- 1Graduate School of Health and Sports Science, Doshisha University, Kyo-Tanabe, Japan
- 2Faculty of Education, Miyagi Gakuin Women’s University, Sendai, Japan
- 3College of Life and Health Sciences, Chubu University, Kasugai, Japan
- 4Sun Chlorella Corp., Kyoto, Japan
- 5Faculty of Health and Sports Science, Doshisha University, Kyo-Tanabe, Japan
Background: Cardiac autonomic function (CAF) decreases with aging, and Acanthopanax senticosus Harms (ASH) consumption reportedly induces anti-stress effects. This study aimed to assess the effect of continuous supplementation of ASH on CAF during resting and standing tests in the elderly population.
Methods: This double-blind, randomized controlled trial was conducted in the morning in a laboratory setting and was carried out between June 2017 and July 2017 at Kambaikan, Doshisha University (Karasuma-higashi-iru, Imadegawa-dori, Kamigyo-ku, Kyoto 602-8580, Japan). In total, 28 community-dwelling elderly individuals (mean ± standard deviation = 72.5 ± 4.5 years) were included. Each subject was instructed to consume ASH or placebo supplements twice daily for 4 weeks. An autonomic reflex orthostatic tolerance recorder was used to measure CAF in pre- and post-intervention phases. Parameters were measured in a seated position and included coefficient of variation of R-R intervals (CVRR), low frequency (LF), high frequency (HF), LF/HF ratio, blood pressure, and heart rate (HR). Changes in each parameter were evaluated before and after standing. All parameters were defined as the difference between the mean value obtained in a standing position for 2 min and that obtained in a 2-min seated position.
Results: A two-way analysis of variance revealed a significant group-time interaction effect on CVRR, HF, and ΔLF/HF ratio. Following the intervention, CVRR, HF, LF/HF ratio, systolic blood pressure (SBP), HR, ΔLF/HF ratio, ΔSBP, and ΔHR improved significantly in the ASH group only.
Conclusions: Four-week supplementation of ASH improved CAF in community-dwelling elderly individuals during resting and standing tests.
Clinical Trial Registration: https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000031218, UMIN Clinical Trials Registry (UMIN000027251).
1 Introduction
The heart rate (HR) is not a constant parameter, and fluctuations occur every beat. Evaluating the beat-to-beat variation in the HR, a parameter referred to as HR variability (HRV), is a reliable and non-invasive approach for assessing cardiac autonomic function (CAF) (1–3). CAF decreases with age (4, 5). Low CAF values are associated with morbidity and mortality following myocardial infarction (6), sudden death (7), and heart disease severity (8). In a 10-year cohort study conducted among community-dwelling elderly individuals, Mäkikallio et al. reported that CAF was an independent predictor of sudden cardiac death (9). Therefore, CAF should be maintained within a normal state in elderly individuals.
Decreased CAF is related to the onset of dizziness (10). Owing to gravitational force, immediately after assuming a standing position, 300 ml–800 ml of blood pools in the skeletal muscles of the lower body. As a consequence, the venous return of blood to the heart decreases, and this results in a diminished stroke volume and blood pressure. In response to this, cardiac sympathetic nervous system (SNS) activity increases, while cardiac parasympathetic nervous system (PNS) activity decreases. These autonomic adjustments increase the HR and cardiac contractility and restore blood pressure to a lower level. However, in elderly individuals with low CAF, this compensation mechanism is not effective during standing. As a result, dizziness is a common finding in elderly individuals (11). A previous epidemiologic survey revealed that approximately 20% of elderly persons aged 65 years and older have experienced dizziness while standing up (12). Ooi et al. also reported that more than 50% of the elderly residents of nursing homes have experienced dizziness (13). Thus, apparent structural disorder in the occurrence of dizziness is a common finding among elderly persons. The occurrence of dizziness while standing is a predictor of vascular death (14) and increases the risk of falls among elderly individuals (15). A clinical practice guideline established by the European Society of Cardiology has also stated that the symptoms of orthostatic hypotension (OH), such as dizziness, are related to unconsciousness (16). Melillo et al. suggested that CAF levels that can increase the risk of falls could reliably be detected (17), and another study reported similar results (18). To prevent falls, a stable CAF level should be maintained in elderly individuals.
Acanthopanax senticosus Harms (ASH) is a plant belonging to the Araliaceae family that grows abundantly in various regions of Russia, China, Korea, Southeast Asia, and North Japan (19). The ASH root bark has traditionally been used for nutritional fortification, and ASH supplementation has an anti-stress effect during cold-water immersion restraint in rats (20). In addition, Hartz et al. demonstrated that in patients who experienced chronic fatigue for 6 months, chronic fatigue significantly improved after 2 months of ASH supplementation (21). These results are supported by several previous studies that investigated the physiological effects of ASH in rats and humans (22–24). In other findings, during the novelty-suppressed feeding test, ASH treatment for 1 week significantly decreased latency to eat and improved SNS and PNS activity (25). This finding suggests that the anxiolytic effects of ASH result from the regulation of autonomic function. Nevertheless, no previous studies have evaluated the effects of ASH supplementation on CAF in humans. Therefore, this study aimed to examine the effects of continuous ASH supplementation on CAF during tests conducted while resting and standing among the community-dwelling elderly population.
