- 1Heart Rhythm Center, Taipei Veterans General Hospital, Taipei, Taiwan
- 2Division of Cardiology, Serdang Hospital, Selangor, Malaysia
- 3Department of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 4Cardiovascular Center, Taichung Veterans General Hospital, Taichung, Taiwan
- 5Department of Medicine, National Chung Hsing University, Taichung, Taiwan
Introduction: Catheter ablation is an effective and safe strategy for treating atrial fibrillation patients. Nevertheless, studies on the long-term outcomes of catheter ablation in patients with dilated cardiomyopathy are limited. This study aimed to assess the electrophysiological characteristics of atrial fibrillation patients with dilated cardiomyopathy and compare the long-term clinical outcomes between patients undergoing catheter ablation and medical therapy.
Method: Patient baseline characteristics and electrophysiological parameters were examined to identify the predictors of atrial fibrillation recurrence following catheter ablation. The clinical outcomes of catheter ablation and medical therapy were compared using the propensity score matched method.
Results: A total of 343 patients were enrolled, with 46 in the catheter ablation group and 297 in the medical therapy group. Among the catheter ablation group, 58.7% (n = 27) had persistent atrial fibrillation. The recurrence rate of atrial arrhythmia was 30.4% (n = 14) after an average follow-up duration of 7.7 years following catheter ablation. The only predictive factor for atrial fibrillation recurrence after catheter ablation was the left atrial diameter. When compared to medical therapy, catheter ablation demonstrated significantly better outcomes in terms of overall survival, freedom from heart failure hospitalization, improvement in left ventricular ejection fraction, and a greater reduction in left ventricular diameter and left atrial diameter after propensity score matching.
Conclusions: Therefore, catheter ablation proves to be effective in providing long-term control of atrial fibrillation in patients with dilated cardiomyopathy. In addition to standard heart failure care, catheter ablation significantly enhanced both morbidity and mortality outcomes and reversed structural remodeling when compared to heart failure medication alone.
1 Introduction
Atrial fibrillation (AF) is one of the most common arrhythmias that predisposes patients to a higher risk of morbidity and mortality. The prevalence of AF is approximately 1% in the United States (1), while it is estimated to be 0.6%–1.5% in the Asian population (2–4).
Dilated cardiomyopathy (DCM) is defined as a dilated left ventricular (LV) chamber with a poor left ventricular ejection fraction (LVEF) in the absence of coronary artery disease, hypertension, valvular disease, or congenital heart disease (5). DCM is a severe cardiac disorder in which structural or functional abnormalities of the heart muscle can lead to substantial morbidity and mortality due to complications such as heart failure (HF) and arrhythmia (6).
AF and HF often coexist, while HF precedes AF leading to a worse outcome (7). When cardiomyopathy and AF occur concurrently, patients may experience worse symptoms and poorer prognosis (8). Previous studies such as AATAC and Castle-AF showed that catheter ablation (CA) is superior to medical therapy (9, 10). A recently published Castle-HTx demonstrated that the combination of CA and guideline-directed medical therapy (GDMT) improves survival in end-stage HF patients (11). However, evidence-based evaluations and previous studies did not specifically address this complex subgroup of patients with AF, poor LVEF, and a dilated LV chamber.
We hypothesized that a vicious cycle exists between AF and LV dysfunction in patients with DCM. AF results in atrial stunning and arrhythmia-induced cardiomyopathy, leading to the deterioration of preexisting LV dysfunction and chamber dilatation (8). The LV dysfunction increases the left atrium (LA) pressure, which causes LA dilatation and AF (12). Based on this theory, CA may provide better outcomes in terms of AF control and the reversal of LV dysfunction and structural remodeling compared to medical therapy. Therefore, this study aimed to examine the long-term clinical outcomes of CA in patients with AF and DCM and compare these outcomes with those of patients who underwent medical therapy without CA.
2 Method
2.1 Study population
This retrospective cohort study included consecutive patients with documented DCM and AF who underwent CA for drug-refractory AF (Group 1) and those who did not undergo CA (Group 2) between January 2001 and November 2021 at Taipei Veterans General Hospital. Data were analyzed between November 1, 2022 and March 31, 2023. The baseline characteristics, echocardiographic parameters, and electrophysiological findings of the patients were assessed. DCM was defined as an LVEF less than 45% and a left ventricular internal dimension in diastole (LVIDd) of more than 5.3 cm for females and 5.9 cm for males, respectively (13). AF was defined based on electrocardiography (ECG) or Holter monitoring. Paroxysmal and persistent AF were defined according to an updated consensus (14).
This study was approved by the Institutional Ethics Review Board of the Taipei Veterans General Hospital (IRB No. 2021-11-015BC). Given the retrospective nature of this study, the requirement for informed consent was waived by the institutional review board.
2.2 Inclusion and exclusion criteria
Patients diagnosed with concomitant DCM and AF were included. Exclusions were made for patients with secondary causes of DCM, such as hypertension, coronary heart disease, congenital heart disease, valvular heart disease, peripartum conditions, alcoholic and metabolic diseases like thyroid disease. Additionally, patients with a history of previous cardiovascular surgeries, Cox maze procedures, in-hospital and out-of-hospital cardiac arrests, a life expectancy of less than one year after diagnosis, and incomplete data were excluded from this study. Patients suspected of arrhythmia-induced cardiomyopathy, showing improved LVEF after rate control were also excluded from this study.
2.3.1 Part I: ablation procedure
The protocols for the CA procedures have been described in our previous studies (15–18). Anti-arrhythmic medications were discontinued for at least five half-lives before the procedure. A 7F decapolar catheter with a 2-mm interelectrode distance and 5-mm spacing between each electrode pair was introduced into the coronary sinus. Transseptal atrial punctures were performed using fluoroscopic landmarks or transesophageal echocardiography and an 8.5F SL-0 sheath was inserted into the left atrium.
