- 1Department of Rehabilitation, The First Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 2Department of Cardiology, The First Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
Background: Exercise training is commonly employed as a efficacious supplementary treatment for individuals suffering from heart failure, but the optimal exercise regimen is still controversial. The objective of the review was to compare the effects of high-intensity interval training (HIIT) and moderate-intensity continuous training (MICT) on the exercise capacity, cardiac function, quality of life (QoL) and heart rate among patients with heart failure with reduced ejection fraction.
Methods: A systematic search was performed using the following eight databases from their inception to July 5, 2023: PubMed, Web of Science, Embase, Cochrane Library, Clinical Trials, China Knowledge Network, Wan fang Data, and the China Biology Medicine databases. The meta-analysis results were presented as mean difference (MD) and 95% confidence interval (CI). The Cochrane Risk of Bias tool was used for the included studies. The Grading of Recommendations Assessment, Development, and Evaluations was used to assess the certainty of evidence.
Results: Thirteen randomized controlled trials were included in the study. The results showed that HIIT had a significant positive effect on peak oxygen uptake (MD = 1.78, 95% CI for 0.80–2.76), left ventricular ejection fraction (MD = 3.13, 95% CI for 1.25–5.02), six-minute walk test (MD = 28.13, 95% CI for 14.56–41.70), and Minnesota Living with Heart Failure Questionnaire (MD = −4.45, 95% CI for −6.25 to −2.64) compared to MICT. However, there were no statistically significant differences observed in resting heart rate and peak heart rate.
Conclusions: HIIT significantly improves peak oxygen uptake, left ventricular ejection fraction, six-minute walk test, and Minnesota Living with Heart Failure Questionnaire in patients with heart failure with reduced ejection fraction. Additionally, HIIT exhibits greater effectiveness in improving peak oxygen uptake among patients with lower body mass index.
Systematic Review Registration: https://www.doi.org/10.37766/inplasy2023.7.0100, identifier (INPLASY2023.7.0100).
1 Introduction
Heart failure (HF) is characterized by typical signs and symptoms due to structural and/or functional cardiac abnormalities. It is commonly characterized by dyspnea, fatigue, and leg swelling, which may be accompanied by peripheral edema and elevated jugular venous pressure (1). The 2016 guidelines of the European Society of Cardiology classify left ventricular ejection fraction (LVEF) ≤ 40% as HF with reduced ejection fraction (HFrEF) (2). Reduction in exercise tolerance has been suggested to predict readmission and mortality in HF patients (3). In 1990, Coats et al. conducted the first study on HF and exercise training, which demonstrated that patients with HF benefit from exercise training by improving their aerobic capacity and physical fitness (4). Exercise training has become a common adjunctive treatment for individuals suffering from HF, as recommended by the American College of Cardiology (ACC) at level 1 (5). Furthermore, Antunes et al. (6, 7) demonstrated that exercise training improved functional capacity, exercise endurance, and quality of life (QoL) for patients with HFrEF.
Among the various training methods utilized for individuals with cardiovascular disease, moderate-intensity continuous training (MICT) is the most frequently used, with an intensity of 50%–60% peak oxygen uptake (Peak VO2) or 50%–75% peak heart rate (HR peak) (6). MICT is characterized by lower intensity, longer duration, and a higher level of safety (7). Recent studies (8, 9) have indicated that high-intensity interval training (HIIT) has the potential to greatly enhance the aerobic capacity of individuals with HF and coronary artery disease. HIIT involves short bursts of intense exercise lasting between 10 s and 5 min, during which individuals reach 80%–90% of their Peak VO2 or 85%–95% of their peak heart rate. These high-intensity periods are interspersed with intervals of low-intensity exercise at less than 45% of Peak VO2 or less than 80% of peak heart rate, or rest (6). HIIT exhibits diversity in its types, incorporates a specific recovery interval, and achieves comparable training effects to MICT within a shorter duration. Additionally, HIIT elicits an intense cardiovascular stimulation and enhances the enjoyment and concentration of exercise (10, 11).
A meta-analysis comparing HIIT and MICT for HF indicated that HIIT yielded superior improvements in Peak VO2 compared to MICT, although no significant difference was observed in the enhancement of QoL (12). Tucker et al. concluded that HIIT was superior to conventional exercise training but not MICT in terms of improving left ventricular ejection fraction (LVEF) and Peak VO2 (13). Heart failure with reduced ejection fraction (HFrEF) is a syndrome characterized by high mortality and morbidity (14). However, there are no sufficient and conclusive studies regarding the impact of HIIT on HFrEF. Furthermore, the majority of published studies comparing HIIT and MICT in HF have primarily focused on heart failure with preserved ejection fraction (HFpEF). Therefore, it is necessary to clarify the clinical effects of HIIT specifically in HFrEF (15–17). Furthermore, it is crucial to consider energy expenditure during training when comparing various exercise regimens. The aim of isocaloric protocols is to equalize energy expenditure during aerobic exercises of varying intensities. Additionally, the enhancement of exercise capacity in individuals with HF is primarily influenced by energy expenditure (18). A previous study by Mansueto et al. discovered that HIIT was superior to MICT in improving Peak VO2, the superiority disappeared in subgroup analyses based on isocaloric protocols, however (19).
