- 1School of Traditional Chinese Medicine, Hunan University of Chinese Medicine, Changsha, Hunan, China
- 2The First Hospital of Hunan University of Chinese Medicine, Changsha, Hunan, China
- 3The Third School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
Objective: The effect of mental disorders (MD) on cardiovascular disease (CVD) remains controversial, and this study aims to analyze the causal relationship between eight MD and CVD by Mendelian randomization (MR).
Methods: Single nucleotide polymorphisms of attention-deficit/hyperactivity disorder (ADHD), anorexia nervosa (AN), anxiety disorder (ANX), autism spectrum disorder (ASD), bipolar disorder (BD), depression, obsessive-compulsive disorder (OCD), schizophrenia (SCZ), and CVD were obtained from UK Biobank and FinnGen. Exposure-outcome causality was tested using inverse variance weighted (IVW), MR-Egger, and weighted median. Horizontal pleiotropy and heterogeneity were assessed by MR-Egger intercept and Cochran's Q, respectively, while stability of results was assessed by leave-one-out sensitivity analysis.
Results: MR analysis showed that ANX (IVW [odds ratio (OR) 1.11, 95% confidence intervals (CI) 1.07–1.15, p < 0.001]; MR-Egger [OR 1.03, 95% CI 0.92–1.14, p = 0.652]; weighted median [OR 1.09, 95% CI 1.03–1.14, p = 0.001]), ASD (IVW [OR 1.05, 95% CI 1.00–1.09, p = 0.039]; MR-Egger [OR 0.95, 95% CI 0.84–1.07, p = 0.411]; weighted median [OR 1.01, 95% CI 0.96–1.06, p = 0.805]), depression (IVW [OR 1.15, 95% CI 1.10–1.19, p < 0.001]; MR-Egger [OR 1.10, 95% CI 0.96–1.26, p = 0.169]; weighted median [OR 1.13, 95% CI 1.08–1.19, p < 0.001]) were significantly associated with increased risk of CVD, whereas ADHD, AN, BD, OCD, and SCZ were not significantly associated with CVD (p > 0.05). Intercept analysis showed no horizontal pleiotropy (p > 0.05). Cochran's Q showed no heterogeneity except for BD (p = 0.035). Sensitivity analysis suggested that these results were robust.
Conclusions: ANX, ASD, and depression are associated with an increased risk of CVD, whereas AN, ADHD, BD, OCD, and SCZ are not causally associated with CVD. Active prevention and treatment of ANX, ASD, and depression may help reduce the risk of CVD.
1 Introduction
Cardiovascular disease (CVD), a circulatory system disease involving lesions of the heart and blood vessels (1, 2), is the leading cause of disability and premature death in adults (3). Epidemiological research showed that in 2019, about 523 million people worldwide had CVD, about 18.6 million people died from CVD, and 23.6 million people were expected to die from CVD annually by 2030 (4, 5). CVD remains a significant threat to human health, and controlling associated risk factors is an essential means of preventing and treating CVD (6). Smoking, obesity, and unhealthy diet are considered to be common risk factors for CVD (7, 8), and the control of these risk factors effectively reduces the risk of CVD (9). In recent years, with the development of the biopsychosocial model, people have become concerned about the influence of psychosocial factors on disease, and there has been growing evidence that mental disorders (MD) may be potential risk factors for CVD (10, 11).
MD are chronic heterogeneous diseases characterized by abnormal mental or behavioral patterns (12, 13). They include attention-deficit/hyperactivity disorder (ADHD), anorexia nervosa (AN), anxiety disorder (ANX), autism spectrum disorder (ASD), bipolar disorder (BD), depression, obsessive-compulsive disorder (OCD), schizophrenia (SCZ), and so on. As a global health problem, MD are characterized by high prevalence and high cost, which brings a huge burden to individuals, families, and society (14). Previous studies have shown that depression increases the risk of adverse cardiovascular events such as acute myocardial infarction, atrial fibrillation, heart failure, and stroke (15, 16). However, the effects of other MD on CVD remain controversial. Given that ADHD, AN, ANX, ASD, BD, depression, OCD, and SCZ are among the most prevalent and debilitating mental health problems, this study employed them as exposure factors to assess the causality between MD and CVD using Mendelian randomization (MR).
2 Materials and methods
2.1 Study design
Mendelian Randomization relied on three basic assumptions: (1) The association assumption: Single nucleotide polymorphisms (SNPs) were strongly associated with exposure factors. (2) The independence assumption: SNPs were independent of confounding factors. (3) The exclusivity assumption: SNPs could not act on outcome variables through pathways other than exposure factors. The MR design for MD and CVD is shown in Figure 1.
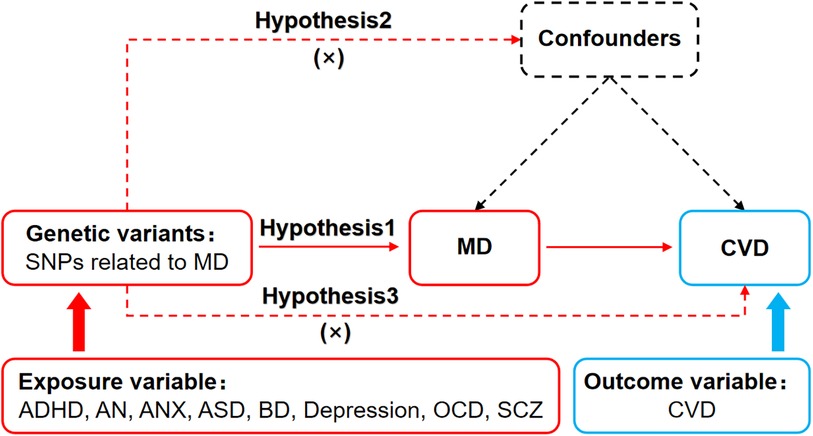
Figure 1. MR design for causal analysis of MD and CVD. MD, mental disorders; CVD, cardiovascular diseases.