In patients with paroxysmal AF, we performed wide antral pulmonary vein isolation (PVI). In patients with non-paroxysmal AF, the ablation approach was described in the previous studies (18). Briefly, if sinus rhythm was not restored after PVI, linear ablation (mitral or roof ablation) was performed in selected patients with documented atypical flutter. Substrate modification was performed when non-pulmonary vein (PV) triggers were identified (17, 19, 20). Cardioversion was performed in cases in which all the above procedures failed to restore sinus rhythm.
Non-PV triggers were mapped and ablated (16). The strategy described earlier was applied to the atrial tachycardia (AT), which lasted for more than one minute. Subsequently, induction of AF was performed. If left atrial flutter or AT was induced and sustained for more than one minute, a re-entry circuit was identified, followed by additional focal or linear ablation.
Cavotricuspid isthmus (CTI) ablation was performed at the end of the procedure in patients with documented or inducible atrial flutter. Bidirectional blocks were achieved during the sinus rhythm.
2.3.2 Part I: follow-up and detection of arrhythmia recurrence after ablation in group 1
After the procedure, patients were scheduled to visit the outpatient clinic for regular follow-up (two weeks, then every 1–3 months after CA). Routine ECG was performed for each outpatient follow-up, and 24-h Holter monitoring was performed at 3, 6, and 12 months. In addition, for patients experiencing symptoms suggestive of recurrence, 24-h Holter monitoring or cardiac event recording was performed to ascertain the types of arrhythmias.
2.3.3 Part II: data collection for the clinical outcome for overall patients
Patients in Group 2 were followed up in the outpatient clinic for HF care (every 2–3 months after the first presentation). Data on patient baseline characteristics, medication history, electrophysiological features, and clinical outcomes, including major adverse cardiovascular events (MACE), changes in LVEF, left atrial diameter (LAD), and HF events, were collected.
The primary outcome was all-cause mortality. The secondary outcomes included cardiovascular mortality, ischemic stroke, myocardial infarction, hospitalization due to HF, changes of mean heart rate, LVEF, LVIDd and LAD. The cardiovascular mortality in the secondary outcome included composite death of acute coronary syndrome, myocardial infarction and heart failure. The mean heart rate was determined by resting heart rate recorded during two consecutive outpatient clinic visits.
The primary and secondary outcomes were identified through the hospital electronic database. The nationally linked electronic database was utilized to ascertain the cause of death for each patient. Phone calls to patients were also conducted to detect events that occurred in other hospitals.
To minimize the impact of confounding factors on clinical characteristics, we employed propensity analysis and matching techniques. We matched one-to-one pairs (Group 1 vs. Group 2) with identical propensity scores (PS) and a 0.01 calliper width. Suitability was assessed by estimating the standardized differences between the two groups in baseline characteristics (age, sex, and heart rate), comorbidities, echocardiographic parameters (LVEF, LVIDd, and LAD), and medications (oral anticoagulant and HF medication).
2.4 Statistical analysis
Categorical variables were presented as frequencies and percentages and compared using the chi-square and Fisher's exact tests. The Student's t-test was used to analyze normally distributed data. Continuous variables are presented as mean ± standard deviation and compared using the Mann–Whitney U test, as the data distribution was not normal. Univariate Cox regression analysis was initially performed. Differences in study endpoints were analyzed using the Kaplan–Meier method with the log-rank test. A p-value of <.05 with a 95% confidence interval was considered statistically significant. All statistical tests were performed using the IBM SPSS Statistics software version 26 (SPSS, Inc., Chicago, IL).
3 Results
A total of 8,507 patients with cardiomyopathy were screened. 343 patients (4.0%) with DCM and concomitant AF were recruited for this study, with 46 patients (13.4%) receiving CA in Group 1 and 297 patients (86.6%) receiving medical therapy in Group 2 (Figure 1).
3.1 Part I study
3.1.1 Baseline characteristics and electrophysiological characteristics
The baseline and electrophysiological characteristics of the patients in Group 1 are shown in Table 1. The mean age was 59.3 ± 10.0 years, with 87.0% (n = 40) male, and the mean heart rate was 88.4 ± 25.8 beats per minute. A total of 8.7% (n = 4) of Group 1 patients were diagnosed with diabetes. The mean CHA2DS2VASc score was 2.0 ± 1.0. Twenty-seven patients (58.7%) had persistent AF. The mean LVEF was 37.0 ± 7.1%, the mean LVIDd was 60.6 ± 5.0 mm, and the mean LAD was 45.1 ± 6.0 mm.
