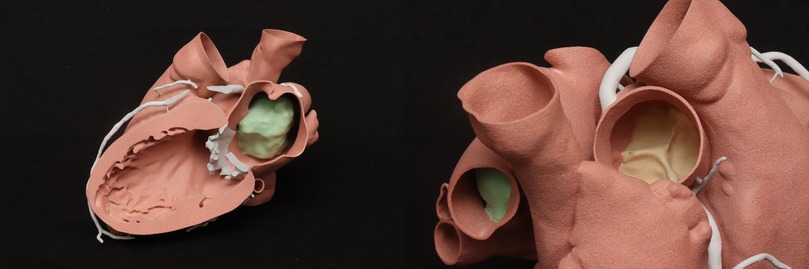
Following the reported imaging findings, a careful surgical plan was discussed with the multidisciplinary team for resection of the mass. A CCTA with a three-dimensional (3D) reconstruction of the mass, including a 3D-printed model of the patient's heart depicting the mass and relationship with adjacent structures, was obtained to help with surgical planning (Figure 3). The 3D CT model redemonstrated a polypoid, heterogeneously enhancing left atrial mass attached to the entire left side of the atrial septum and measuring approximately 44 mm × 28 mm × 40 mm. The appearance on the CCTA and the location were consistent with a cardiac myxoma. The mass occluded the right superior pulmonary vein ostium during the atrial systole phase. A large-caliber sinoatrial artery branch arising from the proximal segment appeared to be the primary vascular supply to the mass. A transesophageal echocardiogram (TEE) was obtained, which confirmed that the mass was not adherent to the right superior pulmonary vein (Supplementary Videos S2, S3). Therefore, the patient was deemed to be a surgical candidate for a minimally invasive robotic approach given the location of the mass and the lack of adherence to the wall of the right superior pulmonary vein.
The patient underwent a robot-assisted minimally invasive mass resection. She was placed on cardiopulmonary bypass via the right femoral artery and vein. Access was obtained and the robot was docked to the right chest. After arresting the heart, we elected to access the left atrium via the right inferior pulmonary vein (as per visualization with the 3D-printed model), the point from which the tumor was furthest away. Upon entering the left atrium, a large tumor was identified, and care was taken not to disrupt the tumor. The mass was visualized and was easily separated from the atrial septum (Supplementary Video S4). A partial thickness of the atrial septum was resected along with the mass. The tumor had a gelatinous quality, consistent with that of a myxoma, and was sent to pathology for confirmation (Supplementary Figure S2). In the frozen section, the tumor was reported to be a highly undifferentiated malignant tumor that was adjacent to the line of resection. By the time the frozen section results became available, the atrium had been closed and the cross-clamp was removed, but the patient remained cannulated. With the findings of the pathology, it was decided to reinitiate the cardiopulmonary bypass and arrest the heart again, in order to resect the atrial septum at the foramen ovale, given the potential malignant nature of the tumor. The systemic margins alongside the septum were sent to pathology for evaluation, and the results were negative for malignancy. The atrial septum was closed with a bovine pericardial patch. The patient was extubated in the operating room and had an uncomplicated recovery in the hospital, following which she was discharged 5 days later. The final pathology report confirmed a cardiac myxoma with no malignancy (Supplementary Figure S3). The margins were uninvolved. The tumor cells were shown to be reactive with antibodies directed against PRKAR1A, in keeping with a non-syndromic cardiac myxoma. No complications were encountered by the patient in the postoperative phase.
Discussion
Cardiac myxomas usually (>90%) occur in an isolated fashion, but rarely, they can also occur in a syndromic context, as part of the Carney complex, an autosomal-dominant condition (3). Cardiac myxomas occurring as part of the Carney complex are termed “syndromic myxomas” and are associated with mutations in PRKAR1A (4). In addition to cardiac myxomas, the syndrome is associated with extracardiac myxomas, endocrinopathy, and spotty skin pigmentation (lentiginoses). Cardiac myxomas occurring in the Carney complex are more likely to occur in atypical (non-left atrial) locations, be multiple, and occur earlier in life. Immunohistochemical staining from our patient's myxoma showed positive results for PRKAR1A, but the patient did not have any dermatological manifestations associated with a Carney complex or a history of other tumors.