Consequently, in order to provide more comprehensive information and guidance for clinical practice, in the meta-analysis, the effects of HIIT and MICT on aerobic exercise capacity, cardiac function, QoL, and heart rate among patients with HFrEF were compared, based on previously conducted randomized controlled trials. Moreover, a sub-analysis of isocaloric exercise training studies is also included in the systematic review.
2 Materials and methods
This systematic review and meta-analysis were conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
2.1 Data source and search strategy
A comprehensive literature search was conducted using the following eight databases from their inception to July 5, 2023: PubMed, Web of Science, Embase, Cochrane Library, Clinical Trials, China Knowledge Network, Wan fang Data, and the China Biology Medicine databases. The following keywords were used as the search terms: “Heart Failure/Cardiac Failure/Heart Decompensation”; “High-Intensity Interval Training/High-Intensity Intermittent Exercise/HIIT/Sprint Interval Training” and their related terms. In addition, all reference lists of eligible studies were reviewed to identify any additional studies that could be included. There are no language restrictions on the search strategy, which is shown in Supplementary Table S1. The systematic review protocol was registered on INPLASY (INPLASY2023.7.0100) and is available on the website inplasy.com (https://www.doi.org/10.37766/inplasy2023.7.0100).
2.2 Inclusion and exclusion criteria
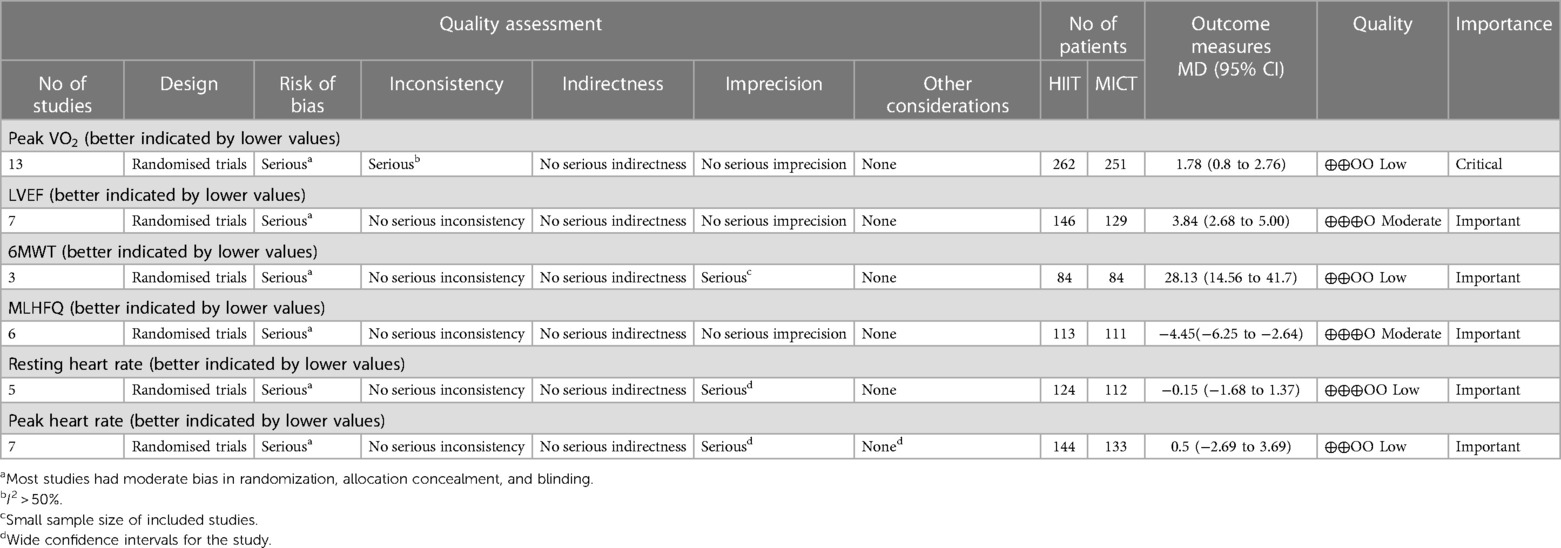
Following the removal of duplicate studies, titles and abstracts of the remaining articles were assessed, and subsequently, their full texts were examined to identify potentially eligible studies. Studies were selected based on the following criteria: (1) Randomized controlled trials (RCTs) involving adult individuals (≥18 years) diagnosed with HF with reduced ejection fraction (LVEF ≤40%); (2) HIIT in the intervention group vs. MICT in the control group; (3) The exercise intensity in both groups met the inclusion criteria established by Weston et al. (20); (4) The outcome measures included at least Peak VO2. Exclusion criteria were: (1) patients with HF with preserved ejection fraction (LVEF >50%) or those with unreported LVEF; (2) incomplete articles, non-randomized controlled trials; (3) not MICT as the control group; (4) missing original data, reviews, meta-analyses, and animal experiments. The primary outcome measure for this review was Peak VO2, and secondary outcomes included six-minute walk test (6MWT), left ventricular ejection fraction (LVEF), Minnesota Living with Heart Failure Questionnaire (MLHFQ), resting heart rate (HR rest), and peak heart rate.