2.2 Data sources
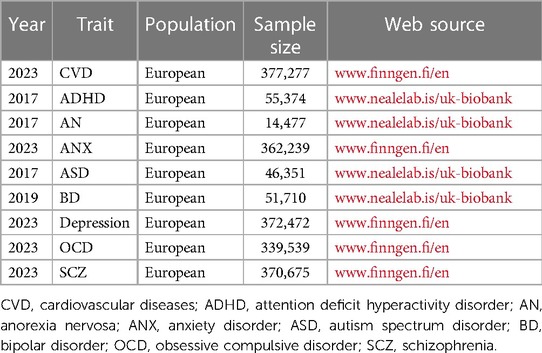
With the exclusion of horizontal pleiotropy, this study selected the latest and largest sample size data for each exposure and outcome. The data for ADHD (dataset number: ieu-a-1183), AN (dataset number: ieu-a-1186), ASD (dataset number: ieu-a-1185), and BD (dataset number: ieu-b-41) were from UK Biobank (www.nealelab.is/uk-biobank), and the data for ANX (dataset number: finn-b-F5_ALLANXIOUS), depression (dataset number: finn-b-F5_DEPRESSIO), OCD (dataset number: finn-b-F5_OCD), SCZ (dataset number: finn-b-F5_SCHZPHR), and CVD (dataset number: finn-b-FG_CVD) were from FinnGen (www.finngen.fi/fi). All the data were obtained from publicly available databases, thus eliminating the need for additional ethical approval.
2.3 Selection of genetic instrument variables
The genome-wide association studies (GWAS) database contained data from eight exposure factors totaling 1990,114 individuals of European ancestry. First, SNPs strongly associated with the exposure factors were screened according to p < 5 × 10–8 to fulfill assumption 1. Second, independent SNPs were further screened according to R2 < 0.001 and kb = 10,000 to avoid potential bias due to linkage disequilibrium. Third, the F-value of each SNP was calculated, and SNPs with F ≤ 10 were excluded. F-value was calculated publicly as . . R2: the cumulative explained variance of the selected IVs on exposure; MAF: the minor allele frequency; β: estimated effect of SNP; N: sample size of the GWAS. Fourth, referring to PhenoScanner (www.phenoscanner.medschl.cam.ac.uk) and related literature to remove SNPs potentially associated with CVD to fulfill assumption 2. Finally, mismatched SNPs were excluded based on the effect allele frequency while harmonizing the allelic orientation. The remaining SNPs were used to perform MR analysis.
2.4 Data analysis
The study followed the STROBE-MR guidelines (17). The MR analysis was performed using the “TwoSampleMR (0.5.7)” package of the R 4.3.1, and inverse variance weighted (IVW), MR-Egger, and weighted median were used to assess causation. IVW is the primary method (18), which achieves unbiased causal estimation without horizontal pleiotropy and is the most informative. MR-Egger and weighted median are complementary methods for MR analysis. MR-Egger provides valid causal estimation in some cases where pleiotropy exists, and weighted median has a lower sensitivity to outliers and measurement errors.
MR results were corrected by using the MR-Pleiotropy RESidual Sum and Outlier method (MR-PRESSO), and MR analysis was re-executed after removing outlier (p < 1) SNPs. Horizontal pleiotropy was assessed using MR-Egger intercept analysis, with p ≥ 0.05 suggesting the absence of horizontal pleiotropy to fulfill assumption 3. Heterogeneity was assessed using Cochran's Q, with p ≥ 0.05 suggesting the absence of heterogeneity. Leave-one-out sensitivity analysis was used to assess the robustness of the results and to clarify the presence of individual SNPs that significantly affected the pooled results.
3 Results
3.1 GWAS data on exposure and outcomes
Details of the GWAS included in this study are shown in Table 1, with data on MD from UK Biobank and FinnGen, including 1990,114 Europeans. Data on CVD were from the FinnGen, including 377,277 Europeans. After excluding the effects of linkage disequilibrium and confounders, the included SNPs are shown in Supplementary Table S1. Subsequently, mismatched SNPs were excluded when harmonizing the allelic orientations of the exposure-SNPs and the outcome-SNPs. And outlier SNPs were excluded in the MR-PRESSO correction. Finnaly, SNPs for causal analysis of MD and CVD are shown in Supplementary Table S2.
3.2 Mr analysis results
The causal relationships of ADHD, AN, ANX, ASD, BD, depression, OCD, and SCZ with CVD were analyzed using MR. The forest plot of the MR analysis is shown in Figure 2, and the effect estimates for each SNP are shown in Figure 3. MR-Egger intercept analysis is shown in Supplementary Table S3, heterogeneity analysis is shown in Supplementary Figure S1; Supplementary Table S4, and sensitivity analysis is shown in Supplementary Figure S2.
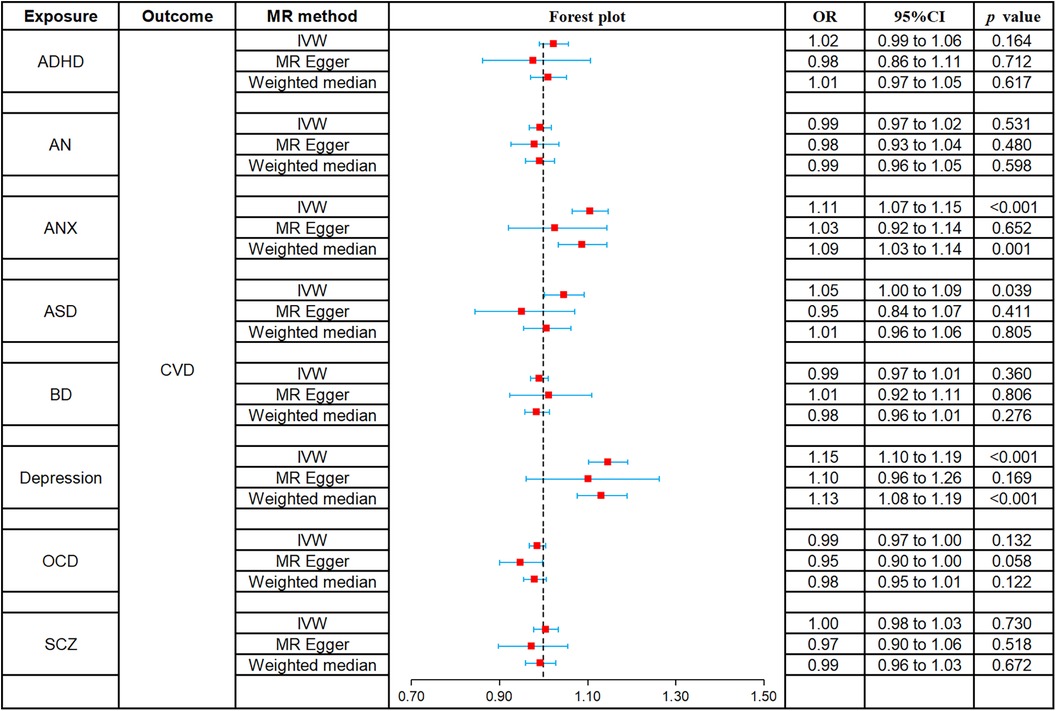
Figure 2. Forest plot of MR analysis on the causal relationship between MD and CVD. (A) ADHD on CVD; (B) AN on CVD; (C) ANX on CVD; (D) ASD on CVD; (E) BD on CVD; (F) Depression on CVD; (G) OCD on CVD; (H) SCZ on CVD. MD, mental disorders; CVD, cardiovascular diseases; ADHD, attention deficit hyperactivity disorder; AN, anorexia nervosa; ANX, anxiety disorder; ASD, autism spectrum disorder; BD, bipolar disorder; OCD, obsessive compulsive disorder; SCZ, schizophrenia.