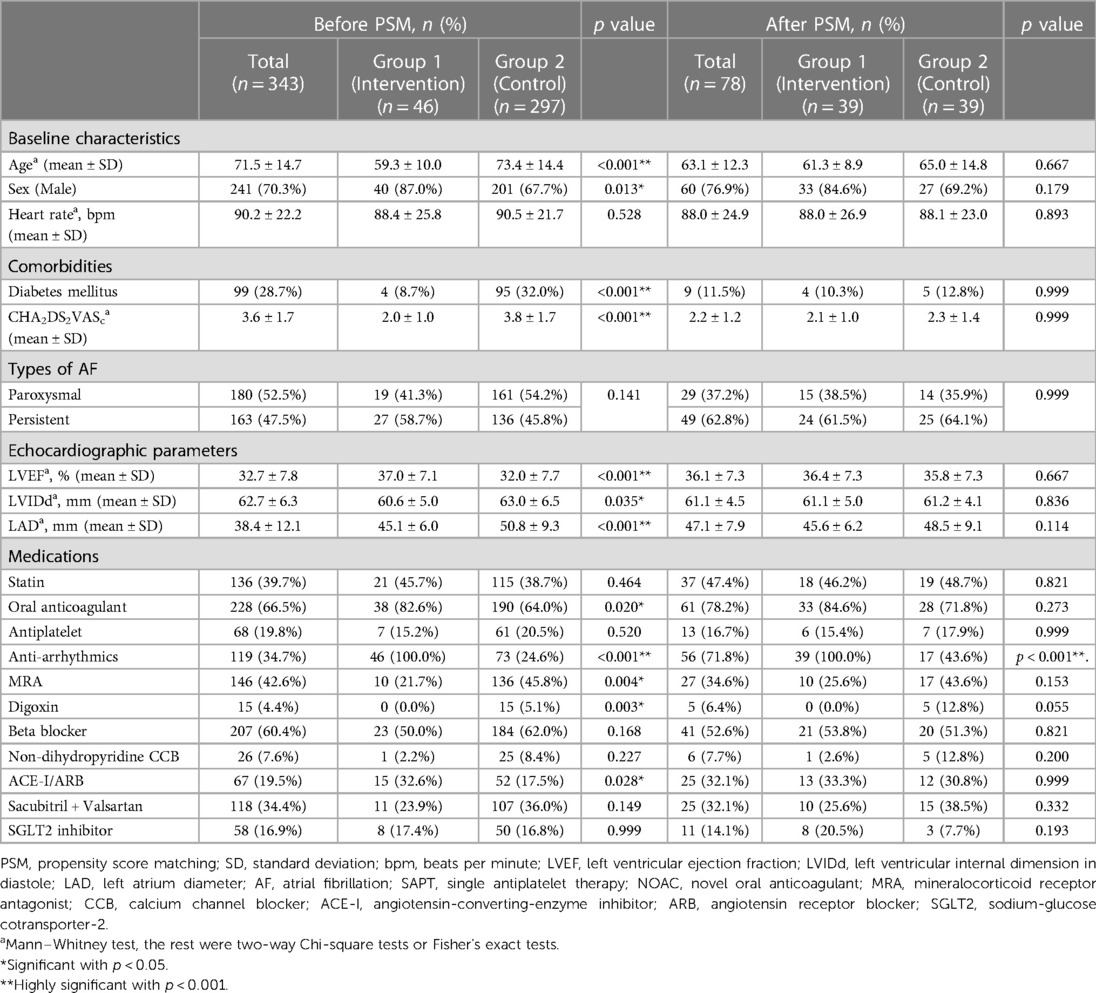
Table 1. Baseline characteristics, comorbidities, echocardiographic parameters and medications before and after propensity score matching.
PVI was successfully performed in all patients in Group 1. CTI ablation, linear ablation and substrate modification were performed in 73.9% (n = 34), 8.7% (n = 4) and 23.9% (n = 11) of patients, respectively. Non-PV triggers were identified in 8.7% (n = 4) of patients. Notably, the non-PV triggers were from the superior vena cava (75%, n = 3), LA anterior wall (50%, n = 2), and LA roof (25%, n = 1).
The overall recurrence rate of any atrial arrhythmia (AF or AT) in Group 1 patients was 30.4% (n = 14) after a mean follow-up duration of 7.7 years after CA. Of note, 21.1% (n = 4) of the patients with paroxysmal AF and 37.0% (n = 10) of the patients with persistent AF developed recurrence. Among the 14 patients with recurrence, 85.8% (n = 12) had recurrent AF, 7.1% (n = 1) had AT recurrence, and 7.1% (n = 1) had atypical atrial flutter recurrence. Of the patients with recurrence, five (35.7%) underwent repeat CA. During the repeat procedures, the cause of recurrence was PV reconnection in 3 patients (60%), non-PV triggers (LA atrial appendage base and superior vena cava) in 2 (40%), and atypical flutter (perimitral and RA free wall reentry) in 1 patient (20%).
No major adverse events were reported in Group 1. Femoral hematoma developed in 4.3% (n = 2) of the patients and 2.2% (n = 1) had pericardial effusion. All patients were managed conservatively. In addition, 2.2% (n = 1) of the patients developed acute HF with pulmonary edema, which resolved after diuretic administration. All the patients who developed complications were discharged after the procedure.
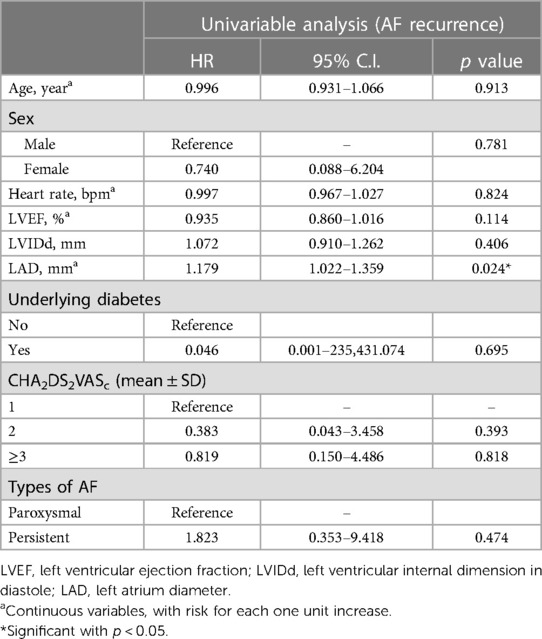
3.1.2 Predictors of AF recurrence
The baseline characteristics, comorbidities, echocardiographic parameters, and ablation modalities were analyzed to predict AF recurrence. The LAD was the only predictor of AF recurrence after a single procedure (HR, 1.179; 95% CI 1.022 to 1.359, p = .024) in univariate Cox regression (Table 2).
3.1.3 Part I study: subgroup analysis of LVEF ≤ 30%
A total of 8 patients (17.4%) in Group 1 had LVEF ≤ 30% prior to ablation. Atrial arrhythmia recurrence occurred in four patients (50%). The types of recurrence included AF (75%, n = 3) and AT (25%, n = 1). In this group, 50% (n = 2) of patients underwent repeat procedures. The causes of recurrence included PV reconnection (n = 1, 50%) and perimitral flutter (n = 1, 50%). There was no significant difference in recurrence between the patients with LVEF > 30% and ≤30% (28.3% vs. 50%, p = .418).
3.2 Part II study
3.2.1 Baseline characteristics
Before PS matching, there were significant differences in the baseline characteristics between Group 1 and Group 2. Group 1 had younger patients, more men, fewer individuals with diabetes and lower CHA2DS2VASc scores. Group 1 patients also had higher LVEF, lower LVIDd, lower LAD, more anticoagulants, antiarrhythmic drugs, and ACEi/ARB, and fewer mineralocorticoid receptor antagonists and digoxin. After PS matching, there was no significant difference between PS-Group 1 and PS-Group 2, except that antiarrhythmic drugs were prescribed more frequently in Group 1 (p < .001) (Table 1).
