
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 14 March 2024
Sec. Cardiovascular Epidemiology and Prevention
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1328087
Background: Many studies have shown that omega-3 fatty acids may play critical roles in cardiovascular diseases. Myocardial infarction (MI) typically results from a thrombotic occlusion of a coronary artery leading to myocardial ischemia. Thus, this study aims to examine the association between omega-3 fatty acids and MI.
Methods: A two-sample Mendelian randomization study was used to explore the causal relationship between circulating omega-3 fatty acids and the risk of MI performed by MR-Egger regression, inverse-variance weighted (IVW), weighted median, and weighted mode.
Results: Five single-nucleotide polymorphisms strongly related to circulating omega-3 fatty acids were selected as instrumental variables from a published genome-wide association study (GWAS) meta-analysis including 13,544 subjects. We extracted summary data for the risk of MI from another GWAS meta-analysis including 171,875 individuals (43,676 cases and 128,199 controls). The genetically predicted lower circulating omega-3 increased the risk of myocardial infarction showed by the results of IVW [odds ratio (OR) = 1.224, 95% CI = 1.045–1.433, P = 0.012], weighted median method (OR = 1.171, 95% CI = 1.042–1.315, P = 0.008), and weighted mode (OR = 1.149, 95% CI = 1.002–1.317, P = 0.117), although the result of MR-Egger was not significant (OR = 0.950, 95% CI = 0.513–1.760, P = 0.880) with a wider confidence interval.
Conclusion: The findings from our Mendelian randomization analysis suggest that the association between omega-3 fatty acid levels and MI is likely causal.
Omega-3 (n-3) fatty acids are a family of polyunsaturated fatty acids (PUFA) that cannot be produced in mammals. The main members of omega-3 fatty acids are eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which must be obtained from the ocean, and linolenic acid, which is obtained from vegetable oils. Omega-3 fatty acids are good for the secondary prevention of coronary artery disease and stroke. The most functionally important n-3 PUFA, EPA and DHA, exist in fish and other seafood (1).
Many long-term prospective cohort studies and meta-analyses have provided supportive evidence that higher intakes of EPA and DHA reduce the risk of developing cardiovascular disease (CVD) in the general population (2, 3). Zhang et al. found the significant association between higher n-3 PUFA intake and decreased total mortality (4). A total of 54,230 men and 30,882 women died during the 6.07 million person-years of follow-up. Compared to the lowest quintiles of fish intake, males and females with the highest quintiles of fish intake had 10% (6%–15%) and 10% (3%–17%) lower CVD mortality, respectively (4).
Acute myocardial infarction (AMI) is a common cardiovascular event that results from a reduction or interruption of blood flow to a portion of the heart, leading to necrosis of heart muscle. Myocardial infarction (MI) leads to a large number of deaths and disabilities and carries a significant socio-economic burden (5). AMI can result in the development of heart failure although the ischemic episode is often not fatal (6). Many studies have studied the relationships between MI and omega-3 fatty acids. Omega-3 fatty acids are believed to protect against MI through potential multiple mechanisms. They reduce triglyceride levels (7), diminishing the risk of atherosclerosis, and exhibit anti-inflammatory properties, lowering the inflammatory response associated with cardiovascular diseases (8). In addition, these acids enhance heart rate variability and decrease platelet aggregation, reducing thrombosis and further mitigating the risk of MI (3). The synergy of these mechanisms underscores the significant role of omega-3 in cardiovascular prevention. However, many trial studies were short in duration and concentrated on patients who had preexisting coronary heart disease (CHD) or high risk for CHD (9), and the background dietary intake of subjects in these trials was unknown, which could bias the results. Therefore, the potential role of omega-3 fatty acids on MI need to be further explored.
Recently, the Mendelian randomization (MR) method, which regards genetic variation as instrumental variables (IVs) replaced the exposure, has been considered an effective measure to explore the causal effect of the exposure on the outcome (10). Moreover, two-sample MR (TSMR) analysis extracted the summary data of the exposure and the outcome from separate samples, elevating the effectiveness (11).
Exploring the factors affecting MI can not only guide the treatment decision but also provide valuable prognostic information. In this research, we extracted the summary data from published large genetic studies to detect whether there was a causal relationship between omega-3 fatty acids and the risk for MI by TSMR analysis.
We employed the MR design to detect the causal effect of circulating omega-3 fatty acids on the risk of myocardial infarction. Remarkably, the MR study should satisfy the following hypotheses (12): (a) IVs should be strongly related to the exposure, and the F statistics will be used to evaluate the association strength; (b) no horizontal pleiotropy exists between IVs and outcome, that the intercept of MR-Egger regression should be non-significant; (c) IVs should not be associated with confounders.