Myxomas are often initially diagnosed by echocardiography. The classic presentation is a mobile mass on a stalk arising from the atrial septum. In our patient, a TEE was done prior to surgery to assess hemodynamic function. A cavitated sessile mass arising from the atrial septum was seen. The mass did not have a stalk and exhibited contrast within the body of the tumor. The lack of a stalk from which the tumor arose and its cavitated appearance were atypical for a myxoma, warranting further investigation and planning prior to resection. The mass also extended upward into the right pulmonary veins, which is extremely uncommon. Cardiac MRI (CMRI) often complements echocardiography and offers improved tissue characterization, with cardiac myxomas typically demonstrating hypointensity on T1 images, hyperintensity on T2 images, and little to no perfusion or late enhancement (5). T1- and T2-weighted double-recovery sequences aid tissue characterization. Furthermore, cine cardiac imaging holds great importance in evaluating atrial myxomas because of their high mobility and their tendency to prolapse through the atrioventricular valve during diastole (6). Contrast-enhanced sequences are crucial in distinguishing myxomas from thrombus, as myxomas typically exhibit minimal or nil enhancement during first-pass perfusion, yet display a more heterogeneous enhancement pattern on late gadolinium enhancement (LGE) imaging (7). In our case, the patient's cardiac MRI scan did show findings consistent with those of a myxoma, but it revealed more enhancement than is typical. Cardiac MRI has shown remarkable accuracy in identifying cardiac masses and effectively differentiating between benign and malignant tumors. However, relying solely on MRI has been associated with occasional instances of inaccurate diagnosis. Therefore, adopting a multimodal imaging approach comprising echocardiography, MRI, CT, and possibly positron emission tomography (PET) imaging offers an optimal approach for a comprehensive evaluation and stratification of cardiac masses (8). Given the atypical characteristics and location of our patient's left atrial mass, a CT angiogram with a 3D reconstruction of the heart depicting the mass was requested to guide the diagnosis and surgical planning. The mass was heterogeneously enhancing and polypoid in shape. The mass did not infiltrate or invade surrounding structures. This observation holds considerable significance as it reduces the likelihood of the tumor being a malignant one (9). However, it did extend to the ostium of the right superior pulmonary vein, occluding it during atrial systole. It is hard to ascertain whether the mass was the source of our patient's symptoms, but these findings could potentially explain the patient's orthostatic syncope and dizziness. A case report in the literature describes a patient presenting with syncope and dyspnea, who was found to have a left atrial myxoma extending into and occluding the left pulmonary veins and causing pulmonary infarction (10). However, in our patient, the mass did not extend or occlude the mitral valve, which made it feasible to adopt a robotic approach. Furthermore, the mass received blood supply from the small sinoatrial node branch of the right coronary artery. This was visualized precisely and with high spatial resolution on the 3D-printed model, which also showed the extent of expansion of the mass into the right upper pulmonary veins (Figure 4). The printed model also provided for visualization of the best approach to enter the left atrium without disruption of the mass. A possible implication of this occurrence is the formation of a fistula between the sinoatrial nodal artery and the right atrium after surgical resection of the atrial myxoma. For this reason, special attention should be paid to ligating neovascularized branches feeding myxomas during the surgical procedure (11).
Conclusion
This study described a peculiar and unique presentation of a left atrial myxoma extending into the right upper pulmonary veins. Multimodal imaging, including 3D reconstruction and printing of the heart and mass, guided the diagnostic approach and successful resection of the mass by robotic intervention.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
EA: Writing – original draft, Writing – review & editing. AAr: Writing – review & editing. MA: Writing – review & editing. EE: Writing – review & editing. AAh: Writing – review & editing. TF: Writing – review & editing. RD: Writing – review & editing. JM: Writing – review & editing. RK: Writing – review & editing. KK: Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1340406/full#supplementary-material
Supplementary Figure S1
Cardiac MRI images depicting (A) T1-weighted and (B) T2-weighted sequences.
Supplementary Figure S2
Gross photograph of a resected cardiac tumor. (A) The tumor has a lobulated morphology and (B) in the cut section has a gelatinous quality.
Supplementary Figure S3
Photomicrograph of a resected cardiac tumor. (A) Hematoxylin and eosin staining discloses bland spindle-shaped cells, occurring singly and in small clusters, proliferating in a myxoid background, consistent with a cardiac myxoma. (B) The neoplastic cells are reactive with antibodies directed against PRKAR1A, suggesting a non-syndromic tumor.
Supplementary Video S1
A four-chamber view shows the entire left atrial septum being engulfed by the large mass and extending up and into the right upper pulmonary vein.
Supplementary Video S2
A TEE of a myxoma four-chamber. (A) A demonstration of the entire left atrial septum being engulfed by the tumor mass. (B) A zoom view showing flow within the mass by a color Doppler.
Supplementary Video S3
A TEE at 70°–88° showing the myxoma filling the right superior pulmonary vein (A); color (B) freely moving and color flow around the mass.