2 Methods
2.1 Participants
This study was conducted in accordance with the Declaration of Helsinki, and ethical approval was obtained from the Research Ethics Review Committee on Human Subjects of Doshisha University (authorization number: 16008). The trial has been registered in the UMIN Clinical Trials Registry (UMIN000027251). Written consent was obtained from all participants. In addition, participants had 1 month to decide on their willingness to participate.
The sample size was based on a previous study design that measured CAF in elderly individuals by postural changes at rest, tilt-up, and tilt-down, as in the present study (26). Therefore, before conducting the experiments, the appropriate sample size was estimated by power analysis using software G∗power (Version 3.1.9.4) for two-way repeated measures analysis of variance. The effect size f was set to 0.4, alpha error probability to 0.05, beta error probability to 0.95, number of groups to 2, and number of measurements to 2. The calculated total sample size was 24; therefore, 30 subjects were recruited with an anticipation of a 20% dropout.
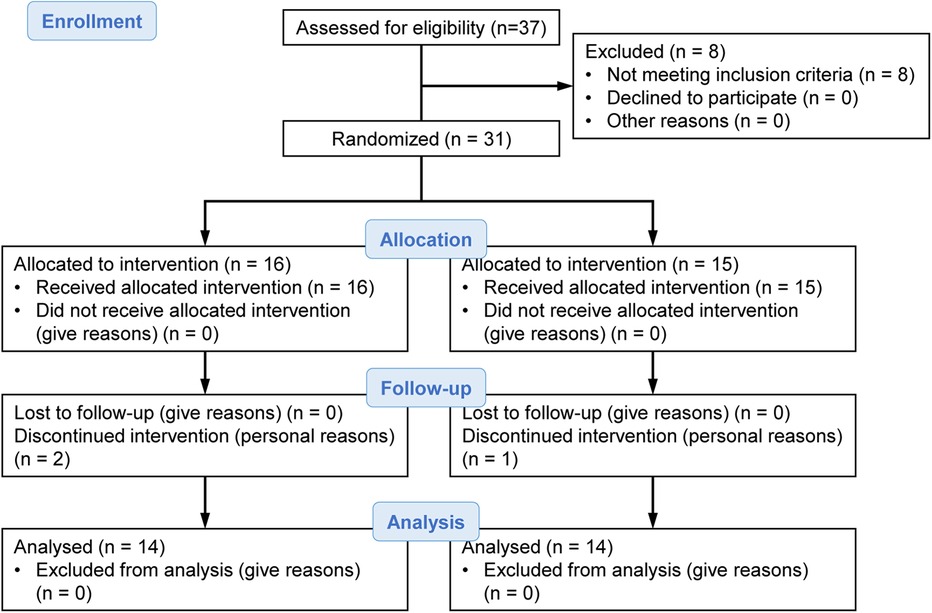
Our laboratory recruited participants by posting notifications on the circular community bulletin board and in the flyer of the local neighborhood association of Kyoto city from the end of May to early June 2017. We had 37 applicants, but this study included 31 community-dwelling elderly individuals aged 65–90 years without hypertension, heart disease, or diabetes. Participants were randomly assigned to two groups of 16 and 15 individuals. During the intervention period, two participants from the group of 16 and one participant from the group of 15 interrupted the intervention for personal reasons. Finally, statistical analysis was performed on the two groups of 14 participants each (Figure 1).
2.2 Intervention methods
In this double-blind, randomized controlled trial, the participants were assigned to either the ASH group [patients receiving ASH supplements (Sun Chlorella Corp., Kyoto, Japan)] or the placebo group (patients receiving placebo supplements). The randomization code was set using computer-generated random numbers by a researcher who was not engaged in running the trial. The group allocation was blinded for both investigators and participants. Each participant was instructed to consume 12 tablets twice daily (after breakfast and dinner) for 4 weeks between the end of June and the end of July 2017. The compliance rate for all participants was 100%.
Plant powder tablets used in this study were prepared from powdered roots of ASH from Heilongjiang, China. The herb specimen was authenticated by the validation test method of the United States Pharmacopeia that assesses the content of eleutheroside B and E. The tablet (Lot No. 024) was provided by Sun Chlorella Co., Ltd. (Kyoto, Japan). We measured each major component in the tablet using HPLC and an ODS column. These tablets primarily comprised isofraxidin (8.3 mg/100 g), eleutheroside B (74.3 mg/100 g), eleutheroside E (58.2 mg/100 g), eleutheroside B1 (8.5 mg/100 g), and chlorogenic acid (318 mg/100 g).
