
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 02 May 2024
Sec. Cardiovascular Epidemiology and Prevention
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1329586
Introduction: Although heart failure (HF) has been linked to bisphenol A (BPA), few studies have investigated the cut-off values for the effects of urinary BPA levels on heart failure risk. The association between urinary BPA levels and HF prognosis has not been investigated.
Methods: This study included 11,849 adults over 20 years old using information from the National Health and Nutrition Examination Survey (NHANES), which was conducted from 2003 to 2016. The relationship between urinary BPA levels and the risk of HF was determined via a multivariable logistic regression model, and restricted cubic spline (RCS) methods were used to determine the cut-off for the effect of BPA levels on HF risk. Based on the available NT-proBNP concentration data from the NHANES (2003–2004), multivariable linear regression was applied to determine the linear association between the NT-proBNP concentration and urinary BPA concentration.
Results: The results revealed a positive correlation between a urinary BPA concentration in the fourth quartile and the occurrence of heart failure [OR 1.49, 95% CI (1.09, 2.04), p = 0.012]. A one-unit increase (1 ng/mg creatinine) in the ln-transformed BPA concentration was linked to a 15% increase in the incidence of HF [OR 1.15, 95% CI (1.03, 1.29), p = 0.014]. The cut-off urinary BPA concentration for HF risk was 1.51 ng/mg creatinine. There was a positive correlation between urinary BPA and NT-proBNP concentrations [β = 0.093, 95% CI (0.014, 0.171), p = 0.02] in males, but there was no linear association [β = 0.040, 95% CI (−0.033, 0.113), p = 0.283] in females.
Discussion: Increased urinary BPA levels are linked to an increased risk of heart failure and poor prognosis. There is a significant increase in the risk of heart failure if the urinary concentration of BPA exceeds 1.51 ng/mg creatinine.
As the terminal stage of a variety of cardiovascular diseases, heart failure (HF) has a high mortality rate and dismal prognosis, with a 5-year mortality rate of 45%–60% (1, 2). Available data show that HF currently affects 64 million people worldwide and results in 10 million lost DALYs (3). The 2022 AHA/CAC/HFSA guidelines emphasize that risk factors such as hypertension, atherosclerosis, diabetes, and obesity contribute to susceptibility to HF (4). In addition to the typical risk factors listed above, previous research has revealed that environmental factors are intimately related to the development of HF. For example, long- or short-term exposure to airborne particulate matter (PM2.5 or PM10) increases the risk of HF (5). Dietary exposure to polychlorinated biphenyls (PCBs) and increased urinary cadmium concentrations have been positively linked to the risk of HF (6, 7). Blood lead levels are inversely correlated with left ventricular stroke volume and ejection fraction (8) and with reduced left ventricular systolic function (9), possibly leading to HF. Therefore, identifying the environmental risk factors associated with the development of HF is critical for its prevention.
Bisphenol A (BPA) is an industrial chemical that is used in the manufacturing of various polymers and resins (10). Some European Union countries have formulated regulations to restrict BPA use. For example, France prohibits the use of BPA in food packaging for consumers of all ages. However, in Belgium, the addition of BPA to specific food contact materials is prohibited only for children. Although BPA use is restricted, BPA has still been detected in the human body in recent years due to its environmental accumulation and bioaccumulation (11, 12). Therefore, the effect of BPA on human health cannot be ignored (13, 14).
Previous research has indicated that BPA can induce a variety of harmful health effects, including endocrine disruption, cytotoxicity, and reproductive toxicity (15). A recent study indicated that bisphenol A (BPA) is associated with an increased risk of HF. Specifically, each incremental increase in the BPA level (µg/g creatinine) corresponds to a 19% increase in the likelihood of HF (16). In an animal model, BPA also caused cardiac cell hypertrophy in mice (17). This evidence suggests that BPA may cause HF. However, few studies have investigated the relationship between urinary BPA levels and HF prognosis. N-terminal pro-B-type natriuretic peptide (NT-proBNP) serves as a clinical indicator of HF prognosis. NT-proBNP is a peptide hormone discharged by ventricular cells in reaction to augmented tension within the ventricular walls (18). Manal et al. reported that carbon monoxide can increase NT-proBNP levels (19). Drug treatment for breast cancer can also cause an increase in NT-proBNP levels (20). However, there are currently no reports about the associations between BPA and NT-proBNP levels.