Figure 3. Scatter plot of MR analysis on the causal relationship between MD and CVD. (A) ADHD on CVD; (B) AN on CVD; (C) ANX on CVD; (D) ASD on CVD; (E) BD on CVD; (F) Depression on CVD; (G) OCD on CVD; (H) SCZ on CVD. MD, mental disorders; CVD, cardiovascular diseases; ADHD, attention deficit hyperactivity disorder; AN, anorexia nervosa; ANX, anxiety disorder; ASD, autism spectrum disorder; BD, bipolar disorder; OCD, obsessive compulsive disorder; SCZ, schizophrenia.
3.2.1 ADHD
All three methods of analysis showed no significant causal relationship between ADHD and CVD: IVW (OR 1.02, 95% CI 0.99–1.06, p = 0.164), MR-Egger (OR 0.98, 95% CI 0.86–1.11, p = 0.712), and weighted median (OR 1.01, 95% CI 0.97–1.05, p = 0.617). Intercept analysis showed no horizontal pleiotropy (p = 0.460), heterogeneity test showed no significant heterogeneity (p = 0.177), and sensitivity analysis suggested the results were robust.
3.2.2 AN
All three methods of analysis showed no significant causal relationship between AN and CVD: IVW (OR 0.99, 95% CI 0.97–1.02, p = 0.531), MR-Egger (OR 0.98, 95% CI 0.93–1.04, p = 0.480), and weighted median (OR 0.99, 95% CI 0.96–1.05, p = 0.598). Intercept analysis showed no horizontal pleiotropy (p = 0.622), heterogeneity test showed no significant heterogeneity (p = 0.582), and sensitivity analysis suggested the results were robust.
3.2.3 ANX
IVW (OR 1.11, 95% CI 1.07–1.15, p < 0.001) and weighted median (OR 1.09, 95% CI 1.03–1.14, p = 0.001) showed that ANX was associated with increased risk of CVD, whereas no such causal relationship was observed for MR-Egger (OR 1.03, 95% CI 0.92–1.14, p = 0.652). Intercept analysis showed no horizontal pleiotropy (p = 0.159), heterogeneity test showed no significant heterogeneity (p = 0.731), and sensitivity analysis suggested the results were robust.
3.2.4 ASD
IVW (OR 1.05, 95% CI 1.00–1.09, p = 0.039) showed that ASD was associated with increased risk of CVD, while MR-Egger (OR 0.95, 95% CI 0.84–1.07, p = 0.411) and weighted median (OR 1.01, 95% CI 0.96–1.06, p = 0.805) did not observe this causal relationship. Intercept analysis showed no horizontal pleiotropy (p = 0.106), heterogeneity test showed no significant heterogeneity (p = 0.059), and sensitivity analysis suggested the results were robust.
3.2.5 BD
All three methods of analysis showed no significant causal relationship between BD and CVD: IVW (OR 0.99, 95% CI 0.97–1.01, p = 0.360), MR-Egger (OR 1.01, 95% CI 0.92–1.11, p = 0.806), and weighted median (OR 0.98, 95% CI 0.96–1.01, p = 0.276). Intercept analysis showed no horizontal pleiotropy (p = 0.643), heterogeneity test showed heterogeneity (p = 0.035), and sensitivity analysis suggested the results were robust.
3.2.6 Depression
Both IVW (OR 1.15, 95% CI 1.10–1.19, p < 0.001) and weighted median (OR 1.13, 95% CI 1.08–1.19, p < 0.001) showed that BD was associated with increased risk of CVD, while MR-Egger (OR 1.10, 95% CI 0.96–1.26, p = 0.169) did not observe such a causal relationship. Intercept analysis showed no horizontal pleiotropy (p = 0.554), heterogeneity test showed no significant heterogeneity (p = 0.063), and sensitivity analysis suggested the results were robust.
3.2.7 OCD
All three methods of analysis showed no significant causal relationship between OCD and CVD: IVW (OR 0.99, 95% CI 0.97–1.00, p = 0.132), MR-Egger (OR 0.95, 95% CI 0.90–1.00, p = 0.058), and weighted median (OR 0.98, 95% CI 0.95–1.01, p = 0.122). Intercept analysis showed no horizontal pleiotropy (p = 0.130), heterogeneity test showed no significant heterogeneity (p = 0.305). Sensitivity analysis suggested that rs80216287 was a significant SNP affecting the combined outcome, but detailed information about it could not be retrieved from the database. Hence, there is no justification for its exclusion.