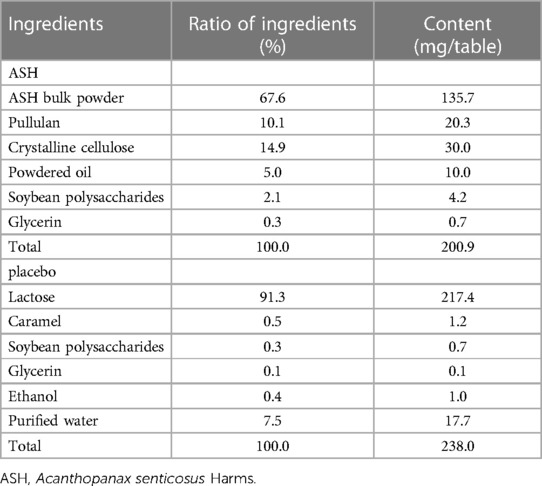
The participants were provided with a pedometer (HJ-720IT, Omron Health Care Corp., Kyoto, Japan) to monitor their average daily step count during the intervention period. Table 1 presents the ingredients of the ASH and placebo supplements, respectively.
2.3 Measurement methods
To measure CAF, an HRV analysis was performed. An autonomic reflex orthostatic tolerance recorder (Kiritsu-Meijin, Crosswell Co., Inc., Yokohama, Japan) was used before and after the 4-week intervention period. This device included an HR monitor (LRR-03, Arm Electronics Corp., Tokyo, Japan) and a blood pressure monitor (TM2584, A&D Company., Ltd., Tokyo, Japan), and recorded the HRV during the R-R intervals on a 2-lead electrocardiogram. The participants were prohibited from performing intense exercise and consuming shellfish, energy drinks, health foods, garlic, and alcohol the day before the measurement. They were instructed to sleep well the day before the measurement, eat breakfast 2 h prior to the measurement, and avoid running while commuting to the laboratory. All measurements were performed in the morning in a laboratory in the Kambaikan at Doshisha University (Karasuma-higashi-iru, Imadegawa-dori, Kamigyo-ku, Kyoto 602-8580, Japan). The participants were allowed to rest sufficiently in a seated position prior to the measurement. During the measurement, the participants were placed in a seated position for 2 min. Subsequently, they were asked to stand up and remain in a standing position for 2 min, followed by a seated position for 1 min (Figure 2). During the measurement, with the aid of an electronic metronome, the breathing of the participants was maintained at 15 breaths/min to reduce the influence of breathing on the results. Using the obtained data, a time domain analysis (27–30) and power spectrum analysis (31, 32) were conducted following the MemCalc method, a new technique for time series analyses.
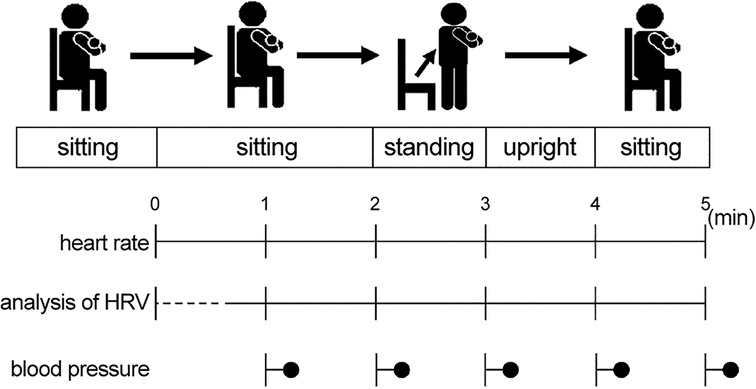
Figure 2. Measurement methods. The participants assumed a seated position for 2 min. Subsequently, they were asked to stand up and remain in a standing position for 2 min, followed by a seated position for 1 min. HRV, heart rate variability.
2.4 Measurement of baseline characteristics and HRV
The participants' age, sex, height, weight, and body mass index were obtained. The binary sex categorization (male/female) in this study was determined based on physical and physiological characteristics. Furthermore, we measured the coefficient of variation of the R-R intervals (CVRR), low-frequency (LF: 0.04–0.15 Hz) rate, high-frequency (HF: 0.15–0.40 Hz) rate, LF/HF ratio, systolic blood pressure (SBP) level, diastolic blood pressure (DBP) level, mean blood pressure (MBP) level, and HR. The mean values of these measurement items were assessed while the participants were in a seated position for 2 min. The LF was used as an index of PNS activity and SNS activity. The HF was used as an index of PNS activity. The LF/HF ratio was used as an index of SNS activity (33–35). The changes in each parameter were evaluated before and after standing. The ΔCVRR, ΔLF, ΔHF, ΔLF/HF ratio, ΔSBP, ΔDBP, ΔMBP, and ΔHR were defined as the difference between the mean values obtained in a standing position for 1 min and those obtained in a seated position for 2 min (e.g., ΔCVRR = CVRR in a standing position for 1 min—CVRR in a seated position for 2 min).
2.5 Statistics
The values are expressed as mean ± SD. The statistical differences between the groups were assessed using Student's unpaired t-test and a χ2-test before the intervention. A two-way analysis of variance with repeated measures was used to assess the interaction effects on the measured parameters between the ASH and placebo groups. Parameters for which the ANOVA results were significant were compared to baseline values for the ASH and placebo groups using a paired Student's t-test. All statistical analyses were performed using SPSS statistics 25 (IBM, Tokyo, Japan). A p-value of <0.05 was considered significant.