The general population from the NHANES was used in this study to investigate the association between urinary BPA concentrations and HF risk. Subsequently, the association between BPA and NT-proBNP concentrations was explored for the first time to investigate the link between BPA concentrations and HF prognosis. The different effects and associations in males and females were investigated by performing a stratified analysis by sex.
Our research data were derived from the NHANES, an ongoing cross-sectional study conducted to monitor the health of noninstitutionalized civilians in the United States since 1999. The survey includes questionnaire interviews concerning demographic, socioeconomic, dietary, and health characteristics, as well as physical examinations and laboratory tests. Serum, plasma, and urine samples are collected from the participants and processed in mobile examination centres (MECs). Additional details regarding the survey are available in the online resources of the NHANES (www.cdc.gov/nchs/nhanes.htm). The NHANES has received appropriate ethical approval, and research concerning the population aligns with ethical standards.
We aggregated NHANES data spanning from 2003 to 2016, aiming to optimize the sample size to include available urinary BPA concentration and HF data. In total, 11,849 participants were included in the analysis of urinary BPA levels and HF risk after excluding 31,837 participants under 20 years old, 27,305 participants without urinary BPA concentration data and 67 participants without HF outcome data. Then, we obtained data from the year in which the BNP test data were obtained. NT-proBNP data were available for 2,262 NHANES participants from 2003 to 2004. In addition, we excluded individuals without HF outcome data (N = 880), and 1,382 participants were included in the association analysis of urinary BPA and NT-proBNP concentrations. A flowchart of the data integration and analysis process is shown in Figure 1.
Heart failure was defined by self-reported physician diagnoses and medical status questionnaires completed during individual interviews. Participants were identified as having HF if they responded affirmatively to the structured question. Comprehensive information regarding the question is available in the Supplementary Materials. Individuals who answered that they did not know whether they had been diagnosed with congestive heart failure (CHF) were excluded from our study. The CHF outcome was converted into a dichotomous variable.
Total (free and conjugated) urinary BPA was one of the biological variables evaluated in the NHANES. Spot urine samples were collected from participants during the interview period. Prior to testing, the urine samples were frozen and preserved on dry ice before being transported to the National Environmental Health Center. BPA concentrations were measured in urine samples from a one-third random subsample of the participants. The measurement methods have been described previously (21). Detailed measurements are available in the NHANES Laboratory Procedures Manual (22). Considering the effect of other urinary components and urinary dilution, the urinary BPA concentration was adjusted by urinary creatinine (BPA/Cr), which is a common method for assessing chemical exposure (23). When the detectable concentration of BPA was less than the lower limit of detection (LLOD) (0.4 ng/ml), the value was replaced by the square root of 2.
Blood samples taken during the NHANES (1999–2004) were used for NT-proBNP laboratory testing. The NT-proBNP data included in our study can be referred to as the NHANES 2003–2004 laboratory data. For detailed testing methodologies, please see the Supplementary Materials. NT-proBNP remains stable in samples subjected to up to four freeze-thaw cycles or in frozen samples previously stored at 4°C for 24 h (24). These measurement and storage conditions ensure the stability of NT-proBNP.
Some variables were chosen as potential confounding factors in our study, including demographic information (age, sex, race, socioeconomic status, and education level), health-related factors [body mass index (BMI), alcohol consumption, and physical activity level], and metabolite levels (serum cotinine and urinary creatinine). The serum cotinine concentration was considered an indicator of smoking status and exposure to secondhand smoke. The questionnaire used for the baseline interview yielded comprehensive information. Comprehensive information about the covariates examined in this study can be found in the Supplementary Materials.
The baseline characteristics of the participants are reported according to the incidence of HF. Categorical variables are presented as proportions (%), whereas continuous variables are presented as the means with standard deviations or medians with interquartile ranges (IQRs). Differences in continuous and categorical variables between the groups were assessed using the Mann–Whitney U test and chi-squared test, respectively.
Due to the skewed distribution, the natural logarithm (ln) was used to transform urinary BPA (ng/mg, creatinine) and NT-proBNP (pg/ml) concentrations. We constructed a multivariable logistic regression model to estimate the association between urinary BPA concentrations (continuously distributed or categorized into quartiles) and HF risk after we adjusted for potential confounding variables (details of the covariates selected in this study are described in the Supplementary Material). The outcomes reveal the effect estimates (ORs) and their corresponding 95% confidence intervals (CIs) for each quartile in relation to the reference quartile (Q1 group). Furthermore, we conducted a sex-stratified analysis to explore sex differences between males and females. The RCS model was used to visualize the exposure-response relationship between urinary BPA concentrations and HF risk and the cut-off values. We used multivariate linear regression to determine the relationship between the HF prognosis biomarker (NT-proBNP concentration) and urinary BPA concentration.
