- 1Clinical Pharmacology and Pharmacometrics, Graduate School of Pharmaceutical Sciences, Chiba University, Chiba, Japan
- 2Early Development, Astellas Pharma Inc., Tokyo, Japan
- 3Department of Preventive Medicine and Public Health, Keio University School of Medicine, Tokyo, Japan
- 4Department of Cardiology, International University of Health and Welfare Narita Hospital, Chiba, Japan
- 5Department of Pharmacology, Graduate School of Medicine, Chiba University, Chiba, Japan
Background: The aim of this study was to identify significant factors affecting the effectiveness of exercise training using information of the HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) study.
Methods: Background factors influencing the effect of exercise training were comprehensively surveyed for 2,130 patients by multivariable Cox regression analysis with the stepwise variable selection, and only significant factors were selected that were statistically distinguished from dummy noise factors using the Boruta method.
Results: The analysis suggested that the use of beta-blockers, pulse pressure, hemoglobin level, electrocardiography findings, body mass index, and history of stroke at baseline potentially influenced the exercise effect on all-cause death (AD). Therefore, a hypothetical score to estimate the effect of exercise training was constructed based on the analysis. The analysis suggested that the score is useful in identifying patients for whom exercise training may be significantly effective in reducing all-caused death and hospitalization (ADH) as well as AD. Such a subpopulation accounted for approximately 40% of the overall study population. On the other hand, in approximately 45% of patients, the effect of exercise was unclear on either AD or ADH. In the remaining 15% of patients, it was estimated that the effect of exercise might be unclear for ADH and potentially rather increase AD.
Conclusions: This study is the first analysis to comprehensively evaluate the effects of various factors on the outcome of exercise training in chronic heart failure, underscoring the need to carefully consider the patient's background before recommending exercise training. However, it should be noted that exercise training can improve many outcomes in a wide variety of diseases. Therefore, given the limitations involved in post-hoc analyses of a single clinical trial, the characteristics of patients to whom the results of this analysis can be applied need attention, and also further research is necessary on the relationship between the degree of exercise and the outcomes. A new clinical trial would be needed to confirm the factors detected and the appropriateness of the score.
1 Introduction
Exercise training improves mortality, health-related quality of life, and exercise capacity in patients with chronic heart failure (1–6) and is widely recommended as an effective treatment that can be combined with pharmacotherapy such as renin-angiotensin system inhibitors (RASIs, i.e., angiotensin-converting enzyme inhibitor and/or angiotensin II receptor blocker) and beta-blockers (BBs) (7, 8). The Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) study conducted in 2003–2008 is the only large-scale randomized controlled trial that examined the efficacy of exercise training in medically stable patients with chronic heart failure (9). The trial demonstrated that a prescribed exercise training program was associated with a reduction in all-cause death and hospitalization (ADH) rate. This improvement was statistically significant after the adjustment for prognostic factors, and a non-significant reduction in all-cause death (AD), one of the secondary endpoints, was noted regardless of factor adjustment.
In chronic heart failure, reducing the cardiac load is essential for successful treatment (10–12). We previously performed a model-based meta-analysis of 61 studies in patients with chronic heart failure and reported that estimated myocardial oxygen consumption, a cardiac load index, correlated excellently with the reduction in mortality after various pharmacotherapies (13). In particular, RASIs and BBs reduced the estimated myocardial oxygen consumption more efficiently than other drug classes, including calcium channel blockers and direct renin inhibitors, which supported the superior prognostic improvement ability of these drugs. In contrast, exercise activates the sympathetic nervous system (14) and increases cardiac load, which contradicts the expected effect of drug therapy (15–17). Therefore, the use of RASIs and BBs may affect exercise training efficacy. The subgroup analysis of the HF-ACTION trial showed that various factors including baseline RASI and BB use did not affect exercise effects. However, assuming that a variety of factors including cardiopulmonary capacity, medical condition, and medical history (18–20) potentially affect exercise effects and that these factors are correlated with each other, there would be limitations in revealing the heterogeneity of exercise effects by simple subgroup analyses.
This study, as a post hoc analysis of individual patient data from the HF-ACTION trial, aimed to hypothetically elucidate the association between various patient characteristics at baseline and the exercise training effects and provide useful information for individualization of exercise training to optimize benefit of patients.
2 Methods
2.1 Study population
The HF-ACTION study was a randomized multicenter trial that evaluated the effectiveness of exercise training vs. usual care in patients with chronic heart failure. The study included patients with stable heart failure with a left ventricular ejection fraction (LVEF) of less than 35% and a New York Heart Association (NYHA) class of II–IV despite optimal heart failure treatment for at least 6 weeks. See the original report for HF-ACTION study for details including exercise training procedures (9).
2.2 Outcomes
The primary endpoints in this analysis were (1) AD; and (2) ADH during the entire follow-up period (up to 4 years; median, 30 months). We adopted an intention-to-treat population analysis.
2.3 Variables
Variables missing in more than 30% of the samples were excluded beforehand and remaining missing data were complemented by a multiple imputation method (21). To avoid obvious multicollinearity, Spearman's rank correlation coefficient of greater than 0.8 for continuous variables and the chi-square value of greater than 300 for categorical variables were excluded. The resulting 73 variables (Supplementary Table S1) were used to in the subsequent Cox analysis to comprehensively explore the interaction effects with exercise training. All continuous variables were transformed into binary variables nearly at the 25th, 50th, and 75th percentiles.
2.4 Cox proportional hazards models
Main effects of each variable and their interaction with exercise training was tested with a univariate Cox analysis, and those with a P-value of less than 0.3 were selected. The stepwise method with a significance level of P = 0.1 for inclusion and of P = 0.05 for exclusion was then applied to the selected variables to develop a full model.
To exclude effects with a potentially high false positive rate (FDR) and obtain a robust final model, we applied the Boruta method to the full model (22). Boruta is a variable selection approach commonly used in the area of machine learning/artificial intelligence, which attempts to select meaningful variables by intentionally adding meaningless variables to the model. Briefly, Boruta first prepares an equal number of dummy variables as candidate real variables. These dummy variables are copies of the real variables, but shuffled in the sample direction and should therefore have no importance in the predictive model. A model is then developed using data containing both real and dummy variables, and the real variables that are more important than the dummy variables are selected. This is repeated N times with a certain degree of randomness. If the importance of a real variable is the same as the corresponding dummy variable (i.e., it is meaningless), the probability that it is selected should be 50%, so the number of times a real variable is selected follows a binomial distribution with p = 0.5 and n = N. Finally, whether the number of times the real variable is selected is sufficiently large is tested based on this binomial distribution at any significance level α. In this analysis, we applied Boruta to each of the main effects and interaction effects in the full model using 1,000 datasets generated by the bootstrap method and excluded effects with FDR greater than 0.05 (i.e., N = 1,000, α = 0.05). The -log(p) value was used as the importance measure of the variables, and the 95th percentile value of the importance of the dummy variables was used as the threshold for the selection of important real variables. To avoid population imbalance, the bootstrapping was performed by stratifying data by the treatment group, medication group, and death or hospitalization at 4 years.