In our study, we regarded single-nucleotide polymorphisms (SNPs) as IVs. SNPs strongly related to circulating omega-3 fatty acids (P < 5 × 10−8) were extracted from a published genome-wide association study (GWAS) meta-analysis in 2016 containing 13,544 subjects of European ancestry (13). This comprehensive meta-analysis encompassed 14 studies, starting from 1972 and spanning across regions including Finland, Helsinki, the Netherlands, and Germany. To escape the bias caused by strong linkage disequilibrium, we stipulated the inclusion criteria r2 < 0.001. Meanwhile, we excluded SNPs with palindrome. For the risk of MI, relevant data were abstracted from another GWAS meta-analysis published in 2015 including 171,875 individuals (43,676 cases and 128,199 controls) of European ancestry, incorporating findings from 48 studies (14). Given that EPA and DHA are the primary constituents of omega-3, we investigated the effects of the included SNPs on EPA and DHA levels (15, 16).
We employed three MR methods to estimate the casual association between circulating omega-3 fatty acids and myocardial infarction risk: MR-Egger regression, inverse-variance weighted (IVW), and weighted median. The results were presented as odds ratio (OR) with 95% confidence intervals (CIs). The IVW method requests total included IVs to be valid and gives the most accurate estimation (17). However, the weighted median and weighted mode method only requires half of included IVs to be valid (18). The MR-Egger regression method does not force the regression equation to go through the origin and gives a relatively wider CI. Moreover, the intercept of the MR-Egger regression equation was performed to evaluate the existence of the horizontal pleiotropy pathway between IVs and the outcome. If the intercept was not significant, we considered there was no horizontal pleiotropy pathway (19). Subsequently, to assess the stability of our results, we removed SNPs in sequence and employed the IVW method to estimate the effects of the remaining IVs. Then, Cochran's Q statistics was used to estimate the heterogeneity among included SNPs (20). To minimize confounding effects, we used the PhenoScanner website to exclude SNPs that were associated with established risk factors for myocardial infarction (such as hypertension, diabetes mellitus, dyslipidemia, kidney dysfunction, and obesity) from our instrumental variables. We defined two-sided P < 0.05 as statistical significance. All statistical analyses were performed by the TwoSampleMR package in the R software (version 3.6.2).
We selected six SNPs that are strongly associated with circulating omega-3 fatty acids (P < 5 × 10−8). Then, we excluded rs145717049, which was not found in the GWAS outcome. Five SNPs (rs11604424, rs1260326, rs143988316, rs174546, and rs1077835), strongly related to circulating omega-3, were finally included in our study as IVs and are detailed in Table 1 and Supplementary Tables S1, S2. Included SNPs explained 1.27% of the variance in circulating omega-3 fatty acids. We ignored the bias caused by weak IVs, for the F statistics far more than 10 (F = 34.9). The results of IVW (OR = 1.224, 95% CI = 1.045–1.433, P = 0.012), weighted median method (OR = 1.171, 95% CI = 1.042–1.315, P = 0.008), and weighted mode (OR = 1.149, 95% CI = 1.002–1.317, P = 0.117) showed that genetically predicted lower circulating omega-3 increased the risk of myocardial infarction, although the result of MR-Egger was not significant (OR = 0.950, 95% CI = 0.513–1.760, P = 0.880) exhibiting a broader confidence interval (Figure 1). The sensitivity analysis performed by the leave-one-out method confirmed the stability of our results (Figure 2). In addition, the intercept of the MR-Egger regression equation suggested an absence of horizontal pleiotropy between the IVs and the outcome, implying that the IVs are likely to influence the risk of myocardial infarction exclusively through the levels of circulating omega-3 (intercept = 0.031, SE = 0.037, P = 0.464) (Figure 3). The funnel plot showed significant heterogeneity among included SNPs (Cochran's Q = 12.211, P = 0.016) (Figure 4).
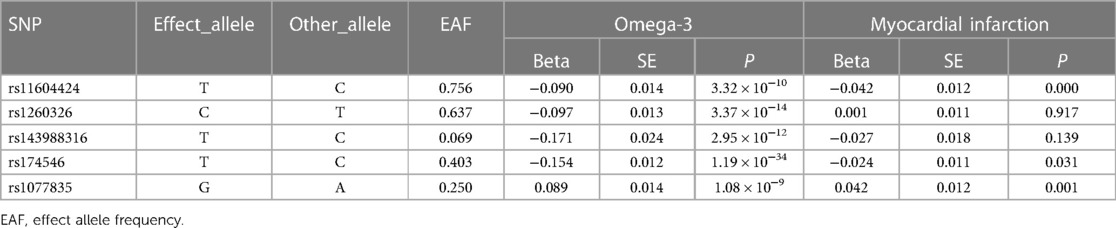
Table 1. Genome-wide significant variants of circulating omega-3 fatty acids and their association with myocardial infarction.