Supplementary Video S4
A robot-assisted minimally invasive resection of an atypical left atrial myxoma.
Abbreviations
3D, three-dimensional; CCTA, coronary computed tomography angiography; CMRI, coronary magnetic resonance imaging; CT, computed tomography; ED, emergency department; MRI, magnetic resonance imaging; SVT, supraventricular tachycardia.
References
1. Tamin SS, Maleszewski JJ, Scott CG, Khan SK, Edwards WD, Bruce CJ, et al. Prognostic and bioepidemiologic implications of papillary fibroelastomas. J Am Coll Cardiol. (2015) 65(22):2420–9. doi: 10.1016/j.jacc.2015.03.569
2. Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma: a series of 112 consecutive cases. Medicine (Baltimore). (2001) 80(3):159–72. doi: 10.1097/00005792-200105000-00002
3. Jain S, Maleszewski JJ, Stephenson CR, Klarich KW. Current diagnosis and management of cardiac myxomas. Expert Rev Cardiovasc Ther. (2015) 13(4):369–75. doi: 10.1586/14779072.2015.1024108
4. Maleszewski JJ, Larsen BT, Kip NS, Castonguay MC, Edwards WD, Carney JA, et al. PRKAR1A in the development of cardiac myxoma: a study of 110 cases including isolated and syndromic tumors. Am J Surg Pathol. (2014) 38(8):1079–87. doi: 10.1097/PAS.0000000000000202
5. Kurmann R, El-Am E, Ahmad A, Abbasi MA, Mazur P, Akiki E, et al. Cardiac masses discovered by echocardiogram; what to do next? Struct Heart. (2023) 7(4):100154. doi: 10.1016/j.shj.2022.100154
6. Abbas A, Garfath-Cox KA, Brown IW, Shambrook JS, Peebles CR, Harden SP. Cardiac MR assessment of cardiac myxomas. Br J Radiol. (2015) 88(1045):20140599. doi: 10.1259/bjr.20140599
7. Fussen S, De Boeck BW, Zellweger MJ, Bremerich J, Goetschalckx K, Zuber M, et al. Cardiovascular magnetic resonance imaging for diagnosis and clinical management of suspected cardiac masses and tumours. Eur Heart J. (2011) 32(12):1551–60. doi: 10.1093/eurheartj/ehr104
8. Giusca S, Kelle S, Korosoglou G. When tissue and outcomes are the issue. Cardiac magnetic resonance for patients with suspected cardiac tumours. Eur Heart J. (2021) 43(1):81–3. doi: 10.1093/eurheartj/ehab625
9. Avranas K, Eisenbach C, Flechtenmacher C, Korosoglou G. Diagnostic pathway from incidental mass to metastatic melanoma. JACC Case Rep. (2024) 29(1):102146. doi: 10.1016/j.jaccas.2023.102146
10. Stevens LH, Hormuth DA, Schmidt PE, Atkins S, Fehrenbacher JW. Left atrial myxoma: pulmonary infarction caused by pulmonary venous occlusion. Ann Thorac Surg. (1987) 43(2):215–7. doi: 10.1016/S0003-4975(10)60401-8
Keywords: atypical myxoma, multimodal imaging, 3D reconstruction, pulmonary veins, cardiac MRI (CMRI), cardiac CT, echocardiography
Citation: Akiki E, Arghami A, Abbasi MA, El-Am EA, Ahmad A, Foley TA, Daly RC, Maleszewski JJ, Kurmann R and Klarich KW (2024) Case Report: A myxoma with a far reach. Front. Cardiovasc. Med. 11:1340406. doi: 10.3389/fcvm.2024.1340406
Received: 17 November 2023; Accepted: 3 January 2024;
Published: 24 January 2024.
Edited by:
Grigorios Korosoglou, GRN Klinik Weinheim, GermanyReviewed by:
Wolfgang Fehske, Independent Researcher, GermanyAlexandros Kallifatidis, St. Luke’s Hospital, Greece
© 2024 Akiki, Arghami, Abbasi, El-Am, Ahmad, Foley, Daly, Maleszewski, Kurmann and Klarich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyle W. Klarich a2xhcmljaC5reWxlQG1heW8uZWR1
 Elias Akiki
Elias Akiki Arman Arghami
Arman Arghami Muhannad A. Abbasi
Muhannad A. Abbasi Edward A. El-Am
Edward A. El-Am Ali Ahmad
Ali Ahmad Reto Kurmann
Reto Kurmann Kyle W. Klarich
Kyle W. Klarich