In the series of variable selection processes, the interaction between the choice of medication at baseline and exercise training was always accounted for by modeling the exercise effect separately for each medication group. The medication was categorized into three groups according to the use of BBs and RASIs at baseline: both BB and RASI, RASI only, and BB only. The exercise effect in the subgroup of patients who were not treated with neither RASI nor BB at baseline was neglected because the sample size was too small (n = 10; 0.5%). Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) were combined as RASIs. The interaction P-value for the medication group and exercise training was assessed posteriori by a likelihood ratio test comparing models with and without splitting the exercise effect according to medication.
To ensure the interpretability of the effects of the exercise training in the final model, all the variables other than the exercise effect were zero-centered before the Cox regression analysis (i.e., subtracting the mean of each variable). This process sets the baseline hazard to the overall mean of the usual care subjects and avoids the main effect of exercise training being estimated as a value in an extremely biased small population without changing the statistical significance of the analysis.
2.5 Scores predicting benefit or harm from exercise training
Based on the results of the Cox proportional hazards model, the predictive score for exercise eligibility was developed incorporating significant interaction terms identified in the final model. For ease of use, all predictors comprising the score were assigned integer points by scaling of the β-coefficients of the final Cox model. The score was designed such that the higher the total score, the more beneficial exercise training would be.
2.6 Software
The PHREG procedure implemented in SAS® 9.4 was used to conduct the Cox proportional hazards model analysis. In addition to SAS, Python 3.8 (Python Software Foundation) was used for the data processing and illustrations. IterativeImputer, implemented in scikit-learn 0.24.2, was used for multiple imputations of missing data.
3 Results
3.1 Model development
All records of eligible patients from the HF-ACTION trial (2,130 of 2,331 patients consented to share for non-commercial uses and allowed for any research purpose) were included in the analyses.
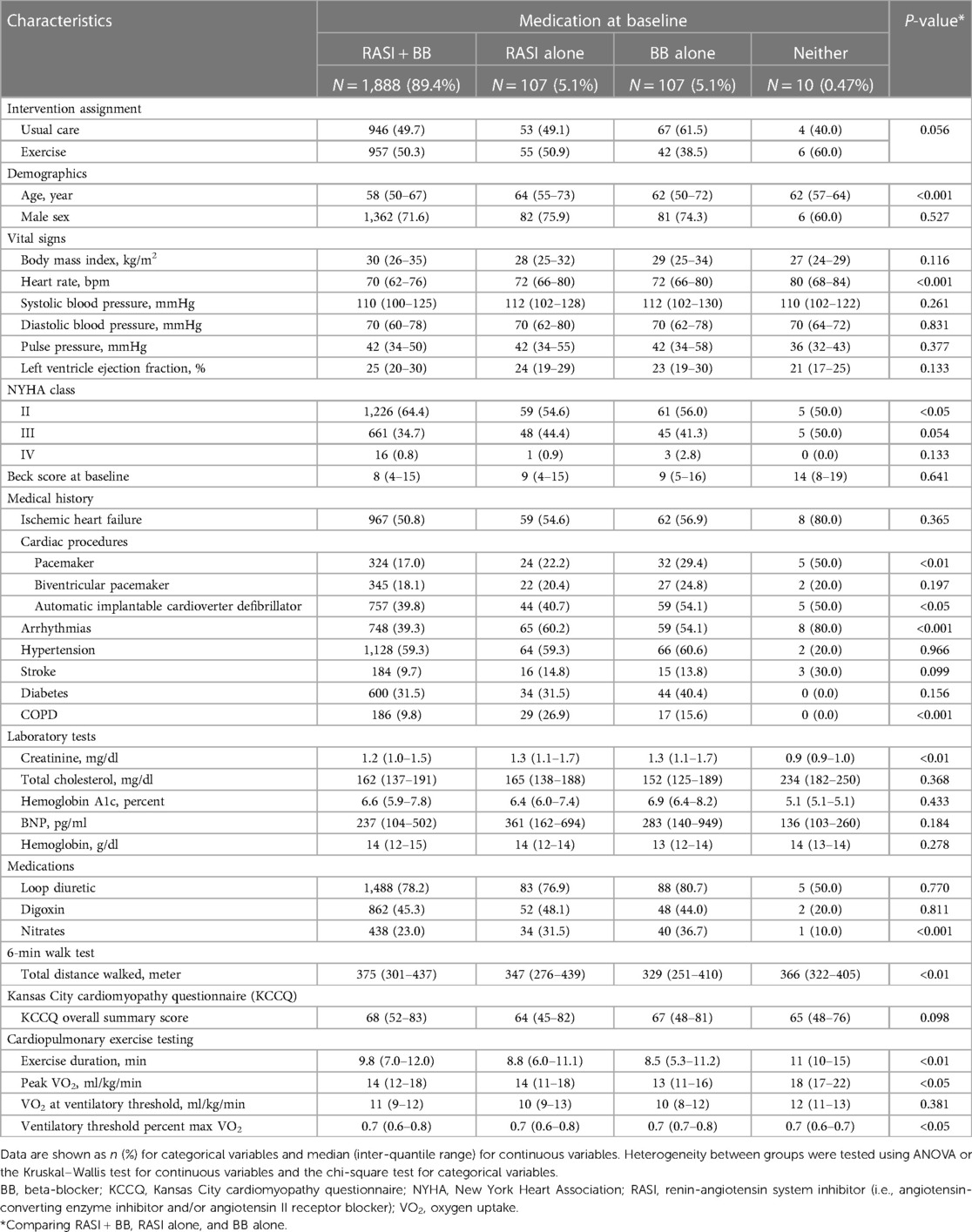
As a result of tentative variable selection using the stepwise method, 32 variables as main effects and 8 variables as interactions with exercise training included in the full model for AD, and 39 variables as main effects and 7 variables as interactions with exercise training were included in the full model for ADH. Of these, 6 main effects and 5 interaction effects in the AD model and 17 main effects and 5 interaction effects in the ADH model were considered significant because the FDR calculated by Boruta was smaller than 0.05 (Figure 1 and Supplementary Figure S1; see also Methods for details).
