- 1Department of Cardiology and Vascular Medicine, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia
- 2Makassar Cardiac Center, Dr. Wahidin Sudirohusodo General Teaching Hospital, Makassar, Indonesia
- 3Department of Public Health and Community Medicine, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia
- 4Heart and Lung Division, Department of Cardiology, University Medical Center Utrecht, Utrecht, Netherlands
- 5Faculty of Medicine, Hasanuddin University, Makassar, Indonesia
- 6Netherlands Heart Institute, Utrecht, Netherlands
- 7Department of Physiology, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia
- 8Doctoral Study Program, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia
Background: Hyperglycemia, characterized by elevated blood glucose levels, is frequently observed in patients with acute coronary syndrome, including ST-elevation myocardial infarction (STEMI). There are conflicting sources regarding the relationship between hyperglycemia and outcomes in STEMI patients. We aimed to compile evidence to assess the association between hyperglycemia and adverse outcomes.
Methods: We conducted a comprehensive search for articles on PubMed and Embase using search strategies which yielded 4,061 articles. After full-text screening, 66 articles were included for systematic review, and 62 articles were further selected for meta-analysis.
Results: The 66 included articles spanned the years 2005–2023. Of these, 45 articles reported admission blood glucose, 13 articles used HbA1c, and 7 articles studied fasting blood glucose. Most studies defined STEMI with primary PCI as their inclusion criteria. Mortality was the most often outcome reported related to hyperglycemia. Overall, 55 (83.3%) studies were at low risk of bias. Both admission and fasting blood glucose were significantly related to short- and long-term mortality after STEMI, with a pooled risk ratio (RR) of 3.02 (95%CI: 2.65–3.45) and 4.47 (95% CI: 2.54–7.87), respectively. HbA1c showed substantial association with long-term mortality (HR 1.69, 95% CI: 1.31–2.18)) with a pooled RR of 1.58 (95% CI 1.26–1.97). In subsequent analyses, admission hyperglycemia was associated with an increased risk of reinfarction (pooled RR 1.69, 95% CI 1.31–2.17), heart failure (pooled RR 1.56, 95% CI: 1.37–1.77), cardiogenic shock (pooled RR 3.68, 95% CI 2.65–5.11), repeat PCI or stent thrombosis (pooled RR 1.99, 95% CI 1.21–3.28), and composite major adverse cardiac and cerebrovascular events (MACCE) (pooled RR 1.99, 95% CI: 1.54–2.58).
Conclusions: Our study demonstrated that hyperglycemia has a strong association with poor outcomes after STEMI. Admission and fasting blood glucose are predictors for short-term outcomes, while HbA1c is more appropriate for predicting longer-term outcomes in STEMI patients.
Systematic Review Registration: PROSPERO 2021 (CRD42021292985).
Introduction
Coronary heart disease (CHD) is a leading cause of morbidity and mortality worldwide. The most common type of CHD is acute myocardial infarction (MI). Each year, it is reported that at least 15% of deaths are caused by MI, with the majority presenting as ST-elevation myocardial infarction (STEMI) (1). To date, there is increasing evidence reporting an association between the incidence of hyperglycemia and poor outcomes in STEMI patients. Several studies have demonstrated that hyperglycemia significantly increased mortality in patients with STEMI, both during the hospital stay and several days or months after the first diagnosis (2–4). Other studies have also reported a significant association between hyperglycemia in STEMI patients and reperfusion failure (5–7).
Hyperglycemia is a frequent condition observed in patients with acute MI, even in the absence of a history of diabetes mellitus. The pathological stress response, a series of neurohormonal reactions activated during acute MI, leads to excessive sympathetic nerve activation, resulting in elevated blood sugar levels (5, 8, 9). However, the precise mechanism by which hyperglycemia contributes to poor outcomes in STEMI patients is currently not clearly understood. Therefore, the primary objective of this systematic review is to summarize the evidence regarding the association between stress-induced hyperglycemia and adverse clinical outcomes in patients with ST-elevation Myocardial Infarction (STEMI). We explicitly formulate the review questions as follows: (1) Does hyperglycemia increase the risk of mortality and major adverse cardiac and cerebrovascular events (MACCE) in patients with STEMI? (2) What is the optimal cutoff point for diagnosing hyperglycemia in critically ill patients, especially those with STEMI?
Methods search strategy
This review was registered in The International Prospective Register of Systematic Reviews (PROSPERO) database (registration number CRD42021292985) and conducted in accordance with the PRISMA guidelines (10). The literature search was performed in December 2021 and additionally in January 2024 to cover the recently updated literature. We utilized the following databases: PubMed (Medline), EMBASE (Ovid), and Web of Science. We employed a combination of MeSH terms and free-text words to identify relevant articles. Additionally, we screened the reference lists of published reviews to identify any additional relevant studies. Detailed information regarding the search strategy is presented below:
#1. Hyperglycemia OR stress hyperglycemia OR high blood sugar OR blood glucose OR diabetes
#2. Coronary heart disease OR Acute coronary syndrome OR acute myocardial infarction OR ST-elevation myocardial infarction.
#3. Prognosis OR outcomes OR adverse events OR MACE OR MACCE OR mortality OR death OR cardiovascular death OR re-hospitalization OR recurrent MI OR re-infarction OR recurrent HF OR stent thrombosis OR repeat PCI OR emergency CABG OR recurrent stroke.
#1 AND #2 AND #3 AND #4 NOT animal.
Our detailed search strategies were provided as (Supplementary Table S1).
Eligibility criteria
Studies meeting the following criteria were included in this systematic review and meta-analysis:
(1) Cohort (prospective or retrospective) or case-control studies conducted in STEMI patients.
(2) Outcomes were clearly defined as follows: mortality (in-hospital, at 30 days, at 6 months, or >6 months after discharge), recurrent MI, stent thrombosis or repeat PCI, emergency CABG, development of heart failure (acute pulmonary edema), cardiogenic shock, stroke, and longer hospitalization.
(3) Blood glucose or hyperglycemia was quantified (at admission, within 24 h, within >24 h of admission, or at another specific time during hospitalization).
(4) Sufficient data on clinical outcomes (mortality, MACCE, or other complications as previously stated), including relative risks (RR), odds risks (OR), Hazard Ratio (HR), and their corresponding 95% confidence interval (CI).
In the case of serial or updated articles based on the same study, only the last published report was selected for analysis, while previous reports were considered supplementary for missing data where applicable. Only full articles in English were included. A manual search and snowballing method for additional relevant studies using references from retrieved articles were also completed. Articles were searched independently by two investigators (AQ and NQ), and all included abstracts were exclusively collected using the Rayyan—Intelligent Systematic Review application (https://www.rayyan.ai) for further screening.
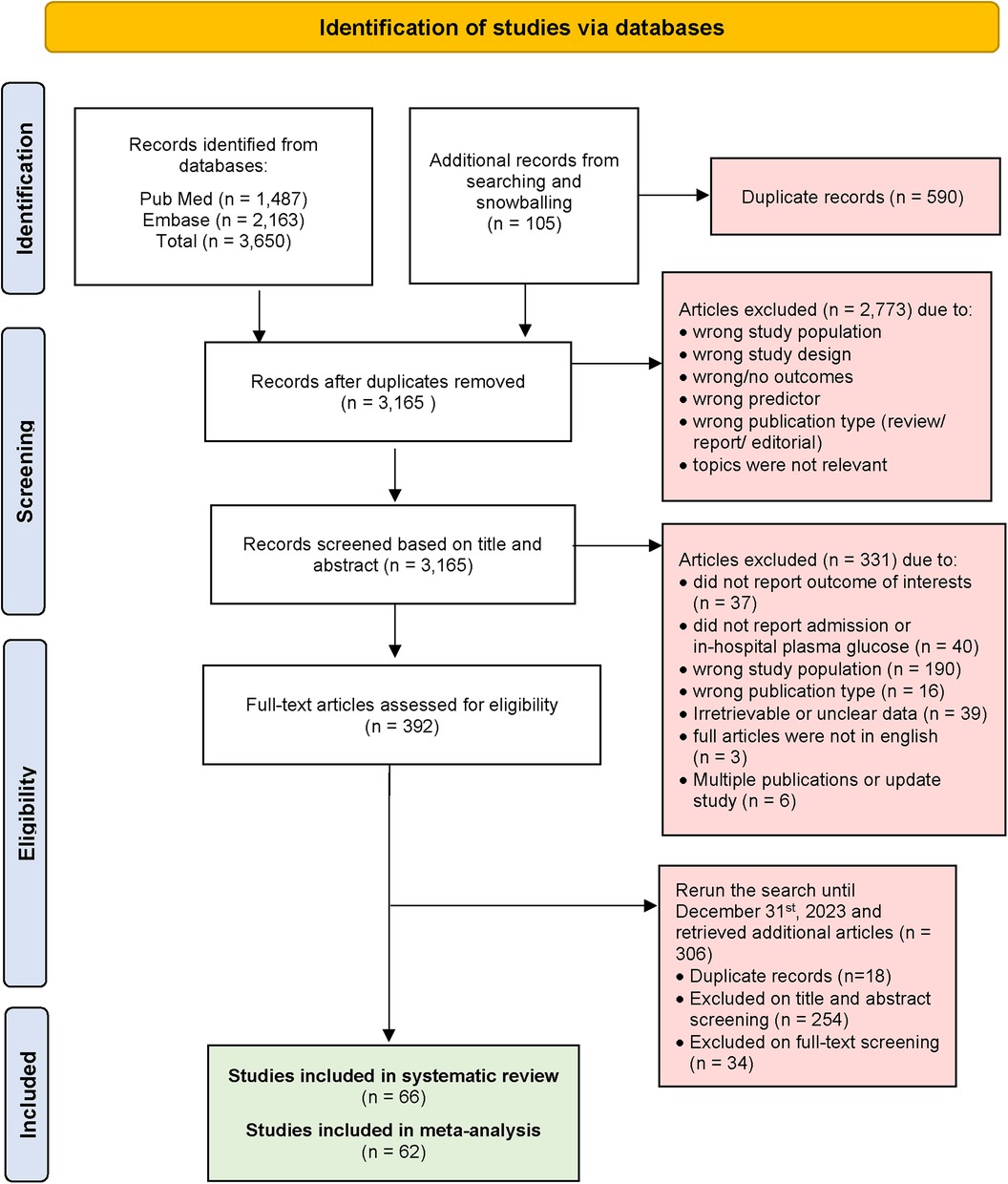
Generally, we excluded studies if the abstract or full-text paper in English were not accessible. Studies were excluded if they lacked sufficient information to calculate RR. Additionally, cohort studies that did not explicitly report the proportion of patients followed up, blood glucose measurement, and those that followed up less than 70% of patients were also excluded. Detailed reasons for study exclusion are clearly reported in the PRISMA flow chart (see Figure 1).
Study selection
Following the literature search, AQ and NQ independently screened the titles and abstracts. Any disagreements were resolved through mutual consensus. Literature studies that met the eligibility criteria were included, while those that did not meet the criteria were excluded with specific reasons provided. Conflicts in selecting the studies were discussed, and additional judgment was provided by the third reviewer (AHA) until a consensus was reached.
Data extraction
Three investigators (AQ, NQ, and AT) independently screened the full-text article, performed data extraction, and assessed the risk of bias in each individual study. Any discrepancies in data were resolved by reviewing the primary data from the original articles. We employed a standardized data extraction approach using a Google form and Microsoft Excel. The extracted variables included the first author's name and year of publication, study design, country, recruitment period, follow-up duration, study population, sample size, male gender, age, hyperglycemia definition and cut off, measured outcomes, and conclusions (OR, RR or HR).
The extracted outcome data, including mortality and MACCE, were analyzed for both hyperglycemic and non-hyperglycemic group. In cases where the included literature study had incomplete or inaccessible data, the study was excluded with the agreement of the reviewers.