Data processing and baseline characteristic analyses were carried out using STATA MP16 (SAS Institute, Cary, NC, USA), and association or mediation analysis was carried out using R 4.1.3 (R-Foundation for Statistical Computing, Vienna, Austria). Every test was two-sided, and a p value of 0.05 or lower was considered to indicate statistical significance.
Table 1 provides comprehensive details regarding the baseline characteristics of the participants. Among the 11,849 participants, 371 participants were diagnosed with heart failure. The participants’ average age was 49 ± 18 years, and the age of the population with HF outcomes was older than that of the healthy population (p < 0.001). A total of 48.7% of the participants were male, and the majority of the participants were non-Hispanic white. A BMI indicating obesity, an educational level less than the 11th grade, a low physical activity level, and a family history of CVD were more common in heart failure patients. The median creatinine-corrected BPA concentration was 1.56 [0.91, 2.80] (ng/mg creatinine), and individuals with HF tended to have higher urinary BPA concentrations.
Table 2 displays the link between urinary BPA concentrations and HF risk. According to the analysis of all individuals’ quartiles, the incidence of HF was positively correlated with a urinary BPA concentration in the fourth quartile [OR 1.49, 95% CI (1.09, 2.04), p = 0.012]. The significant relationship between a urinary BPA concentration in any quartile and HF risk disappeared in females, while a urinary BPA concentration in the second quartile was significantly related to HF risk in males [OR 1.54, 95% CI (1.03, 2.31), p = 0.036]. Additionally, we assessed the relationship between continuous urinary BPA concentrations after ln-transformation and HF outcomes. According to the findings, there was a 15% increase in the incidence of HF for every one-unit (ng/mg creatinine) increase in the ln-transformed urinary BPA concentration [OR 1.15, 95% CI (1.03, 1.29), p = 0.014]. A sex-specific stratified analysis revealed that although the aforementioned association disappeared in males, the ln-transformed BPA concentration was positively associated with HF risk in females [OR 1.20, 95% CI (1.09, 1.43), p = 0.044]. As shown in Figure 2, we used restricted cubic splines to visualize the relationship between urinary BPA concentrations and HF risk. The risk of HF was relatively stable until a urinary BPA/Cr value of approximately 1.51 ng/mg was reached and then started to increase afterwards. Beyond this point, the risk of HF increased, reaching 9.91 ng/mg, after which it plateaued and gradually increased.
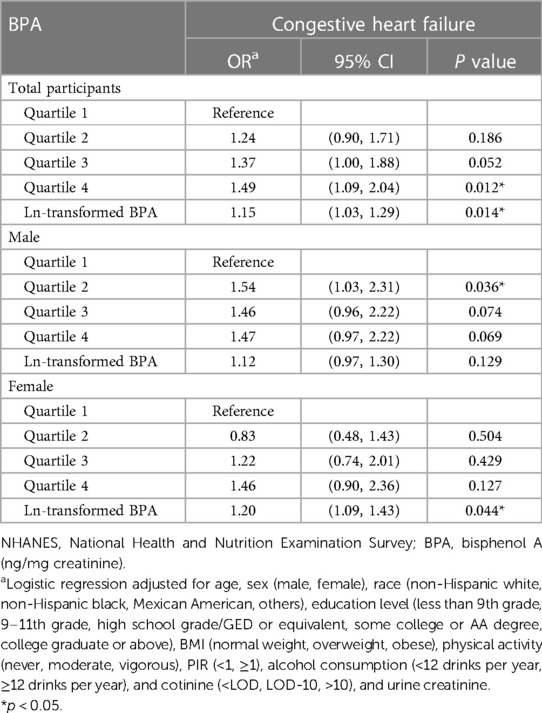
Table 2. Association between urinary bisphenol A (BPA) levels and CVD risk among U.S. adults, NHANES 2003–2016.