2.3 Data extraction and quality assessment
Based on studies that met the inclusion criteria, the following information was extracted: (1) authors of the study and publication year; (2) the mean age, gender, and body mass index (BMI) of the participants; (3) sample size; (4) frequency, intensity, time, and modality of training in the HIIT and MICT groups; (5) mean and standard deviation of the outcome measures. To obtain the original data, we will contact the authors if complete data are not provided in the publication.The risk of bias was assessed by following the Cochrane guidelines for RCTs in several areas: (1) the generation of sequences and allocation concealment; (2) blinding of participants and personnel; (3) blinding of outcome assessment; (4) incomplete outcome data; (5) selective reporting and other potential biases. According to established criteria, the risk of bias was classified as low, uncertain, or high.
2.4 Quality of meta-analysis evidence
Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines were used to assess the quality of evidence for the outcomes. The evaluation included the assessment of five GRADE items: (1) bias risk, (2) inconsistency, (3) indirectness, (4) imprecision, and (5) publication bias (21). There were four levels of certainty of evidence: high, moderate, low, and very low.
2.5 Data synthesis
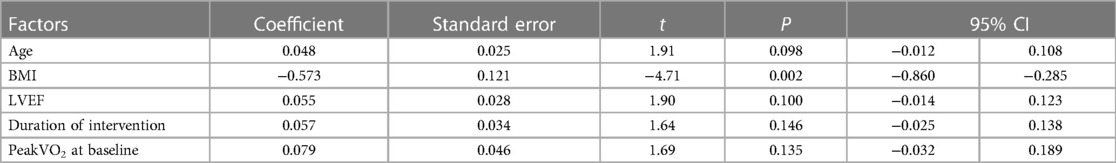
The mean differences (MD) and standard deviations from baseline to endpoint were extracted and included in the database for analysis for each group. The mean difference (MD) and 95% confidence intervals (95% CIs) were utilized for result comparison, but when outcomes were measured in the different ways, the standardized mean difference (SMD) and 95% CIs were used. The analysis was performed using Rev Man 5.3, and heterogeneity was assessed using the I2 statistic. I2 values of 25%, 50%, and 75% indicated low, moderate, and high levels of heterogeneity, respectively (22). If moderate or high heterogeneity (I2 > 50%) was detected, a random-effects model was applied; otherwise, the fixed-effects model was employed. Statistical significance was determined at p < 0.05, and the effect sizes and 95% confidence intervals were graphically presented through forest plots. Additionally, a sensitivity analysis was conducted to assess the impact of potential bias in some included RCTs on the study findings, and studies with factors contributing to heterogeneity were excluded. Moreover, in cases where I2 > 50%, subgroup analyses were performed to investigate potential factors that may contribute to the heterogeneity of the primary outcomes. These factors included age, duration of intervention, and BMI. The purpose of conducting these analyses was to minimize the impact of population baseline information and cumulative effects of interventions on the heterogeneity of results. Furthermore, the meta-regression analyses took into account participants' characteristics such as age, BMI, LVEF, and peak VO2 at baseline, as well as intervention characteristics such as duration, in order to explain potential sources of heterogeneity. Egger's test was used to assess the potential publication bias of these results, with p < 0.1 indicating significant publication bias. When ≥10 studies were included in the meta-analysis, we used the funnel plot (Supplementary Table S2). The analyses were performed using STATA software (Stata/MP 17.0).
3 Results
3.1 Study selection
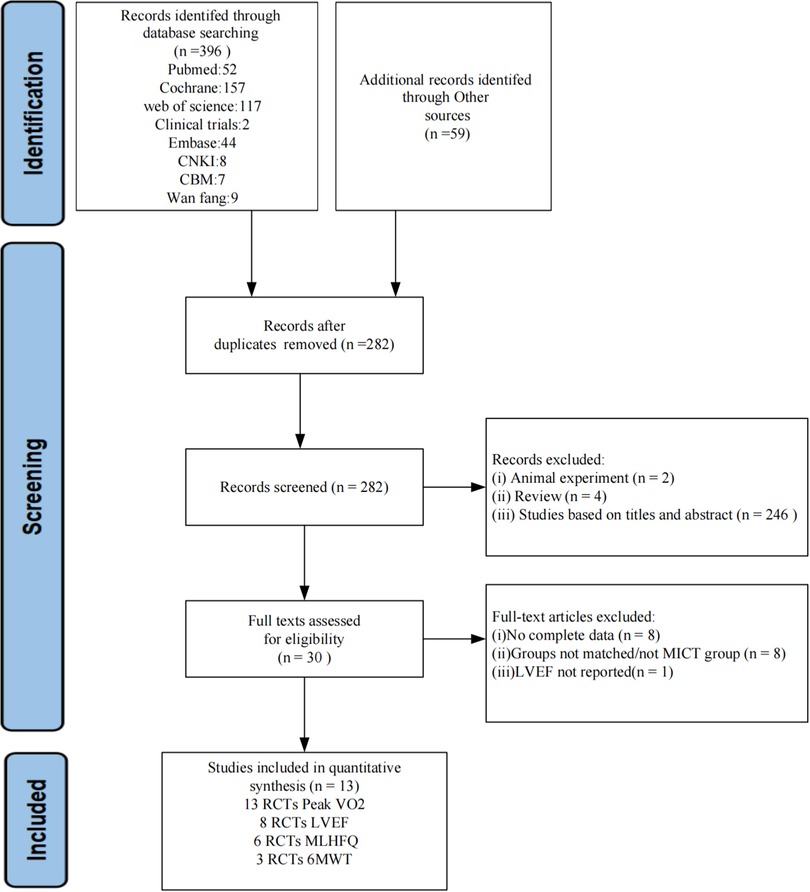
A total of 455 articles were obtained by searching 8 databases and conducting manual searches. After removing duplicates, 282 articles remained. Following a thorough evaluation of the title and abstract, 30 articles were identified as potentially meeting the criteria. Subsequently, we read the full text to further exclude 17 articles, 8 did not have complete data, 8 controls were not in the MICT group, and 1 did not report LVEF. In the end, a total of 13 randomized controlled trials (RCTs) were deemed eligible for inclusion. A flowchart summarizing the study selection process is shown in Figure 1.