Definition
ST-elevation myocardial infarction (STEMI) was defined as the presence of at least two contiguous leads with ST-segment elevation ≥2.5 mm in men under 40 years, ≥ 2 mm in men aged 40 years or older, or ≥1.5 mm in women in leads V2-V3 and/or ≥1 mm in the other leads (11). Patients were classified as having diabetes if they had a reported history of diabetes. Glycated haemoglobin (HbA1C) and blood glucose levels on admission were not integrated because HbA1C was not measured in all studies, and stress glucose concentrations corresponding to diabetes cutoff values (i.e., fasting plasma glucose of 7.0 mmol/L or 2 h glucose 11.1 mmol/L on a 75 g oral glucose tolerance test) were undefined. Hyperglycemia was defined according to the definitions used in individual studies, resulting in varying threshold glucose concentrations to define hyperglycemia across studies.
Quality assessment
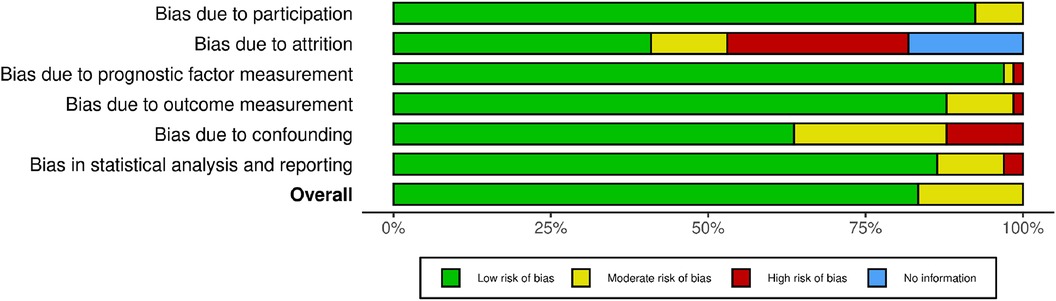
To assess the individual studies, we evaluated the risk of bias using the QUIPS (Quality in Prognosis Study) tool (12). This tool assesses six domains: study participation, study attrition (for cohorts), prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting. Each domain was graded as low, moderate, or high risk of bias, with specific appraisal criteria for each domain. The overall risk of bias for each individual study was graded as follows: high risk of bias (if ≥4 of 6 domains showed high risks), medium risk of bias (if ≥4 of 6 domains showed medium risk), and low risk of bias (if ≥4 of 6 domains showed low risk).
Statistical analysis
We conducted a meta-analysis of the included studies using a random-effects model. Point estimates of RR, OR, or HR, along with their respective 95% confidence intervals (CIs), were pooled from each individual study to assess the association between hyperglycemia and clinical outcomes in STEMI patients. Heterogeneity in effect size estimates across these studies was examined using the Chi- squared test and quantified using the I2 statistic. The I2 statistic, with values ranging from 0 to 100% was classified as follows: low heterogeneity (if I2 < 25%), moderate heterogeneity (if I2 = 25%–49%), and substantial heterogeneity (if I2 > 50%). Publication bias was assessed using a funnel plot and the Egger's regression test, with p < 0.05 considered statistically significant. All data analyses were performed using Review Manager (RevMan) 5.4, SPSS ver. 26 for mac, and RStudio veer. 1.4.1564 for Windows.
Results
Study selection
At initial systematic searching, we retrieved a total of 3,650 articles from PubMed and Embase using the search strategy. An additional 105 articles were identified through snowballing of selected articles and previous systematic reviews. After excluding 590 duplicate articles, 3,165 articles were screened based on their titles and abstracts. This process left us with 392 articles for full-text review. Then we extended our search to include studies published until 31st December 2023, and found 306 articles with 18 duplicates. Articles were excluded if they did not report blood glucose level parameters or outcomes of interest. Additionally, articles were excluded if data from STEMI patients were not extractable. Ultimately, 66 articles were included for systematic review, and after data assessment, 62 articles were included for meta-analysis. A visual representation of the screening process is provided in Figure 1 (PRISMA flow diagram).
Study characteristics
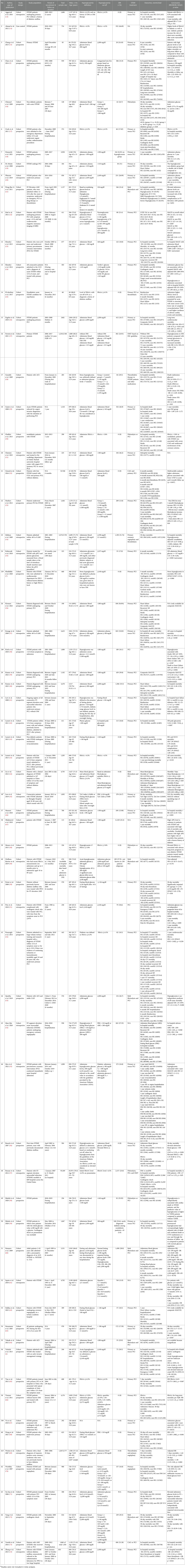
We summarized the 66 included articles in Table 1. The publication years of the included studies ranged from 2005 to 2023. In our additional literature searching from January 2022 to December 2023, we incorporated five articles that were employed in both systematic reviews and meta-analyses (n = 66). Among these, 46 articles were prospective cohort studies, 19 were retrospective cohort studies, and 1 was a case-control study. Hyperglycemia was defined using various parameters: 47 articles used admission blood glucose, 13 used HbA1c, and 7 used fasting blood glucose. Other parameters such as mean and peak glucose, as well as triglyceride-glucose (TyG) index, were also reported in some studies, however we did not include these parameters in our meta-analysis. Most articles reported mortality at different time points (i.e., in-hospital, 30-day, 6-month, >6-month mortality). Other outcomes analyzed in this review included recurrent myocardial infarction, heart failure, stroke, cardiogenic shock, repeat PCI, emergency CABG, and composite MACCE.
Participant's characteristics
In total, 164,927 patients were included in the study, with 72.9% being male, and a mean age of 58.5 years. Only a third of the patients had a known history of diabetes mellitus. The majority of the patients received emergency or primary PCI as the treatment for STEMI. The minimum blood glucose level used as a hyperglycemia cutoff was 110 mg/dl.
Quality assessment
We assessed the quality of the included studies using the Quality of Prognosis Studies (QUIPS) tool, designed for the evaluation of risk of bias in observational cohort studies. The summary of the quality assessment is presented in Figure 2 and Supplementary Figure S1, indicating a range of quality from low to moderate risk.
Hyperglycemia and STEMI outcomes
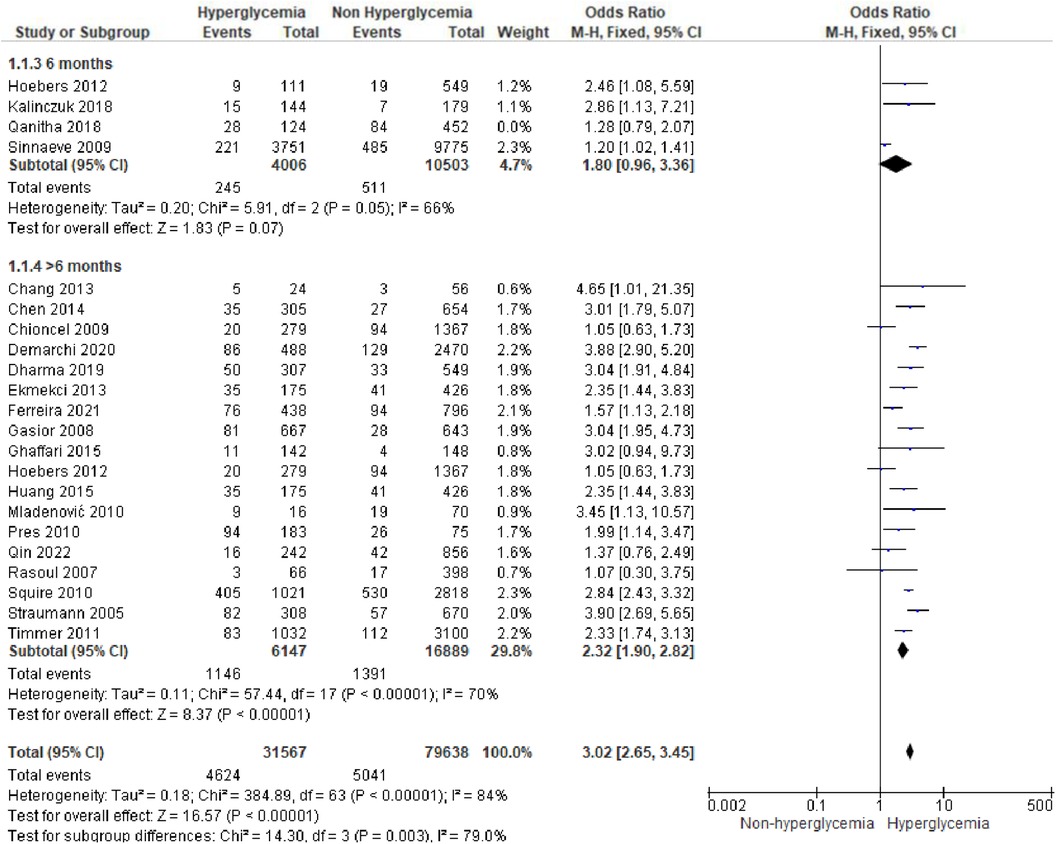
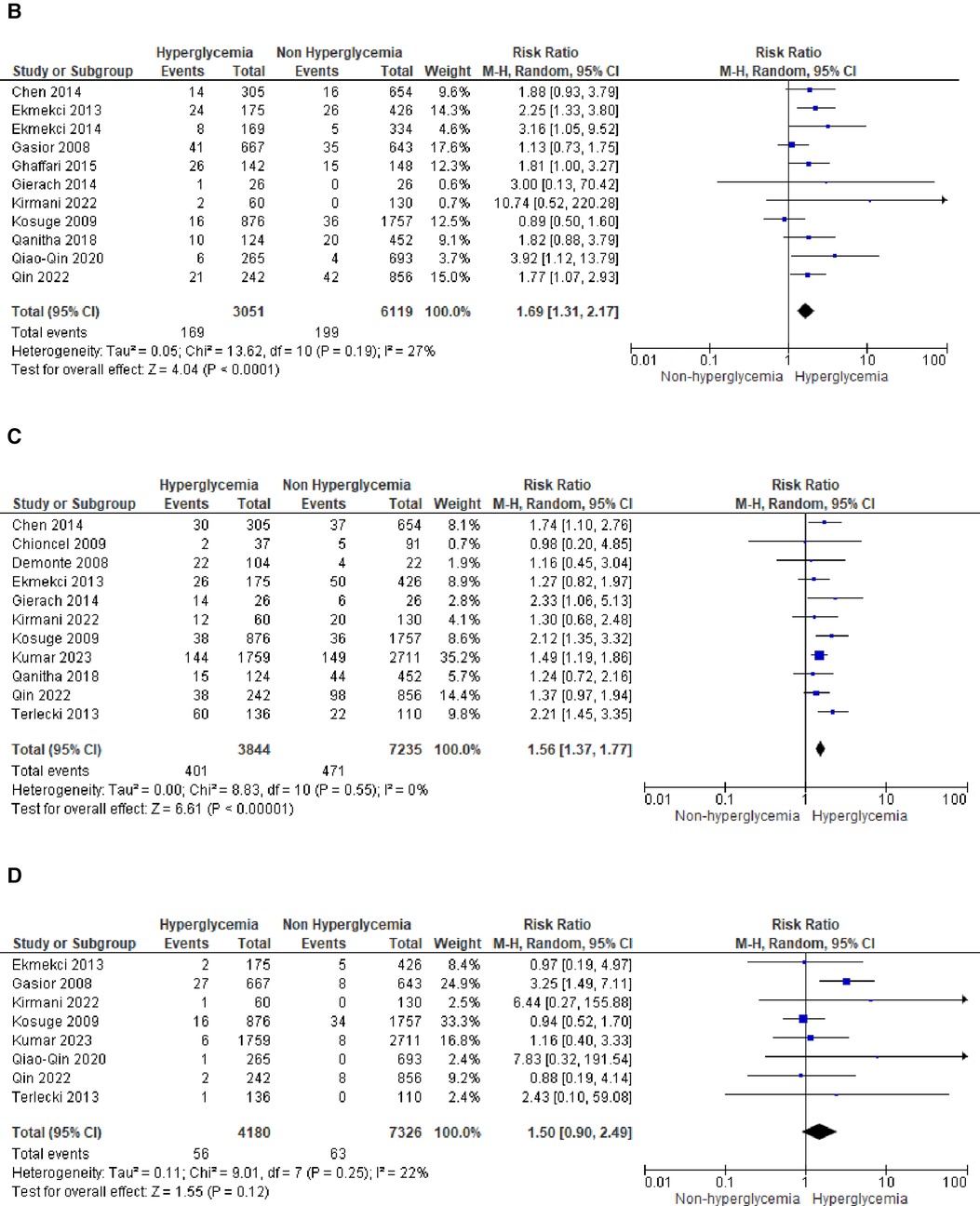
We analyzed outcomes in subgroups based on glucose parameters: admission blood glucose, HbA1c, and fasting blood glucose. Results were presented in forest plots analyzed using random effects. Pooled risk ratio (RR) and 95% confidence interval (CIs) were calculated for each outcome (Figures 3A–C).