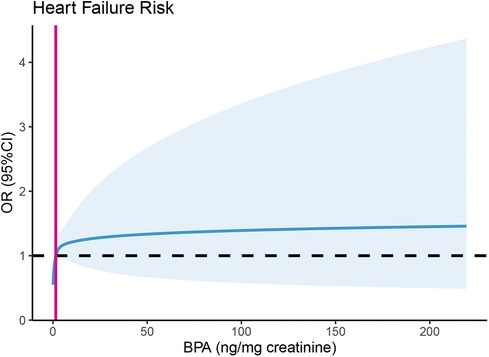
Figure 2. Predicted spline curves for the association between urinary BPA concentrations and heart failure risk using restricted cubic spline (RCS) regression. Restricted cubic spline regression adjusted for age, sex (male, female), race (non-Hispanic white, non-Hispanic black, Mexican American, others), education level (less than 9th grade, 9–11th grade, high school grade/GED or equivalent, some college or AA degree, college graduate or above), BMI (normal weight, overweight, obese), physical activity level (none, moderate, vigorous), PIR (<1, ≥1), alcohol consumption (<12 drinks per year, ≥12 drinks per year), and cotinine (< LOD, LOD-10, >10). NT-proBNP, N-terminal prohormone B-type natriuretic peptide; BPA/Cr, creatinine-corrected urinary BPA.
The multivariate linear regression results showed that the concentration of NT-proBNP was positively related to the concentration of urinary BPA [β = 0.053, 95% CI (0.006, 0.099), p = 0.028]. A positive association between urinary BPA and NT-proBNP concentrations was detected in males [β = 0.093, 95% CI (0.014, 0.171), p = 0.02], while no linear association between BPA and NT-proBNP concentrations was detected in females [β = 0.040, 95% CI (−0.033, 0.113), p = 0.283].
This study revealed that BPA concentrations are associated with HF risk, and the cut-off urinary BPA concentration for increased HF risk was above 1.51 ng/mg creatinine. BPA concentrations were also shown to be associated with NT-proBNP concentrations for the first time, highlighting the poor prognosis of heart failure patients with BPA exposure.
Although previous studies have demonstrated that BPA exposure is associated with several cardiovascular disease (CVD) outcomes (25), such as myocardial infarction (MI), coronary heart disease (CHD) and hypertension, there are currently few reports on the associations between BPA and HF. The results of a meta-analysis incorporating multiple epidemics also revealed an association between an increased urinary BPA concentration (adjusted by urinary creatinine) and an increased risk of HF (23). In addition, compared to the highest and lowest levels of exposure to the BPA substitute bisphenol F, exposure to bisphenol F increased the risk of HF by 15% (26). According to the findings in the present study, the risk of HF increased by 15% for every one-unit (ng/mg creatinine) increase in the ln BPA/Cr ratio, which is consistent with previous findings. However, the association between urinary BPA concentrations and HF risk differed according to sex. In contrast to the findings of Li et al. (16), the ln-transformed BPA/Cr ratio was positively associated with heart failure risk in females, while the aforementioned association disappeared in males. This opposite conclusion may be due to the varying covariates used in the logistic regression model. In addition, the included studies were cross-sectional studies, which may have led to bias, and subsequent cohort studies are needed to verify the causal relationship. In addition, the nonlinear association between urinary BPA concentrations and heart failure risk may be because BPA, as an environmental pollutant, has a toxic effect threshold at which heart failure risk increases. The threshold for urinary BPA, below which there is no heightened risk of heart failure, was determined to be 1.51 ng/mg creatinine—a value closely approximating the median urinary BPA level within this study population. The plateau in the increased risk of heart failure is potentially associated with direct myocardial injury induced by BPA beyond a urinary concentration of 9.91 ng/mg creatinine.
At present, although there are reports of an association between BPA and heart failure, these cross-sectional studies cannot confirm a causal relationship. A mechanistic study revealed that BPA exposure may cause HF. The expression of mast cell markers increased significantly in mice treated with 25 μg/L BPA in drinking water compared to those treated with 0 μg/L BPA (17). Other mechanistic studies have shown that mast cells promote heart remodelling and fibrosis (27, 28). This evidence suggested that BPA may cause cardiac remodelling and fibrosis and further increase HF risk. Another study revealed that exposure to BPA led to prolonged PR segments and decreased epicardial conduction velocity in the heart in mice (29). After exposure to BPA, the vitality of heart cells decreases (30). This experimental evidence suggests that BPA can cause cardiac dysfunction, which may lead to HF. The sex specificity of BPA exposure and HF risk was reported in several previous animal studies. Yan et al. (31) revealed a more evident oestrogen-like effect of BPA in female mice, but no observable response was found in male mice. More epidemiological and experimental research is needed to explore the sex-specific impact of BPA exposure on the incidence of heart failure.