3.2 Study characteristics
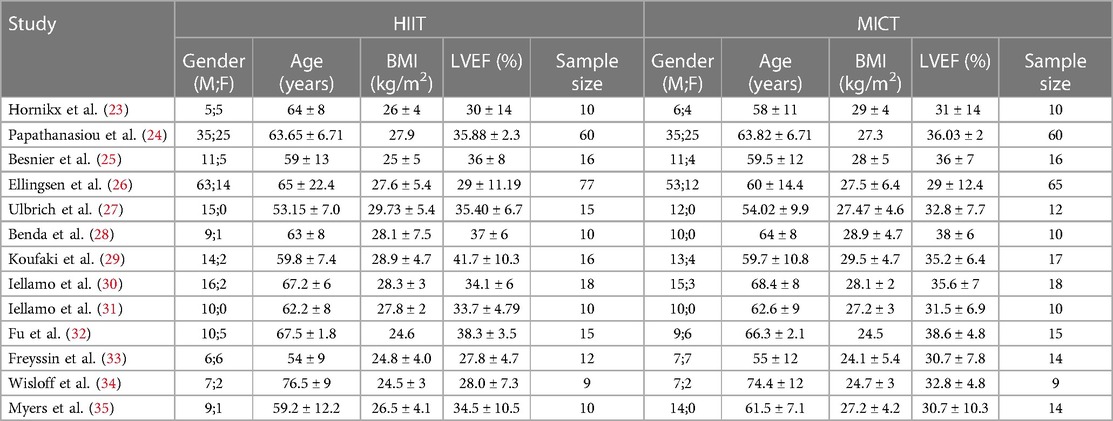
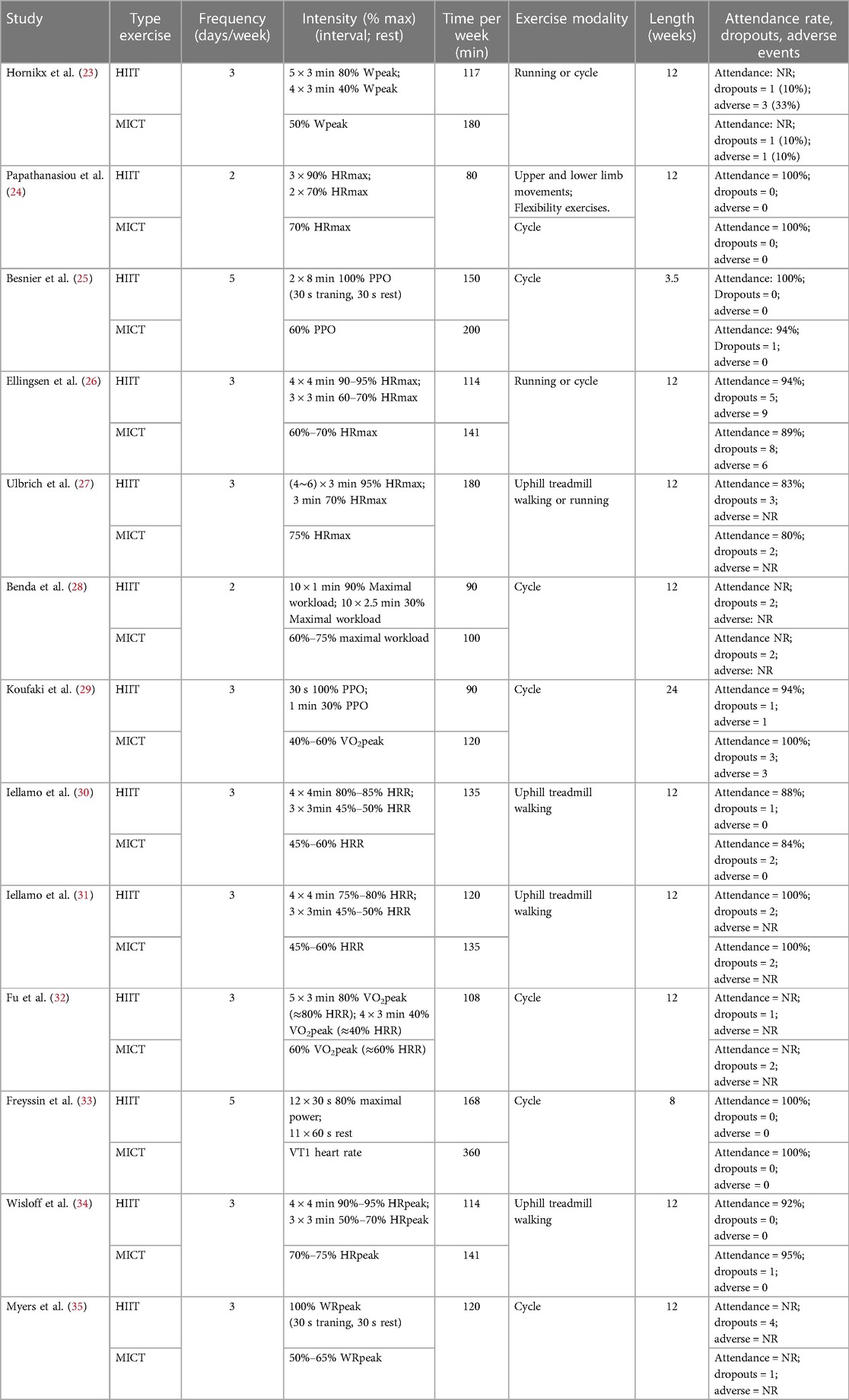
A total of thirteen studies (23–35) were conducted on patients with reduced ejection fraction in HF, with a total of 513 participants, of whom 454 (88%) were male. Among the participants, 262 underwent HIIT training, while 251 completed MICT training. There was an average age of 63 years and a BMI of 27 kg/m2 among the participants. The mean LVEF at baseline was 34%, and the study lasted from 3.5 weeks to 6 months. Based on all the studies included in the analysis, Peak VO2 was assessed using the cardiopulmonary exercise testing (CPET). The primary exercise modalities employed were cycling and uphill treadmill walking, and the exercise intensity was predominantly measured as a percentage of peak power or peak heart rate. However, two studies (30, 31) utilized a percentage of heart rate