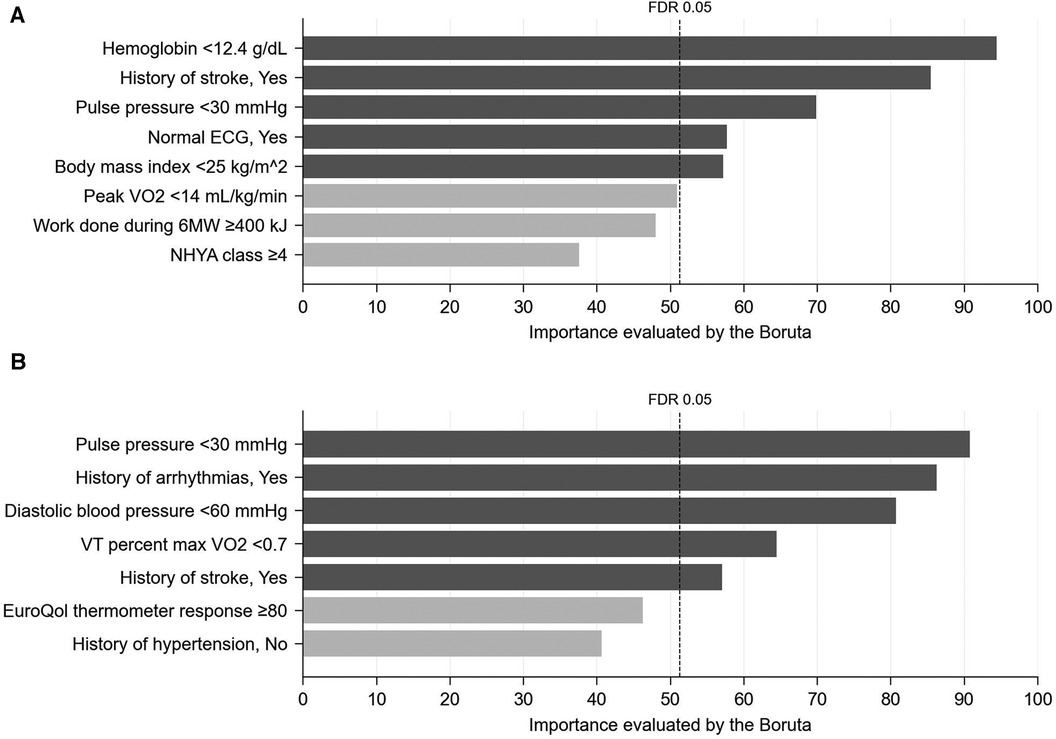
Figure 1. Importance (probability of selection, %) of interaction effects with exercise training assessed using 1,000 bootstrap datasets by the Boruta method (see methods for details). (A) AD, (B) ADH. FDR, false discovery rate.
3.2 Model interpretation
The overall hazard ratios (HRs) of the exercise effect calculated from the final Cox models were 0.92 (95% CI, 0.81–1.04) for AD and 0.88 (95% CI, 0.82–0.94) for ADH, which was consistent with the original analysis of the HF-ACTION trial reporting that exercise training significantly reduced the risk of ADH with adjustment but not that of AD (9).
Forest plots, Figures 2, 3, summarize the significant influence of various patient characteristics at baseline on the effectiveness of exercise training identified by the multivariate Cox proportional hazards model for AD and ADH, respectively. The effects of exercise on AD differed significantly depending on medication at baseline (interaction P = 0.005, Figure 2). A tendency toward beneficial exercise effects was estimated in the subgroups taking BBs at baseline, i.e., in patients taking BBs alone (HR, 0.59; 95% CI, 0.36–0.92) and in patients taking both RASIs and BBs (HR, 0.91; 95% CI, 0.82–1.02), whereas exercise training was potentially associated with an increased risk of AD in patients not treated with BBs at baseline, i.e., taking RASIs alone (HR, 4.48; 95% CI, 3.05–6.31). For ADH, on the other hand, the interaction between exercise training and medication at baseline was not statistically significant (interaction P = 0.236, Figure 3). For both endpoints, five factors other than medication were identified as significantly interacting with exercise training. Interactions of pulse pressure of <30 mmHg and history of stroke with exercise training were identified in both AD and ADH, with the former associated with adverse exercise effects and, conversely, the latter with beneficial exercise effects. Other factors associated with increased exercise effectiveness included a body mass index (BMI) of <25 kg/m2 and normal electrocardiography (ECG) findings for AD, vs. a diastolic blood pressure (DBP) of <60 mmHg for ADH. As factors reducing exercise effectiveness, a hemoglobin of <12.4 g/dl for AD, vs. a history of arrhythmia and a VT percent max VO2 of <0.7 for ADH were identified.