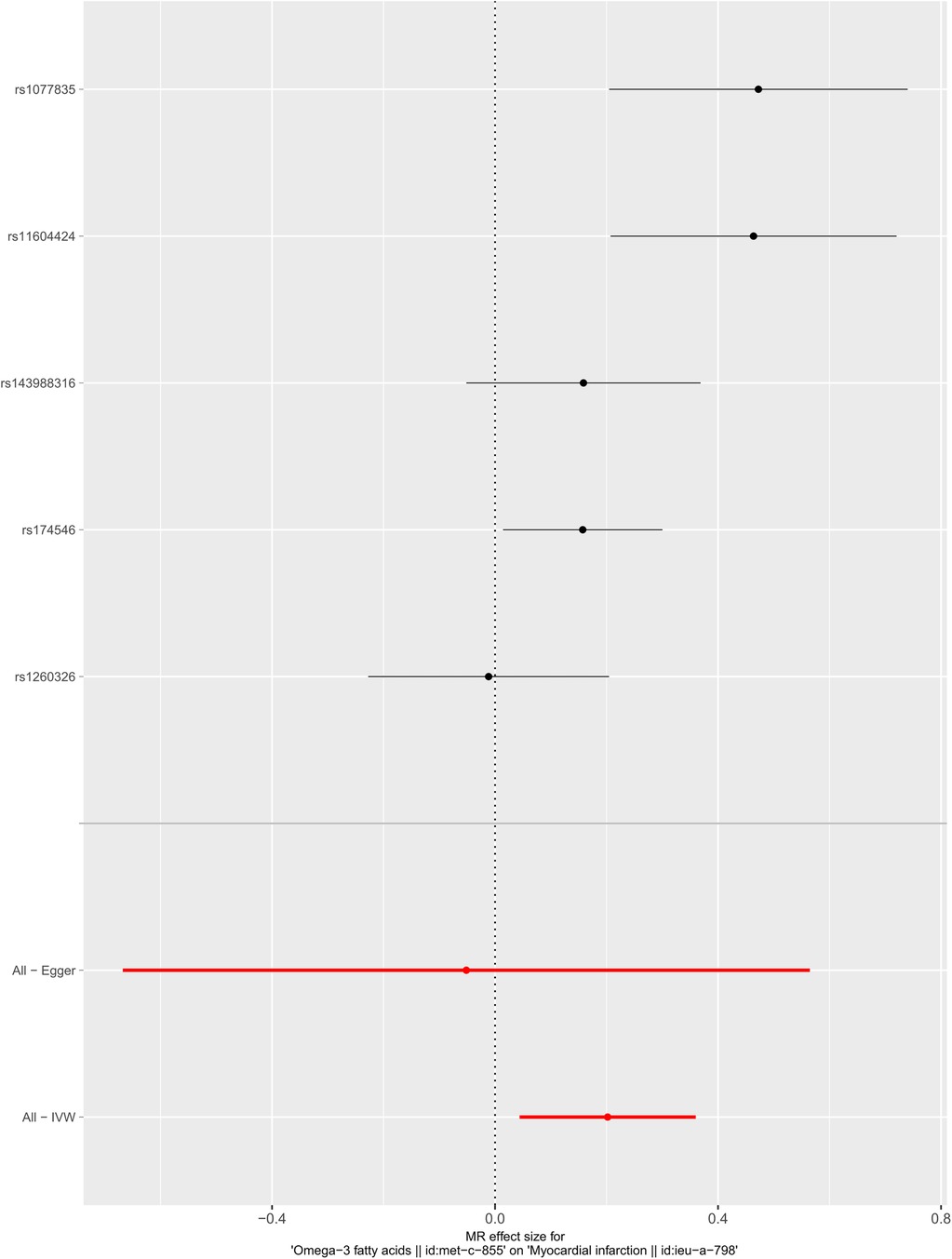
Figure 1. Forest plot of the casual association between circulating omega-3 fatty acids and the risk of myocardial infarction.
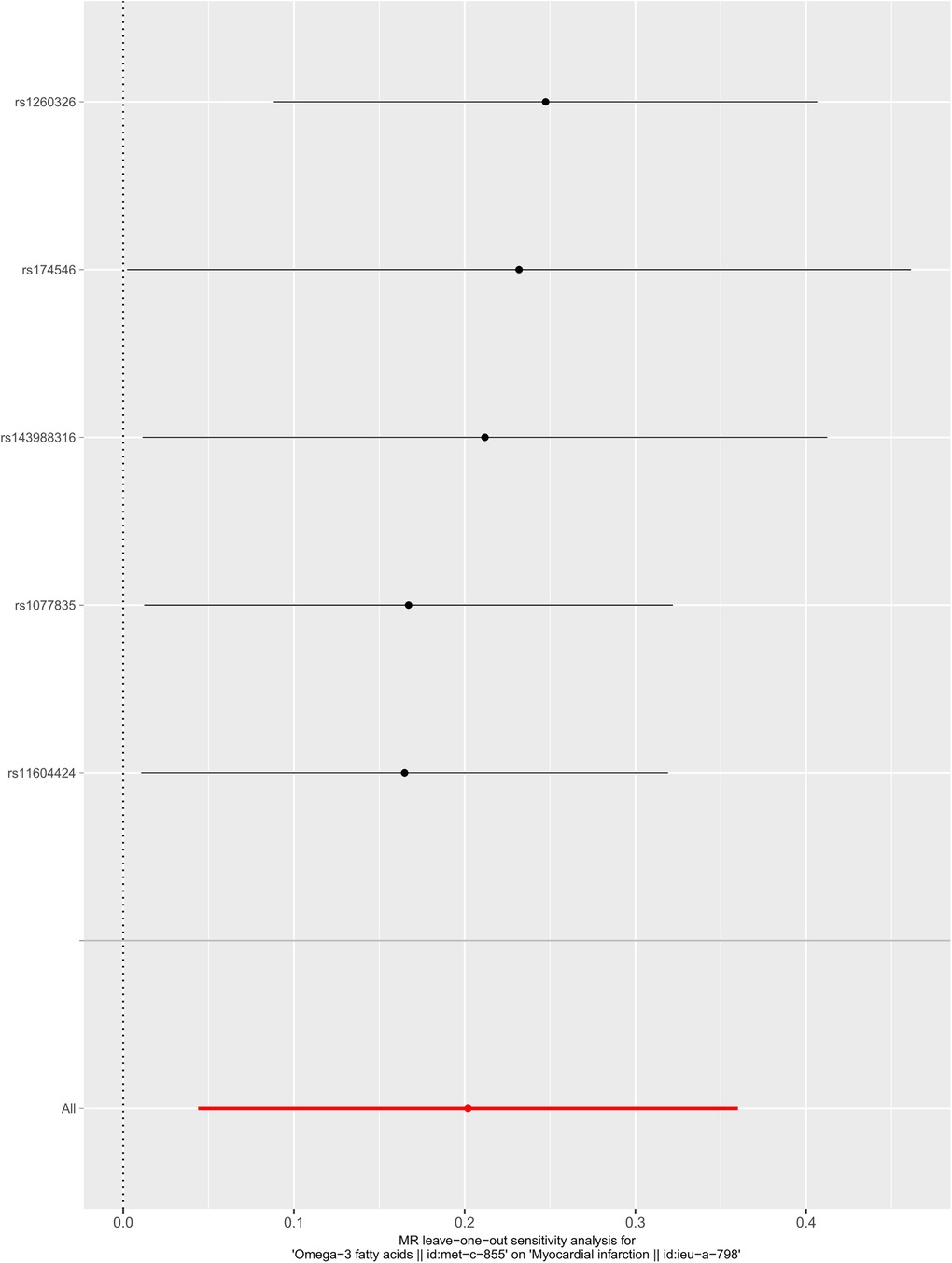
Figure 2. Sensitivity analysis of the casual