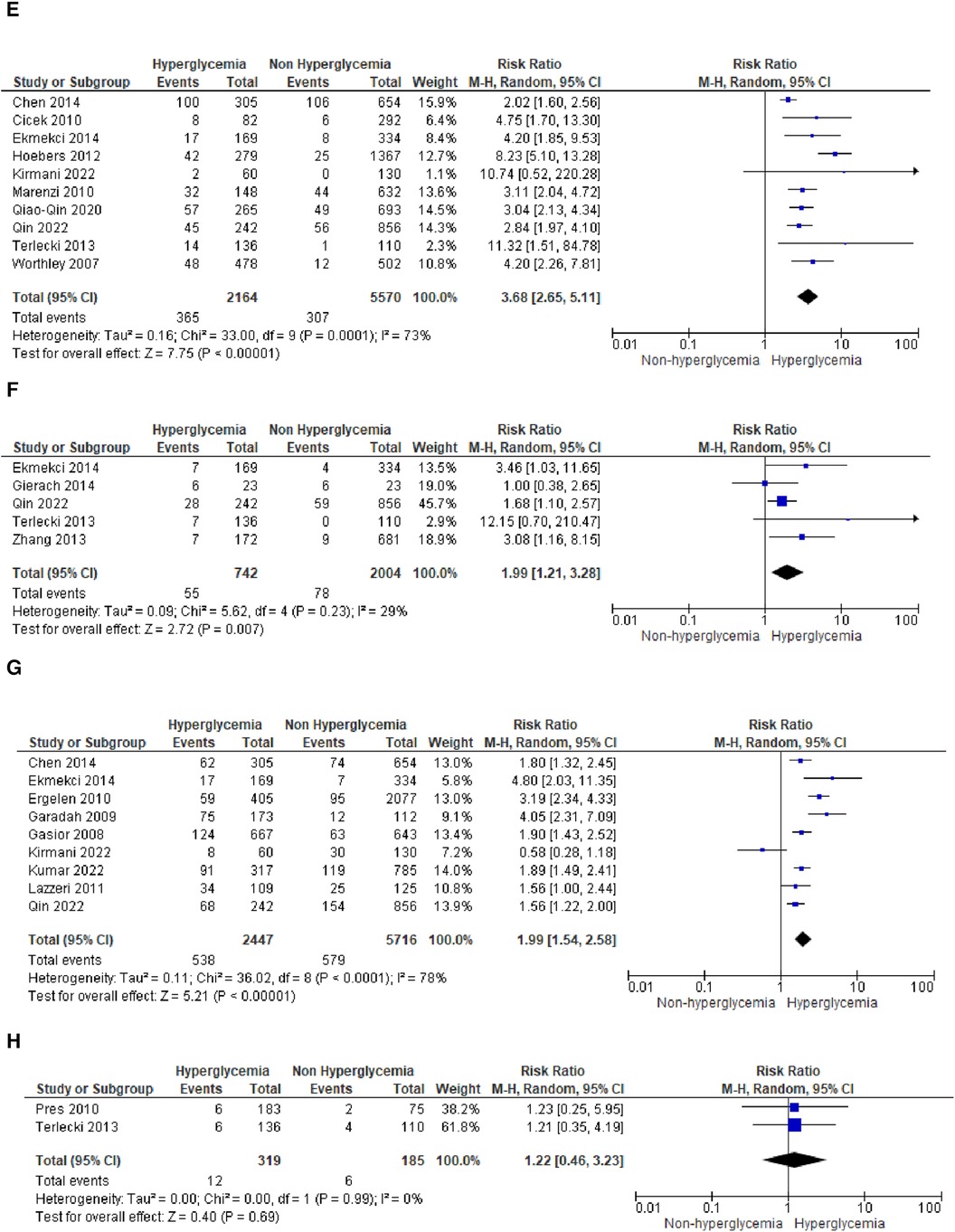
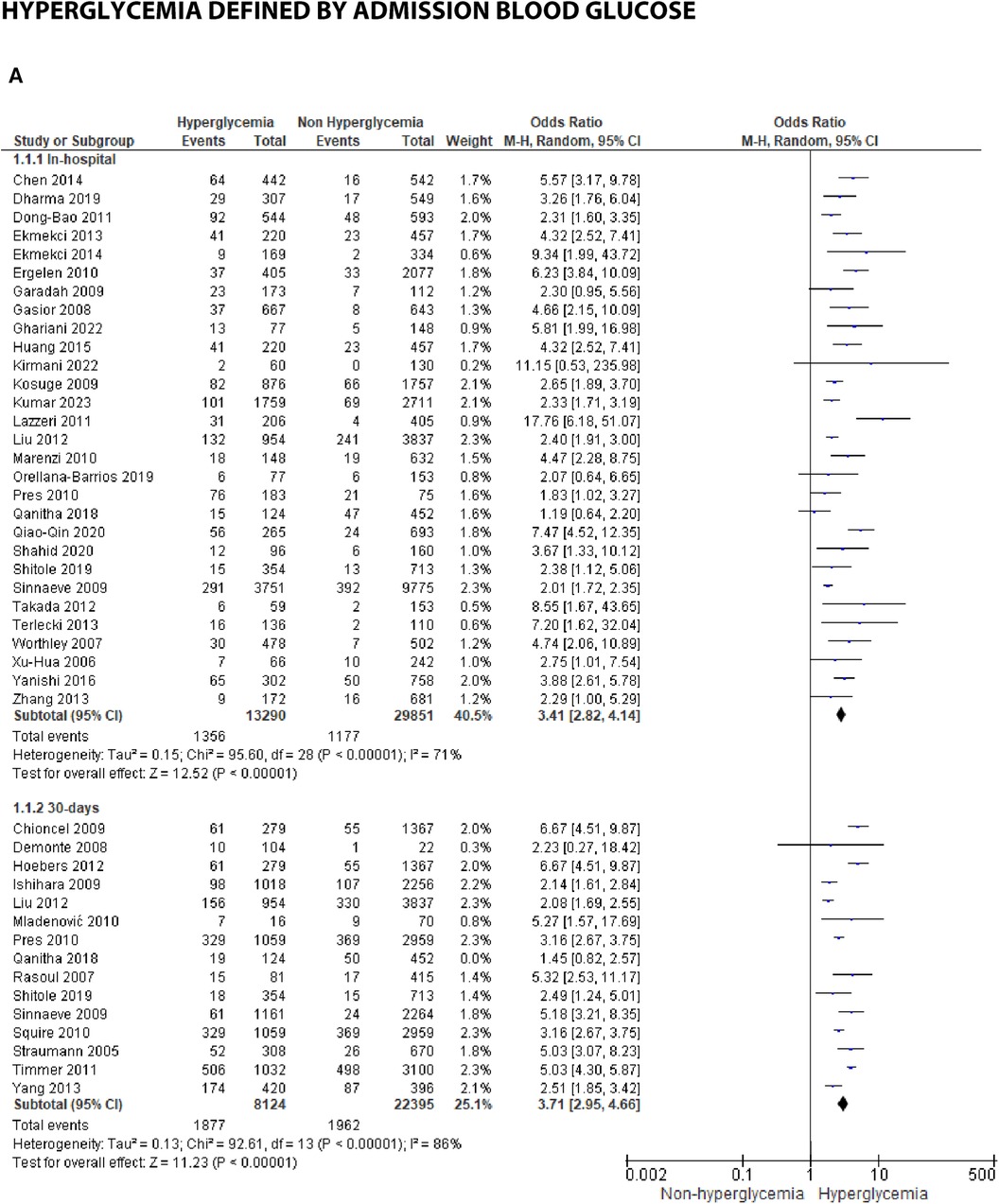
Figure 3A. Forest plot of studies using admission blood glucose as a parameter for hyperglycemia with outcomes of (A) mortality (in-hospital, 30 days, 6 months, and >6 months), (B) reinfarction or recurrent MI, (C) heart failure, (D) stroke, (E) cardiogenic shock, (F) repeat PCI or stent thrombosis, (G) composite MACCE, and (H) emergency CABG.
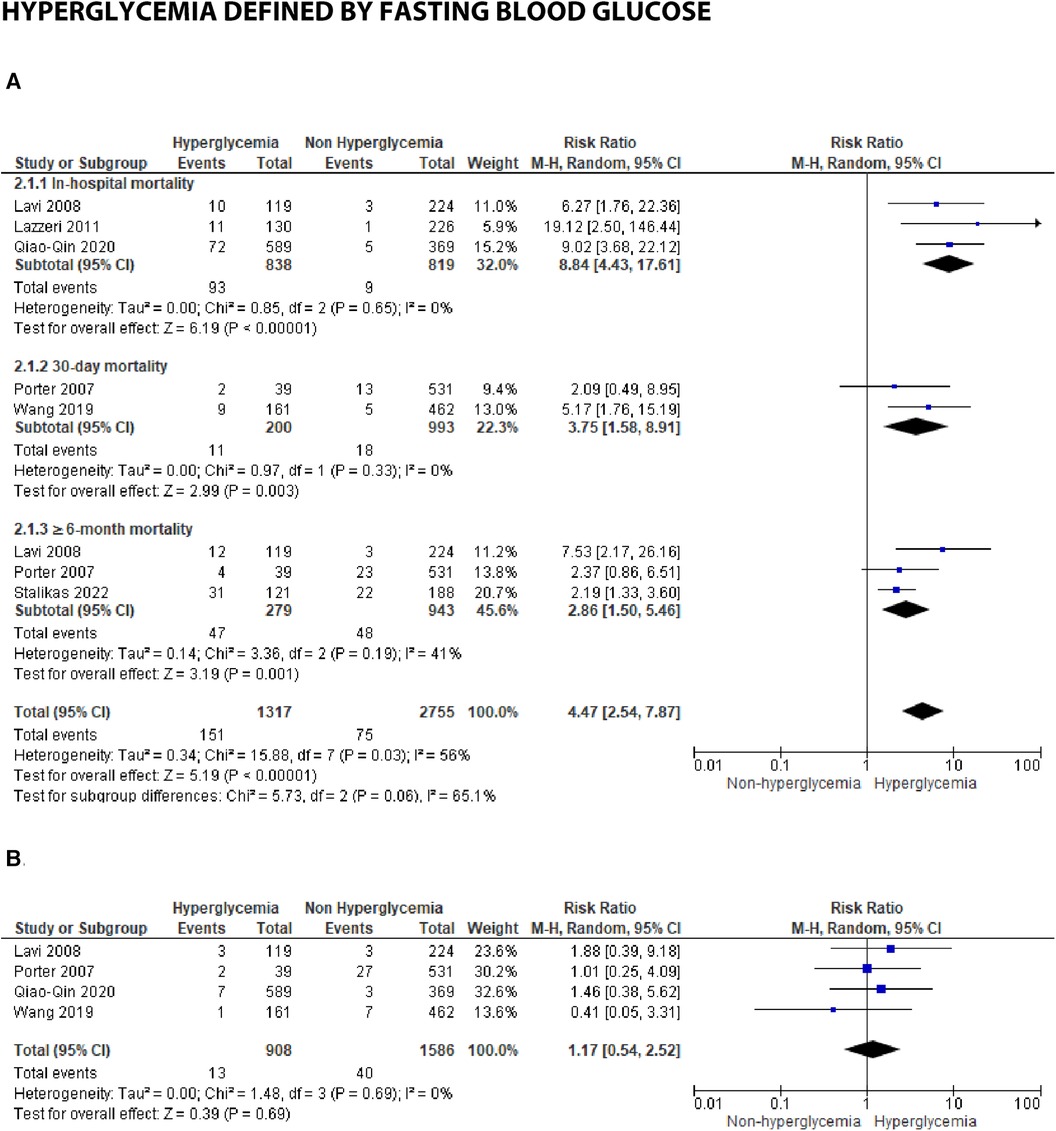
Figure 3B. Forest plot of studies using fasting blood glucose as a parameter for hyperglycemia with outcomes of (A) mortality (in-hospital, 30 days, and ≥6 months), and (B) reinfarction or recurrent MI.