reserve (HRR), while one study (32) employed a percentage of Peak VO2. Table 1 shows the baseline demographic and clinical characteristics of the study participants. Table 2 summarizes the exercise training protocols for the HIIT and MICT groups.
3.3 Quality and bias assessment in the included studies
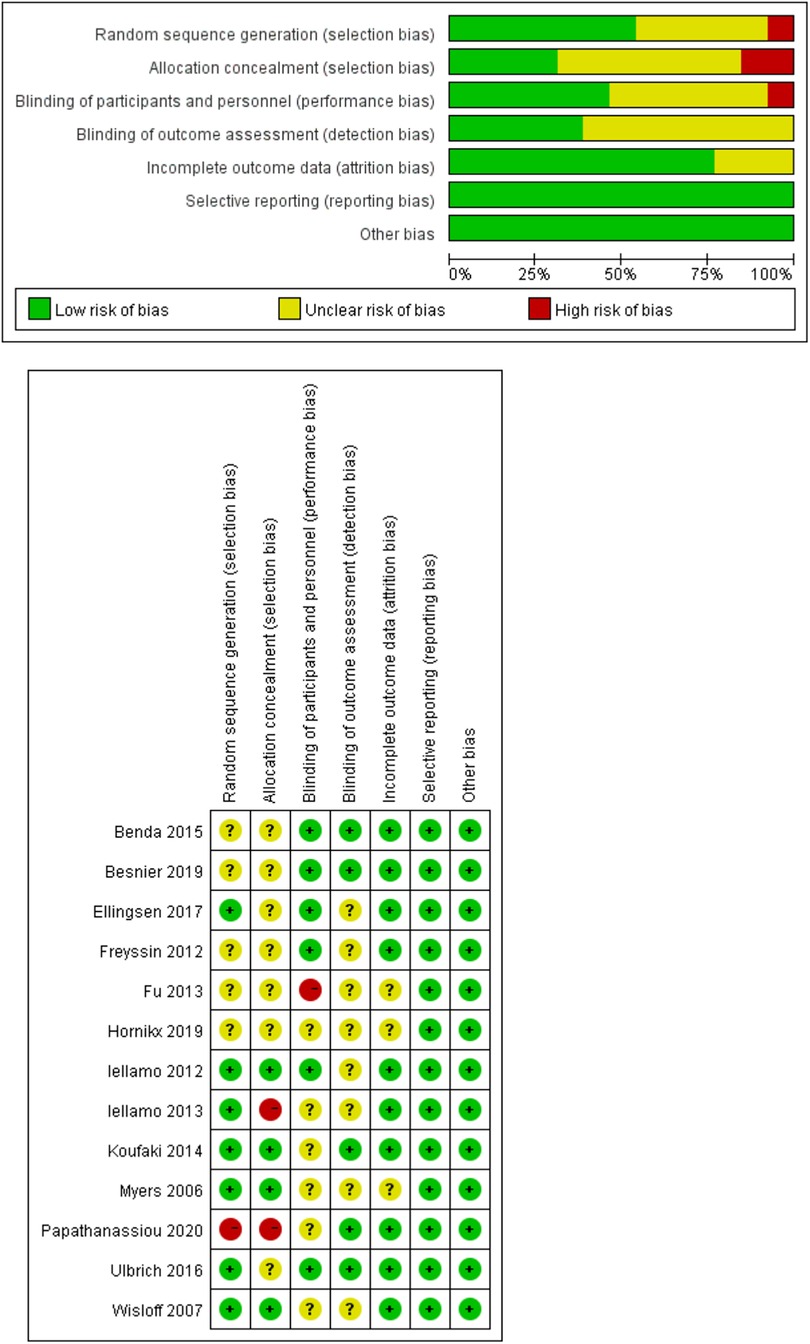
It is worth noting that all studies included in the analysis used randomization. However, only 7 (54%) of these studies provided a description of the method used for random sequence generation. Additionally, 4 (7.5%) studies reported allocation concealment, 12 (92%) studies reported blinding of participants. Notably, none of the studies employed blinding for the participants. Moreover, 5 (38%) studies reported blinding of outcome measures, 3 (23%) studies had unclear completeness of outcome data, and none of the studies reported data selectively (Figure 2).