association between circulating omega-3 fatty acids and the risk of myocardial infarction.
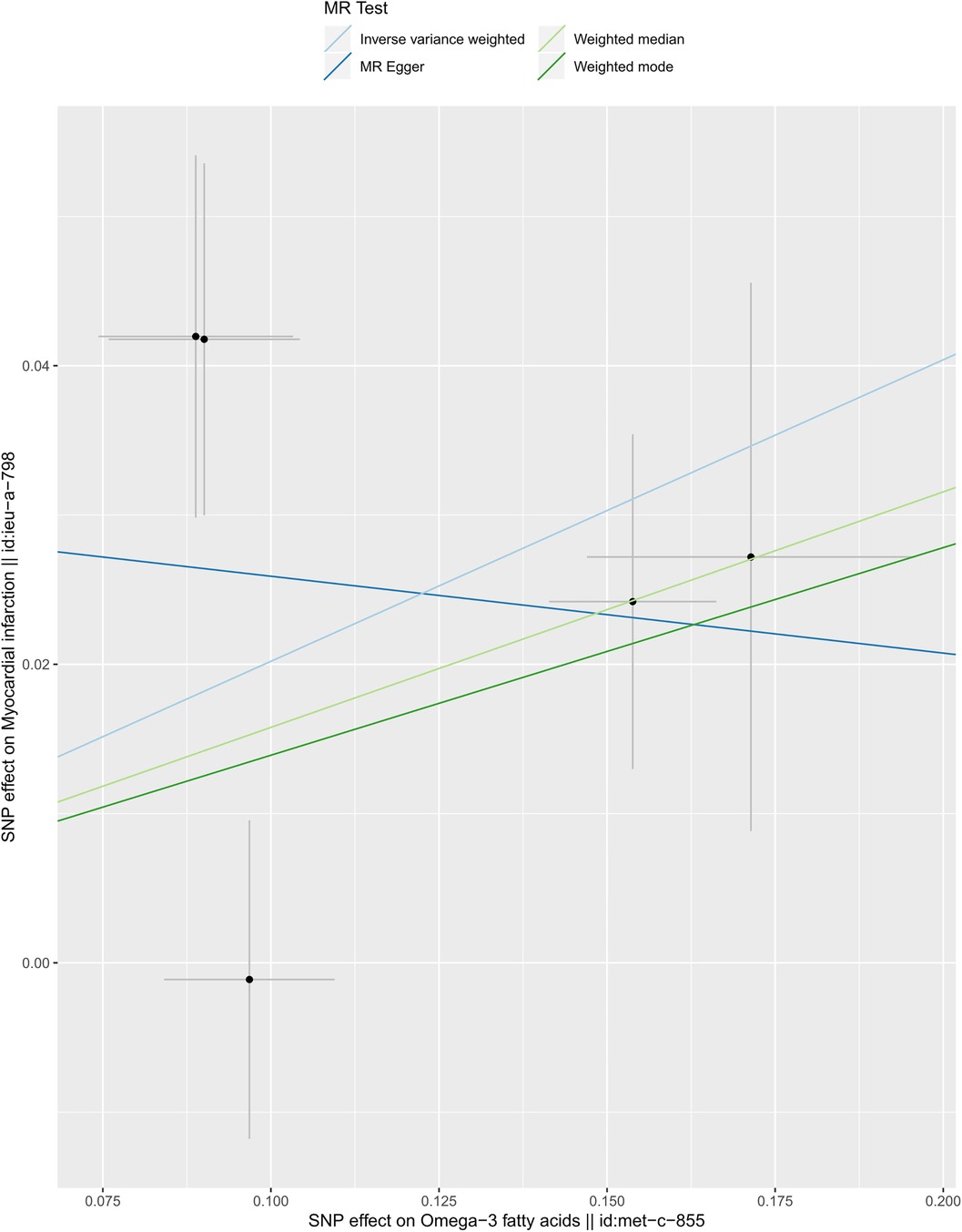
Figure 3. Scatter plot of the casual association between circulating omega-3 fatty acids and the risk of myocardial infarction.