An elevated NT-proBNP level provides evidence for the prognosis of HF patients. In this study, we found that BPA was associated with HF and increased NT-proBNP levels, which indicated a poor prognosis for patients with HF. The sex specificity of the association between urinary BPA and NT-proBNP concentrations was reported for the first time, in which a significant association was observed for males but not for females. However, sex differences in this association need to be further investigated. This research approach may provide a possible paradigm for studying exposure to environmental chemicals and HF incidence. Many studies have shown an association between chemical treatment and HF (32–35). However, there has been no simultaneous research on the association between these environmental chemicals and NT-proBNP concentrations. Therefore, this is also the first study to analyse the relationships between exposure to environmental chemicals and NT-proBNP concentrations and HF risk.
This study has the following strengths. First, for the first time, we reported an association between urinary BPA and NT-proBNP concentrations, and similar to the findings of previous studies, we also observed an association between urinary BPA concentrations and HF risk. The positive correlation observed between urinary bisphenol A (BPA) and NT-proBNP concentrations underscores the link between urinary BPA concentrations and the risk of heart failure. Second, we identified the cut-off urinary BPA concentration, indicating no heightened risk of heart failure.
However, this study also has several limitations. First, because this study was cross-sectional in nature, it was impossible to determine whether an increased BPA concentration causes HF or whether HF causes an increase in the urinary BPA concentration. In addition, there is a lack of strong evidence for an association between urinary BPA and NT-proBNP concentrations, and additional epidemiological and experimental studies are needed to verify this conclusion. We summarized the mechanistic studies previously reported in the Discussion section. Second, several factors, such as genetic factors, were not considered. Due to the presence of genetic factors, some people are susceptible to HF. However, due to the lack of complete genetic factor information in the NHANES, we could not perform covariate correction to reduce this bias, which needs to be addressed in future research. Third, other potential biomarkers of HF were not included in the present study, mainly because of the unavailability of related data. The role of other heart markers could be investigated in future research once the data are released by the NHANES. Finally, the exposure level of BPA may change dynamically, possibly due to changes in exposure sources. This study used the urinary BPA concentration to represent a possible bias in BPA exposure. In future research, additional time points are needed to represent stable BPA exposure.
Our study revealed the relationship between urinary BPA concentrations and HF risk. The cut-off BPA concentration for HF risk was 1.51 ng/mg creatinine. Urinary BPA concentrations were associated with increased plasma NT-proBNP concentrations, indicating a poor prognosis in HF patients.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
YM: Data curation, Methodology, Writing – original draft. HH: Conceptualization, Writing – review & editing. HQ: Validation, Writing – review & editing. YW: Writing – review & editing, Methodology, Supervision, Project administration, Validation. ZG: Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1329586/full#supplementary-material
1. Becher PM, Lund LH, Coats AJS, Savarese G. An update on global epidemiology in heart failure. Eur Heart J. (2022) 43(32):3005–7. doi: 10.1093/eurheartj/ehac248
2. Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. (2002) 347(18):1397–402. doi: 10.1056/NEJMoa020265
3. Joshi SS, Miller MR, Newby DE. Air pollution and cardiovascular disease: the Paul Wood lecture, British cardiovascular society 2021. Heart. (2022) 108(16):1267–73. doi: 10.1136/heartjnl-2021-319844
4. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2022) 145(18):e895–e1032. doi: 10.1161/cir.0000000000001063