3.2.2 Primary and secondary outcome
The overall mortality rate was 20.1% (n = 69). The causes of death included cardiovascular-related death in 69.6% (n = 48), sepsis-related death in 26.2% (n = 18), ischemic stroke in 1.4% (n = 1), 1.4% (n = 1) pulmonary embolism, and 1.4% (n = 1) upper gastrointestinal bleeding.
Kaplan–Meier analysis of survival from all-cause mortality showed significantly better survival in Group 1 than in Group 2 before (p < .001) and after PS matching (p = .009) (Figure 2).
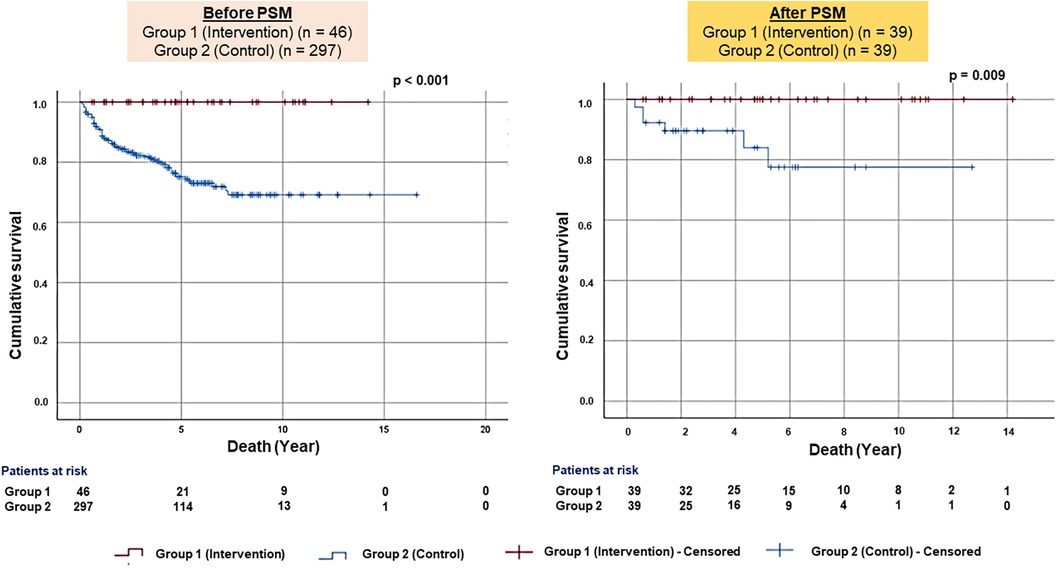
Figure 2. Kaplan–Meier survival plots for all-cause mortality. The figure presented the Kaplan–Meier survival plots for all-cause mortality before (left panel) and after the PS-matched (right panel) patients.
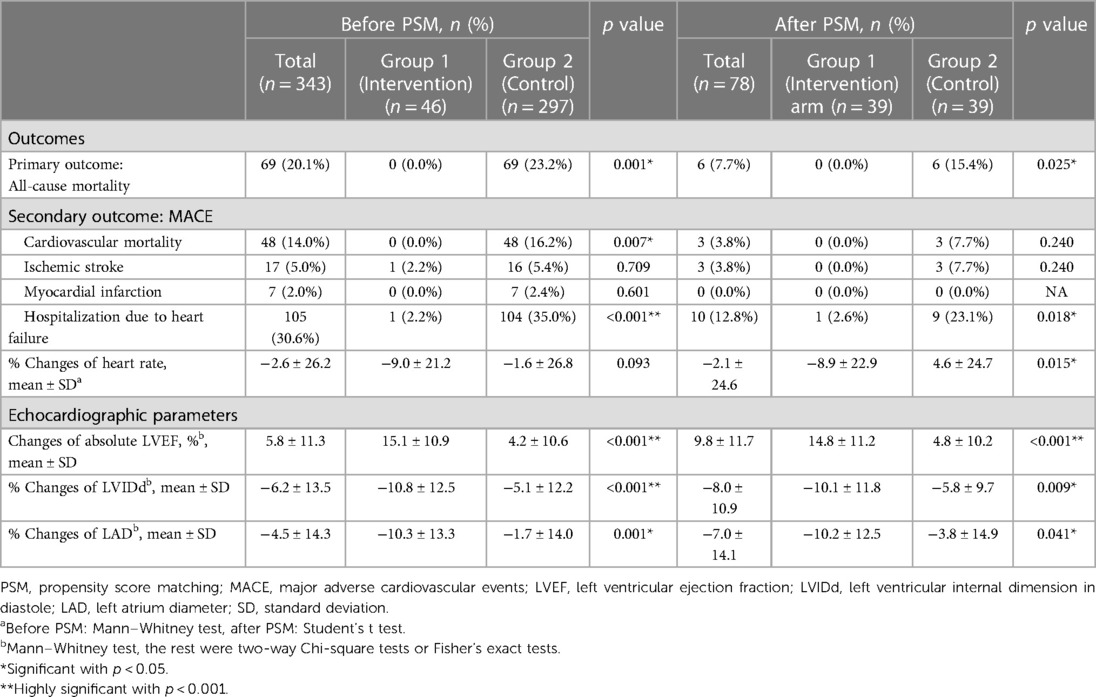
The heart rate was decreased in both Group 1 and Group 2 without significant difference before PS matching (reduction of heart rate: −9.0 ± 21.2% vs. −1.6 ± 26.8%, p = .093). After PS matching, there was a statistically higher reduction of heart rate in PS-Group 1 compared to PS-Group 2 (−8.9 ± 22.9% vs. 4.6 ± 24.7%, p = 0.015) (Figures 3g,h; Table 3).