3 Results
3.1 Baseline characteristics and daily step counts during the intervention period
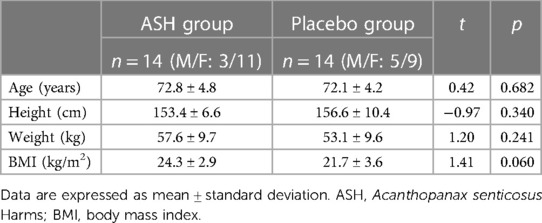
During the intervention period, three participants withdrew from the study for personal reasons, leaving a total of 28 participants [mean ± standard deviation (SD) = 72.5 ± 4.5 years] for analysis. No significant differences were observed in male-to-female ratio and any of the measurement items between the ASH and placebo groups before the intervention (Tables 2, 3). During the intervention period, no significant differences were observed in the daily step counts between the ASH (5,985 ± 2,167 steps/day) and placebo (6,803 ± 2,833 steps/day) groups.
3.2 Changes in measurement items before and after the intervention
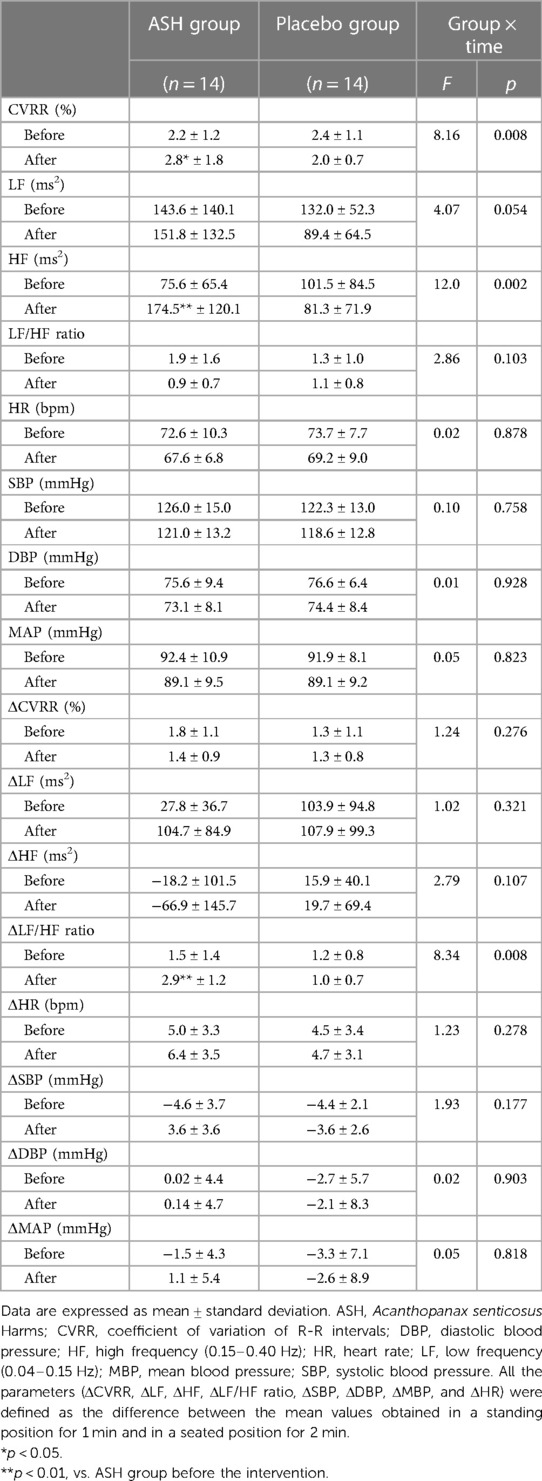
The two-way analysis of variance revealed significant group-time interactions in the CVRR, HF, and ΔLF/HF ratio (Table 3). Following the intervention, the CVRR, HF, and ΔLF/HF ratio significantly improved in the ASH group (Table 3). Similar changes were not observed in the placebo group.
4 Discussion
In this study, significant group-time interactions were observed in the CVRR, HF, and ΔLF/HF ratio Following the intervention period, in the ASH group, the CVRR, HF, and ΔLF/HF ratio significantly increased. In several previous studies, CAF decreased with age (36, 37). However, low CAF results not only from aging but also various diseases; patients with obesity and diabetes have a low CAF (38–40). In the Framingham Heart Study, low HF was associated with all-cause mortality in elderly individuals, even after adjusting the prevalence for age, sex, smoking history, diabetes, and heart disease (41). Therefore, maintaining normal CAF states among elderly individuals is important, as CAF reduction is associated with various pathologies and mortality. Notably, the CVRR, HF, and ΔLF/HF ratio were significantly increased in the ASH group after the intervention period. Therefore, continuous ASH supplementation may potentially improve CAF in a resting state among elderly individuals. The HR is controlled by PNS and SNS activities. In a resting state, PNS activity increases, while SNS activity decreases. In an active state, SNS activity increases, while PNS activity decreases. The PNS and SNS enable HR to fluctuate appropriately during various human activities (42, 43). In this study, the HF increased in the ASH group following the intervention period. Considering that the HF was used as an index of PNS activity, this suggests improvement in resting state HR.