5. Konduracka E, Rostoff P. Links between chronic exposure to outdoor air pollution and cardiovascular diseases: a review. Environ Chem Lett. (2022) 20(5):2971–88. doi: 10.1007/s10311-022-01450-9
6. Åkesson A, Donat-Vargas C, Berglund M, Glynn A, Wolk A, Kippler M. Dietary exposure to polychlorinated biphenyls and risk of heart failure—a population-based prospective cohort study. Environ Int. (2019) 126:1–6. doi: 10.1016/j.envint.2019.01.069
7. Sears CG, Eliot M, Raaschou-Nielsen O, Poulsen AH, Harrington JM, Howe CJ, et al. Urinary cadmium and incident heart failure: a case-cohort analysis among never-smokers in Denmark. Epidemiology. (2022) 33(2):185–92. doi: 10.1097/ede.0000000000001446
8. Chen Z, Huo X, Zhang S, Cheng Z, Huang Y, Xu X. Relations of blood lead levels to echocardiographic left ventricular structure and function in preschool children. Chemosphere. (2021) 268:128793. doi: 10.1016/j.chemosphere.2020.128793
9. Yang WY, Zhang ZY, Thijs L, Cauwenberghs N, Wei FF, Jacobs L, et al. Left ventricular structure and function in relation to environmental exposure to lead and cadmium. J Am Heart Assoc. (2017) 6(2):e004692. doi: 10.1161/jaha.116.004692
10. Ma Y, Liu H, Wu J, Yuan L, Wang Y, Du X, et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ Res. (2019) 176:108575. doi: 10.1016/j.envres.2019.108575
11. Cimmino I, Fiory F, Perruolo G, Miele C, Beguinot F, Formisano P, et al. Potential mechanisms of bisphenol A (BPA) contributing to human disease. Int J Mol Sci. (2020) 21(16):5761. doi: 10.3390/ijms21165761
12. Sousa S, Maia ML, Delerue-Matos C, Calhau C, Domingues VF. The role of adipose tissue analysis on environmental pollutants biomonitoring in women: the European scenario. Sci Total Environ. (2022) 806(Pt 4):150922. doi: 10.1016/j.scitotenv.2021.150922
13. Kahn LG, Philippat C, Nakayama SF, Slama R, Trasande L. Endocrine-disrupting chemicals: implications for human health. Lancet Diabetes Endocrinol. (2020) 8(8):703–18. doi: 10.1016/s2213-8587(20)30129-7
14. Mustieles V, D'Cruz SC, Couderq S, Rodríguez-Carrillo A, Fini JB, Hofer T, Steffensen IL, Dirven H, Barouki R, Olea N, Fernández MF, David A. Bisphenol A and its analogues: a comprehensive review to identify and prioritize effect biomarkers for human biomonitoring. Environ Int. 2020;144:105811. doi: 10.1016/j.envint.2020.105811
15. Chen D, Kannan K, Tan H, Zheng Z, Feng YL, Wu Y, et al. Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity-A review. Environ Sci Technol. (2016) 50(11):5438–53. doi: 10.1021/acs.est.5b05387
16. Cai S, Rao X, Ye J, Ling Y, Mi S, Chen H, et al. Relationship between urinary bisphenol a levels and cardiovascular diseases in the U.S. adult population, 2003–2014. Ecotoxicol Environ Saf. (2020) 192:110300. doi: 10.1016/j.ecoenv.2020.110300
17. Bruno KA, Mathews JE, Yang AL, Frisancho JA, Scott AJ, Greyner HD, et al. BPA alters estrogen receptor expression in the heart after viral infection activating cardiac mast cells and T cells leading to perimyocarditis and fibrosis. Front Endocrinol (Lausanne). (2019) 10:598. doi: 10.3389/fendo.2019.00598
18. Goetze JP, Bruneau BG, Ramos HR, Ogawa T, de Bold MK, de Bold AJ. Cardiac natriuretic peptides. Nat Rev Cardiol. (2020) 17(11):698–717. doi: 10.1038/s41569-020-0381-0
19. Abdel Aziz MH, El Dine F, Hussein H, Abdelazeem AM, Sanad IM. Prediction of troponin I and N-terminal pro-brain natriuretic peptide levels in acute carbon monoxide poisoning using advanced electrocardiogram analysis, Alexandria, Egypt. Environ Sci Pollut Res Int. (2021) 28(35):48754–66. doi: 10.1007/s11356-021-14171-3
20. Rüger AM, Schneeweiss A, Seiler S, Tesch H, van Mackelenbergh M, Marmé F, et al. Cardiotoxicity and cardiovascular biomarkers in patients with breast cancer: data from the GeparOcto-GBG 84 trial. J Am Heart Assoc. (2020) 9(23):e018143. doi: 10.1161/jaha.120.018143
21. McGuinn LA, Ghazarian AA, Joseph Su L, Ellison GL. Urinary bisphenol A and age at menarche among adolescent girls: evidence from NHANES 2003–2010. Environ Res. (2015) 136:381–6. doi: 10.1016/j.envres.2014.10.037
22. Centers for Disease Control and Prevention. Laboratory Procedure Manual (2013). Available online at: https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/labmethods/eph_g_met.pdf (Accessed April 17, 2024).