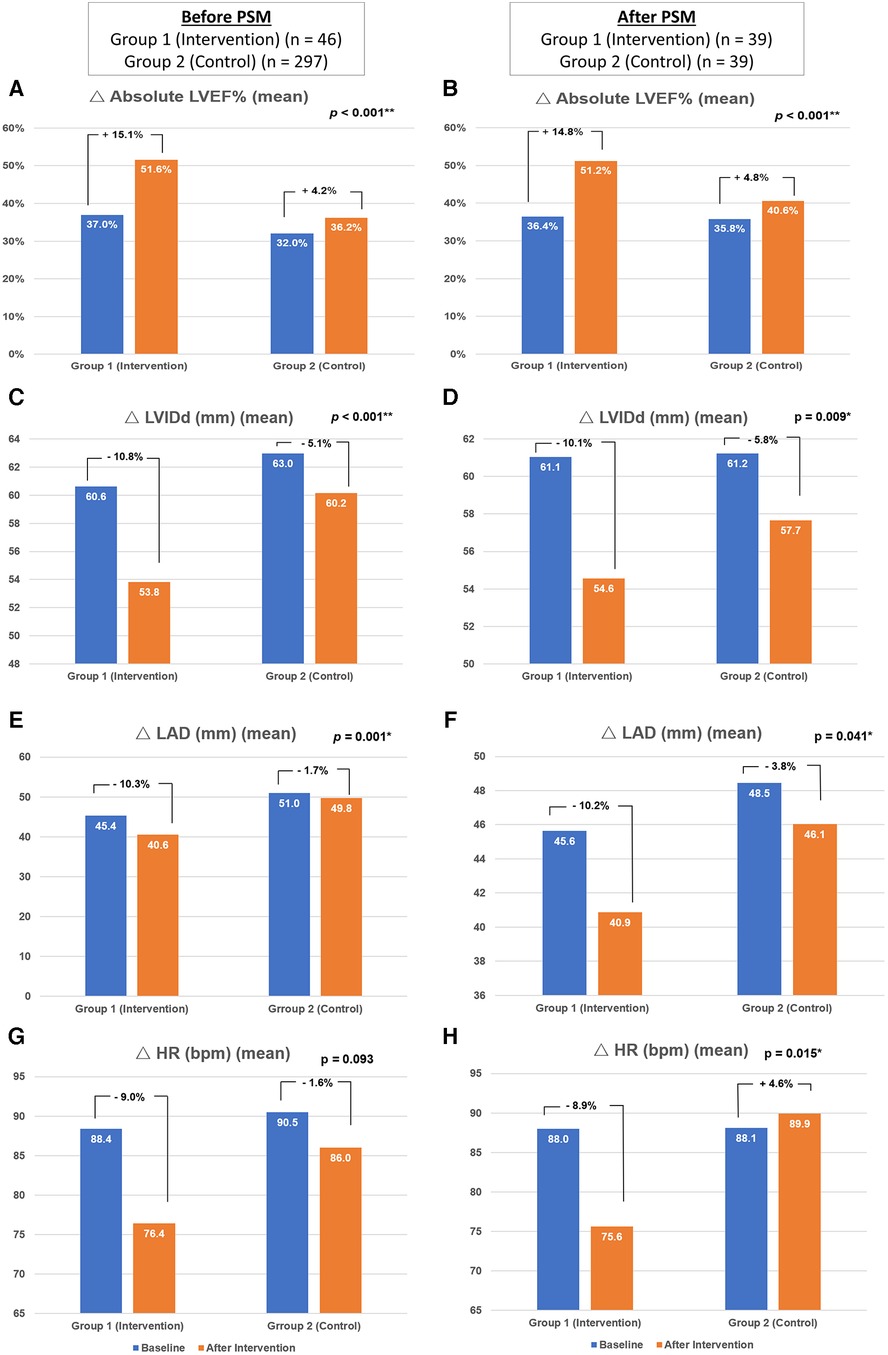
Figure 3. Changes in the echocardiographic parameters and heart rate. The figure demonstrated the changes of absolute left ventricle ejection fraction (A,B), left ventricular internal diameter in diastole (C,D), left atrium diameter (E,F) and heart rate (G,H) of respondents before (n = 343) and after (n = 39) propensity score matching. PSM, indicates propensity score matching; LVEF, left ventricular ejection fraction; LVIDd, left ventricular internal dimension in diastole; LAD, left atrium diameter, bpm: beats per minute.
As for the secondary outcome, the cardiovascular mortality rate was 14% (n = 48) and the incidence of HF hospitalization was 30.6% (n = 105) across all patients in Groups 1 and 2. Before PS matching, Group 1 patients exhibited a lower incidence of cardiovascular mortality [Group 1 vs. Group 2: 0% (n = 0) vs. 16.2% (n = 48), p = .007] and HF hospitalization [Group 1 vs. Group 2: 2.2% (n = 1) vs. 35.0% (n = 104), p < .001]. After PS matching, there were still fewer HF hospitalizations in PS-Group 1 than in PS-Group 2 patients [PS-Group 1 vs. PS-Group 2: 2.6% (n = 1) vs. 23.1% (n = 9), p = .018]. There were no significant differences in the incidences of ischemic stroke or myocardial infarction between the two groups (Table 3).
Regarding the echocardiographic parameters, there was a significant improvement in Group 1 compared to Group 2. Before PS matching, there was a better improvement of absolute LVEF in Group 1 than in Group 2 (15.1 ± 10.9% vs. 4.2 ± 10.6%, p < .001). The reduction of LVIDd (−10.8 ± 12.5% vs. −5.1 ± 12.2%, p < .001) and LAD (−10.3 ± 13.3% vs. −1.7 ± 14.0%, p = .001) were also more significant in Group 1 than in Group 2. After PS matching, the increment of absolute LVEF remained more significant in PS-Group 1 than in PS-Group 2 (14.8 ± 11.2% vs. 4.8 ± 10.2%, p < .001). The reduction of LVIDd (−10.1 ± 11.8% vs. −5.8 ± 9.7%, p = .009) and LAD (−10.2 ± 12.5% vs. −3.8 ± 14.9%, p = .041) were also more significant in PS-Group 1 compared to PS-Group 2 (Table 3; Figures 3a–f).