Furthermore, CAF is associated with blood pressure regulation (44). In a previous study, reduced CAF was associated with the incidence of hypertension in men during a follow-up period of 4 years, even after adjusting for factors associated with hypertension (45). Additionally, individuals with high SBP and DBP levels exhibit decreased PNS activity and increased SNS activity during the resting state (46, 47). Based on the above findings, continuous ASH supplementation can properly regulate not only the CAF level but also blood pressure levels in elderly individuals.
When an adult assumes a standing position, 300 ml–800 ml of blood pools in the lower extremities, and the venous return of blood to the heart and blood pressure decrease (48, 49). In this situation, the SNS and PNS decrease the blood pressure level (33). In contrast, CAF may not be effective when elderly individuals and patients with diabetes assume a standing position, and blood pressure may not return to normal (4). Therefore, the incidence of dizziness is relatively high among elderly individuals and patients with diabetes. Kawaguchi et al. clarified that, when used as an index of SNS activity, the LF/HF ratio increased significantly in young participants immediately after assuming a standing position, while it only increased in elderly participants following a delay after assuming a standing position (50). Our results revealed significant group-time interactions in the CVRR, HF, and ΔLF/HF ratio. A decrease in PNS activity and an increase in SNS activity are related to an elevation in HR and blood pressure levels (10, 51). Therefore, continuous ASH supplementation increased the CAF and blood pressure responsiveness among elderly participants while they were in a standing position.
In the ASH group, an increase in the HF while in a resting state significantly improved the ΔLF/HF ratio. Taylor et al. clarified that HF decreases immediately after a healthy adult performs an exercise, but the decrease is mild in elderly individuals. A significant positive correlation was previously observed between the HF at rest and the ΔHR and ΔLF/HF ratio from a resting to an active state (52). Accordingly, the decrease in the HF at rest contributes to the decrease in the reactivity of the HR and SNS activity while standing. Based on the above findings, the increase in the HF at rest in the ASH group contributed to the improvement in the ΔLF/HF ratio reported in this study. Additionally, a previous study examining the effects of hot spring foot bathing on CAF among frail, elderly Japanese individuals using the same standing test as in this study found no improvement in CAF. Therefore, we suggest that ASH intake among elderly individuals is an effective means of improving CAF (53).
In previous studies, treatment with ASH reduced the cardiovascular responses to stress in healthy young individuals (24), and ASH treatment for 1 week significantly increased the HF in rats during an improved elevated beam-walking test (25). However, only a few studies have investigated the effects of continuous ASH supplementation on CAF and no study has analyzed the effects of ASH supplementation on CAF in humans. Our results suggest that continuous ASH supplementation may improve CAF during resting and standing states in elderly individuals.
4.1 Limitations and strengths of the trial
Certain limitations must be taken into account for the interpretation and generalization of this study's results, such as the relatively small sample size and brief study duration. Therefore, future research should be conducted to recruit a larger sample of participants. In addition, longer duration trials of adequate sample size with a clinically significant margin should be designed to determine the equivalence of the interventions. Secondly, the mechanism behind the improvement in CAF was not clearly explained by our results, similar to previous studies. However, ASH plays a role in the body's coping mechanism during stress via a brain noradrenergic mechanism. Notably, the main components of ASH, which include syringin, chlorogenic acid, eleutheroside E, and isofraxidin, were involved in that action (54–56).
Further, the sudden drop in blood pressure levels when assuming a standing position is not necessarily caused by a decrease in CAF, and several factors, such as the progression of atherosclerosis and reduced baroreceptor sensitivity, have an effect (10, 49, 57, 58). Recent evidence indicates that ASH has the potential to improve blood pressure (BP) and arterial stiffness via endothelial eNOS activation in healthy adults who smoke and have a tendency toward elevated BP or blood lipid parameters (59). Therefore, the improvement in blood pressure responsiveness during the standing test in our study may not have been due to an improvement in CAF. However, CAF reduction and the occurrence of dizziness are associated with various pathologies and mortality, and CAF should be maintained at a normal state in elderly individuals. The results of this study have elucidated the effects of ASH on CAF during resting and standing states among elderly individuals and have demonstrated the potential clinical applicability of ASH.
In conclusion, this study examined the effects of continuous ASH supplementation on CAF during resting and standing tests in community-dwelling elderly individuals. The results of this study have demonstrated that supplementation with ASH over a 4-week period improved the CAF and blood pressure responsiveness in community-dwelling elderly individuals during resting and standing tests.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Research Ethics Review Committee on Human Subjects of Doshisha University; authorization number: 16008. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TS: Writing – review & editing, Data curation. TA: Data curation, Writing – review & editing. YI: Data curation, Writing – review & editing. KO: Data curation, Writing – review & editing. MF: Resources, Writing – original draft. EO: Resources, Writing – original draft. KI: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by Sun Chlorella Corporation, which supplied the supplements used in this study.
Acknowledgments
The authors are grateful to Dr. Keisuke Komura (Meijo University) for technical assistance.