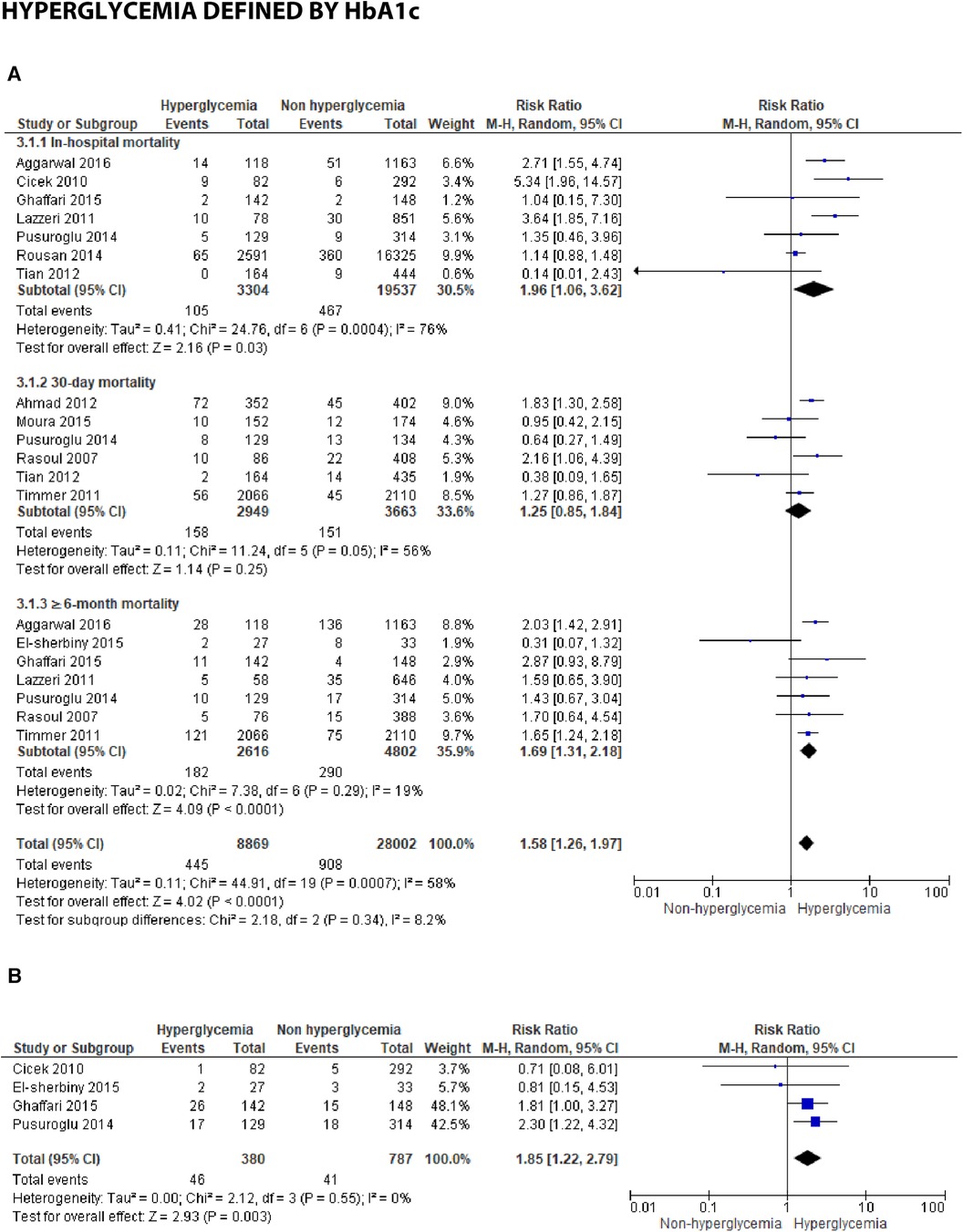
Figure 3C. Forest plot of studies using HbA1c as a parameter for hyperglycemia with outcomes of (A) mortality (in-hospital, 30 days, and ≥6 months), and (B) reinfarction or recurrent MI.
Hyperglycemia defined by admission blood glucose
(A) Mortality
Mortality outcomes in STEMI patients, using admission blood glucose as the predictor, were categorized into subgroups based on the time frame. Twenty-nine included studies assessing in-hospital mortality outcomes revealed a pooled risk ratio of 3.41 (2.82–4.14), underscoring the association between elevated admission blood glucose levels and the risk of in-hospital mortality. However, there was significant heterogeneity among studies in this subgroup. For 30-day mortality, 14 studies were included, yielding a pooled risk ratio of 3.71 (2.95–4.66) with I2 = 86% indicating substantial heterogeneity among these studies. Similarly, for 6-month and >6-month mortality, both displayed consistent pooled risk ratios exceeding 1 with heterogeneity ranging between 66%–70%. Consequently, admission blood glucose exhibited a significant association with mortality across all time frames. However, when subjected to subgroup analysis, we identified an I2 value of 79.0% with p = 0.003, indicating considerable heterogeneity.
(B) Recurrent MI
The pooled risk ratio for recurrent MI and admission blood glucose levels demonstrated a statistically significant result of 1.69 (1.31, 2.17) with I2 = 27% (p = 0.19), indicating only slight heterogeneity among the included studies.
(C) Heart failure
In the case of heart failure, admission blood glucose also exhibited a pooled risk ratio of 1.56 (1.37, 1.77) with I2 = 0% (p = 0.55) and a Z-score for overall effect of 6.61 (p < 0.001). This outcome indicates a robust association between admission blood glucose levels and heart failure within the homogeneous STEMI population.
(D) Stroke
There was no observed association between stroke and admission blood glucose levels, as indicated by the pooled risk ratio of 1.50 (0.90, 2.49) and the overall effect (Z = 1.55, p = 0.12). The total combined events from both groups amounted to only 109.
(E) Cardiogenic shock
A pooled RR of 3.68 (2.65, 5.11) was identified for cardiogenic shock following STEMI. However, a notable level of heterogeneity was observed among the included studies, with I2 of 73%.
(F) Repeat PCI
The likelihood of requiring repeat percutaneous coronary intervention (PCI) was higher within the slightly heterogeneous STEMI population with elevated admission blood glucose levels. This was evident from the analysis of this outcome subgroup, which yielded a pooled RR of 1.99 (1.21, 3.28).
(G) Composite MACCE
Nine of the included studies combined adverse effects following STEMI into the MACCE group. It was determined that MACCE and admission blood glucose were significantly correlated, with a total pooled RR of 1.99 (1.545, 2.58), and a heterogeneity level of I2 = 78% (p < 0.001).
(H) Emergency CABG
Two of the included studies analyzed emergency CABG as the outcome of interest. Admission blood glucose was shown to be not correlated emergency CABG, with a total pooled RR of 1.22 (0.46, 3.23), and a heterogeneity level of I2 = 0% (p = 0.99).
Hyperglycemia defined by fasting blood glucose
Seven studies reported fasting blood glucose (FBG) as their parameter. Further analysis using meta-analysis explored the association between fasting blood glucose and in-hospital, 30-day, and ≥6-month mortality, as well as recurrent myocardial infarction.
(A) Mortality
Three studies were included in the subgroup assessing in-hospital mortality. The calculated pooled RR was 8.84 (4.43, 17.61) with minimal heterogeneity (I2 = 0%, p = 0.65). Despite the smaller number of patients and studies compared to admission blood glucose, the risk ratio between FBG and in-hospital mortality was higher, approximately 2–3 times that of admission blood glucose's risk.
Similarly, the pooled risk ratios in the 30-day and ≥6-month mortality subgroups were higher compared to admission blood glucose, as depicted in the forest plot [30-days: 3.75 (1.58, 8.91) and ≥6 months: 2.86 (1.50, 5.46)]. Heterogeneity among the included studies in these subgroups was lower when compared to the same groups using admission blood glucose as the parameter (I2 = 0%–41%).
(B) Reinfarction or recurrent MI
No significant association was found between FBG and recurrent myocardial infarction, with a total pooled RR of 1.17 (0.54, 2.52). Only four studies reported recurrent myocardial infarction for this blood glucose parameter, and low heterogeneity was observed among these studies (I2 = 0%, p = 0.69.)
Hyperglycemia defined by HbA1c
(A) Mortality
In studies employing HbA1c, several time points were reported, including in-hospital, 30-day, and ≥6-month mortality. An association was identified between HbA1c and in-hospital mortality, with a pooled RR of 1.96 (1.06, 3.62). However, the test revealed substantial heterogeneity, with an I2 = 76% (p = 0.0004), among the included studies. Additionally, for 30-day mortality, the result was insignificant with a pooled RR of 1.25 (0.85, 1.84) and high heterogeneity (I2 = 56%, p = 0.05). Furthermore, a significant association was found between increased HbA1c and mortality over 6 months post-STEMI, with a pooled RR of 1.69 (1.31, 2.18) and low heterogeneity (I2 = 19%, p = 0.0007) among the studies. When analyzing the overall pooled risk ratio, HbA1c demonstrated a statistically significant association with overall mortality, with an RR of 1.58 (1.26, 1.97). Subgroup difference testing reported no difference between different mortality periods (I2 = 8.2%, p = 0.34).
(B) ≥6-month recurrent myocardial infarction
An association was identified between the incidence of ≥6-month reinfarction and HbA1c, with a pooled risk ratio 1.85 (1.22, 2.79), within the homogenous studies (I2 = 0%, p = 0.55).
Publication bias
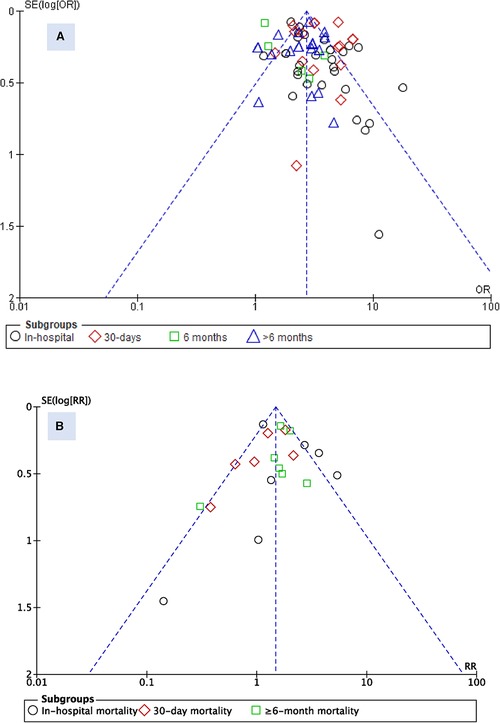
To assess for publication bias between studies, a funnel plot was used for visual assessment. While there is a probability of underreporting of studies with lower risk ratios, no substantial asymmetry was observed for the admission blood glucose parameter (Figure 4). However, for HbA1c, underreporting of studies with higher risk ratios was probable, particularly in the in-hospital mortality subgroup.