3.2.8 SCZ
All three methods of analysis showed no significant causal relationship between SCZ and CVD: IVW (OR 1.00, 95% CI 0.98–1.03, p = 0.730), MR-Egger (OR 0.97, 95% CI 0.90–1.06, p = 0.518), and weighted median (OR 0.99, 95% CI 0.96–1.03, p = 0.672). Intercept analysis showed no horizontal pleiotropy (p = 0.420), heterogeneity test showed no significant heterogeneity (p = 0.278), and sensitivity analysis suggested the results were robust.
4 Discussion
CVD, a group of diseases that involve pathological changes in the heart and systemic vasculature (19), is one of the leading causes of increased mortality worldwide (20). With the development of the biopsychosocial model, more and more researchers have found a close link between psychological factors and CVD (21). Studies have shown that MD may be an independent risk factor for CVD (22). They increase the morbidity and mortality of CVD patients and reduce the quality of survival in CVD patients (22, 23).
Previous MR analyses have found that ANX is linked to an elevated risk of coronary heart disease, myocardial infarction, and heart failure (24); ASD is associated with an increased risk of atrial fibrillation (25); depression is correlated with an increased risk of coronary heart disease and myocardial infarction (26); and SCZ is linked to an increased risk of heart failure (27). While these studies delve into the impact of specific types of MD on distinct cardiovascular events, they lack a comprehensive assessment of the different types of MD and the overall risk of CVD. In this study, we conducted a comprehensive assessment of different MD types on the overall risk of CVD, using MD such as ADHD, AN, ANX, ASD, BD, depression, OCD, and SCZ as exposure factors and CVD as the outcome variable. The MR analysis revealed a significant causal relationship of depression, ANX, and ASD with CVD, whereas no significant causal relationship of ADHD, AN, BD, OCD, and SCZ with CVD. These results were free of horizontal pleiotropy and heterogeneity and were confirmed to be robust through sensitivity analysis. This study provides additional support for the association of ANX, ASD, and depression with cardiovascular risk compared to previous findings, while refuting the notion of SCZ significantly affecting the overall CVD risk. It means that SCZ may only elevate the risk of heart failure but has a non-significant effect on the overall cardiovascular risk. In addition, this study points out that ADHD, AN, BD, and SCZ are not associated with the overall risk of CVD.
A previous cohort study has shown that depression increases the risk of CVD. A cohort study of Chinese older adults showed a 5% increased risk of CVD in those with depression compared with those without depression (28). A study in Hong Kong has shown that patients with depression have a significantly increased risk of CVD, and this association was more pronounced in women and individuals over 65 years of age (29). In a recent study, the degree of depression was also positively associated with the risk of CVD, with the more depressed patients being more likely to develop CVD (30). A recent meta-analysis showed that depression was a significant risk factor for CVD, which not only increases the incidence of stroke, myocardial infarction, and congestive heart failure, but also increases cardiovascular-related and all-cause mortality rates (31). These pieces of evidence suggest that depressed patients have a higher risk of nonfatal CVD and fatal CVD (32). Furthermore, being female, over 65 years of age, and having major depression may strengthen this association.
At present, the mechanisms associated with depression and CVD have not been fully elucidated, while some studies have suggested that inflammatory responses associated with the immune system are a comorbidity mechanism for depression and CVD (33). Some drugs with anti-inflammatory effects alleviate the clinical manifestations of patients with depression and CVD (34). There was also evidence that the cortisol-regulated stress inflammatory response in depressed patients is a crucial mechanism in the pathogenesis of CVD (35). Plasma cortisol levels are maintained high in depressed patients. The sensitivity of immune cells to cortisol is reduced due to the stimulation of persistently high cortisol levels, which leads to an increased susceptibility to CVD (35, 36). Moreover, cortisol has a vasoconstrictive effect and elevates blood pressure, and sustained high levels of cortisol are prone to vascular damage and plaque formation, resulting in an increased risk of CVD (37).
The topic of ANX and CVD has always been controversial. An early meta-analysis showed that ANX increased the risk of coronary heart disease and cardiovascular mortality in patients (38), but very few included studies in this analysis controlled for the depression variable. However, some researchers did not find a significant effect of ANX on CVD after adjusting for the interference of depression. In a Greek cohort study, Kyrou I et al. (39) found that depression increased cardiovascular events, whereas ANX had no significant effect on cardiovascular events. In a study of older Americans, Karlsen et al. (40) reported that ANX was not associated with coronary heart disease or cerebrovascular disease. Intriguingly, there is evidence that ANX may even be a protective factor for CVD when potential risk factors are excluded. In a cross-sectional study in Taiwan, Huang et al. (41) found that older adults with ANX but not depression had a significantly lower risk of developing coronary heart disease or hypertension than healthy individuals in the same age group. Langvik et al. (42) showed in a study that ANX reduced the risk of acute myocardial infarction when controlling for depression. Parker G et al. (43) noted that generalized anxiety disorder (GAD) significantly improved cardiac prognosis in patients with acute coronary syndromes. More and more researchers have realized that comorbid depression is an essential factor influencing the causal relationship between ANX and CVD. Batelaan et al. (44) conducted a meta-analysis of 14 clinical studies that excluded or controlled for cases of depression and found that ANX was strongly associated with increased cardiovascular risk. This meta-analysis supports our findings, and our results point to ANX as a potential risk factor for CVD.
The current research indicates that the mechanism of ANX-mediated CVD may be related to the stimulation of the nervous system and the induction of an inflammatory response. ANX induces CVD by stimulating the amygdala, hippocampus, medial prefrontal cortex, and the autonomic nervous system. First, ANX affects the survival and growth of hippocampal neurons, leading to hippocampal atrophy, which can cause a decrease in cardiac ejection fraction and increase N-terminal prohormone of brain natriuretic peptide (NT-proBNP) (45). Second, ANX stimulates the amygdala, which affects blood pressure and heart rate (46). Third, ANX affects cardiac autonomic regulation by stimulating the medial prefrontal cortex (47, 48). The decreased autonomic nervous system regulation of heart rate is one of the most critical risk factors for adverse cardiovascular events (49). It has also been shown that ANX may affect the normal functioning of the cardiovascular system through the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis, thereby increasing the risk of myocardial ischemia, arrhythmia, and sudden cardiac death (50). Moreover, the inflammatory response is closely associated with MD and CVD and may play a role in the comorbidity of ANX and CVD (51). Related studies have shown that ANX patients have higher C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) (52, 53). These inflammatory factors are also associated with developing diseases such as atherosclerosis, heart failure, and unstable angina pectoris (54).