3.2.3 Subgroup analysis LVEF ≤ 30%
In the subgroup analysis of the patients with LVEF ≤ 30% (8 patients in Group 1 and 121 patients in Group 2), there was less incidence of hospitalization due to HF in Group 1 when compared with Group 2 [0% (n = 0) vs. 43.8% (n = 53), p = .021]. There were no significant differences in the overall mortality, cardiovascular mortality, ischemic stroke, or myocardial infarction in this subgroup analysis.
4 Discussion
4.1 Main findings
The present study has several main findings. First, CA for AF in patients with DCM can achieve a good AF-freedom outcome (30.4% recurrence rate) in long-term follow-up. Second, the LAD was the only factor that predicted AF recurrence in patients with DCM. Third, CA significantly improved clinical outcomes and reversed structural remodeling in both LA and LV.
4.2 Recurrence of AF in DCM patients after CA
The overall AF recurrence rate in patients with DCM after CA was 30.4%. In previous studies with ten-year follow-up, the recurrence of AF after the index AF procedure was 42% and 84% in patients with paroxysmal and persistent AF, respectively (21, 22). The recurrence rate was lower in the CA group than in the medical therapy group. This finding requires further studies with a larger number of patients. Additionally, PV reconnection is a major cause of AF recurrence after repeated procedures. This result is in line with those of previous studies (21, 22) as the major triggers of PV in both paroxysmal and persistent AF (23). The results of the present study highlighted the benefits of CA in patients with DCM and AF. Further prospective studies are required to determine the advantages of CA in this cohort.
4.3 Predictor of recurrence in DCM patients after CA
In the present study, LAD was the only predictor of atrial tachyarrhythmia recurrence in patients with DCM. This result is consistent with those of previous studies (21, 22, 24). LA dilatation is more than just an increment in size, it is also a manifestation of LA fibrosis and remodeling. These are the substrates for AF occurrence, and advanced stages of fibrosis and remodeling are also considered important factors in predicting recurrence after catheter ablation (25–28).
4.4 Survival in DCM patients after CA
The prevalence of DCM with AF in our registry was 4.0%, similar to real-world data of 5.9% by Buckley et al. (29) To the best of our knowledge, this study is the first to demonstrate improvements in survival rates and overall echocardiographic parameters among patients with AF and DCM after receiving CA compared to medical therapy, at a mean follow-up of 7.7 years.
Compared to anti-arrhythmic drugs, CA was proven to reduce mortality and HF hospitalization in HF patients. Previous landmark studies include AATAC, Castle-AF and Castle-HTx delineated the superiority of CA while compared to medical therapy in AF and HF patients (9, 10, 30). However, these studies did not address DCM in particular.
Furthermore, Zhao et al. demonstrated that CA improved heart failure in DCM and AF patients during the early stages, but this improvement was not sustained beyond three years (31). Another study by Rillig et al. showed improvements in LVEF and a reduction in LAD among AF and DCM patients who received CA, but statistically not significant in the reduction of LVIDd (32). In the same study, a higher mortality rate was observed in the DCM group compared to those with arrhythmia-induced cardiomyopathy. Thus, in the current study, CA significantly reduced all-cause mortality, cardiovascular death, and HF hospitalization in patients with DCM and AF compared with those treated with medication alone.
The outcomes of DCM have been poor in the past. A previous study demonstrated that ACEi/ARB and beta-blockers improved the survival rates of patients with DCM from 55% to 87% over a mean follow-up period of 8 years (33). The survival rate with medical therapy was 79.9% in the current group of patients with DCM and AF, which was comparable to that reported in a previous study (8, 12). Further prospective randomized studies were required to prove the benefit of AF ablation regarding the clinical outcome in DCM patients.
4.5 Benefit of combination of CA and medication in DCM with AF patients
The present study demonstrated a significant increase in LVEF and a reduction in both LVIDd and LAD in patients with DCM after CA for AF. LVEF improvement was in line with the previous studies (10, 11, 30). As mentioned previously, GDMT has been proven to improve long-term outcomes and reduce mortality in patients with DCM (33, 34).
After PS matching, there were no discernible differences in the usage of oral anticoagulants and SGLT2 inhibitors between patients receiving CA and medical therapy. However, clinicians may incline to administer more intensive treatments to patients in the CA patients, which could potentially impact the long-term outcomes.
In addition, heart rate strongly predicts cardiovascular outcomes in patients with DCM (34). However, optimal rate control using medication alone for DCM remains a challenge. In this study, there was a significant reduction in heart rate in patients with CA compared to those receiving medication only. Therefore, in the present study, adjuvant CA in addition to GDMT improved structural remodeling and long-term outcomes.
4.6 Limitations
This study has several noteworthy limitations. Since data were retrospectively collected from a hospital electronic database, some comorbidities may have been underreported. Second, this single-center study conducted in Taiwan may not represent the general population with DCM and AF. Third, relatively small sample size in the patients receiving CA and a selection bias might have existed despite with PS matching because of the retrospective nature of the study. Although the PS matching method might adjust the differences in patient characteristics, but could not eliminate the selection bias, especially considering procedure-related outcomes. Fourth, advancement in AF ablation technologies and emergence of new medications such as sacubitril/valsartan or SGLT2 inhibitor may affect the outcomes in this very long-term follow up populations. Further studies with a standardized protocol for medication and ablation strategies are warranted. Fifth, patients who received CA may be subjected to more intensive monitoring, integrated care and treatment. These strategies might translate into a better outcome in CA arm. Lastly, strict screening and protocols such as genetic screening, endomyocardial biopsy or cardiac magnetic resonance imaging were not mandatory for all patients. This may affect the result of the study.
5 Conclusion
In addition to conventional HF treatment, CA is effective for long-term AF and HF control in patients with DCM. Compared to medical therapy, adjuvant CA significantly improves overall survival, reduces cardiovascular mortality, and improves structural remodeling in the LA and LV in patients with AF and DCM.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Ethics Review Board of the Taipei Veterans General Hospital (IRB No. 2021-11-015BC). The studies were conducted in accordance with the local legislation and institutional requirements. Informed consent was waived for this study.