Summary of admission blood glucose's predicting accuracy
Efforts were made to determine the optimal cut-off for estimating mortality outcomes. The cut-off values used in the included studies to diagnose hyperglycemia within the populations were applied. It was discovered from the summary ROC curve that the AUC for admission blood glucose is 0.688, indicating that admission blood glucose is a reliable predictor for mortality outcome. The summary estimate reveals a sensitivity of 0.594 and a 1-specificity of 0.304, signifying that when using the optimal blood glucose cut-offs included, there is a 59.4% chance of correctly predicting outcomes in those with hyperglycemia, but a 30.4% tendency to incorrectly estimate the probability of those outcomes (Figure 5).
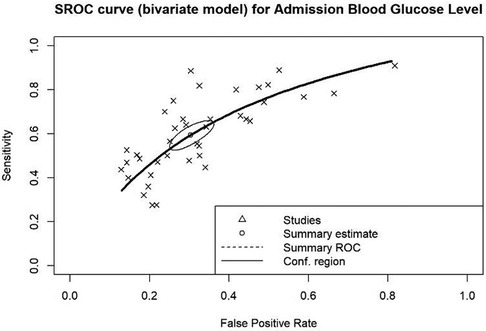
Figure 5. Summary receiver operating characteristics (ROC) curve for overall admission blood glucose levels (AUC: 0.688).
Discussion
Summary of main findings
After conducting a comprehensive review of the full-text articles, we identified 47 studies that utilized admission blood glucose, 13 studies that relied on HbA1c, and 7 articles that examined fasting blood glucose. The majority of the studies focused on primary PCI as their inclusion criteria, making it outside the scope of this systematic review to compare clinical outcomes between patients who underwent primary PCI and those who received optimal medical treatment. The most commonly reported outcomes related to hyperglycemia were in-hospital and 30-day mortality. Both admission and fasting blood glucose levels were significantly associated with in-hospital and short-term mortality following STEMI.
Among the 164,927 STEMI patients included, hyperglycemia upon admission was significantly related to all-cause mortality, irrespective of the follow-up period, with an RR of 3.02 (95%CI: 2.65–3.45). A more detailed analysis revealed that admission hyperglycemia independently predicted all-cause mortality, including in-hospital (RR 3.41, 95% CI: 2.82–4.14), at 30 days (RR 3.71, 95% CI: 2.95–4.66), at 6 months (RR 1.80, 95% CI: 0.96–3.36), and over 6 months of follow-up (RR 2.32, 95% CI: 1.90–2.82). These findings align with a previous systematic review by Capes et al. [NO_PRINTED_FORM] confirming the significant impact of admission blood glucose levels on short-term outcomes in myocardial infarction patients. Notably, even in non-diabetic patients, higher HbA1c levels were associated with increased mortality (74).
In this meta-analysis, we explored the association between hyperglycemia, as indicated by three clinically relevant parameters (i.e., admission, fasting, and Hb1Ac) and a broader range of clinical outcomes, some of which have not been previously reported in a single systematic review. We observed a significant relation between admission hyperglycemia and other short-term outcomes, including reinfarction, heart failure, cardiogenic shock, repeat PCI, and composite MACCE. However, our analysis did not find a significant link between admission hyperglycemia and stroke.
Utilizing FBG as a parameter of hyperglycemia showed similar effects on short-term outcomes, but no significant association was found between FBG and recurrent MI at 6 months. Our findings align with Li et al.'s previous review, which suggested that admission blood glucose levels are influenced by both acute physiological stress and chronic baseline glycemic levels, particularly in patients with known diabetes mellitus (21). We agree with this assessment, as our results using HbA1c as an indicator of acute hyperglycemia showed a significant association between hyperglycemia and mortality over a 6-month period, with a pooled RR of 1.69 (95% CI: 1.31–2.18). However, this association was not observed in short-term outcomes.
We conducted a subgroup analysis to examine the association between admission blood glucose and mortality, stratifying patients into those with and without DM. Our supplementary analysis revealed an elevated risk of mortality in both patient groups when presenting with hyperglycemia on admission, specifically observing a 46% increase in patients with DM and a 71% increase in patients without DM (Supplementary Figures S2,S3). Notably, the heightened mortality risk in patients with DM was observed primarily in the short term, but not in the longer observation term (>30-days). Numerous studies incorporated into our systematic review have indicated that, following multivariable analysis, elevated admission blood glucose levels in patients without DM emerged as an independent predictor of mortality. Conversely, this independent predictive association was not observed in patients with DM (18, 29, 34, 72). Direct comparison between DM vs. non-DM in STEMI patients who suffered from stresss hyperglycemia is shown in Supplementary Figure S4.
Noteworthy among these findings is the study conducted by Eitel et al. (22), which identified a correlation between higher myocardial injury and patients without DM. Additionally, the investigation by Ferreira et al (27) highlighted a noteworthy shift in the association between admission blood glucose levels and mortality risk among patients without DM, aligning more closely with those observed in patients with DM during long-term observations. In their recent meta-analysis, Karakasis et al. (75) proposed that the stress hyperglycemia ratio (SHR) might serve as a superior predictor compared to individual hyperglycemia parameters in assessing the association with MACE and mortality, regardless of the time period and diabetes status.
While we acknowledge the potential advantages of SHR, single hyperglycemia parameters such as admission blood glucose levels, fasting blood glucose, and HbA1c hold widespread recognition and routine utilization in clinical practice. These established metrics are familiar to clinicians, seamlessly integrated into diagnostic and management processes for cardiovascular events like ST-elevation myocardial infarction (STEMI), and readily available in standardized clinical settings. Although SHR offers the potential for more robust analysis and longer-term decision-making, the use of single hyperglycemia predictors remains advantageous for rapid and efficient clinical decisions in time-sensitive situations. This nuanced approach allows for a balanced integration of both methodologies, catering to the diverse needs of clinical practice.
Defining hyperglycemia and the cutoff level
Although numerous studies on stress hyperglycemia in the context of myocardial infarction have been published, there is currently no universally accepted definition for stress hyperglycemia in this setting. Previous studies has often relied on admission blood glucose for defining hyperglycemia (2, 3, 14–18). The most widely accepted definition for admission blood glucose is the first collected blood glucose measurement within 24 h of hospital admission. However, the specific cutoff point used to define hyperglycemia in STEMI patients has varied among previous studies.
In 2008, the American Heart Association (AHA) Scientific Statement on Hyperglycemia and Acute Coronary Syndrome suggested using a cutoff point of >140 mg/dl for admission blood glucose levels to define hyperglycemia in such cases (76). A prior meta-analysis by Capes et al. indicated that among non-diabetic patients with acute myocardial infarction, those with an admission glucose level >110 mg/dl had a 3.9 times higher relative risk of in-hospital mortality compared with the normoglycemic patients. Additionally, among diabetic patients with acute myocardial infarction, an increased risk of in-hospital mortality was observed only in those with admission glucose levels ≥180 mg/dl (74). Despite several studies investigating the effects of hyperglycemia on cardiovascular outcomes, a consensus on the definition or cut-off for acute hyperglycemia in myocardial infarction patients has not been reached (5).
In this systematic review, the included studies employed various cutoff points for admission (random) blood glucose: 19 studies used a level of ≥198 mg/dl (11.0 mmol/L); 7 studies used ≥180 mg/dl (10 mmol/L); 13 studies used ≥140 mg/dl (7.8 mmol/L); 2 studies used ≥126 mg/dl (7 mmol/L), and 1 study used cutoff >110 mg/dl. Additionally, 7 studies used a cutoff of ≥126 (7 mmol/L) for FBG. For the parameter of HbA1c, 6 studies employed a cut off of ≥6.5%, and 1 study used a cutoff of ≥5.5%. The selection of cutoff values for admission hyperglycemia is in line with the guidelines provided by the American Diabetes Association (ADA), which recommends initiating insulin therapy for persistent hyperglycemia >180 mg/dl (10 mmol/L) (74).
Based on these findings, we suggest adopting a lower cutoff point of ≥140 mg/dl (7.8 mmol/L) for non-diabetic patients as the definition of admission blood glucose hyperglycemia. For diabetic patients, a higher cutoff point of ≥180 mg/dl (10 mmol/L) could be considered. In the case of FBG, we recommend using a cutoff point of ≥126 mg/dl (7 mmol/L) to quantify stress hyperglycemia.
The variation in cutoff values for admission glucose and their predictive accuracy for adverse outcomes between diabetic and non-diabetic patients with acute myocardial infarction may be attributed to differences in baseline glucose metabolic status. Admission glucose levels are influenced by both acute physiological stress and chronic baseline glycemic levels, particularly in patients with established diabetes mellitus. Several criteria were used in this review to define diabetes mellitus, including fasting blood glucose ≥126 mg/dl (7 mmol/L), 2-h post-prandial blood glucose ≥200 mg/dl (11.1 mmol/L), HbA1c ≥ 6.5%, and classic symptoms of hyperglycemia with random blood glucose ≥200 mg/dl (11.1 mmol/L), or a self-reported history of diabetes or the use of hypoglycemic agents.
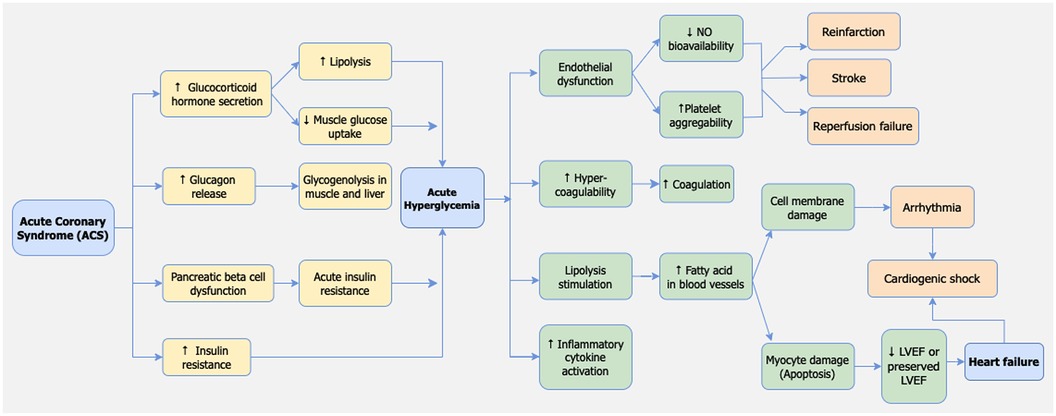
Pathophysiology of stress-induced hyperglycemia in STEMI
Hyperglycemia is a frequent response in patients with STEMI, even among those without a previous history of diabetes mellitus (77). Several mechanisms underlie the occurrence of hyperglycemia in myocardial infarction patients. The primary mechanism involves the activation of the sympathetic nervous system and heightened activity of the hypothalamic-pituitary axis, leading to the production of catecholamines and glucocorticoids, in the form of cortisol (78). Additionally, increased sympathetic nerve activity triggers the release of glucagon, which stimulates glycogenolysis in muscles and the liver, resulting in the breakdown of glycogen into glucose that enters the circulation (79, 80).
Other contributing mechanisms include dysfunction of pancreatic beta cells and insulin resistance due to acute stress conditions. The precise pathophysiology of these mechanisms remains unclear (78). However, previous studies have indicated a link between acute stress conditions during myocardial infarction and beta cell dysfunction, leading to reduced insulin production and increased insulin resistance. It is possible that hyperglycemia is merely an expression of stress and a consequence of the acute-phase reaction in the clinical setting of acute myocardial infarction. This reaction involves a series of neurohormonal responses activated during MI, leading to the overactivation of sympathetic nerves. Concurrently, the activation of stress-induced cortisol, noradrenaline, growth hormone, and glucagon release can disrupt glucose homeostasis and insulin secretion, resulting in insulin insufficiency and acute hyperglycemia. This mechanism is illustrated in Figure 6.
Mechanism on how hyperglycemia adversely affect the STEMI outcomes
Hyperglycemia is a common occurrence during the early stages of STEMI, even in patients with no previous history of diabetes mellitus. Increased sympatho-adrenergic activation and dysregulation of adrenergic receptors, such as beta-adrenergic receptors modulated by G protein coupled receptor kinase (GRK) (81), can contribute to various complications, including heart failure and arrhythmias (9).