Conflict of interest
MF and EO are employees of the Sun Chlorella Corporation and provided information about the supplements used in this study. Furthermore, the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. (1981) 213:220–2. doi: 10.1126/science.6166045
2. Moritani T, Hayashi T, Shinohara M, Mimasa F, Shibata M. Comparison of sympatho-vagal function among diabetic patients, normal controls and endurance athletes by heart rate spectral analysis. J Sports Med Sci. (1993) 7:31–9. https://ndlsearch.ndl.go.jp/en/books/R100000136-I1572824499522327296
3. Task force of the European Society of Cardiology and the North American society of pacing and electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur Heart J. (1996) 17:354–81. doi: 10.1093/oxfordjournals.eurheartj.a014868
4. Shannon DC, Carley DW, Benson H. Aging of modulation of heart rate. Am J Physiol. (1987) 253:H874–7. doi: 10.1152/ajpheart.1987.253.4.H874
5. Fukusaki C, Kawakubo K, Yamamoto Y. Assessment of the primary effect of aging on heart rate variability in humans. Clin Auton Res. (2000) 10:123–30. doi: 10.1007/BF02278016
6. Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. (1987) 59:256–62. doi: 10.1016/0002-9149(87)90795-8
7. Taylor JA, Hand GA, Johnson DG, Seals DR. Augmented forearm vasoconstriction during dynamic exercise in healthy older men. Circulation. (1992) 86:1789–99. doi: 10.1161/01.CIR.86.6.1789
8. Hayano J, Sakakibara Y, Yamada M, Ohte N, Fujinami T, Yokoyama K, et al. Decreased magnitude of heart rate spectral components in coronary artery disease. Its relation to angiographic severity. Circulation. (1990) 81:1217–24. doi: 10.1161/01.CIR.81.4.1217
9. Mäkikallio TH, Huikuri HV, Mäkikallio A, Sourander LB, Mitrani RD, Castellanos A, et al. Prediction of sudden cardiac death by fractal analysis of heart rate variability in elderly subjects. J Am Coll Cardiol. (2001) 37:1395–402. doi: 10.1016/S0735-1097(01)01171-8
10. Bloomfield DM, Kaufman ES, Bigger JT, Fleiss J, Rolnitzky L, Steinman R. Passive head-up tilt and actively standing up produce similar overall changes in autonomic balance. Am Heart J. (1997) 134:316–20. doi: 10.1016/S0002-8703(97)70140-6
11. Hopson JR, Rea RF, Kienzle MG. Alterations in reflex function contributing to syncope: orthostatic hypotension, carotid sinus hypersensitivity and drug-induced dysfunction. Herz. (1993) 18:164–74. PMID: 8330851
12. Robbins AS, Rubenstein LZ. Postural hypotension in the elderly. J Am Geriatr Soc. (1984) 32:769–74. doi: 10.1111/j.1532-5415.1984.tb04178.x
13. Ooi WL, Hossain M, Lipsitz LA. The association between orthostatic hypotension and recurrent falls in nursing home residents. Am J Med. (2000) 108:106–11. doi: 10.1016/S0002-9343(99)00425-8
14. Luukinen H, Koski K, Laippala P, Kivelä SL. Prognosis of diastolic and systolic orthostatic hypotension in older persons. Arch Intern Med. (1999) 159:273–80. doi: 10.1001/archinte.159.3.273
15. O’Sullivan SS, Massey LA, Williams DR, Silveira-Moriyama L, Kempster PA, Holton JL, et al. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain. (2008) 131:1362–72. doi: 10.1093/brain/awn065
16. Task Force for the Diagnosis and Management of Syncope, European Society of Cardiology (ESC), European Heart Rhythm Association (EHRA), Heart Failure Association (HFA), Heart Rhythm Society (HRS), Moya A, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. (2009) 30:2631–71. doi: 10.1093/eurheartj/ehp290
17. Melillo P, Jovic A, De Luca N, Pecchia L. Automatic classifier based on heart rate variability to identify fallers among hypertensive subjects. Healthc Technol Lett. (2015) 2:89–94. doi: 10.1049/htl.2015.0012
18. Sannino G, Melillo P, Stranges S, De Pietro G, Pecchia L. Blood pressure drop prediction by using HRV measurements in orthostatic hypotension. J Med Syst. (2015) 39:143. doi: 10.1007/s10916-015-0292-5
19. Huang L, Zhao H, Huang B, Zheng C, Peng W, Qin L. Acanthopanax senticosus: review of botany, chemistry and pharmacology. Pharmazie. (2011) 66:83–97. PMID: 21434569
20. Fujikawa T, Yamaguchi A, Morita I, Takeda H, Nishibe S. Protective effects of Acanthopanax senticosus Harms from hokkaido and its components on gastric ulcer in restrained cold water stressed rats. Biol Pharm Bull. (1996) 19:1227–30. doi: 10.1248/bpb.19.1227
21. Hartz AJ, Bentler S, Noyes R, Hoehns J, Logemann C, Sinift S, et al. Randomized controlled trial of Siberian ginseng for chronic fatigue. Psychol Med. (2004) 34:51–61. doi: 10.1017/S0033291703008791
22. Jin L, Wu F, Li X, Li H, Du C, Jiang Q, et al. Anti-depressant effects of aqueous extract from Acanthopanax senticosus in mice. Phytother Res. (2013) 27:1829–33. doi: 10.1002/ptr.4938
23. Nishibe S, Kinoshita H, Takeda H, Okano G. Phenolic compounds from stem bark of Acanthopanax senticosus and their pharmacological effect in chronic swimming stressed rats. Chem Pharm Bull. (Tokyo). (1990) 38:1763–5. doi: 10.1248/cpb.38.1763
24. Facchinetti F, Neri I, Tarabusi M. Eleutherococcus senticosus reduces cardiovascular stress response in healthy subjects: a randomized, placebo-controlled trial. Stress Health. (2002) 18:11–7. doi: 10.1002/smi.914
25. Miyazaki S, Oikawa H, Takekoshi H, Hoshizaki M, Ogata M, Fujikawa T. Anxiolytic effects of Acanthopanax senticosus harms occur via regulation of autonomic function and activate hippocampal BDNF–TrkB signaling. Molecules. (2018) 24:132. doi: 10.3390/molecules24010132
26. Matsugi A, Nagino K, Shiozaki T, Okada Y, Mori N, Nakamura J, et al. No impact of stochastic galvanic vestibular stimulation on arterial pressure and heart rate variability in the elderly population. Front Hum Neurosci. (2021) 15:646127. doi: 10.3389/fnhum.2021.646127
27. Moritani T. Sympatho-vagal activities of NIDDM patients during exercise as determined by heart rate spectral analysis. In: Kawamori R, Vranic M, Horton ES, Kubota M, editors. Glucose Fluxes, Exercise and Diabetes. Great Britain: Smith-Gordon (1995). p. 91–6.