Author contributions
Y-KS: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. C-YL: Conceptualization, Resources, Supervision, Validation, Writing – review & editing. F-PC: Conceptualization, Investigation, Resources, Writing – review & editing. Y-JL: Methodology, Supervision, Writing – review & editing. S-LC: Supervision, Writing – review & editing. L-WL: Supervision, Validation, Writing – review & editing. Y-FH: Project administration, Supervision, Writing – review & editing. J-NL: Methodology, Supervision, Writing – review & editing. T-YC: Resources, Validation, Writing – review & editing. T-CT: Methodology, Supervision, Writing – review & editing. LK: Methodology, Supervision, Validation, Writing – review & editing. C-IW: Investigation, Validation, Writing – review & editing. C-ML: Investigation, Validation, Writing – review & editing. S-HL: Methodology, Validation, Visualization, Writing – review & editing. G-YL: Investigation, Software, Writing – review & editing. M-JK: Investigation, Methodology, Validation, Writing – review & editing. S-JW: Formal Analysis, Investigation, Writing – review & editing. JB: Investigation, Software, Writing – review & editing. Y-SH: Investigation, Resources, Writing – review & editing. NN: Resources, Validation, Writing – review & editing. S-AC: Conceptualization, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the Biosense Webster IIS (C2304900), Ministry of Science and Technology (MOST 110-2314-B-A49A-541-MY3, MOST 111-2314-B-075-007-MY3); and Taipei Veterans General Hospital (grant no. C19-027). C-YL was the recipient of the funding award. The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. (2001) 285(18):2370–5. doi: 10.1001/jama.285.18.2370
2. Chao TF, Liu CJ, Tuan TC, Chen TJ, Hsieh MH, Lip GYH, et al. Lifetime risks, projected numbers, and adverse outcomes in Asian patients with atrial fibrillation: a report from the Taiwan nationwide AF cohort study. Chest. (2018) 153(2):453–66. doi: 10.1016/j.chest.2017.10.001
3. Inoue H, Fujiki A, Origasa H, Ogawa S, Okumura K, Kubota I, et al. Prevalence of atrial fibrillation in the general population of Japan: an analysis based on periodic health examination. Int J Cardiol. (2009) 137(2):102–7. doi: 10.1016/j.ijcard.2008.06.029
4. Yap KB, Ng TP, Ong HY. Low prevalence of atrial fibrillation in community-dwelling Chinese aged 55 years or older in Singapore: a population-based study. J Electrocardiol. (2008) 41(2):94–8. doi: 10.1016/j.jelectrocard.2007.03.012
5. Mahmaljy H, Yelamanchili VS, Singhal M. Dilated Cardiomyopathy. Treasure Island, FL: StatPearls (2022).
6. Schultheiss HP, Fairweather D, Caforio ALP, Escher F, Hershberger RE, Lipshultz SE, et al. Dilated cardiomyopathy. Nat Rev Dis Primers. (2019) 5(1):32. doi: 10.1038/s41572-019-0084-1
7. Pallisgaard J, Greve AM, Lock-Hansen M, Thune JJ, Fosboel EL, Devereux RB, et al. Atrial fibrillation onset before heart failure or vice versa: what is worst? A nationwide register study. Europace. (2023) 25(2):283–90. doi: 10.1093/europace/euac186
8. Huizar JF, Ellenbogen KA, Tan AY, Kaszala K. Arrhythmia-induced cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73(18):2328–44. doi: 10.1016/j.jacc.2019.02.045
9. Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. (2016) 133(17):1637–44. doi: 10.1161/CIRCULATIONAHA.115.019406
10. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. (2018) 378(5):417–27. doi: 10.1056/NEJMoa1707855
11. Sohns C, Fox H, Marrouche NF, Crijns H, Costard-Jaeckle A, Bergau L, et al. Catheter ablation in end-stage heart failure with atrial fibrillation. N Engl J Med. (2023) 389(15):1380–9. doi: 10.1056/NEJMoa2306037
12. Cha YM, Redfield MM, Shen WK, Gersh BJ. Atrial fibrillation and ventricular dysfunction: a vicious electromechanical cycle. Circulation. (2004) 109(23):2839–43. doi: 10.1161/01.CIR.0000132470.78896.A8
13. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2015) 28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003
14. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2021) 42(5):373–498. doi: 10.1093/eurheartj/ehaa612
15. Chen SA, Hsieh MH, Tai CT, Tsai CF, Prakash VS, Yu WC, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. (1999) 100(18):1879–86. doi: 10.1161/01.CIR.100.18.1879
16. Lin WS, Tai CT, Hsieh MH, Tsai CF, Lin YK, Tsao HM, et al. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation. (2003) 107(25):3176–83. doi: 10.1161/01.CIR.0000074206.52056.2D
17. Lin YJ, Tai CT, Chang SL, Lo LW, Tuan TC, Wongcharoen W, et al. Efficacy of additional ablation of complex fractionated atrial electrograms for catheter ablation of nonparoxysmal atrial fibrillation. J Cardiovasc Electrophysiol. (2009) 20(6):607–15. doi: 10.1111/j.1540-8167.2008.01393.x
18. Lo LW, Lin YJ, Chang SL, Hu YF, Chao TF, Chung FP, et al. Predictors and characteristics of multiple (more than 2) catheter ablation procedures for atrial fibrillation. J Cardiovasc Electrophysiol. (2015) 26(10):1048–56. doi: 10.1111/jce.12748
19. Chang TY, Lin CY, Lin YJ, Wu CI, Chang SL, Lo LW, et al. Long-term outcome of patients with long-standing persistent atrial fibrillation undergoing ablation guided by a novel high-density panoramic mapping system: a propensity score matching study. Heart Rhythm O2. (2022) 3(3):269–78. doi: 10.1016/j.hroo.2022.04.003