23. Moon S, Yu SH, Lee CB, Park YJ, Yoo HJ, Kim DS. Effects of bisphenol A on cardiovascular disease: an epidemiological study using national health and nutrition examination survey 2003–2016 and meta-analysis. Sci Total Environ. (2021) 763:142941. doi: 10.1016/j.scitotenv.2020.142941
24. Hezzell MJ, Boswood A, Lötter N, Elliott J. The effects of storage conditions on measurements of canine N-terminal pro-B-type natriuretic peptide. J Vet Cardiol. (2015) 17(1):34–41. doi: 10.1016/j.jvc.2014.10.002
25. Lakind JS, Goodman M, Mattison DR. Bisphenol A and indicators of obesity, glucose metabolism/type 2 diabetes and cardiovascular disease: a systematic review of epidemiologic research. Crit Rev Toxicol. (2014) 44(2):121–50. doi: 10.3109/10408444.2013.860075
26. Moreno-Gómez-Toledano R. Relationship between emergent BPA-substitutes and renal and cardiovascular diseases in adult population. Environ Pollut. (2022) 313:120106. doi: 10.1016/j.envpol.2022.120106
27. Coronado MJ, Brandt JE, Kim E, Bucek A, Bedja D, Abston ED, et al. Testosterone and interleukin-1β increase cardiac remodeling during coxsackievirus B3 myocarditis via serpin A 3n. Am J Physiol Heart Circ Physiol. (2012) 302(8):H1726–36. doi: 10.1152/ajpheart.00783.2011
28. Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Davis SE, Gatewood SJ, et al. Interferon-gamma protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-beta 1, interleukin-1 beta, and interleukin-4 in the heart. Am J Pathol. (2004) 165(6):1883–94. doi: 10.1016/s0002-9440(10)63241-5
29. Posnack NG, Jaimes R 3rd, Asfour H, Swift LM, Wengrowski AM, Sarvazyan N, et al. Bisphenol A exposure and cardiac electrical conduction in excised rat hearts. Environ Health Perspect. (2014) 122(4):384–90. doi: 10.1289/ehp.1206157
30. Yujiao C, Meng Z, Shanshan L, Wei W, Yipeng W, Chenghong Y. Exposure to bisphenol A induces abnormal fetal heart development by promoting ferroptosis. Ecotoxicol Environ Saf. (2023) 255:114753. doi: 10.1016/j.ecoenv.2023.114753
31. Yan S, Chen Y, Dong M, Song W, Belcher SM, Wang HS. Bisphenol A and 17β-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS One. (2011) 6(9):e25455. doi: 10.1371/journal.pone.0025455
32. Shah AS, Langrish JP, Nair H, McAllister DA, Hunter AL, Donaldson K, et al. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet. (2013) 382(9897):1039–48. doi: 10.1016/s0140-6736(13)60898-3
33. Huang M, Jiao J, Zhuang P, Chen X, Wang J, Zhang Y. Serum polyfluoroalkyl chemicals are associated with risk of cardiovascular diseases in national US population. Environ Int. (2018) 119:37–46. doi: 10.1016/j.envint.2018.05.051
34. Peters JL, Perlstein TS, Perry MJ, McNeely E, Weuve J. Cadmium exposure in association with history of stroke and heart failure. Environ Res. (2010) 110(2):199–206. doi: 10.1016/j.envres.2009.12.004
Keywords: bisphenol A, heart failure, NT-proBNP, cut-off, NHANES
Citation: Ma Y, Huang H, Qian H, Wu Y and Gao Z (2024) Association of urinary bisphenol A levels with heart failure risk in U.S. adults from the NHANES (2003–2016). Front. Cardiovasc. Med. 11:1329586. doi: 10.3389/fcvm.2024.1329586
Received: 30 October 2023; Accepted: 10 April 2024;
Published: 2 May 2024.
Edited by:
Maria Concetta Pastore, University of Siena, ItalyReviewed by:
Velmurugan Ganesan, KMCH Research Foundation, India© 2024 Ma, Huang, Qian, Wu and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhe Gao ZHJnYW96aGVAMTYzLmNvbQ== Yanhu Wu d3V5YW5odUBuam11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.