28. Pfeifer MA, Cook D, Brodsky J, Tice D, Reenan A, Swedine S, et al. Quantitative evaluation of cardiac parasympathetic activity in normal and diabetic man. Diabetes. (1982) 31:339–45. doi: 10.2337/diab.31.4.339
29. Wheeler T, Watkins PJ. Cardiac denervation in diabetes. Br Med J. (1973) 4:584–6. doi: 10.1136/bmj.4.5892.584
30. Ewing DJ, Neilson JM, Travis P. New method for assessing cardiac parasympathetic activity using 24 h electrocardiograms. Heart. (1984) 52:396–402. doi: 10.1136/hrt.52.4.396
31. Parati G, Pomidossi G, Casadei R, Groppelli A, Trazzi S, Di Rienzo M, et al. Role of heart rate variability in the production of blood pressure variability in man. J Hypertens. (1987) 5:557–60. doi: 10.1097/00004872-198710000-00008
32. Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ. Hemodynamic regulation: investigation by spectral analysis. Am J Physiol. (1985) 249:H867–75. doi: 10.1152/ajpheart.1985.249.4.H867
33. Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, Gordon D, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. (1985) 248:H151–3. doi: 10.1152/ajpheart.1985.248.1.H151
34. Nagai N, Matsumoto T, Kita H, Moritani T. Autonomic nervous system activity and the state and development of obesity in Japanese school children. Obes Res. (2003) 11:25–32. doi: 10.1038/oby.2003.6
35. Shihara N, Yasuda K, Moritani T, Ue H, Adachi T, Tanaka H, et al. The association between Trp64Arg polymorphism of the β3-adrenergic receptor and autonomic nervous system activity. J Clin Endocrinol Metab. (1999) 84:1623–7. doi: 10.1210/jcem.84.5.5701
36. Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol. (1998) 31:593–601. doi: 10.1016/S0735-1097(97)00554-8
37. Zhang J. Effect of age and sex on heart rate variability in healthy subjects. J Manipulative Physiol Ther. (2007) 30:374–9. doi: 10.1016/j.jmpt.2007.04.001
38. Masaoka S, Lev-Ran A, Hill LR, Vakil G, Hon EH. Heart rate variability in diabetes: relationship to age and duration of the disease. Diabetes Care. (1985) 8:64–8. doi: 10.2337/diacare.8.1.64
39. Malpas SC, Maling TJ. Heart-rate variability and cardiac autonomic function in diabetes. Diabetes. (1990) 39:1177–81. doi: 10.2337/diab.39.10.1177
40. Matsumoto T, Miyawaki T, Ue H, Kanda T, Zenji C, Moritani T. Autonomic responsiveness to acute cold exposure in obese and non-obese young women. Int J Obes Relat Metab Disord. (1999) 23:793–800. doi: 10.1038/sj.ijo.0800928
41. Tsuji H, Venditti FJ, Manders ES, Evans JC, Larson MG, Feldman CL, et al. Reduced heart rate variability and mortality risk in an elderly cohort. The framingham heart study. Circulation. (1994) 90:878–83. doi: 10.1161/01.CIR.90.2.878
42. Fujii N, Nabekura Y, Gwon O, Yamazaki F, Homma S, Ikegami H, et al. Heart rate and plasma catecholamines responses to exercise at various intensities. Jpn J Phys Fit Sports Med. (1992) 41:313–21. doi: 10.7600/jspfsm1949.41.313
43. Robinson BF, Epstein SE, Beiser GD, Braunwald E. Control of heart rate by the autonomic nervous system: studies in man on the interrelation between baroreceptor mechanisms and exercise. Circ Res. (1966) 19:400–11. doi: 10.1161/01.RES.19.2.400
44. Julius S. Autonomic nervous system dysregulation in human hypertension. Am J Cardiol. (1991) 67:3B–7B. doi: 10.1016/0002-9149(91)90813-Z
45. Singh JP, Larson MG, Tsuji H, Evans JC, O'Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension. (1998) 32:293–7. doi: 10.1161/01.HYP.32.2.293
46. Hojo Y, Noma S, Ohki T, Nakajima H, Satoh Y. Autonomic nervous system activity in essential hypertension: a comparison between dippers and non-dippers. J Hum Hypertens. (1997) 11:665–71. doi: 10.1038/sj.jhh.1000515