At this stage, there are a few studies on the correlation between ASD and CVD, which may be related to the verbal communication deficits of ASD patients. Heffernan et al. (55) found that decreased physical activity and weight gain were prevalent in children with ASD, and these factors led to increased arterial stiffness, which adversely affected cardiovascular health. Related studies have shown that children with ASD have higher levels of homocysteine (HCY) (56), which are positively correlated with carotid artery intima-media thickness (CA-IMT), atherogenic index, left ventricular mass index (LVMI), and blood pressure (57) and are also strongly associated with the severity of heart failure (58). In addition, Imre Yetkin et al. (59) found that Childhood Autism Rating Scale (CARS) scores were positively correlated with vessel diameter, carotid intima-media thickness (cIMT), and intima-media thickness/diameter ratio (IDR) values in children with ASD, suggesting that CVD risk may increase with the severity of ASD. These pieces of evidence support ASD as a potential risk factor for CVD, which is consistent with our findings. Interestingly, a recent study has found that the mechanism of comorbidity between ASD and CVD may be related to dysregulated phosphate metabolism (60), which provides a new direction for future research.
We did not find a causal relationship of AN, ADHD, BD, OCD, and SCZ with CVD in this study, which is quite different from the results of previous cohort studies. The current mainstream view is that AN, ADHD, BD, OCD, and SCZ may be potential risk factors for CVD. A relevant study has shown that bradycardia and QT interval prolongation are the most common adverse cardiovascular events in patients with AN and that AN patients with concomitant eating disorders also have a higher risk of acute cardiac disease (61). A UK cohort study showed that ADHD significantly increased the risk of CVD, particularly hemorrhagic stroke, cardiac arrest, and atherosclerosis (62). A cross-sectional study found that patients with BD had a significantly higher risk of developing CVD over a 10-year period, which was more pronounced in patients with depressive phases (63). Isomura K et al. (64) noted that patients with OCD had a higher risk of CVD, which might be related to decreased parasympathetic function and abnormal sympathetic responses in OCD patients (65). Veeneman et al. (27) found that SCZ was associated with heart failure and cardiovascular death, which might be a potential risk factor for CVD. Although these pieces of evidence point to the possibility that MD such as AN, ADHD, BD, OCD, and SCZ may be associated with an increased risk of CVD, there have been some reports to the contrary. Kalla et al. (66) found that older patients with AN had a lower incidence of coronary heart disease and heart failure compared with the general population over 65. Kittel-Schneider et al. (67) concluded that there was currently insufficient evidence to argue for an association between ADHD and CVD, and potential cardiovascular risks from drug therapy could not be excluded (68). Therefore, the causal relationship of AN, ADHD, BD, OCD, and SCZ with CVD is inconclusive, and we expect more researchers to continue exploring it in the future.
In summary, this MR analysis supports the notion that depression, ANX, and ASD as potential risk factors for CVD. It underscores the connection between mental health and CVD, suggesting that proactive psychological screening and intervention may help to mitigate an individual's CVD risk. Therefore, we recommend that cardiovascular physicians endeavor to focus on psychological and mental well-being of their patients. Ultimately, these efforts have the potential to enhance the health and well-being of individuals, families, and communities by alleviating the burden of CVD and its related complications.
However, our study has some limitations. First, the data used in the study were exclusively derived from European populations, and the findings primarily explained the impact of MD on CVD risk in European populations. They did not apply to Asian, African, and Latino populations. Second, the study focused on the overall effect of MD on CVD and did not clarify the effect of MD on specific diseases such as coronary heart disease, hypertension, and heart failure. Third, this study only explained the effects of depression, ANX, and ASD on CVD but not reflect the specific effects of different levels of depression, ANX, and ASD on CVD. Fourth, although the sensitivity analysis suggested that the results were robust, the influence of study design, investigative population, and selection of specific genetic variants cannot be excluded. These factors may have contributed to the MR analysis not identifying an association between AN, ADHD, BD, OCD, SCZ, and CVD risk.
Given these limitations, we expect future studies to continue to improve: First, research centers should be established in Asia, Africa, and Latin America to explore the causal relationship between MD and CVD across different ethnicities. This will provide a more comprehensive data source for MR studies. Second, a more extensive stratified cohort study should be conducted to control for the variables of interest and explore the specific effects of different types and degrees of MD on the risk of CVD. Third, basic research is needed to explore the potential mechanisms by which depression, ANX, and ASD increase the risk of CVD in different populations.
5 Conclusion
This MR analysis showed that depression, ANX, and ASD were associated with an increased risk of CVD, whereas there was no causal relationship of AN, ADHD, BD, OCD, and SCZ with CVD. It implies that the active prevention and treatment of depression, ANX, and ASD may help reduce CVD risk. More studies are needed to explore the causal relationship and mechanism between MD and CVD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
YY: Conceptualization, Data curation, Supervision, Writing – original draft. XY: Methodology, Supervision, Writing – original draft. JW: Data curation, Methodology, Writing – original draft. GH: Data curation, Formal Analysis, Writing – original draft. SB: Formal Analysis, Methodology, Writing – original draft. RY: Formal Analysis, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by The Key Support Project of the Regional Innovation and Development Joint Fund of the National Natural Science Foundation of China [U21A20411].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1329463/full#supplementary-material
Abbreviations
ADHD, attention-deficit/hyperactivity disorder; AN, anorexia nervosa; ANX, anxiety disorder; ASD, autism spectrum disorder; BD, bipolar disorder; CVD, cardiovascular disease; GAD, generalized anxiety disorder; GWAS, genome-wide association studies; IVW, inverse variance weighted; MD, mental disorders; MR, Mendelian randomization; MR-PRESSO, MR-pleiotropy residual sum and outlier method; OCD, obsessive-compulsive disorder; SCZ, schizophrenia; SNP, single nucleotide polymorphism.