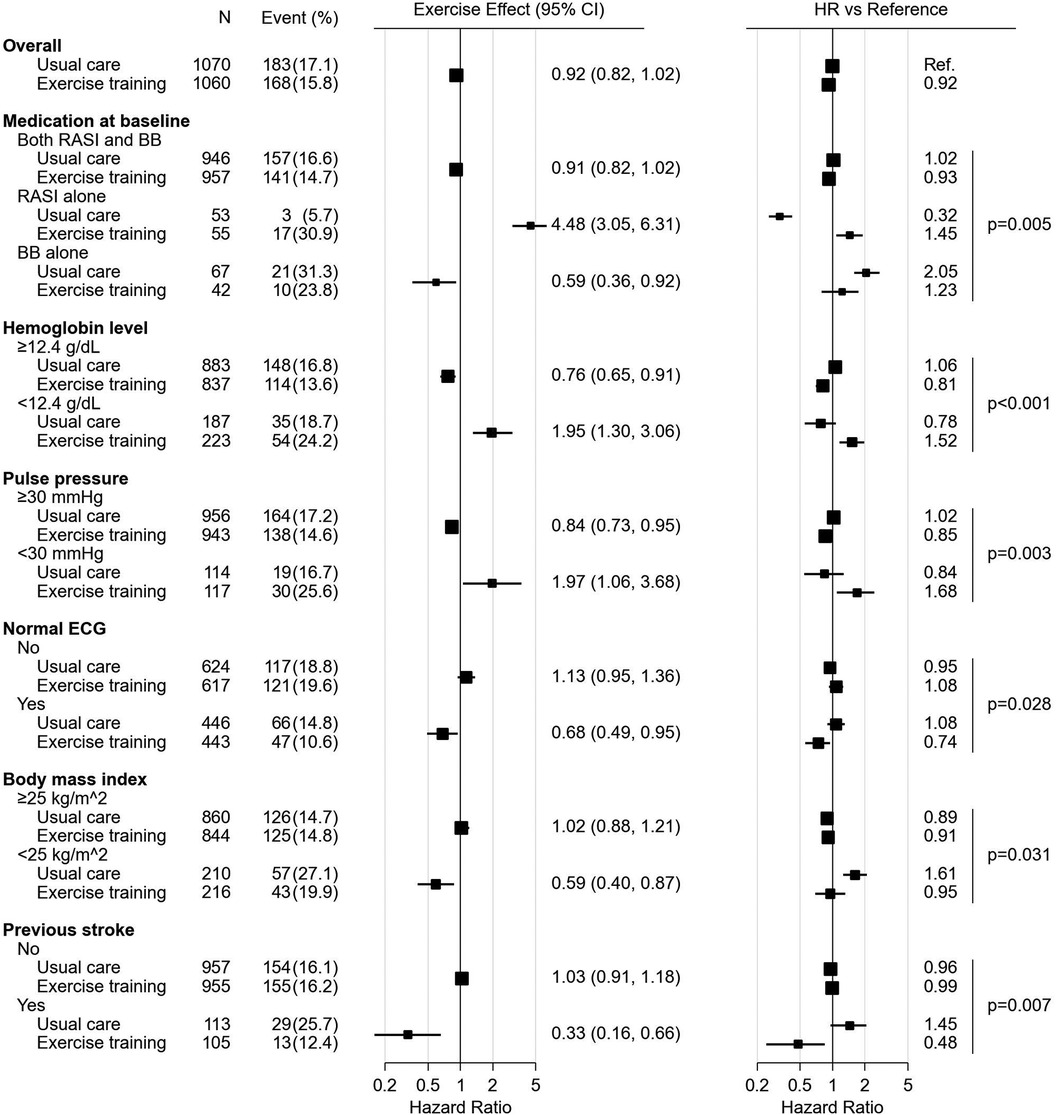
Figure 2. The forest plot represents influence of medication and other characteristics on exercise effect for all-cause death. All the HRs, effects and their CIs were calculated from the analysis of 1,000 bootstrap datasets based on the multivariate Cox proportional hazards model analysis. The hazard ratios of subgroups are presented versus the overall mean of the usual care subjects. The marker size is calculated to be proportional to the square root of the number of subjects. BB, beta-blocker; CI, confidence interval; ECG, electrocardiogram; HR, hazard ratio; RASI, renin-angiotensin system inhibitor.
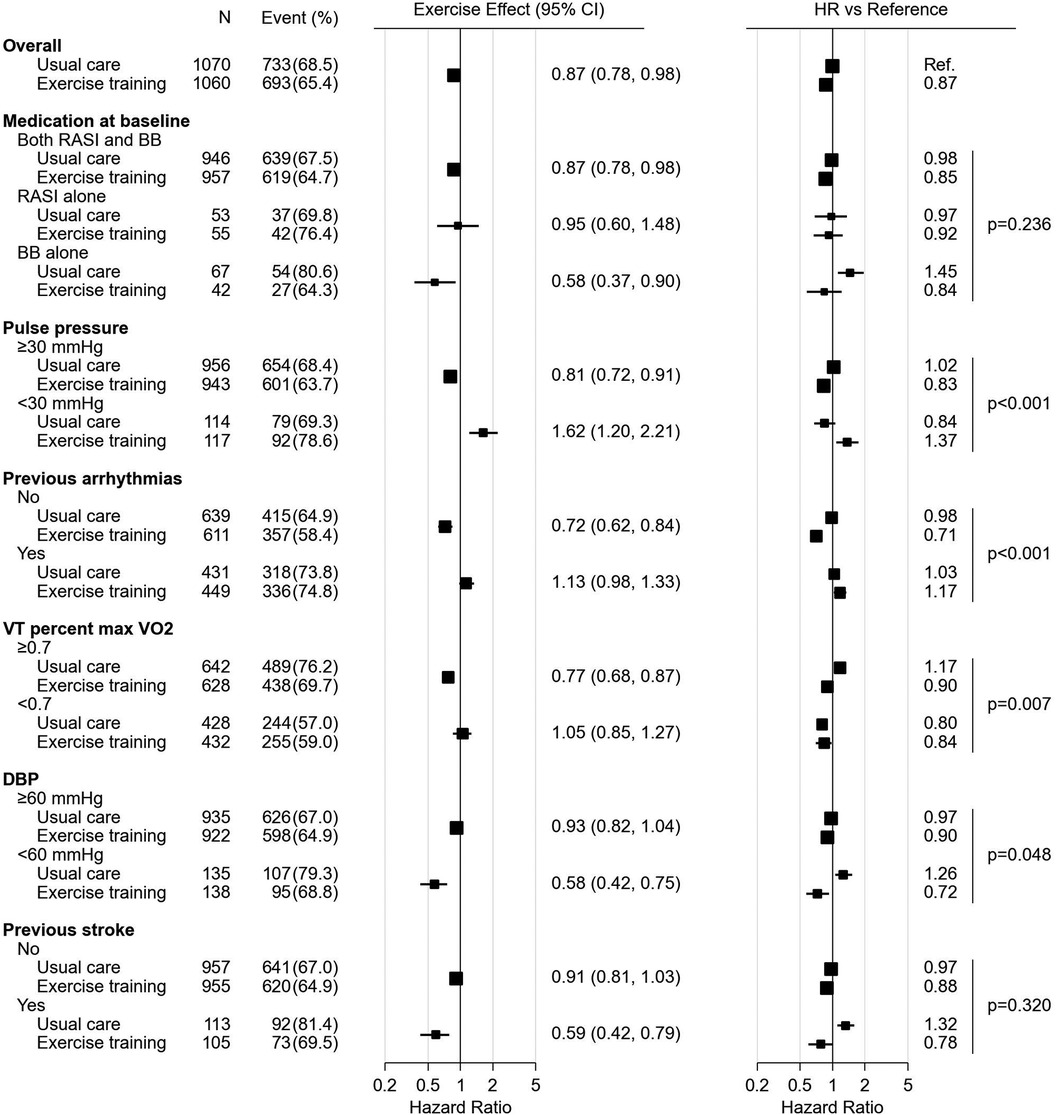
Figure 3. The forest plot represents influence of medication and other characteristics on exercise effect for all-cause death or hospitalization. All the HRs, effects and their CIs were calculated from the analysis of 1,000 bootstrap datasets based on the multivariate Cox proportional hazards model analysis. The figure was prepared in the same format as Figure 1. BB, beta-blocker; CI, confidence interval; ECG, electrocardiogram; HR, hazard ratio; RASI, renin-angiotensin system inhibitor; VO2, oxygen uptake.