Several mechanisms have been proposed to explain why hyperglycemia serves as a significant predictor of poor outcomes in STEMI patients, including arrhythmias, heart failure, and hospital death. First, Pres et al. reported that acute hyperglycemia in STEMI patients leads to vascular endothelial dysfunction (51). Similar findings were observed by Dong Bao et al. who demonstrated that endothelial dysfunction in the presence of acute hyperglycemia reduces the bioavailability of Nitric oxide (NO) and increases platelet aggregability. These effects can contribute to reperfusion failure in STEMI patients with acute hyperglycemia (21). Second, Kocas et al. reported that hyperglycemia in STEMI patients causes an increase in prothrombin fragments and factor VII levels while inhibiting tissue plasminogen activator. This leads to blood hypercoagulability in patients with hyperglycemia, further contributing to reperfusion failure (5). Third, hyperglycemia may reflect relative insulin deficiency, resulting in increased lipolysis and elevated levels of circulating free fatty acids. Pres et al. reported that hyperglycemia stimulates lipolysis, thereby increasing free fatty acid in blood flow. This increase in free fatty acids in the bloodstream can be toxic to the ischemic or infarcted myocardium, exacerbating myocyte injury, calcium overload, and arrhythmias. Insulin deficiency also reduces the myocardium's ability to utilize glucose anaerobically (51).
Furthermore, hyperglycemia-induced damage to the myocardium can lead to contractility disorders, adversely affecting the already ischemic or infarcted myocardium and reducing the left ventricular ejection fraction (LVEF) (51). In addition, Chen et al. reported that hyperglycemia is associated with increased activity of inflammatory cytokines, contributing to damage in heart muscle cells (Figure 6) (3).
Paradoxically, Kocas et al. reported that hypoglycemia in STEMI patients could also increase the risk of death. This suggests a U-shaped correlation between blood glucose levels and STEMI outcomes, where both hyperglycemia and hypoglycemia are associated with poor outcomes (5). This is supported by the study by Ishihara et al. who reported that hypoglycemia would adversely impact on STEMI patients outcomes. There are several factors contributing to hypoglycemia in STEMI patients, including the effects of anti-diabetic medications, hepatic gluconeogenesis dysfunction, and relative adrenal insufficiency (82). However, it's important to note that in our systematic review, we specifically focused on examining the relationship between hyperglycemia and outcomes in STEMI patients.
In experimental models, hyperglycemia has been associated with various detrimental effects. It leads to increased expression of adhesion molecules, enhances leukocyte adhesion to capillary walls, exacerbates free-radical-related reperfusion injury, and induces excessive apoptosis of endothelial cells. Furthermore, hyperglycemia augments the formation of microthrombi by increasing platelet aggregation and elevates circulating cytokine levels (including IL-6, IL-18, TNF- α, and CCL-2) contributing to instability in atheromatous plaques. Hyperglycemia may also disrupt endothelium-dependent vasodilatation and impair endogenous fibrinolysis (as depicted in Figure 7) (84). In addition to stress-induced hyperglycemia, long-lasting hyperglycemia, primarily stemming from underlying glucose metabolism abnormalities, can play a significant role in the progression of atherosclerosis, potentially leading to more extensive coronary artery disease. This persistent hyperglycemia can result in diffuse endothelial dysfunction, affecting both diastolic and systolic function in addition to the infarcted myocardium (85).
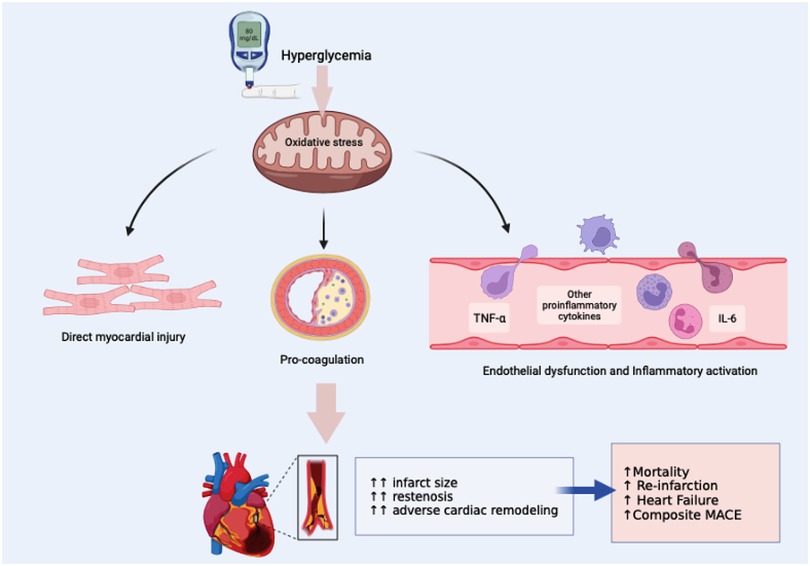
Figure 7. Mechanisms underlying the adverse effects of hyperglycemia in STEMI (figure modified from Li et al. (83)).
Hyperglycemia has secondary effects that include prothrombotic and proinflammatory effects. It can lead to the formation of thrombi in microvasculature, potentially accelerating remodeling of the surrounding myocardium while impeding collateral vascular development. Additionally, the recovery of stunned myocardium may be more challenging in a hyperglycemic environment, as hyperglycemia has been shown to be associated with several markers indicative of left ventricular dysfunction (85). Acute hyperglycemia aggravates platelet-dependent thrombus formation, attenuates endothelium-dependent vasodilatation, and reduces collateral blood flow by negatively affecting nitric oxide availability. These changes are linked to microvascular dysfunction before reperfusion and could influence the outcomes of interventions.
The impact of managing hyperglycemia on long-term patient outcomes has been explored in various studies. Rigorous glycemic control has demonstrated its effectiveness in reducing multiple organ failure, infection, and mortality both in the short and long term (86). However, navigating the complexities of hyperglycemia management in hospitalized patients, including those with diabetes, is intricate, and the treatments effect on long-term outcomes is influenced by various factors. For instance, findings from the Stroke Hyperglycemia Insulin Network Effort (SHINE) trial revealed that, among patients with acute ischemic stroke and hyperglycemia, the difference in functional outcomes between intensive and standard glucose control for up to 72 h was not statistically significant (87). Additionally, evidence from the The Diabetes Control and Complications Trial (DCCT), Epidemiology of Diabetes Interventions and Complications (EDIC), and the UK Prospective Diabetes Study (UKPDS) cohorts suggested that early control of hyperglycemia in recently diagnosed diabetes has a lasting impact on long-term outcomes (88). Hence, while intense glycemic control has demonstrated advantages in certain studies, the intricacies of hyperglycemia management and its implications for long-term outcomes represent a complex and evolving area of research.
To our knowledge, this is the first review to utilize a comprehensive meta-analysis to assess various clinical outcomes in STEMI patients. The interpretations derived from this review can generally apply to diverse populations as we collected studies from various countries. In this systematic review, we did not impose a fixed timeframe for measuring secondary outcomes such as reinfarction, recurrent MI, incidence of HF, cardiogenic shock, and stroke. The included studies reported varied time points from short-term (in-hospital and 30-day) to long-term (≥6 months), which potentially introducing variability and heterogeneity into the estimated relative risk values.
In conclusion, hyperglycemia has a strong association with adverse outcomes following STEMI. Admission and FBG levels may serve as predictors for short-term outcomes in STEMI patients, whereas HbA1c may be a more suitable indicator for forecasting longer-term outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
AA: Conceptualization, Formal Analysis, Funding acquisition, Resources, Supervision, Writing – review & editing, Project administration. NQ: Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing, Project administration. IM: Resources, Supervision, Validation, Writing – review & editing. AZ: Data curation, Formal Analysis, Investigation, Writing – original draft. MC: Data curation, Supervision, Validation, Writing – review & editing. PD: Methodology, Resources, Supervision, Validation, Writing – review & editing. AQ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgements
The authors gratefully thank to Mr. Dian Sidik Arsyad for providing the figure of summary ROC analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1303685/full#supplementary-material
References
1. Jayaraj JC, Davatyan K, Subramanian SS, Priya J. Epidemiology of myocardial infarction [Internet]. IntechOpen. (2019) 10. doi: 10.5772/intechopen.74768
2. Orellana-Barrios MA, Fries JW, Nugent K, Shurmur S, Meyerrose G. Evaluation of hemoglobin a1c and admission blood glucose as predictors of in-hospital and 12-month mortality in patients with stemi. J Am Coll Cardiol 2016;67:498. doi: 10.1016/S0735-1097(16)30499-5
3. Chen P-C, Chua S-K, Hung H-F, Huang C-Y, Lin C-M, Lai S-M, et al. Admission hyperglycemia predicts poorer short- and long-term outcomes after primary percutaneous coronary intervention for ST-elevation myocardial infarction. J Diabetes Investig. (2014) 5:80–6. doi: 10.1111/jdi.12113
4. Terlecki M, Bednarek A, Kawecka-Jaszcz K, Czarnecka D, Bryniarski L. Acute hyperglycaemia and inflammation in patients with ST segment elevation myocardial infarction. Kardiol Pol. (2013) 71:260–7. doi: 10.5603/KP.2013.0038
5. Kocas C, Abaci O, Halil GS, Arslan S, Cetinkal G, Bostan C, et al. Admission hyperglycemia is associated with failed reperfusion following fibrinolytic therapy in patients with STEMI: results of a retrospective study. Am J Cardiovasc Drugs. (2015) 15:35–42. doi: 10.1007/s40256-014-0097-9
6. Planer D, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Xu K, et al. Impact of hyperglycemia in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention: the HORIZONS-AMI trial. Int J Cardiol. (2013) 167:2572–9. doi: 10.1016/j.ijcard.2012.06.054
7. Khalfallah M, Abdelmageed R, Elgendy E, Hafez YM. Incidence, predictors and outcomes of stress hyperglycemia in patients with ST elevation myocardial infarction undergoing primary percutaneous coronary intervention. Diab Vasc Dis Res. (2020) 17:1479164119883983. doi: 10.1177/1479164119883983
8. Sarlak H, Akhan M, Cakar M, Kurt O, Arslan E, Balta S. Admission hyperglycemia may be the result of counterregulatory hormones during acute coronary syndrome events. Angiology. (2014) 65:160. doi: 10.1177/0003319713498150
9. Huang B, Wang X, Yang Y, Zhu J, Liang Y, Tan H, et al. Association of admission glycaemia with high grade atrioventricular block in ST-segment elevation myocardial infarction undergoing reperfusion therapy: an observational study. Medicine (Baltimore). (2015) 94:e1167. doi: 10.1097/MD.0000000000001167
10. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
11. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Euroheartj. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
12. Grooten WJA, Tseli E, Äng BO, Boersma K, Stålnacke B-M, Gerdle B, et al. Elaborating on the assessment of the risk of bias in prognostic studies in pain rehabilitation using QUIPS-aspects of interrater agreement. Diagn Progn Res. (2019) 3(5):1–11. doi: 10.1186/s41512-019-0050-0
13. Aggarwal B, Shah GK, Randhawa M, Ellis SG, Lincoff AM, Menon V. Utility of glycated hemoglobin for assessment of glucose metabolism in patients with ST-segment elevation myocardial infarction. Am J Cardiol. (2016) 117:749–53. doi: 10.1016/j.amjcard.2015.11.060