References
1. Martins B, Ferreira D, Neto C, Abelha A, Machado J. Data mining for cardiovascular disease prediction. J Med Syst. (2021) 45:6. doi: 10.1007/s10916-020-01682-8
2. Liu MN, Luo G, Gao WJ, Yang SJ, Zhou H. miR-29 family: a potential therapeutic target for cardiovascular disease. Pharmacol Res. (2021) 166:105510. doi: 10.1016/j.phrs.2021.105510
3. O’Sullivan JW, Raghavan S, Marquez-Luna C, Luzum JA, Damrauer SM, Ashley EA, et al. Polygenic risk scores for cardiovascular disease: a scientific statement from the American heart association. Circulation. (2022) 146:e93–e118. doi: 10.1161/CIR.0000000000001077
4. Kralj V, Brkić Biloš I. Morbidity and mortality from cardiovascular diseases. Cardiol Croat. (2013) 8:373–8. doi: 10.15836/ccar.2013.373
5. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
6. Oseran A, Wasfy JH. Cardiovascular disease prevention: the role of policy interventions. Curr Treat Options Cardiovasc Med. (2017) 19:43. doi: 10.1007/s11936-017-0545-3
7. Puska P, Jaini P. The North Karelia project: prevention of cardiovascular disease in Finland through population-based lifestyle interventions. Am J Lifestyle Med. (2020) 14:495–9. doi: 10.1177/1559827620910981
8. Balakumar P, Maung-U K, Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol Res. (2016) 113:600–9. doi: 10.1016/j.phrs.2016.09.040
9. De Backer G. Epidemiology and prevention of cardiovascular disease: quo vadis? Eur J Prev Cardiol. (2017) 24:768–72. doi: 10.1177/2047487317691875
10. Shen Q, Mikkelsen DH, Luitva LB, Song H, Kasela S, Aspelund T, et al. Psychiatric disorders and subsequent risk of cardiovascular disease: a longitudinal matched cohort study across three countries. EClinicalMedicine. (2023) 61:102063. doi: 10.1016/j.eclinm.2023.102063
11. Sowden GL, Huffman JC. The impact of mental illness on cardiac outcomes: a review for the cardiologist. Int J Cardiol. (2009) 132:30–7. doi: 10.1016/j.ijcard.2008.10.002
12. Vigo D, Thornicroft G, Atun R. Estimating the true global burden of mental illness. Lancet Psychiatry. (2016) 3:171–8. doi: 10.1016/S2215-0366(15)00505-2
13. Ni P, Ma Y, Chung S. Mitochondrial dysfunction in psychiatric disorders. Schizophr Res. (2022) S0920-9964(22):00333–4. doi: 10.1016/j.schres.2022.08.027
14. Baker M, Hong SI, Kang S, Choi DS. Rodent models for psychiatric disorders: problems and promises. Lab Anim Res. (2020) 36:9. doi: 10.1186/s42826-020-00039-z
15. Vyas A, Desai R, Patel V, Bansal P, Jain A, Gupta T, et al. Rising burden of cardiovascular disease risk factors and acute cardiac events in young adults with comorbid depression: a comparison nationwide US cohorts hospitalized 10-years apart. Curr Probl Cardiol. (2023) 48:101755. doi: 10.1016/j.cpcardiol.2023.101755
16. Yoo TK, Han KD, Rhee EJ, Lee WY. Impact of mental disorders on the risk of heart failure among Korean patients with diabetes: a cohort study. Cardiovasc Diabetol. (2023) 22:115. doi: 10.1186/s12933-023-01809-4
17. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. Jama. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
18. 1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. (2015) 526:68–74. doi: 10.1038/nature15393
19. Gao J, Hou T. Cardiovascular disease treatment using traditional Chinese medicine: mitochondria as the achilles’ heel. Biomed Pharmacother. (2023) 164:114999. doi: 10.1016/j.biopha.2023.114999
20. Soppert J, Lehrke M, Marx N, Jankowski J, Noels H. Lipoproteins and lipids in cardiovascular disease: from mechanistic insights to therapeutic targeting. Adv Drug Deliv Rev. (2020) 159:4–33. doi: 10.1016/j.addr.2020.07.019
21. Rødevand L, Tesli M, Andreassen OA. Cardiovascular disease risk in people with severe mental disorders: an update and call for action. Curr Opin Psychiatry. (2022) 35:277–84. doi: 10.1097/YCO.0000000000000797
22. Piña IL, Di Palo KE, Ventura HO. Psychopharmacology and cardiovascular disease. J Am Coll Cardiol. (2018) 71:2346–59. doi: 10.1016/j.jacc.2018.03.458
23. Darabi Z, Najafi F, Safari-Faramani R, Salimi Y. Controlled direct effect of psychiatric disorders on cardiovascular disease: evidence from a large kurdish cohort. BMC Cardiovasc Disord. (2020) 20:501. doi: 10.1186/s12872-020-01794-6
24. Peng B, Meng H, Guo L, Zhu J, Kong B, Qu Z, et al. Anxiety disorder and cardiovascular disease: a two-sample Mendelian randomization study. ESC Heart Fail. (2024) 11(2):1174–81. doi: 10.1002/ehf2.14676
25. Sun X, Chen L, Wang Z, Lu Y, Chen M, He Y, et al. Association of autism Spectrum disorder, neuroticism, and subjective well-being with cardiovascular diseases: a two-sample Mendelian randomization study. Front Cardiovasc Med. (2021) 8:676030. doi: 10.3389/fcvm.2021.676030
26. Lu Y, Wang Z, Georgakis MK, Lin H, Zheng L. Genetic liability to depression and risk of coronary artery disease, myocardial infarction, and other cardiovascular outcomes. J Am Heart Assoc. (2021) 10:e017986. doi: 10.1161/JAHA.120.017986
27. Veeneman RR, Vermeulen JM, Abdellaoui A, Sanderson E, Wootton RE, Tadros R, et al. Exploring the relationship between schizophrenia and cardiovascular disease: a genetic correlation and multivariable Mendelian randomization study. Schizophr Bull. (2022) 48:463–73. doi: 10.1093/schbul/sbab132