Since the identification of the medication group as a factor significantly influencing the effect of exercise training on AD was completely unexpected, patient characteristics were analyzed for each medication subgroup. There were no distinct differences in patient characteristics between the medication subgroups (Table 1). The distribution of model-predicted individual risks (sum of log partial hazards) calculated from the main effects excluding exercise and medication at baseline also did not explain the differences in exercise effects by medication group (Supplementary Figure S2).
The parameter estimates for the final models for AD and ADH were shown in Supplementary Tables S2, S3, respectively. Forest plots for the main effects in the models for AD and ADH were shown in Supplementary Figures S3, S4, respectively. To understand the results of the Cox analysis more intuitively, the cumulative incidence curves for AD and ADH according to the baseline medication group are shown in Supplementary Figures S5, S6, respectively.
3.3 Score for predicting exercise eligibility
We developed a hypothetical clinical score, which estimates a patient's eligibility for exercise training according to a total of six predictors: treated with BBs (+3), pulse pressure ≥30 mmHg (+2), hemoglobin ≥12.4 g/dl (+2), history of stroke (+2), normal ECG findings (+1), and BMI <25 kg/m2 (+1) (Table 2). The score considered all the interaction effects identified in the final Cox model for AD, and for ease of understanding, all variables were assigned integer points by scaling the β coefficients while maintaining the gradient of the effects. Figures 4A,B show the distribution of patients by score (histogram), as well as the 3-year AD- or ADH-free survival rate, respectively, for patients belonging to each score who received usual care (lines with circles) or exercise training (lines with squares).
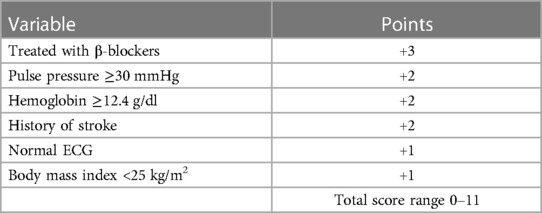
Table 2. Hypothetical Predictive Score for Exercise Eligibility in Patients with Chronic Heart Failure.
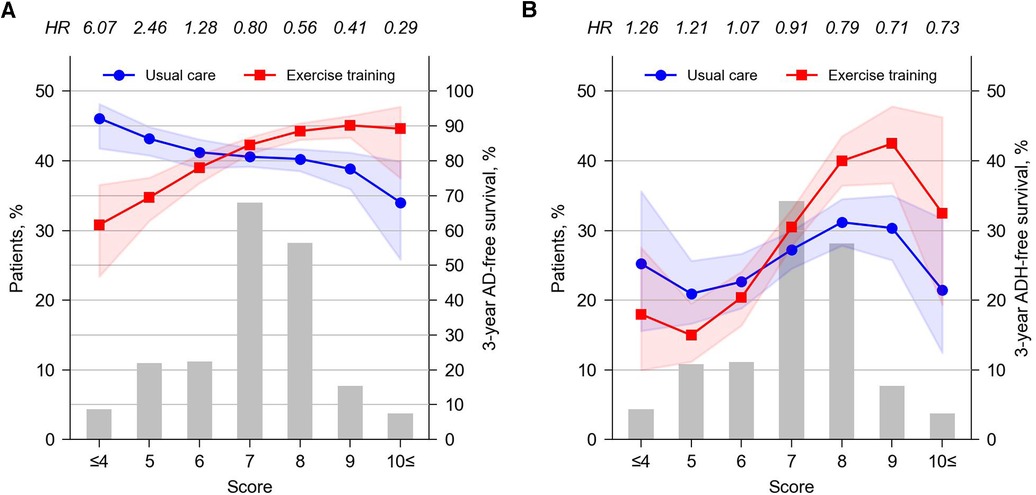
Figure 4. Patient distribution by score (left axis) and 3-year AD-free (A) or ADH-free (B) survival rates by intervention at each score (right axis). The survival rates per score and intervention were calculated using a Cox model accounting for predicted score (continuous variable) and intervention and their interaction, developed separately from the main analysis using Python's lifeline package (version 0.27.7). The values above the top axis indicate the HR for AD or ADH at each score (exercise training vs. usual care). Shared areas around the lines indicates the 95% CI. CI, confidence interval; HR, hazard ratio.
Score analysis showed that exercise training was clearly beneficial for AD in 39.6% of the total study population (i.e., patients with a score of 8 or higher) and in 45.3% of patients (i.e., those with scores of 6 or 7), exercise had no clear benefit on AD. Exercise training was estimated to increase AD in 15.3% of patients (i.e., patients with a score of 5 or lower) (Figure 4A). Note that although we used the results for AD to develop the presented score, an independent score developed from the results for ADH was also considered in a preliminary analysis (Supplementary Figure S7). When ADH score was used, again, exercise was predicted to be effective in reducing ADH only in approximately 40% of the study population (i.e., patients with a score of 4 or higher; Supplementary Figure S7C). However, such a subpopulation did not present a statistically significant exercise effect on AD (Supplementary Figure S7B), which was inferior to the results from the AD score, where patients who were predicted to benefit from exercise training in terms of AD also benefited in terms of ADH (Figures 4A,B). Similar discrepancies are also found in the subgroup for which exercise is predicted to be not beneficial by AD scores (i.e., the bottom 15% of AD scores), but the impact of such discrepancies would be less than sacrificing the discrimination of the top 40% of scores. Thus, the AD score was considered more discriminative and of greater clinical benefit for both AD and ADH.
4 Discussion
4.1 Main findings
To the best of our knowledge, this is the first analysis to comprehensively evaluate the effects of various factors on the outcome of exercise training in patients with chronic heart failure. The HF-ACTION trial demonstrated that compared to the usual care group, exercise training did not statistically affect the rate of AD but did reduce the rate of ADH (9). On the other hand, the present analysis suggested that the effects of exercise training may not necessarily apply uniformly to all patients with chronic heart failure. In this analysis, we paid best efforts to avoid detecting meaningless covariates by considering correlation between the covariates and applying the Boruta method. However, Boruta is a heuristic; there are no strict guarantees about its output although it is an interesting statistical tool. In addition, this analysis is post-hoc in design and the risk of confounding should always be considered. Even though, this study is expected to provide useful information that may contribute to the individualization of exercise therapy for patients of chronic heart failure.