20. Lin YJ, Lo MT, Chang SL, Lo LW, Hu YF, Chao TF, et al. Benefits of atrial substrate modification guided by electrogram similarity and phase mapping techniques to eliminate rotors and focal sources versus conventional defragmentation in persistent atrial fibrillation. JACC Clin Electrophysiol. (2016) 2(6):667–78. doi: 10.1016/j.jacep.2016.08.005
21. Cheng WH, Lo LW, Lin YJ, Chang SL, Hu YF, Hung Y, et al. Ten-year ablation outcomes of patients with paroxysmal atrial fibrillation undergoing pulmonary vein isolation. Heart Rhythm. (2019) 16(9):1327–33. doi: 10.1016/j.hrthm.2019.03.028
22. Tsai TY, Lo LW, Cheng WH, Liu SH, Lin YJ, Chang SL, et al. 10-year outcomes of patients with non-paroxysmal atrial fibrillation undergoing catheter ablation. Circ J. (2022) 87(1):84–91. doi: 10.1253/circj.CJ-22-0062
23. Santangeli P, Zado ES, Hutchinson MD, Riley MP, Lin D, Frankel DS, et al. Prevalence and distribution of focal triggers in persistent and long-standing persistent atrial fibrillation. Heart Rhythm. (2016) 13(2):374–82. doi: 10.1016/j.hrthm.2015.10.023
24. Benali K, Barre V, Hermida A, Galand V, Milhem A, Philibert S, et al. Recurrences of atrial fibrillation despite durable pulmonary vein isolation: the PARTY-PVI study. Circ Arrhythm Electrophysiol. (2023) 16(3):e011354. doi: 10.1161/CIRCEP.122.011354
25. Zhuang J, Wang Y, Tang K, Li X, Peng W, Liang C, et al. Association between left atrial size and atrial fibrillation recurrence after single circumferential pulmonary vein isolation: a systematic review and meta-analysis of observational studies. Europace. (2012) 14(5):638–45. doi: 10.1093/europace/eur364
26. Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. (1995) 92(7):1954–68. doi: 10.1161/01.CIR.92.7.1954
27. Janse MJ. Why does atrial fibrillation occur? Eur Heart J. (1997) 18(Suppl C):C12–8. doi: 10.1093/eurheartj/18.suppl_C.12
28. Kuppahally SS, Akoum N, Badger TJ, Burgon NS, Haslam T, Kholmovski E, et al. Echocardiographic left atrial reverse remodeling after catheter ablation of atrial fibrillation is predicted by preablation delayed enhancement of left atrium by magnetic resonance imaging. Am Heart J. (2010) 160(5):877–84. doi: 10.1016/j.ahj.2010.07.003
29. Buckley BJR, Harrison SL, Gupta D, Fazio-Eynullayeva E, Underhill P, Lip GYH. Atrial fibrillation in patients with cardiomyopathy: prevalence and clinical outcomes from real-world data. J Am Heart Assoc. (2021) 10(23):e021970. doi: 10.1161/JAHA.121.021970
30. Fujimoto H, Doi N, Okayama S, Naito M, Kobori A, Kaitani K, et al. Long-term prognosis of patients undergoing radiofrequency catheter ablation for atrial fibrillation: comparison between heart failure subtypes based on left ventricular ejection fraction. Europace. (2022) 24(4):576–86. doi: 10.1093/europace/euab201
31. Zhao L, Xu K, Jiang W, Zhou L, Wang Y, Zhang X, et al. Long-term outcomes of catheter ablation of atrial fibrillation in dilated cardiomyopathy. Int J Cardiol. (2015) 190:227–32. doi: 10.1016/j.ijcard.2015.04.186
32. Rillig A, Makimoto H, Wegner J, Lin T, Heeger C, Lemes C, et al. Six-year clinical outcomes after catheter ablation of atrial fibrillation in patients with impaired left ventricular function. J Cardiovasc Electrophysiol. (2015) 26(11):1169–79. doi: 10.1111/jce.12765
33. Merlo M, Pivetta A, Pinamonti B, Stolfo D, Zecchin M, Barbati G, et al. Long-term prognostic impact of therapeutic strategies in patients with idiopathic dilated cardiomyopathy: changing mortality over the last 30 years. Eur J Heart Fail. (2014) 16(3):317–24. doi: 10.1002/ejhf.16
34. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 79(17):1757–80. doi: 10.1016/j.jacc.2021.12.011
Keywords: atrial fibrillation, dilated cardiomyopathy, catheter ablation, mortality, clinical outcomes
Citation: Siow Y-K, Lin C-Y, Chung F-P, Lin Y-J, Chang S-L, Lo L-W, Hu Y-F, Liao J-N, Chang T-Y, Tuan T-C, Kuo L, Wu Cheng-I, Liu C-M, Liu S-H, Li G-Y, Kuo M-J, Wu S-J, Bautista JA, Huang Y-S, Nguyen DSN and Chen S-A (2024) Catheter ablation in patients with atrial fibrillation and dilated cardiomyopathy. Front. Cardiovasc. Med. 11:1305485. doi: 10.3389/fcvm.2024.1305485
Received: 1 October 2023; Accepted: 3 January 2024;
Published: 16 January 2024.
Edited by:
Harry Boardman, Milton Keynes University Hospital, United KingdomReviewed by:
Lingling Wu, University of Alabama at Birmingham, United StatesChun-Li Wang, Linkou Chang Gung Memorial Hospital, Taiwan
© 2024 Siow, Lin, Chung, Lin, Chang, Lo, Hu, Liao, Chang, Tuan, Kuo, Wu, Liu, Liu, Li, Kuo, Wu, Bautista, Huang, Nguyen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chin-Yu Lin clouaa@gmail.com
†These authors have contributed equally to this work
Abbreviations ACEi, angiotensin-converting enzyme inhibitors; AF, atrial fibrillation; ARB, angiotensin receptor blockers; AT, atrial tachycardia; CA, catheter ablation; CTI, cavotricuspid isthmus; DCM, dilated cardiomyopathy; ECG, electrocardiography; GDMT, guideline-directed medical therapy; HF, heart failure; LA, left atrium; LAD, left atrial diameter; LV, left ventricular; LVEF, left ventricular ejection fraction; LVIDd, left ventricular internal dimension in diastole; MACE, major adverse cardiovascular events; PS, propensity scores; PV, pulmonary vein; PVI, pulmonary vein isolation.
 Yoon-Kee Siow1,2,†
Yoon-Kee Siow1,2,†