28. Jin B, Zhang H, Song F, Wu G, Yang H. Interaction of sleep duration and depression on cardiovascular disease: a retrospective cohort study. BMC Public Health. (2022) 22:1752. doi: 10.1186/s12889-022-14143-3
29. Zhang Y, Li X, Chan VKY, Luo H, Chan SSM, Wong GHY, et al. Depression duration and risk of incident cardiovascular disease: a population-based six-year cohort study. J Affect Disord. (2022) 305:188–95. doi: 10.1016/j.jad.2022.03.005
30. Staff TE, O’Leary M, Fretts AM. Depression, physical activity, and incident cardiovascular disease among American Indians: the strong heart family study. Psychiatry Res Commun. (2023) 3:100125. doi: 10.1016/j.psycom.2023.100125
31. Krittanawong C, Maitra NS, Qadeer YK, Wang Z, Fogg S, Storch EA, et al. Association of depression and cardiovascular disease. Am J Med. (2023) 136:881–95. doi: 10.1016/j.amjmed.2023.04.036
32. Inoue K, Beekley J, Goto A, Jeon CY, Ritz BR. Depression and cardiovascular disease events among patients with type 2 diabetes: a systematic review and meta-analysis with bias analysis. J Diabetes Complications. (2020) 34:107710. doi: 10.1016/j.jdiacomp.2020.107710
33. Khandaker GM, Zuber V, Rees JMB, Carvalho L, Mason AM, Foley CN, et al. Shared mechanisms between coronary heart disease and depression: findings from a large UK general population-based cohort. Mol Psychiatry. (2020) 25:1477–86. doi: 10.1038/s41380-019-0395-3
34. Zuzarte P, Duong A, Figueira ML, Costa-Vitali A, Scola G. Current therapeutic approaches for targeting inflammation in depression and cardiovascular disease. Curr Drug Metab. (2018) 19:674–87. doi: 10.2174/1389200219666180305143501
35. Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci USA. (2012) 109:5995–9. doi: 10.1073/pnas.1118355109
36. Shao M, Lin X, Jiang D, Tian H, Xu Y, Wang L, et al. Depression and cardiovascular disease: shared molecular mechanisms and clinical implications. Psychiatry Res. (2020) 285:112802. doi: 10.1016/j.psychres.2020.112802
37. Sher L. Type D personality: the heart, stress, and cortisol. QJM. (2005) 98:323–9. doi: 10.1093/qjmed/hci064
38. Roest AM, Martens EJ, de Jonge P, Denollet J. Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol. (2010) 56:38–46. doi: 10.1016/j.jacc.2010.03.034
39. Kyrou I, Kollia N, Panagiotakos D, Georgousopoulou E, Chrysohoou C, Tsigos C, et al. Association of depression and anxiety status with 10-year cardiovascular disease incidence among apparently healthy Greek adults: the ATTICA study. Eur J Prev Cardiol. (2017) 24:145–52. doi: 10.1177/2047487316670918
40. Karlsen HR, Saksvik-Lehouillier I, Stone KL, Schernhammer E, Yaffe K, Langvik E. Anxiety as a risk factor for cardiovascular disease independent of depression: a prospective examination of community-dwelling men (the MrOS study). Psychol Health. (2021) 36:148–63. doi: 10.1080/08870446.2020.1779273
41. Huang KL, Su TP, Chen TJ, Chou YH, Bai YM. Comorbidity of cardiovascular diseases with mood and anxiety disorder: a population based 4-year study. Psychiatry Clin Neurosci. (2009) 63:401–9. doi: 10.1111/j.1440-1819.2009.01974.x
42. Langvik E, Nordahl HM. Anhedonic depression, history of depression, and anxiety as gender-specific risk factors of myocardial infarction in healthy men and women: the HUNT study. Health Psychol Open. (2014) 1:2055102914557658. doi: 10.1177/2055102914557658
43. Parker G, Hyett M, Hadzi-Pavlovic D, Brotchie H, Walsh W. GAD is good? Generalized anxiety disorder predicts a superior five-year outcome following an acute coronary syndrome. Psychiatry Res. (2011) 188:383–9. doi: 10.1016/j.psychres.2011.05.018
44. Batelaan NM, Seldenrijk A, Bot M, van Balkom AJLM, Penninx BWJH. Anxiety and new onset of cardiovascular disease: critical review and meta-analysis. Br J Psychiatry. (2016) 208:223–31. doi: 10.1192/bjp.bp.114.156554
45. Mueller K, Thiel F, Beutner F, Teren A, Frisch S, Ballarini T, et al. Brain damage with heart failure: cardiac biomarker alterations and gray matter decline. Circ Res. (2020) 126:750–64. doi: 10.1161/CIRCRESAHA.119.315813
46. Chen X, Xu L, Li Z. Autonomic neural circuit and intervention for comorbidity anxiety and cardiovascular disease. Front Physiol. (2022) 13:852891. doi: 10.3389/fphys.2022.852891
47. de la Cruz F, Schumann A, Köhler S, Reichenbach JR, Wagner G, Bär K-J. The relationship between heart rate and functional connectivity of brain regions involved in autonomic control. Neuroimage. (2019) 196:318–28. doi: 10.1016/j.neuroimage.2019.04.014
48. Schumann A, de la Cruz F, Köhler S, Brotte L, Bär K-J. The influence of heart rate variability biofeedback on cardiac regulation and functional brain connectivity. Front Neurosci. (2021) 15:691988. doi: 10.3389/fnins.2021.691988
49. Gorman JM, Sloan RP. Heart rate variability in depressive and anxiety disorders. Am Heart J. (2000) 140:77–83. doi: 10.1067/mhj.2000.109981
50. Cohen BE, Edmondson D, Kronish IM. State of the art review: depression, stress, anxiety, and cardiovascular disease. Am J Hypertens. (2015) 28:1295–302. doi: 10.1093/ajh/hpv047
51. Tayefi M, Shafiee M, Kazemi-Bajestani SMR, Esmaeili H, Darroudi S, Khakpouri S, et al. Depression and anxiety both associate with serum level of hs-CRP: a gender-stratified analysis in a population-based study. Psychoneuroendocrinology. (2017) 81:63–9. doi: 10.1016/j.psyneuen.2017.02.035