4.2 Exercise effect and taking BBs
In patients with chronic heart failure, exercise training improves functional capacity, increases muscle strength, and improves quality of life (1–5, 23–25). However, exercise could excessively increase cardiac workload by increasing heart rate via sympathetic nervous system activation (14, 26) and may also induce arrhythmia when the intensity is increased (27). This study suggested that the effect of exercise training on ADH was larger in patients taking BBs at baseline, which may have occurred because BBs suppressed excessive sympathetic nervous system activation by exercise training and reduced the increased demand for myocardial energy due to increased heart rate (16). BBs may also have prevented sudden death by suppressing the onset of ventricular arrhythmias (28–30). A previous model-based meta-analysis showed that cardiac load reduction by BB was associated with improved prognosis of patients with chronic heart failure (13). Taken together, the present analysis indicated that BBs may play an important role in maximizing benefits of exercise training and improving tolerance.
4.3 Exercise effect and patient backgrounds
In addition to medication, the study identified several factors that could influence the effects of exercise training. In particular, pulse pressure, the difference between systolic and DBP, was identified as one of the most significant factors in both analyses for AD and ADH, with lower values being associated with adverse exercise effects. Similarly, low hemoglobin levels were identified as a factor negatively affecting exercise effectiveness. It is known that in patients with advanced chronic heart failure with reduced cardiac contractility, pulse pressure decreases due to a reduced systolic blood pressure (31). It has also been reported that patients with chronic heart failure complicated by anemia have a reduced oxygen supply to the heart and a poorer prognosis compared to patients without anemia (32–36). Given these pathological changes, our results likely reflect an increased risk of death and hospitalization in these patients due to the cardiac overload caused by exercise.
Other important factors identified as affecting exercise effects included abnormal ECG findings/arrhythmia and BMI. Our results suggest that exercise training is not recommended in patients with abnormal ECG findings or arrhythmia, which seems reasonable considering the risk of sudden death (27). With regard to BMI, the risk of AD was decreased by exercise training in patients with a BMI of <25 kg/m2, suggesting that maintaining one's muscle strength through exercise may contribute to the prevention of cachexia or inhibition of its progression (37–40). The present results overall are consistent with general knowledge on the pathogenesis of chronic heart failure. A history of stroke was a positive effect on exercise but it was also a factor that increased the risk of AD. This result is difficult to explain currently but it might be a confounding of use of antithrombotic drugs, because patients with a history of stroke were more likely to be prescribed antithrombotic drugs (41–44).
4.4 Factors influencing outcomes
In addition to the interactions with exercise, many prognostic factors have been reported for chronic heart failure. O'Connor et al. reported that exercise duration in the CPX test, BUN, and sex were important for AD and ADH based on the analysis of patient information of the HF-ACTION trial (45). In both studies, exercise capacity, renal function, and sex were selected. Overall, although this study is novel in that it includes multiple interactions with exercise, the analysis of other prognostic factors was considered consistent with that of the previous studies.
4.5 Exercise effect and taking RASIs and other medications
The present analysis suggested that exercise is not beneficial in patients taking only RASIs. In fact, the usual care group without exercise training taking only RASIs was associated with the lowest risk for AD in the study. This trend was consistent for ACEIs and ARBs that comprise RASI; no deaths occurred in subjects taking ARB alone in the absence of exercise training (Supplementary Table S4). Since RASI could alter serum potassium by affecting aldosterone secretion, it is not impossible that this could have caused some systematic change in the risk of events in chronic heart failure patients (7, 46). However, it is inappropriate to judge the superiority or inferiority of medications rather than their effect on exercise training based on the results of the present analysis. The HF-ACTION trial was randomized for exercise but not for medications, and only a small number of patients (5.1% of the total) took RASI alone at baseline. In fact, the current chronic heart failure guidelines recommend the combined use of RASIs and BBs as first-line therapy based on the solid evidences (7, 46).
Since the HF-ACTION trial was conducted in 2003–2008, the medication algorithms for chronic heart failure differed from the current ones. Sodium-glucose cotransporter-2 inhibitors and angiotensin receptor-neprilysin inhibitors (ARNIs) have since been demonstrated (47) to improve chronic heart failure prognosis (7, 46). Although the impact of these therapies on exercise training could not be evaluated in this study, given the similarity in the action between RASIs and ARNIs, similar caution might be required for patients who take ARNIs without BBs.
Regarding mineralocorticoid antagonists, spironolactone and eplerenone were prescribed at baseline in 42% and 3% of patients, respectively. Variables related to mineralocorticoids were included in the analysis but was not selected for the final model.
4.6 Exercise Recommendation based on the score
A hypothetical score was constructed based on the results of Cox proportional hazards analysis in terms of AD to categorize patients for whom exercise training is recommended or not recommended. The study may inform the extent to which prognosis changes when patients in each score do or do not perform exercise training, along with their uncertainty (Figure 3). Patients with a score of 8 or higher are considered important to ensure exercise training, whereas patients with intermediate scores of 6–7, the outcome may be affected little from the decision on exercise training. Exercise prescription may need to be carefully avoided for patients with scores of 5 or less. This analysis implied that 40% of the entire study population appeared to clearly benefit from exercise training, while the effect of exercise was unclear in 45% of patients and increased AD in the remaining 15% of patients. Given that exercise training is widely recommended in the current management of chronic heart failure (7), the proposed score would be clinically meaningful. However, these have not yet been validated by multiple studies. Therefore, their generalizability to chronic heart failure patients remains to be confirmed in the future.
4.7 Limitations
The population included in this analysis consisted of patients with stable chronic heart failure, a reduced left ventricular ejection fraction (≤35%), NYHA class II–IV despite treatment, and the ability to perform exercise. While we believe that the analysis in this study is appropriate for these patients, it may not be applicable to other populations, such as those using medications that were not approved at the time the HF-ACTION trial was conducted, those who had a recent cardiovascular event or comorbidities or limitations that could interfere with exercise training. In addition, the appropriateness of exercise training may vary greatly by exercise type and intensity. If exercise is anticipated to be not beneficial, future studies would be needed to examine the effects of reducing exercise intensity. Given the limitations involved in post-hoc analyses of a single clinical trial, new exercise trials in patients with HF would be necessary to be conducted to confirm the factors detected and the appropriateness of the score.
5 Conclusions
These results highlighted that the effects of exercise training may not necessarily apply uniformly to all patients with chronic heart failure. The choice of exercise training in patients with chronic heart failure requires careful judgment that considers patient condition and also possibly medications. Further validation of this study would be required in the future.