3.4 Quality of meta-analysis evidence
The detailed results of the GRADE assessment are presented in Table 3, Peak VO2, 6MWT, resting heart rate, and peak heart rate are determined to have low quality evidence. LVEF and MLHFQ, however, are determined to have moderate quality evidence.
3.5 Results of the meta-analysis
3.5.1 Analysis of peak VO2
Thirteen studies (23–35) assessed the Peak VO2 as outcome.
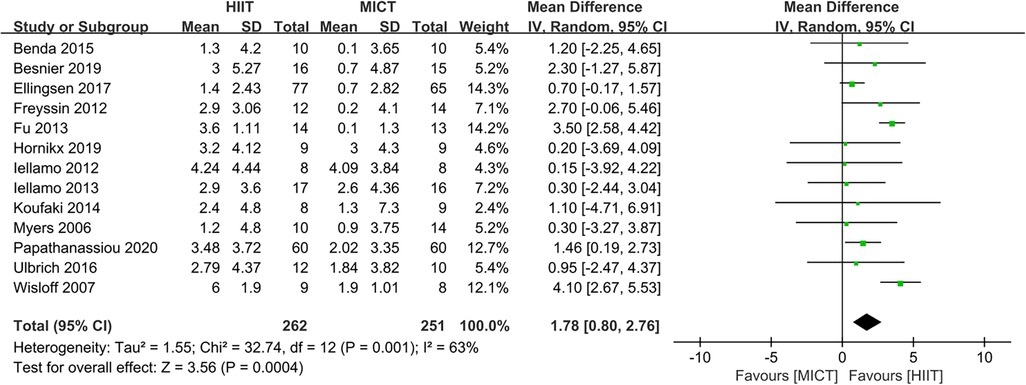
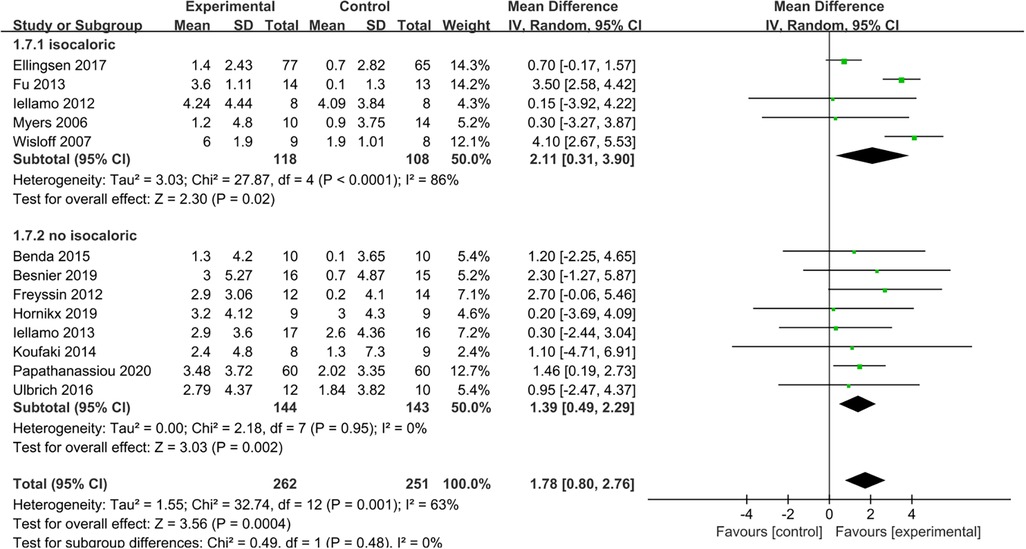
The results indicated that HIIT significantly increased the Peak VO2 compared to MICT (MD = 1.78, 95% CI for 0.80–2.76; 513 participants; 13 studies; I2 = 63%; P = 0.0004; Figure 3). Only 5 of the studies (26, 31, 32, 34, 35) used an isocaloric exercise protocol, in contrast to 8 studies (23–25, 27–30, 33) that didn't report whether the HIIT and MICT protocols were isocaloric. Subgroup analyses demonstrated that HIIT significantly enhanced Peak VO2 compared to MICT in studies involving isocaloric exercise training (MD = 2.11, 95% CI for 0.31–3.90; 226 participants; 5 studies; I2 = 86%; p = 0.02) as well as studies not involving isocaloric exercise training (MD = 1.39, 95% CI for 0.49–2.29; 287 participants; 8 studies; I2 = 0%; p = 0.002) (Figure 4).