14. Ahmad S, Ahmad S, Kashif M. Increasing HbA1C level; A prognostic indicator of increased thirty days mortality in patients of ST-segment elevation in myocardial infarction. Pak J Med Health Sci. (2012) 6:724–6. https://www.pjmhsonline.com/2012/july_sep/pdf/724%20%20%20Increasing%20HbA1C%20Level.pdf;%20a%20Prognostic%20Indicator%20of%20Increased%20Thirty%20Days%20Mortality%20in%20Pts%20of%20ST%20Segment%20Elevation%20in%20MI.pdf
15. Chang J, Zhang G, Zhang L, Hou Y-P, Liu X-L, Zhang L. High admission glucose levels increase fas apoptosis and mortality in patients with acute ST-elevation myocardial infarction: a prospective cohort study. Cardiovasc Diabetol. (2013) 12:171. doi: 10.1186/1475-2840-12-171
16. Chioncel V, Mincu D, Anastasiu M, Sinescu C. The prognostic value of blood glucose level on admission in non-diabetic patients with acute myocardial infarction. J Med Life. (2009) 2:271–8.20112471
17. Cicek G, Uyarel H, Ergelen M, Ayhan E, Abanonu GB, Eren M, et al. Hemoglobin A1c as a prognostic marker in patients undergoing primary angioplasty for acute myocardial infarction. Coron Artery Dis. (2011) 22:131–7. doi: 10.1097/MCA.0b013e328342c760
18. Demarchi A, Cornara S, Somaschini A, Fortuni F, Mandurino-Mirizzi A, Crimi G, et al. Has hyperglycemia a different prognostic role in STEMI patients with or without diabetes? Nutr Metab Cardiovasc Dis. (2021) 31:528–31. doi: 10.1016/j.numecd.2020.09.005
19. De Monte A, Perkan A, Vitrella G, Rakar S, Della Grazia E, Salvi A, et al. Impact of hyperglycemia on clinical outcome in patients undergoing percutaneous coronary intervention (PCI) for ST-segment elevation myocardial infarction (STEMI). Eur J Intern Med. (2008) 19:S45–6. doi: 10.1016/S0953-6205(08)60151-X
20. Dharma S, Mahavira A, Haryono N, Sukmawan R, Dakota I, Siswanto BB, et al. Association of hyperglycemia and final TIMI flow with one-year mortality of patients with acute ST-segment elevation myocardial infarction undergoing primary PCI. Int J Angiol. (2019) 28:182–7. doi: 10.1055/s-0039-1691811
21. Li D, Hua Q, Guo J, Li H, Chen H, Zhao S. Admission glucose level and in-hospital outcomes in diabetic and non-diabetic patients with ST-elevation acute myocardial infarction. Intern Med. (2011) 50:2471–5. doi: 10.2169/internalmedicine.50.5750
22. Eitel I, Hintze S, de Waha S, Fuernau G, Lurz P, Desch S, et al. Prognostic impact of hyperglycemia in nondiabetic and diabetic patients with ST-elevation myocardial infarction: insights from contrast-enhanced magnetic resonance imaging. Circ Cardiovasc Imaging. (2012) 5:708–18. doi: 10.1161/CIRCIMAGING.112.974998
23. Ekmekci A, Uluganyan M, Tufan F, Uyarel H, Karaca G, Kul S, et al. Impact of admission blood glucose levels on prognosis of elderly patients with ST elevation myocardial infarction treated by primary percutaneous coronary intervention. J Geriatr Cardiol. (2013) 10:310–6. doi: 10.3969/j.issn.1671-5411.2013.04.002
24. Ekmekci A, Cicek G, Uluganyan M, Gungor B, Osman F, Ozcan KS, et al. Admission hyperglycemia predicts inhospital mortality and major adverse cardiac events after primary percutaneous coronary intervention in patients without diabetes mellitus. Angiology. (2014) 65:154–9. doi: 10.1177/0003319713488930
25. El-Sherbiny I, Nabil B, Saber T, Abdelgawad FE. Impact of admission glycosylated hemoglobin A1c on angiographic characteristics and short term clinical outcomes of nondiabetic patients with acute ST-segment elevation myocardial infarction. Cardiol Res Pract. (2015) 2015:1–5. doi: 10.1155/2015/274892
26. Ergelen M, Uyarel H, Cicek G, Isik T, Osmonov D, Gunaydin ZY, et al. Which is worst in patients undergoing primary angioplasty for acute myocardial infarction? Hyperglycaemia? Diabetes mellitus? Or both? Acta Cardiol. (2010) 65:415–23. doi: 10.2143/AC.65.4.2053900
27. Ferreira JA, Baptista RM, Monteiro SR, Gonçalves FM, Monteiro PF, Gonçalves LM. Admission hyperglycemia and all-cause mortality in diabetic and non-diabetic patients with acute myocardial infarction: a tertiary center analysis. Intern Emerg Med. (2021) 16:2109–19. doi: 10.1007/s11739-021-02693-0
28. Garadah TS, Kassab S, Al-Shboul QM, Alawadi A. The threshold of admission glycemia as a predictor of adverse events in diabetic and non-diabetic patients with acute coronary syndrome. Clin Med Cardiol. (2009) 3:29–36. doi: 10.4137/CMC.S2289
29. Gasior M, Pres D, Stasik-Pres G, Lech P, Gierlotka M, Hawranek M, et al. Effect of blood glucose levels on prognosis in acute myocardial infarction in patients with and without diabetes, undergoing percutaneous coronary intervention. Cardiol J. (2008) 15:422–30.18810716
30. Ghaffari S, Niafar F, Separham A, Niafar M, Pourafkari L, Nader ND. Association between HbA1c levels with severity of coronary artery disease and short-term outcomes of acute ST-elevation myocardial infarction in nondiabetic patients. Ther Adv Cardiovasc Dis. (2015) 9:305–13. doi: 10.1177/1753944715585500
31. Ghariani A, Mosrati H, Abdessalem MAB, Bouraoui H, Romdhane AF, Ammar F, et al. Prognostic impact of diabetes mellitus on patients managed by urgent percutaneous coronary intervention. Tunisie Medicale. (2022) 100:143–8.35852249
32. Gierach J, Gierach M, Świątkiewicz I, Woźnicki M, Grześk G, Sukiennik A, et al. Admission glucose and left ventricular systolic function in non-diabetic patients with acute myocardial infarction. Heart Vessels. (2016) 31:298–307. doi: 10.1007/s00380-014-0610-8
33. Hoebers LP, Damman P, Claessen BE, Vis MM, Baan JJ, van Straalen JP, et al. Predictive value of plasma glucose level on admission for short and long term mortality in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Am J Cardiol. (2012) 109:53–9. doi: 10.1016/j.amjcard.2011.07.067
34. Ishihara M, Kojima S, Sakamoto T, Kimura K, Kosuge M, Asada Y, et al. Comparison of blood glucose values on admission for acute myocardial infarction in patients with versus without diabetes mellitus. Am J Cardiol. (2009) 104:769–74. doi: 10.1016/j.amjcard.2009.04.055
35. Kalińczuk Ł, Zieliński K, Pręgowski J, Przyłuski J, Karcz M, Bekta P, et al. Higher admission glycaemia independently of diagnosed or unrecognised diabetes mellitus is a risk factor for failed myocardial tissue reperfusion and higher mortality after primary angioplasty. Kardiol Pol. (2018) 76:594–601. doi: 10.5603/KP.a2017.0258
36. Kirmani T, Singh M, Kumar S, Kumar K, Parkash O, Sagar Yasmin F, Khan F, Chughtai N, Asghar MS. Plasma random glucose levels at hospital admission predicting worse outcomes in STEMI patients undergoing PCI. Ann Med Surg (Lond) 2022;78:103857. doi: 10.1016/j.amsu.2022.103857
37. Kosuge M, Kimura K, Morita S, Kojima S, Sakamoto T, Ishihara M, et al. Combined prognostic utility of white blood cell count, plasma glucose, and glomerular filtration rate in patients undergoing primary stent placement for acute myocardial infarction. Am J Cardiol. (2009) 103:322–7. doi: 10.1016/j.amjcard.2008.09.079
38. Kruk M, Przyłuski J, Kalińczuk L, Pregowski J, Kaczmarska E, Petryka J, et al. Clustering of admission hyperglycemia, impaired renal function and anemia and its impact on in-hospital outcomes in patients with ST-elevation myocardial infarction. Atherosclerosis. (2010) 209:558–64. doi: 10.1016/j.atherosclerosis.2009.09.074
39. Kumar R, Shah JA, Solangi BA, Ammar A, Kumar M, Khan N, et al. The burden of short-term Major adverse cardiac events and its determinants after emergency percutaneous coronary revascularization: a prospective follow-up study. J Saudi Heart Assoc. (2022) 34:72–81. doi: 10.37616/2212-5043.1299
40. Kumar R, Ammar A, Kumar A, Ali A, Talpur MFH, Rahooja K, et al. Acute hyperglycemia, a rabble-rouser or innocent bystander? A prospective analysis of clinical implications of acute hyperglycemia in STE-ACS patients. BMC Cardiovasc Disord. (2023) 23:406. doi: 10.1186/s12872-023-03440-3
41. Lavi S, Kapeliovich M, Gruberg L, Roguin A, Boulos M, Grenadier E, et al. Hyperglycemia during acute myocardial infarction in patients who are treated by primary percutaneous coronary intervention: impact on long-term prognosis. Int J Cardiol. (2008) 123:117–22. doi: 10.1016/j.ijcard.2006.11.222
42. Lazzeri C, Valente S, Chiostri M, Attaná P, Picariello C, Gensini GF. The prognostic role of in-hospital peak glycemia in stemi patients with and without diabetes. Acta Diabetol. (2012) 49:379–86. doi: 10.1007/s00592-011-0343-4
43. Lazzeri C, Valente S, Chiostri M, Attanà P, Picariello C, Gensini GF. The glucose dysmetabolism in the acute phase of non-diabetic ST-elevation myocardial infarction: from insulin resistance to hyperglycemia. Acta Diabetol. (2013) 50:293–300. doi: 10.1007/s00592-011-0325-6
44. Liu Y, Yang Y-M, Zhu J, Tan H-Q, Liang Y, Li J-D. Haemoglobin A(1c), acute hyperglycaemia and short-term prognosis in patients without diabetes following acute ST-segment elevation myocardial infarction. Diabet Med. (2012) 29:1493–500. doi: 10.1111/j.1464-5491.2012.03641.x
45. Luo E, Wang D, Yan G, Qiao Y, Liu B, Hou J, et al. High triglyceride-glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc Diabetol. (2019) 18:150. doi: 10.1186/s12933-019-0957-3
46. Marenzi G, De Metrio M, Rubino M, Lauri G, Cavallero A, Assanelli E, et al. Acute hyperglycemia and contrast-induced nephropathy in primary percutaneous coronary intervention. Am Heart J. (2010) 160:1170–7. doi: 10.1016/j.ahj.2010.09.022
47. Mladenović V, Zdravković V, Jović M, Vucić R, Irić-Cupić V, Rosić M. Influence of admission plasma glucose level on short- and long-term prognosis in patients with ST-segment elevation myocardial infarction. Vojnosanit Pregl. (2010) 67:291–5. doi: 10.2298/VSP1004291M
48. Moura FA, Figueiredo VN, Teles BSBS, Barbosa MA, Pereira LR, Costa APR, et al. Glycosylated hemoglobin is associated with decreased endothelial function, high inflammatory response, and adverse clinical outcome in non-diabetic STEMI patients. Atherosclerosis (2015) 243:124–30. doi: 10.1016/j.atherosclerosis.2015.09.004
49. Orellana-Barrios M, Fries J, Nugent K, Shurmur S. Glycated hemoglobin, admission blood glucose delta, and associated mortality in patients with acute ST-segment elevation myocardial infarction. Proc (Bayl Univ Med Cent). (2019) 32:325–30. doi: 10.1080/08998280.2019.1606614
50. Porter A, Assali A, Zahalka A, Iakobishvili Z, Brosh D, Lev E, et al. Impaired fasting glucose and outcomes of ST-elevation acute coronary syndrome treated with primary percutaneous intervention among patients without previously known diabetes mellitus. Am Heart J. (2008) 155:284–9. doi: 10.1016/j.ahj.2007.10.010
51. Pres D, Gąsior M, Strojek K, Gierlotka M, Hawranek Michałand Lekston A, Wilczek K, et al. Blood glucose level on admission determines in-hospital and long-term mortality in patients with ST-segment elevation myocardial infarction complicated by cardiogenic shock treated with percutaneous coronary intervention. Kardiol Pol. (2010) 68:743–51.20648428