Data availability statement
The datasets presented in this article are not readily available because the data analyzed in this study was obtained from National Heart, Lung, and Blood Institute with determined procedure and permission of National Heart, Lung, and Blood Institute. Requests to access the datasets should be directed to https://biolincc.nhlbi.nih.gov/home/. Further enquiries can be directed to the corresponding author.
Ethics statement
This is a post-hoc analysis of a clinical study which was completed in accordance with the local legislation and institutional requirements. This study used anonymized human data and was approved by the ethical review board of the Graduate School of Pharmaceutical Sciences at Chiba University. Written informed consent for participation was not required newly from the participants or participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YS: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. HY: Formal Analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. SG: Investigation, Writing – review & editing. HS: Supervision, Writing – review & editing. HH: Supervision, Writing – review & editing. YS: Supervision, Writing – review & editing. YF: Supervision, Writing – review & editing. NA: Supervision, Writing – review & editing. AH: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the AMED under Grant number JP21mk0101159.
Acknowledgments
This manuscript was prepared using HF-ACTION Research Materials obtained from the BioLINCC data base of NHLBI Biologic Specimen and Data Repository Information Coordinating Center (Bethesda, MD, USA) and does not necessarily reflect the opinions or views of the HF-ACTION or the NHLBI.
Conflict of interest
YS and SG are employees of Nihon Servier Co. Ltd. and Astellas Pharma Inc., respectively; however, Nihon Servier Co. Ltd., and Astellas Pharma Inc., were not involved in this analysis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1330235/full#supplementary-material
References
1. Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation. (1999) 99:1173–82. doi: 10.1161/01.cir.99.9.1173
2. Piña IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, et al. Exercise and heart failure: a statement from the American heart association committee on exercise, rehabilitation, and prevention. Circulation. (2003) 107:1210–25. doi: 10.1161/01.cir.0000055013.92097.40
3. Kavanagh T, Myers MG, Baigrie RS, Mertens DJ, Sawyer P, Shephard RJ. Quality of life and cardiorespiratory function in chronic heart failure: effects of 12 months’ aerobic training. Heart. (1996) 76:42–9. doi: 10.1136/hrt.76.1.42
4. Sarullo FM, Gristina T, Brusca I, Milia S, Raimondi R, Sajeva M, et al. Effect of physical training on exercise capacity, gas exchange and N-terminal pro-brain natriuretic peptide levels in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. (2006) 13:812–7. doi: 10.1097/01.hjr.0000238396.42718.61
5. Piepoli MF, Davos C, Francis DP, Coats AJS, ExTraMATCH Collaborative. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). Br Med J. (2004) 328:189. doi: 10.1136/bmj.37938.645220.EE
6. Sagar VA, Davies EJ, Briscoe S, Coats AJS, Dalal HM, Lough F, et al. Exercise-based rehabilitation for heart failure: systematic review and meta-analysis. Open Heart. (2015) 2:e000163. doi: 10.1136/openhrt-2014-000163
7. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol. (2013) 62:e147–239. doi: 10.1016/j.jacc.2013.05.019
8. Demopoulos L, Yeh M, Gentilucci M, Testa M, Bijou R, Katz SD, et al. Nonselective beta-adrenergic blockade with carvedilol does not hinder the benefits of exercise training in patients with congestive heart failure. Circulation. (1997) 95:1764–7. doi: 10.1161/01.cir.95.7.1764
9. O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. (2009) 301:1439–50. doi: 10.1001/jama.2009.454
10. Barrese V, Taglialatela M. New advances in beta-blocker therapy in heart failure. Front Physiol. (2013) 4:323. doi: 10.3389/fphys.2013.00323. eCollection 2013.24294204
11. Yabe Y, Morishita T. Systemic and coronary hemodynamic effects of beta-adrenoceptor blocking agents in coronary artery disease. Jpn Heart J. (1987) 28:675–86. doi: 10.1536/ihj.28.675
12. Holubarsch C, Hasenfuss G, Thierfelder L, Just H. Energetic consequences of substances currently used or recommended for long-term treatment of chronic heart failure. Basic Res Cardiol. (1991) 86(Suppl 1):107–12.1827977
13. Takaoka R, Soejima Y, Guro S, Yoshioka H, Sato H, Suzuki H, et al. Model-based meta-analysis of changes in circulatory system physiology in patients with chronic heart failure: mBMA of circulatory system physiology in CHF. CPT Pharmacometrics Syst Pharmacol. (2021) 10:1081–91. doi: 10.1002/psp4.12676
14. Nakamura Y, Yamamoto Y, Muraoka I. Autonomic control of heart rate during physical exercise and fractal dimension of heart rate variability. J Appl Physiol (1985). (1993) 74:875–81. doi: 10.1152/jappl.1993.74.2.875
15. Grassi G, Quarti-Trevano F, Esler MD. Sympathetic activation in congestive heart failure: an updated overview. Heart Fail Rev. (2021) 26:173–82. doi: 10.1007/s10741-019-09901-2
16. Sabbah HN. Biologic rationale for the use of beta-blockers in the treatment of heart failure. Heart Fail Rev. (2004) 9:91–7. doi: 10.1023/B:HREV.0000046363.59374.23
17. Wang Y, Seto S, Golledge J. Angiotensin II, sympathetic nerve activity and chronic heart failure. Heart Fail Rev. (2014) 19:187–98. doi: 10.1007/s10741-012-9368-1
18. Fleg JL. Exercise therapy for older heart failure patients. Heart Fail Clin. (2017) 13:607–17. doi: 10.1016/j.hfc.2017.02.012
19. Luo N, Merrill P, Parikh KS, Whellan DJ, Piña IL, Fiuzat M, et al. Exercise training in patients with chronic heart failure and atrial fibrillation. J Am Coll Cardiol. (2017) 69:1683–91. doi: 10.1016/j.jacc.2017.01.032
20. Mentz RJ, Schulte PJ, Fleg JL, Fiuzat M, Kraus WE, Piña IL, et al. Clinical characteristics, response to exercise training, and outcomes in patients with heart failure and chronic obstructive pulmonary disease: findings from heart failure and A controlled trial investigating outcomes of exercise training (HF-ACTION). Am Heart J. (2013) 165:193–9. doi: 10.1016/j.ahj.2012.10.029