52. Pitsavos C, Panagiotakos DB, Papageorgiou C, Tsetsekou E, Soldatos C, Stefanadis C. Anxiety in relation to inflammation and coagulation markers, among healthy adults: the ATTICA study. Atherosclerosis. (2006) 185:320–6. doi: 10.1016/j.atherosclerosis.2005.06.001
53. Einvik G, Hrubos-Strøm H, Randby A, Nordhus IH, Somers VK, Omland T, et al. Major depressive disorder, anxiety disorders, and cardiac biomarkers in subjects at high risk of obstructive sleep apnea. Psychosom Med. (2011) 73:378–84. doi: 10.1097/PSY.0b013e318219e64e
54. Hohensinner PJ, Niessner A, Huber K, Weyand CM, Wojta J. Inflammation and cardiac outcome. Curr Opin Infect Dis. (2011) 24:259–64. doi: 10.1097/QCO.0b013e328344f50f
55. Heffernan KS, Columna L, Russo N, Myers BA, Ashby CE, Norris ML, et al. Brief report: physical activity, body mass Index and arterial stiffness in children with autism spectrum disorder: preliminary findings. J Autism Dev Disord. (2018) 48:625–31. doi: 10.1007/s10803-017-3358-z
56. Altun H, Kurutaş EB, Şahin N, Güngör O, Fındıklı E. The levels of vitamin D, vitamin D receptor, homocysteine and Complex B vitamin in children with autism Spectrum disorders. Clin Psychopharmacol Neurosci. (2018) 16:383–90. doi: 10.9758/cpn.2018.16.4.383
57. Metwalley KA, Farghaly HS, Abdelhamid A. Homocysteine level in children with classic congenital adrenal hyperplasia: relationship to carotid intimal wall thickness and left ventricular function. Horm Res Paediatr. (2018) 90:228–35. doi: 10.1159/000492900
58. El-Amrousy D, Hassan S, Hodeib H. Prognostic value of homocysteine and highly sensitive cardiac troponin T in children with acute heart failure. J Saudi Heart Assoc. (2018) 30:198–204. doi: 10.1016/j.jsha.2017.11.007
59. Imre Yetkin D, Sizer E, Tolu Gökhaner Y, Büyükdemirci E, Atlı A. Assessment of sonographic carotid parameters in autism spectrum disorder: a comparative case control study. Dev Neurorehabil. (2023) 26:172–9. doi: 10.1080/17518423.2023.2181417
60. Brown RB. Dysregulated phosphate metabolism in autism spectrum disorder: associations and insights for future research. Expert Rev Mol Med. (2023) 25:e20. doi: 10.1017/erm.2023.15
61. Giovinazzo S, Sukkar SG, Rosa GM, Zappi A, Bezante GP, Balbi M, et al. Anorexia nervosa and heart disease: a systematic review. Eat Weight Disord. (2019) 24:199–207. doi: 10.1007/s40519-018-0567-1
62. Thapar AK, Riglin L, Blakey R, Collishaw S, Davey Smith G, Stergiakouli E, et al. Childhood attention-deficit hyperactivity disorder problems and mid-life cardiovascular risk: prospective population cohort study. Br J Psychiatry. (2023) 223:472–7. doi: 10.1192/bjp.2023.90
63. Slomka JM, Piette JD, Post EP, Krein SL, Lai Z, Goodrich DE, et al. Mood disorder symptoms and elevated cardiovascular disease risk in patients with bipolar disorder. J Affect Disord. (2012) 138:405–8. doi: 10.1016/j.jad.2012.01.005
64. Isomura K, Sidorchuk A, Brander G, Jernberg T, Rück A, Song H, et al. Risk of specific cardiovascular diseases in obsessive-compulsive disorder. J Psychiatr Res. (2021) 135:189–96. doi: 10.1016/j.jpsychires.2020.12.066
65. Sandhya M, Mittal S, Kathrotia R, Rawat VS, Singh Y, Srikant S, et al. Cardiovascular autonomic function tests in patients of obsessive-compulsive disorder: a cross-sectional study. Indian J Psychol Med. (2022) 44:30–6. doi: 10.1177/02537176211042805
66. Kalla A, Krishnamoorthy P, Gopalakrishnan A, Garg J, Patel NC, Figueredo VM. Gender and age differences in cardiovascular complications in anorexia nervosa patients. Int J Cardiol. (2017) 227:55–7. doi: 10.1016/j.ijcard.2016.11.209
67. Kittel-Schneider S, Reif A. [Adult attention deficit hyperactivity disorder and comorbidity: new findings on epidemiological and genetic factors]. Nervenarzt. (2020) 91:575–82. doi: 10.1007/s00115-020-00900-5
68. Hennissen L, Bakker MJ, Banaschewski T, Carucci S, Coghill D, Danckaerts M, et al. Cardiovascular effects of stimulant and non-stimulant medication for children and adolescents with ADHD: a systematic review and meta-analysis of trials of methylphenidate, amphetamines and atomoxetine. CNS Drugs. (2017) 31:199–215. doi: 10.1007/s40263-017-0410-7
Keywords: mental disorders, cardiovascular disease, risk factor, Mendelian randomization, depression, anxiety disorder, autism spectrum disorder
Citation: Yu Y, Yang X, Wu J, Hu G, Bai S and Yu R (2024) A Mendelian randomization study of the effect of mental disorders on cardiovascular disease. Front. Cardiovasc. Med. 11:1329463. doi: 10.3389/fcvm.2024.1329463
Received: 1 November 2023; Accepted: 16 May 2024;
Published: 3 June 2024.
Edited by:
Dexter Canoy, Newcastle University, United KingdomReviewed by:
Ilan Merdler, MedStar Washington Hospital Center, United StatesNouran Sorour, University of Massachusetts Medical School, United States
Qingshan Geng, Guangdong Provincial People’s Hospital, China
© 2024 Yu, Yang, Wu, Hu, Bai and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Yu, eXVyb25nMTk2OTA1QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Yunfeng Yu
Yunfeng Yu Xinyu Yang
Xinyu Yang Jingyi Wu
Jingyi Wu Gang Hu
Gang Hu Siyang Bai
Siyang Bai Rong Yu1,2*
Rong Yu1,2*