47. Wu JS, Lu FH, Yang YC, Lin TS, Chen JJ, Wu CH, et al. Epidemiological study on the effect of pre-hypertension and family history of hypertension on cardiac autonomic function. J Am Coll Cardiol. (2008) 51:1896–901. doi: 10.1016/j.jacc.2007.12.053
48. Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. (2011) 21:69–72. doi: 10.1007/s10286-011-0119-5
49. Gupta V, Lipsitz LA. Orthostatic hypotension in the elderly: diagnosis and treatment. Am J Med. (2007) 120:841–7. doi: 10.1016/j.amjmed.2007.02.023
50. Kawaguchi T, Uyama O, Konishi M, Nishiyama T, Iida T. Orthostatic hypotension in elderly persons during passive standing: a comparison with young persons. J Gerontol A Biol Sci Med Sci. (2001) 56:M273–80. doi: 10.1093/gerona/56.5.M273
51. Agiovlasitis S, Collier SR, Baynard T, Echols GH, Goulopoulou S, Figueroa A, et al. Autonomic response to upright tilt in people with and without down syndrome. Res Dev Disabil. (2010) 31:857–63. doi: 10.1016/j.ridd.2010.03.002
52. Taylor JA, Hayano J, Seals DR. Lesser vagal withdrawal during isometric exercise with age. J Appl Physiol. (1995) 79:805–11. doi: 10.1152/jappl.1995.79.3.805
53. Kawamura K, Shimasaki H, Deguchi A, Hamaguchi H, Arai H. The effect of natural hot spring foot baths on falls, physical function and autonomic function in frail older adults. Jpn J Fall Prev. (2015) 2:35–43. doi: 10.11335/tentouyobou.2.1_35
54. Ito R, Lee ACH. The role of the hippocampus in approach-avoidance conflict decision-making: evidence from rodent and human studies. Behav Brain Res. (2016) 313:345–57. doi: 10.1016/j.bbr.2016.07.039
55. Soya H, Deocaris CC, Yamaguchi K, Ohiwa N, Saito T, Nishijima T, et al. Extract from Acanthopanax senticosus harms (Siberian ginseng) activates NTS and SON/PVN in the rat brain. Biosci Biotechnol Biochem. (2008) 72:2476–80. doi: 10.1271/bbb.80209
56. Mahady GB, Gyllenhaal C, Fong HHS, Farnsworth NR. Ginsengs: a review of safety and efficacy. Nutr Clin Care. (2000) 3:90–101. doi: 10.1046/j.1523-5408.2000.00020.x
57. Kobayashi Y, Fujikawa T, Kobayashi H, Sumida K, Suzuki S, Kagimoto M, et al. Relationship between arterial stiffness and blood pressure drop during the sit-to-stand test in patients with diabetes mellitus. J Atheroscler Thromb. (2017) 24:147–56. doi: 10.5551/jat.34645
58. Mattace-Raso FUS, van den Meiracker AH, Bos WJ, van der Cammen TJ, Westerhof BE, Elias-Smale S, et al. Arterial stiffness, cardiovagal baroreflex sensitivity and postural blood pressure changes in older adults: the Rotterdam study. J Hypertens. (2007) 25:1421–6. doi: 10.1097/HJH.0b013e32811d6a07
Keywords: Acanthopanax senticosus Harms, cardiac autonomic function, standing test, elderly individuals, dizziness
Citation: Sato T, Aoki T, Ito Y, Oishi K, Fujishima M, Okumura E and Ishii K (2024) Effects of continuous supplementation of Acanthopanax senticosus Harms on the cardiac autonomic function of community-dwelling elderly individuals during resting and standing tests: a randomized controlled trial. Front. Cardiovasc. Med. 11:1336676. doi: 10.3389/fcvm.2024.1336676
Received: 17 November 2023; Accepted: 26 February 2024;
Published: 8 March 2024.
Edited by:
Gen-Min Lin, Hualien Armed Forces General Hospital, TaiwanReviewed by:
Makoto Ayabe, Okayama Prefectural University, JapanMorimasa Kato, Yamagata Prefectural Yonezawa Nutrition University, Japan
© 2024 Sato, Aoki, Ito, Oishi, Fujishima, Okumura and Ishii. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kojiro Ishii a2lzaGlpQG1haWwuZG9zaGlzaGEuYWMuanA=
 Takeru Sato1
Takeru Sato1 Masaki Fujishima
Masaki Fujishima Kojiro Ishii
Kojiro Ishii