21. van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. (2011) 45:1–67. doi: 10.18637/jss.v045.i03
22. Kursa MB, Rudnicki WR. Feature selection with the boruta package. J Stat Softw. (2010) 36:1–13. doi: 10.18637/jss.v036.i11
23. Hambrecht R, Niebauer J, Fiehn E, Kälberer B, Offner B, Hauer K, et al. Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J Am Coll Cardiol. (1995) 25:1239–49. doi: 10.1016/0735-1097(94)00568-B
24. Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J Am Coll Cardiol. (2007) 49:2329–36. doi: 10.1016/j.jacc.2007.02.055
25. Ohtsubo M, Yonezawa K, Nishijima H, Okita K, Hanada A, Kohya T, et al. Metabolic abnormality of calf skeletal muscle is improved by localised muscle training without changes in blood flow in chronic heart failure. Heart. (1997) 78:437–43. doi: 10.1136/hrt.78.5.437
26. Samejima H, Omiya K, Uno M, Inoue K, Tamura M, Itoh K, et al. Relationship between impaired chronotropic response, cardiac output during exercise, and exercise tolerance in patients with chronic heart failure. Jpn Heart J. (2003) 44:515–25. doi: 10.1536/jhj.44.515
27. Atkins JM, Matthews OA, Blomqvist CG, Mullins CB. Incidence of arrhythmias induced by isometric and dynamic exercise. Br Heart J. (1976) 38:465–71. doi: 10.1136/hrt.38.5.465
28. CIBIS-II Investigators and Committees. The cardiac insufficiency bisoprolol study II (CIBIS-II): a randomised trial. Lancet. (1999) 353:9–13. doi: 10.1016/S0140-6736(98)11181-9
29. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. carvedilol heart failure study group. N Engl J Med. (1996) 334:1349–55. doi: 10.1056/NEJM199605233342101
30. MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet. (1999) 353:2001–7. doi: 10.1016/S0140-6736(99)04440-2
31. Tremblay-Gravel M, Khairy P, Roy D, Leduc H, Wyse DG, Cadrin-Tourigny J, et al. Systolic blood pressure and mortality in patients with atrial fibrillation and heart failure: insights from the AFFIRM and AF-CHF studies. Eur J Heart Fail. (2014) 16:1168–74. doi: 10.1002/ejhf.168
32. McCullough PA, Barnard D, Clare R, Ellis SJ, Fleg JL, Fonarow GC, et al. Anemia and associated clinical outcomes in patients with heart failure due to reduced left ventricular systolic function. Clin Cardiol. (2013) 36:611–20. doi: 10.1002/clc.22181
33. Kajimoto K, Sato N, Takano T, Investigators of the Acute Decompensated Heart Failure Syndromes (ATTEND) registry. Association between anemia, clinical features and outcome in patients hospitalized for acute heart failure syndromes. Eur Heart J Acute Cardiovasc Care. (2015) 4:568–76. doi: 10.1177/2048872614554199
34. Yamauchi T, Sakata Y, Takada T, Nochioka K, Miura M, Tadaki S, et al. Prognostic impact of anemia in patients with chronic heart failure-with special reference to clinical background: report from the CHART-2 study. Circ J. (2015) 79:1984–93. doi: 10.1253/circj.CJ-15-0174
35. Kılıçgedik A, Dündar C, Tigen MK. Anemia in heart failure. Anadolu Kardiyol Derg. (2012) 12:65–70. doi: 10.5152/akd.2012.011
36. Klutstein MW, Tzivoni D. Anaemia and heart failure: aetiology and treatment. Nephrol Dial Transplant. (2005) 20:vii7–10. doi: 10.1093/ndt/gfh1100
37. Anker SD, Swan JW, Volterrani M, Chua TP, Clark AL, Poole-Wilson PA, et al. The influence of muscle mass, strength, fatigability and blood flow on exercise capacity in cachectic and non-cachectic patients with chronic heart failure. Eur Heart J. (1997) 18:259–69. doi: 10.1093/oxfordjournals.eurheartj.a015229
38. Lena A, Ebner N, Anker MS. Cardiac cachexia. Eur Heart J Suppl. (2019) 21:L24–7. doi: 10.1093/eurheartj/suz241
39. Lenk K, Erbs S, Höllriegel R, Beck E, Linke A, Gielen S, et al. Exercise training leads to a reduction of elevated myostatin levels in patients with chronic heart failure. Eur J Prev Cardiol. (2012) 19:404–11. doi: 10.1177/1741826711402735
40. Murad K, Kitzman DW. Frailty and multiple comorbidities in the elderly patient with heart failure: implications for management. Heart Fail Rev. (2012) 17:581–8. doi: 10.1007/s10741-011-9258-y
41. Massie BM, Collins JF, Ammon SE, Armstrong PW, Cleland JGF, Ezekowitz M, et al. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the warfarin and antiplatelet therapy in chronic heart failure (WATCH) trial. Circulation. (2009) 119:1616–24. doi: 10.1161/CIRCULATIONAHA.108.801753
42. Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. (2011) 365:981–92. doi: 10.1056/NEJMoa1107039
43. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. (2011) 365:883–91. doi: 10.1056/NEJMoa1009638
44. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. (2013) 369:2093–104. doi: 10.1056/NEJMoa1310907
45. O'Connor CM, Whellan DJ, Wojdyla D, Leifer E, Clare RM, Ellis SJ, et al. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: the HF-ACTION predictive risk score model. Circ Heart Fail. (2012) 5:63–71. doi: 10.1161/CIRCHEARTFAILURE.111.963462
46. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
Keywords: exercise training, chronic heart failure, HF-ACTION, risk factors, Cox regression analysis, Boruta method, predictive scores
Citation: Soejima Y, Yoshioka H, Guro S, Sato H, Hatakeyama H, Sato Y, Fujimoto Y, Anzai N and Hisaka A (2024) Exercise training outcomes in patients with chronic heart failure with reduced ejection fraction depend on patient background. Front. Cardiovasc. Med. 11:1330235. doi: 10.3389/fcvm.2024.1330235
Received: 30 October 2023; Accepted: 17 January 2024;
Published: 31 January 2024.
Edited by:
Erberto Carluccio, Heart Failure Unit, ItalyReviewed by:
Ricardo Mourilhe-Rocha, Rio de Janeiro State University, BrazilKevin Shah, The University of Utah, United States
© 2024 Soejima, Yoshioka, Guro, Sato, Hatakeyama, Sato, Fujimoto, Anzai and Hisaka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akihiro Hisaka aGlzYWthQGNoaWJhLXUuanA=
†Present Address: Yukako Soejima, Nihon Servier Co., Ltd., Tokyo, Japan
‡These authors have contributed equally to this work and share first authorship
 Yukako Soejima
Yukako Soejima Hideki Yoshioka1,‡
Hideki Yoshioka1,‡ Yasunori Sato
Yasunori Sato Naohiko Anzai
Naohiko Anzai Akihiro Hisaka
Akihiro Hisaka