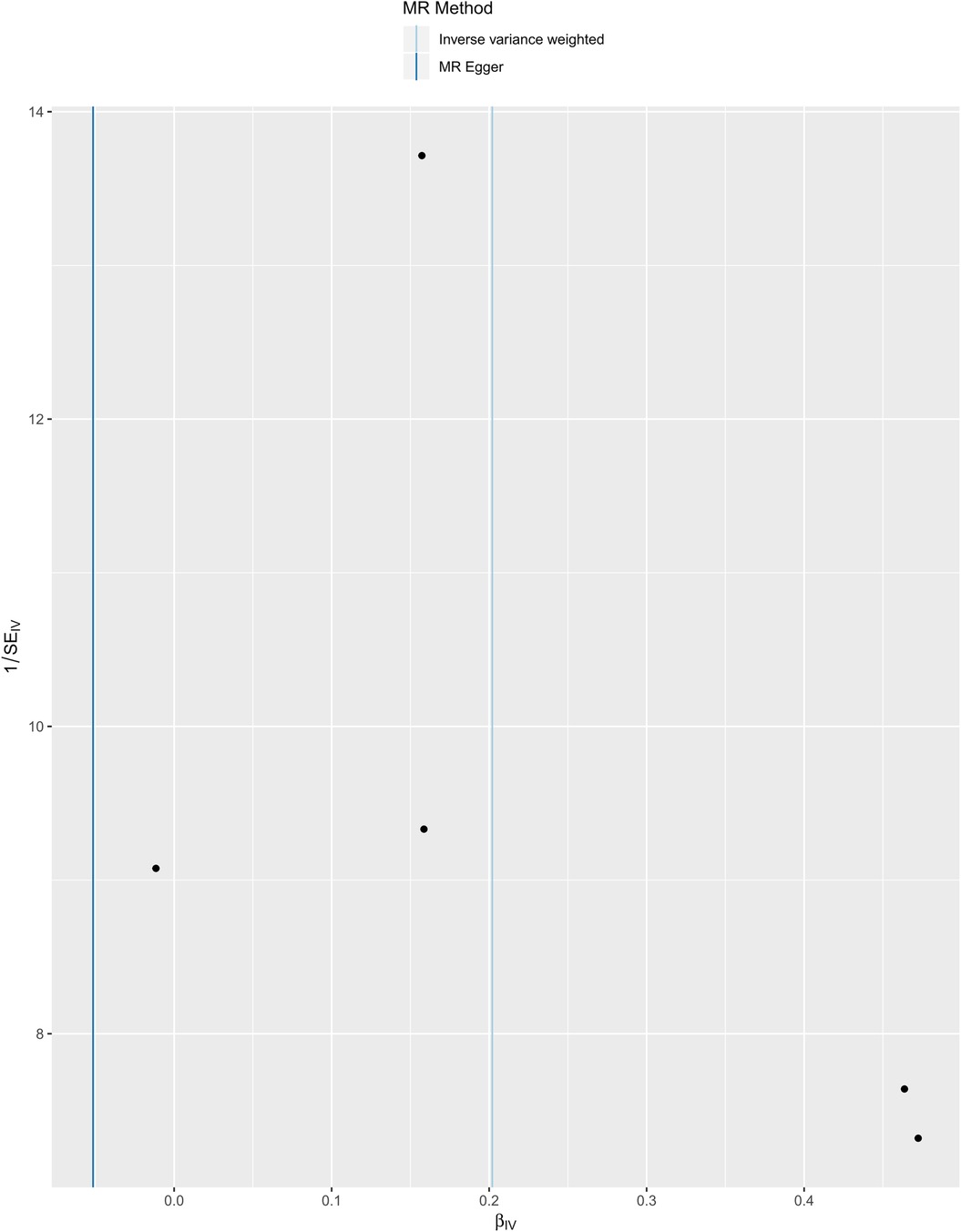
Figure 4. Funnel plot of the casual association between circulating omega-3 fatty acids and the risk of myocardial infarction.