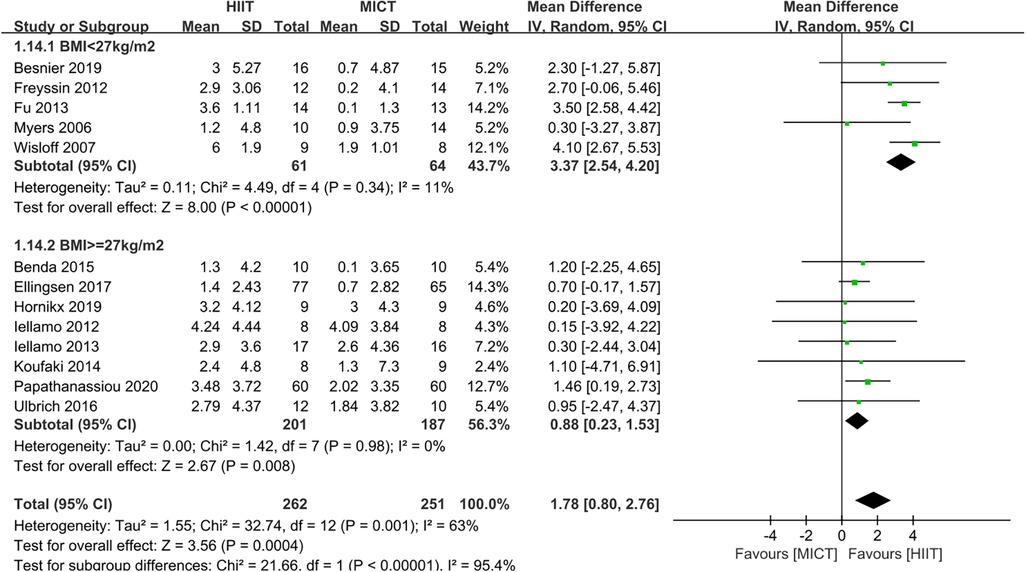
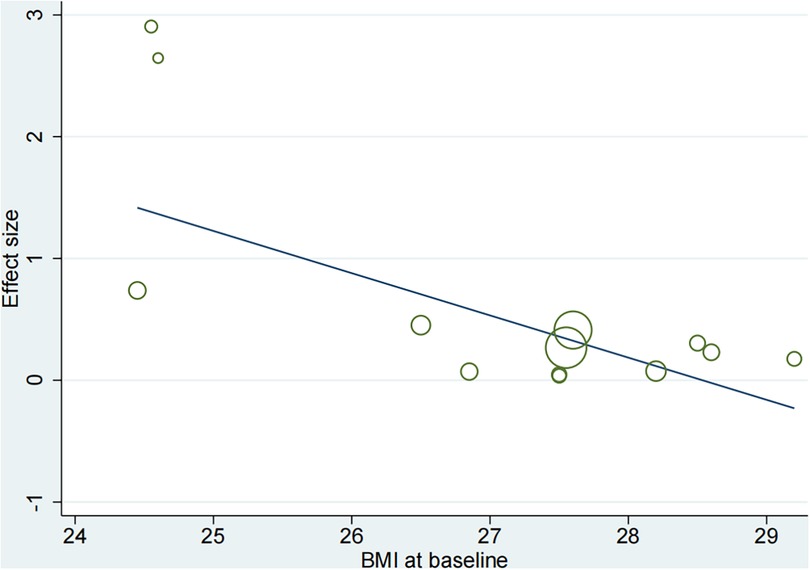
Based on the mean age of the participants, subgroup analyses demonstrated that HIIT significantly improved the Peak VO2 compared to MICT in patients aged ≤ 60 years old (MD = 1.67, 95% CI for 0.09–3.24; 120 participants; 5 studies; I2 = 0%; p = 0.04) and patients aged > 60 years old (MD = 2.07, 95% CI: 1.56–2.57; participants = 393; 8 studies; I2 = 77%; p < 0.00001) (Supplementary Figure S1). Subgroup analyses according to the duration of intervention showed that HIIT substantially increased the Peak VO2 compared to MICT in both interventions lasting <12weeks (MD = 2.55, 95% CI for 0.37–4.73; 57 participants; 2 studies; I2 = 0%; P = 0.02) and those lasting ≥12 weeks (MD = 1.65, 95% CI for 0.55–2.75; 456 participants; 11 studies; I2 = 69%; P = 0.003) (Supplementary Figure S2). In subgroup analyses considering BMI, the meta-analyses indicated that HIIT led to a significant increase in Peak VO2 compared to MICT in patients with BMI <27 kg/m2 (MD = 3.37, 95% CI for 2.54–4.20; 125 participants; 5 studies; I2 = 11%; P < 0.00001) and patients with BMI ≥27 kg/m2 (MD = 0.88, 95% CI for 0.23–1.53; 388 participants; 8 studies; I2 = 0%; P = 0.008; Figure 5). The BMI of the study participants was determined to be the source of heterogeneity based on the subgroup analyses described above. Furthermore, meta-regressions were conducted to examine the relationship between the difference in peak VO2 and the background factors of the patients. There was no significant correlation observed between post-intervention differences in peak VO2 and age (≤60 years or >60 years), LVEF (<35% or ≥35%), duration of intervention (<12 weeks or ≥12 weeks), or peak VO2 at baseline (<16 ml/kg/min or ≥16 ml/kg/min). However, the difference in peak VO2 between HIIT and MICT showed a negative correlation with BMI (<27 kg/m2 or ≥27 kg/m2) (r = −0.57; p = 0.002; 95% CI: −0.86 to −0.29), (Table 4, Figure 6), which further validates the results of the subgroup analysis.