52. Pusuroglu H, Akgul O, Cakmak HA, Erturk M, Surgit O, Celik O, et al. Long-term prognostic value of admission haemoglobin A1c(HbA1c) levels in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Postepy w Kardiologii Interwencyjnej. (2014) 10:166–74. doi: 10.5114/pwki.2014.45143
53. Qanitha A, Uiterwaal CSPM, Henriques JPS, Mappangara I, Idris I, Amir M, et al. Predictors of medium-term mortality in patients hospitalised with coronary artery disease in a resource-limited South-East Asian setting. Open Heart. (2018) 5(2):e000801. doi: 10.1136/openhrt-2018-000801
54. Qin Y, Yan G, Qiao Y, Wang D, Luo E, Hou J, et al. Predictive value of random blood glucose versus fasting blood glucose on in-hospital adverse events in patients with ST-segment elevation acute myocardial infarction. BMC Cardiovasc Disord. (2020) 20:95. doi: 10.1186/s12872-020-01394-4
55. Qin Y, Qiao Y, Wang D, Tang C, Yan G. Admission hyperglycemia is associated with global registry of acute coronary events score and complications following acute myocardial infarction during 1-year follow-up. Angiology. (2022) 73:165–76. doi: 10.1177/00033197211039915
56. Rasoul S, Ottervanger JP, Bilo HJG, Timmer JR, van‘t Hof AWJ, Dambrink JHE, et al. Glucose dysregulation in nondiabetic patients with ST-elevation myocardial infarction: acute and chronic glucose dysregulation in STEMI. Neth J Med. (2007) 65:95–100.17387235
57. Rousan TA, Pappy RM, Chen AY, Roe MT, Saucedo JF. Impact of diabetes mellitus on clinical characteristics, management, and in-hospital outcomes in patients with acute myocardial infarction (from the NCDR). Am J Cardiol. (2014) 114:1136–44. doi: 10.1016/j.amjcard.2014.07.031
58. Shahid M, Zarif HMA, Farid MS, Abid MS, Akhtar B, Khan MR. Prognostic value of hyperglycemia on admission on in-hospital outcomes in patients presenting with ST-elevation myocardial infarction. Cureus. (2020) 12:e7024. doi: 10.7759/cureus.7024
59. Shitole S, Srinivas V, Berkowitz J, Shah T, Park M, Herzig S, et al. Hyperglycaemia, adverse outcomes and impact of intravenous insulin therapy in patients presenting with acute ST-elevation myocardial infarction in a socioeconomically disadvantaged urban setting: the montefiore STEMI registry. Endocrinol Diabetes Metab. (2020) 3:e00089. doi: 10.1002/edm2.89
60. Sinnaeve P, Steg P, Fox K, Van de Werf F, Montalescot G, Granger C, et al. Association of elevated fasting glucose with increased short-term and 6-month mortality in ST-segment elevation and non-ST-segment elevation acute coronary syndromes: the global registry of acute coronary events. Arch Intern Med. (2009) 169:402–9. doi: 10.1001/archinternmed.2008.572
61. Squire IB, Nelson CP, Ng LL, Jones DR, Woods KL, Lambert PC. Prognostic value of admission blood glucose concentration and diabetes diagnosis on survival after acute myocardial infarction: results from 4702 index cases in routine practice. Clin Sci (Lond). (2010) 118:527–35. doi: 10.1042/CS20090322
62. Stalikas N, Papazoglou AS, Karagiannidis E, Panteris E, Moysidis D, Daios S, et al. Association of stress induced hyperglycemia with angiographic findings and clinical outcomes in patients with ST—elevation myocardial infarction. Cardiovasc Diabetol. 21(1):140. doi: 10.1186/s12933-022-01578-6
63. Straumann E, Kurz DJ, Muntwyler J, Stettler I, Furrer M, Naegeli B, et al. Admission glucose concentrations independently predict early and late mortality in patients with acute myocardial infarction treated by primary or rescue percutaneous coronary intervention. Am Heart J. (2005) 150:1000–6. doi: 10.1016/j.ahj.2005.01.033
64. Takada J, Ramos R, Roza L, Avakian S, Ramires J, Mansur Ade P. In-hospital death in acute coronary syndrome was related to admission glucose in men but not in women. Cardiovasc Diabetol. (2012) 11:47. doi: 10.1186/1475-2840-11-47
65. Tian L, Zhu J, Liu L, Liang Y, Li J, Yang Y. Hemoglobin A1c and short-term outcomes in patients with acute myocardial infarction undergoing primary angioplasty: an observational multicenter study. Coron Artery Dis. (2013) 24:16–22. doi: 10.1097/MCA.0b013e32835b3971
66. Timmer JR, Hoekstra M, Nijsten MWN, van der Horst ICC, Ottervanger JP, Slingerland RJ, et al. Prognostic value of admission glycosylated hemoglobin and glucose in nondiabetic patients with ST-segment-elevation myocardial infarction treated with percutaneous coronary intervention. Circulation. (2011) 124:704–11. doi: 10.1161/CIRCULATIONAHA.110.985911
67. Vis M, Engström A, Sjauw K, Tjong F, Baan J J, Koch K, et al. Plasma glucose and not hemoglobin or renal function predicts mortality in patients with STEMI complicated with cardiogenic shock. J Cardiovasc Med (Hagerstown). (2010) 11:827–31. doi: 10.2459/JCM.0b013e32833cdc6d
68. Wang H, Zhang Y, Shen Z, Fang L, Liu Z, Zhang S. Prognostic value of fasting glucose on the risk of heart failure and left ventricular systolic dysfunction in non-diabetic patients with ST-segment elevation myocardial infarction. Front Med. (2021) 15:70–8. doi: 10.1007/s11684-020-0749-x
69. Weston C, Walker L, Birkhead J. Early impact of insulin treatment on mortality for hyperglycaemic patients without known diabetes who present with an acute coronary syndrome. Heart. (2007) 93:1542–6. doi: 10.1136/hrt.2006.108696
70. Worthley M, Shrive F, Anderson T, Traboulsi M. Prognostic implication of hyperglycemia in myocardial infarction and primary angioplasty. Am J Med. (2007) 120:643.e1–7. doi: 10.1016/j.amjmed.2006.06.043
71. Shen X, Jia S, Li H. The influence of admission glucose on epicardial and microvascular flow after primary angioplasty. Chin Med J (Engl). (2006) 119:95–102. doi: 10.1097/00029330-200601020-00002
72. Yang J, Song P, Song Y, Hahn J, Choi S, Choi J, et al. Prognostic value of admission blood glucose level in patients with and without diabetes mellitus who sustain ST segment elevation myocardial infarction complicated by cardiogenic shock. Crit Care. (2013) 17:R218. doi: 10.1186/cc13035
73. Yanishi K, Nakamura T, Nakanishi N, Yokota I, Zen K, Yamano T, et al. A simple risk stratification model for ST-elevation myocardial infarction (STEMI) from the combination of blood examination variables: acute myocardial infarction-Kyoto multi-center risk study group. PLoS One. (2016) 11:e0166391. doi: 10.1371/journal.pone.0166391
74. Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. (2000) 355:773–8. doi: 10.1016/S0140-6736(99)08415-9
75. Karakasis P, Stalikas N, Patoulias D, Pamporis K, Karagiannidis E, Sagris M, et al. Prognostic value of stress hyperglycemia ratio in patients with acute myocardial infarction: a systematic review with Bayesian and frequentist meta-analysis. Trends Cardiovasc Med. (2023). doi: 10.1016/j.tcm.2023.11.006
76. Deedwania P, Kosiborod M, Barrett E, Ceriello A, Isley W, Mazzone T, Raskin P. Hyperglycemia and acute coronary syndrome: a scientific statement from the American heart association diabetes committee of the council on nutrition, physical activity, and metabolism. Circulation. 2008 117(12):1610–9. doi: 10.1161/CIRCULATIONAHA.107.188629
77. Ishihara M. Acute hyperglycemia in patients with acute myocardial infarction. Circ J. (2012) 76:563–71. doi: 10.1253/circj.CJ-11-1376
78. Angeli F, Reboldi G, Poltronieri C, Lazzari L, Sordi M, Garofoli M, et al. Hyperglycemia in acute coronary syndromes: from mechanisms to prognostic implications. Ther Adv Cardiovasc Dis. (2015) 9:412–24. doi: 10.1177/1753944715594528
79. Inzucchi SE. Hyperglycaemia and its therapy during acute coronary syndromes. Diab Vasc Dis Res. (2008) 5:259. doi: 10.3132/dvdr.2008.037
80. Lønborg J, Vejlstrup N, Kelbæk H, Nepper-Christensen L, Jørgensen E, Helqvist S, et al. Impact of acute hyperglycemia on myocardial infarct size, area at risk, and salvage in patients with STEMI and the association with exenatide treatment: results from a randomized study. Diabetes. (2014) 63:2474–85. doi: 10.2337/db13-1849
81. Santulli G, Campanile A, Spinelli L, di Panzillo E A, Ciccarelli M, Trimarco B, et al. G protein-coupled receptor kinase 2 in patients with acute myocardial infarction. Am J Cardiol. (2011) 107:1125–30. doi: 10.1016/j.amjcard.2010.12.006
82. Ishihara M, Kagawa E, Inoue I, Kawagoe T, Shimatani Y, Kurisu S, et al. Impact of admission hyperglycemia and diabetes mellitus on short- and long-term mortality after acute myocardial infarction in the coronary intervention era. Am J Cardiol. (2007) 99:1674–9. doi: 10.1016/j.amjcard.2007.01.044
83. Li M, Chen G, Feng Y, He X. Stress induced hyperglycemia in the context of acute coronary syndrome: Definitions, interventions, and underlying mechanisms. Front Cardiovasc Med. (2021) 8:1–12. doi: 10.3389/fcvm.2021.676892
84. Funk SD, Yurdagul AJ, Orr AW. Hyperglycemia and endothelial dysfunction in atherosclerosis: lessons from type 1 diabetes. Int J Vasc Med. (2012) 2012:569654. doi: 10.1155/2012/569654
85. Vis MM, Sjauw KD, van der Schaaf RJ, Baan JJ, Koch KT, DeVries JH, et al. In patients with ST-segment elevation myocardial infarction with cardiogenic shock treated with percutaneous coronary intervention, admission glucose level is a strong independent predictor for 1-year mortality in patients without a prior diagnosis of di. Am Heart J. (2007) 154:1184–90. doi: 10.1016/j.ahj.2007.07.028
86. Leite SA, Locatelli SB, Niece SP, Oliveira AR, Tockus D, Tosin T. Impact of hyperglycemia on morbidity and mortality, length of hospitalization and rates of re-hospitalization in a general hospital setting in Brazil. Diabetol Metab Syndr. (2010) 2:49. doi: 10.1186/1758-5996-2-49
87. Johnston KC, Bruno A, Pauls Q, Hall CE, Barrett KM, Barsan W, et al. Intensive vs standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: the SHINE randomized clinical trial. JAMA. (2019) 322:326–35. doi: 10.1001/jama.2019.9346
Keywords: adverse outcomes, hyperglycemia, MACCE, mortality, STEMI, revascularization
Citation: Alkatiri AH, Qalby N, Mappangara I, Zainal ATF, Cramer MJ, Doevendans PA and Qanitha A (2024) Stress hyperglycemia and poor outcomes in patients with ST-elevation myocardial infarction: a systematic review and meta-analysis. Front. Cardiovasc. Med. 11:1303685. doi: 10.3389/fcvm.2024.1303685
Received: 28 September 2023; Accepted: 15 February 2024;
Published: 11 March 2024.
Edited by:
Dennis W. T. Nilsen, Stavanger University Hospital, NorwayReviewed by:
Hugo Ten Cate, Maastricht University Medical Centre, NetherlandsNikolaos Stalikas, University General Hospital of Thessaloniki AHEPA, Greece
© 2024 Alkatiri, Qalby, Mappangara, Zainal, Cramer, Doevendans and Qanitha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andriany Qanitha YS5xYW5pdGhhQHVuaGFzLmFjLmlk
†These authors share first authorship
 Abdul Hakim Alkatiri1,2,†
Abdul Hakim Alkatiri1,2,† Nurul Qalby
Nurul Qalby Maarten J. Cramer
Maarten J. Cramer Pieter A. Doevendans
Pieter A. Doevendans Andriany Qanitha
Andriany Qanitha