The current study performed a Mendelian randomization study to explore the causal relationship between circulating omega-3 and the risk of MI and demonstrated that lower circulating omega-3 increased the risk of MI. However, several studies have showed inconsistent results (21, 22). Del et al. analyzed the data from a global consortium of 19 studies comprised of 16 countries, 45,637 unique individuals, 8,000 first CHD events, and 7,157 non-fatal MI events (21). The results showed that the omega-3 biomarkers DHA, plant-derived α-linolenic acid (ALA), and docosapentaenoic acid (DPA) were related to a lower risk of fatal CHD (21). However, no significant relationships were observed between non-fatal MI and omega-3 biomarkers (21). Another study, which included 68,680 patients and reported 7,044 deaths, 1,150 sudden deaths, 3,993 cardiac deaths, and 1,837 MI, also showed that omega-3 fatty acid supplementation was not related to a lower risk of all-cause mortality and MI (22). Two recent studies also reached a similar result that supplementing omega-3 fatty acids did not reduce the incidence of major cardiovascular events or cancer (23, 24). However, in these studies, the heterogeneity of the subjects and the varying levels of dietary background intake may lead to negative results.
There is, of course, a large body of evidence supporting our findings that omega-3 fatty acids have cardioprotective effects (3, 25–27). The GISSI-Prevenzione trial in 1999 showed that omega-3 decreased the risk of death and non-fatal acute MI in patients with recent MI (<3 months) (28). A meta-analysis of 15,806 CHD patients found that omega-3 fatty acids reduce the risk of non-fatal and fatal MI by 20% and 30%, respectively (29). A study published in 2007 also showed that, in Japanese hypercholesterolaemic patients, EPA may be a promising treatment for preventing major coronary events, especially non-fatal coronary events (30). A meta-analysis performed by Casula et al. included 15,348 patients with a history of CVD and demonstrated that supplementation of a high-dose omega-3 fatty acid had significant protective effects on the onset of MI, sudden death, and cardiac death (31). In 2018, the American Heart Association (AHA) published guidance in support of the dietary intake of fish in primary prevention (32). A multi-center randomized trial, published in 2019, certified that the risk of ischemic events was decreased in the icosapent ethyl group than in the placebo group, even when triglyceride levels were high in the study group (1). A meta-analysis, published in 2019, combined data from 13 RCTs and showed that supplementation of omega-3 reduced the MI risk even after exclusion of REDUCE-IT (33).
Reduced blood pressure, triglyceride levels, and low heart rate variability achieved RR reductions for myocardial infarction (34–36), and platelet oxidative stress leads to increased platelet adhesion to damaged endothelial cells, which contributes to the progression of injury (37). Omega-3 fatty acids have anti-inflammatory properties and may modulate the inflammatory response (38, 39); EPA and DHA can reduce blood pressure (40), platelet aggregation (41), triglyceride (42), heart rate (43), and increase heart rate variability (44). As a consequence, all of these properties can reduce the risk of MI. In the era of precision, medicine may aid in the management of MI.
Although our study first provided a causal association that genetically predicted lower circulating omega-3 fatty acids increases the risk of myocardial infarction, there were some limitations that should be noticed. First, our study only paid attention to subjects of European ancestry, which would limit our results to apply to other race. Second, limited SNPs were selected as IVs in our study, which explained the limited heritable variance of the circulating omega-3 fatty acids. Third, there has not been an appropriate method to precisely evaluate the third assumption of the MR study, which might cause some bias. Finally, although our study theoretically proved the causal relationship between omega-3 fatty acids and myocardial infarction, further population studies are needed to demonstrate this finding.
This study highlights the potential value of omega-3 fatty acids in reducing the risk of myocardial infarction. These findings provide a significant basis for nutrition-based preventive strategies. Consequently, further clinical trials are essential to validate these preliminary findings and ensure the translation of research into effective dietary guidelines.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Ethics Committee of the Second People's Hospital of Hefei (Approval No: 2020-ke-058). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because the GWAS data used in our study were downloaded from the GWAS Catalog (https://www.ebi.ac.uk/gwas/) and IEU OpenGWAS (https://gwas.mrcieu.ac.uk/). Accession numbers are GCST90132738 and ieu-a-798.
WW: Data curation, Formal Analysis, Investigation, Software, Visualization, Writing – original draft, Writing – review & editing, Methodology. LY: Writing – review & editing. JZ: Writing – review & editing. HX: Data curation, Formal Analysis, Investigation, Software, Visualization, Writing – original draft, Writing – review & editing.
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1328087/full#supplementary-material.
1. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. (2019) 380(1):11–22. doi: 10.1056/NEJMoa181279230415628
2. Calder PC. n-3 fatty acids and cardiovascular disease: evidence explained and mechanisms explored. Clin Sci (Lond). (2004) 107(1):1–11. doi: 10.1042/CS2004011915132735
3. Innes JK, Calder PC. Marine omega-3 (N-3) fatty acids for cardiovascular health: an update for 2020. Int J Mol Sci. (2020) 21(4):1362. doi: 10.3390/ijms2104136232085487
4. Zhang Y, Zhuang P, He W, Chen JN, Wang WQ, Freedman ND, et al. Association of fish and long-chain omega-3 fatty acids intakes with total and cause-specific mortality: prospective analysis of 421 309 individuals. J Intern Med. (2018) 284(4):399–417. doi: 10.1111/joim.1278630019399
5. Kristensen SD, Laut KG, Fajadet J, Kaifoszova Z, Kala P, Di Mario C, et al. Reperfusion therapy for ST elevation acute myocardial infarction 2010/2011: current status in 37 ESC countries. Eur Heart J. (2014) 35(29):1957–70. doi: 10.1093/eurheartj/eht52924419804
6. Bahit MC, Kochar A, Granger CB. Post-myocardial infarction heart failure. JACC Heart Fail. (2018) 6(3):179–86. doi: 10.1016/j.jchf.2017.09.01529496021
7. Backes J, Anzalone D, Hilleman D, Catini J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. (2016) 15(1):118. doi: 10.1186/s12944-016-0286-427444154
8. Shibabaw T. Omega-3 polyunsaturated fatty acids: anti-inflammatory and anti-hypertriglyceridemia mechanisms in cardiovascular disease. Mol Cell Biochem. (2021) 476(2):993–1003. doi: 10.1007/s11010-020-03965-733179122
9. Elagizi A, Lavie CJ, O'Keefe E, Marshall K, O'Keefe JH, Milani RV. An update on Omega-3 polyunsaturated fatty acids and cardiovascular health. Nutrients. (2021) 13(1):204. doi: 10.3390/nu1301020433445534
10. Bennett DA, Holmes MV. Mendelian randomisation in cardiovascular research: an introduction for clinicians. Heart. (2017) 103(18):1400–7. doi: 10.1136/heartjnl-2016-31060528596306
11. Pierce BL, Burgess S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol. (2013) 178(7):1177–84. doi: 10.1093/aje/kwt08423863760
12. Dan YL, Wang P, Cheng Z, Wu Q, Wang XR, Wang DG, et al. Circulating adiponectin levels and systemic lupus erythematosus: a two-sample Mendelian randomization study. Rheumatology (Oxford). (2021) 60(2):940–6. doi: 10.1093/rheumatology/keaa50632944772
13. Kettunen J, Demirkan A, Wurtz P, Draisma HH, Haller T, Rawal R, et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat Commun. (2016) 7:11122. doi: 10.1038/ncomms1112227005778
14. Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, et al. A comprehensive 1,000 genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. (2015) 47(10):1121–30. doi: 10.1038/ng.339626343387
15. Shin SY, Fauman EB, Petersen AK, Krumsiek J, Santos R, Huang J, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. (2014) 46(6):543–50. doi: 10.1038/ng.298224816252
16. Richardson TG, Leyden GM, Wang Q, Bell JA, Elsworth B, Davey Smith G, et al. Characterising metabolomic signatures of lipid-modifying therapies through drug target Mendelian randomisation. PLoS Biol. (2022) 20(2):e3001547. doi: 10.1371/journal.pbio.300154735213538
17. Yuan S, Kar S, Carter P, Vithayathil M, Mason AM, Burgess S, et al. Is type 2 diabetes causally associated with cancer risk? Evidence from a two-sample Mendelian randomization study. Diabetes. (2020) 69(7):1588–96. doi: 10.2337/db20-008432349989
18. Bowden J, Davey SG, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40(4):304–14. doi: 10.1002/gepi.2196527061298
19. Choi Y, Lee SJ, Spiller W, Jung KJ, Lee JY, Kimm H, et al. Causal associations between serum bilirubin levels and decreased stroke risk: a two-sample Mendelian randomization study. Arterioscler Thromb Vasc Biol. (2020) 40(2):437–45. doi: 10.1161/ATVBAHA.119.31305531801373
20. Mokry LE, Ross S, Timpson NJ, Sawcer S, Davey SG, Richards JB. Obesity and multiple sclerosis: a Mendelian randomization study. PLoS Med. (2016) 13(6):e1002053. doi: 10.1371/journal.pmed.100205327351487
21. Del GL, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, et al. Omega-3 polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med. (2016) 176(8):1155–66. doi: 10.1001/jamainternmed.2016.292527357102
22. Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. (2012) 308(10):1024–33. doi: 10.1001/2012.jama.1137422968891
23. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. (2019) 380(1):23–32. doi: 10.1056/NEJMoa181140330415637
24. Kalstad AA, Myhre PL, Laake K, Tveit SH, Schmidt EB, Smith P, et al. Effects of n-3 fatty acid supplements in elderly patients after myocardial infarction: a randomized, controlled trial. Circulation. (2021) 143(6):528–39. doi: 10.1161/CIRCULATIONAHA.120.05220933191772
25. Innes JK, Calder PC. The differential effects of eicosapentaenoic acid and docosahexaenoic acid on cardiometabolic risk factors: a systematic review. Int J Mol Sci. (2018) 19(2):532. doi: 10.3390/ijms1902053229425187
26. Harris WS, Del GL, Tintle NL. The Omega-3 index and relative risk for coronary heart disease mortality: estimation from 10 cohort studies. Atherosclerosis. (2017) 262:51–4. doi: 10.1016/j.atherosclerosis.2017.05.00728511049
27. Itakura H, Yokoyama M, Matsuzaki M, Saito Y, Origasa H, Ishikawa Y, et al. Relationships between plasma fatty acid composition and coronary artery disease. J Atheroscler Thromb. (2011) 18(2):99–107. doi: 10.5551/jat.587621099130
28. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. (1999) 354(9177):447–55. doi: 10.1016/S0140-6736(99)07072-510465168
29. Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med. (2002) 112(4):298–304. doi: 10.1016/S0002-9343(01)01114-711893369
30. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. (2007) 369(9567):1090–8. doi: 10.1016/S0140-6736(07)60527-317398308
31. Casula M, Soranna D, Catapano AL, Corrao G. Long-term effect of high dose omega-3 fatty acid supplementation for secondary prevention of cardiovascular outcomes: a meta-analysis of randomized, placebo controlled trials [corrected]. Atheroscler Suppl. (2013) 14(2):243–51. doi: 10.1016/S1567-5688(13)70005-923958480
32. Rimm EB, Appel LJ, Chiuve SE, Djousse L, Engler MB, Kris-Etherton PM, et al. Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: a science advisory from the American Heart Association. Circulation. (2018) 138(1):e35–47. doi: 10.1161/CIR.000000000000057429773586
33. Hu Y, Hu FB, Manson JE. Marine omega-3 supplementation and cardiovascular disease: an updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J Am Heart Assoc. (2019) 8(19):e13543. doi: 10.1161/JAHA.119.013543
34. Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. (2016) 387(10017):435–43. doi: 10.1016/S0140-6736(15)00805-326559744
35. Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. (2007) 298(3):299–308. doi: 10.1001/jama.298.3.29917635890
36. Fang SC, Wu YL, Tsai PS. Heart rate variability and risk of all-cause death and cardiovascular events in patients with cardiovascular disease: a meta-analysis of cohort studies. Biol Res Nurs. (2020) 22(1):45–56. doi: 10.1177/109980041987744231558032
37. Fuentes E, Moore-Carrasco R, de Andrade PA, Trostchansky A. Role of platelet activation and oxidative stress in the evolution of myocardial infarction. J Cardiovasc Pharmacol Ther. (2019) 24(6):509–20. doi: 10.1177/107424841986143731280622
38. Verveniotis A, Siasos G, Oikonomou E, Tsigkou V, Papageorgiou N, Zaromitidou M, et al. The impact of omega 3 fatty acids in atherosclerosis and arterial stiffness: an overview of their actions. Curr Pharm Des. (2018) 24(17):1865–72. doi: 10.2174/138161282466618032109502229564974
39. Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. (2017) 45(5):1105–15. doi: 10.1042/BST2016047428900017
40. Guo XF, Li KL, Li JM, Li D. Effects of EPA and DHA on blood pressure and inflammatory factors: a meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. (2019) 59(20):3380–93. doi: 10.1080/10408398.2018.149290129993265
41. Phang M, Lincz LF, Garg ML. Eicosapentaenoic and docosahexaenoic acid supplementations reduce platelet aggregation and hemostatic markers differentially in men and women. J Nutr. (2013) 143(4):457–63. doi: 10.3945/jn.112.17124923390192
42. Mason RP, Libby P, Bhatt DL. Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arterioscler Thromb Vasc Biol. (2020) 40(5):1135–47. doi: 10.1161/ATVBAHA.119.31328632212849
43. von Schacky C. A review of omega-3 ethyl esters for cardiovascular prevention and treatment of increased blood triglyceride levels. Vasc Health Risk Manag. (2006) 2(3):251–62. doi: 10.2147/vhrm.2006.2.3.25117326331
Keywords: omega-3 fatty acids, myocardial infarction, Mendelian randomization study, SNPs, instrumental variables
Citation: Wang W, Yang L, Zhang J and Xiang H (2024) Decreased circulating omega-3 fatty acids increase the risk of myocardial infarction: a two-sample Mendelian randomization study. Front. Cardiovasc. Med. 11:1328087. doi: 10.3389/fcvm.2024.1328087
Received: 10 November 2023; Accepted: 5 March 2024;
Published: 14 March 2024.
Edited by:
Sebhat Erqou, Brown University, United StatesReviewed by:
Rahul Mallick, University of Eastern Finland, Finland© 2024 Wang, Yang, Zhang and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyun Xiang eGh5OTMxOEAxMjYuY29t
Abbreviations AMI, acute myocardial infarction; CIs, confidence intervals; CVD, cardiovascular disease; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; IVW, inverse-variance weighted; MI, myocardial infarction; MR, Mendelian randomization; IVs, instrumental variables; SNPs, single-nucleotide polymorphisms; TSMR, two-sample MR
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.