3.5.2 Analysis of left ventricular ejection fraction
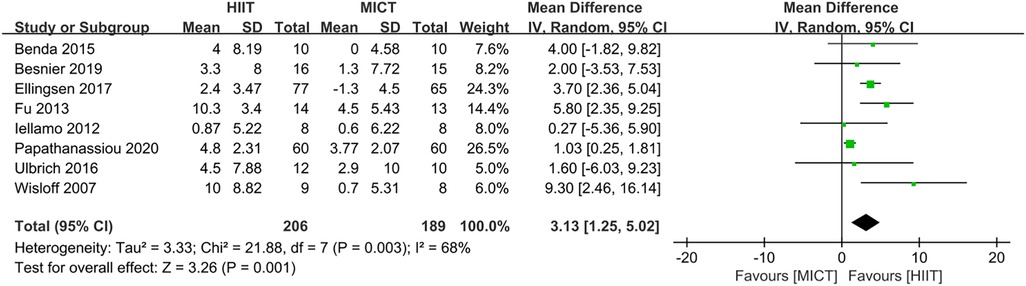
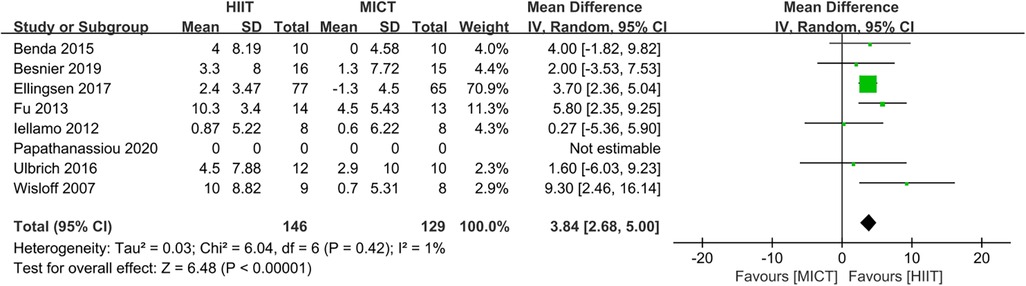
Eight studies (24–28, 31, 32, 34) evaluated the LVEF as outcome. In comparison to MICT, HIIT demonstrated a significant increase in LVEF (MD = 3.13, 95% CI for 1.25–5.02; 395 participants; 8 studies; I2 = 68%; P = 0.001; Figure 7). A sensitivity analysis was conducted, suggesting that the study conducted by Papathanasiou et al. (24) may be the source of heterogeneity. Heterogeneity was significantly decreased by excluding this study (MD = 3.84, 95% CI for 2.68–5.00; 275 participants; 7 studies; I2 = 1%; P < 0.00001; Figure 8).
3.5.3 Analysis of six-minute walk test
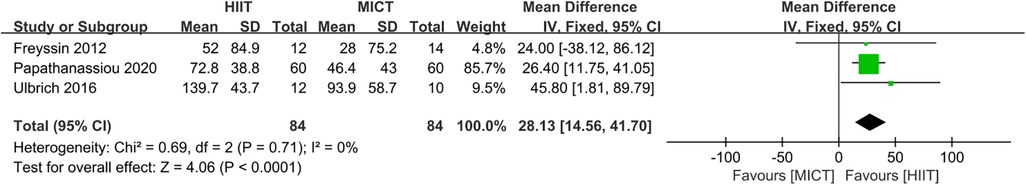
Three studies (24, 27, 33) assessed the 6MWT as outcome. HIIT led to significantly greater improvements in 6MWT compared to MICT (MD = 28.13, 95% CI for 14.56–41.70; 168 participants; 3 studies; I2 = 0%; P < 0.0001; Figure 9).
3.5.4 Analysis of Minnesota living with heart failure questionnaire
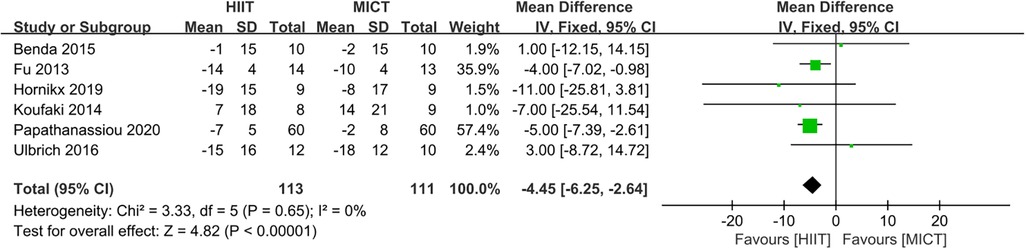
Six studies (23, 24, 27–29, 32) evaluated the MLHFQ as a measure of QoL. HIIT was superior to MICT for improving MLHFQ. (MD = −4.45, 95% CI for −6.25 to −2.64; 224 participants; 6 studies; I2 = 0%; P < 0.00001; Figure 10).
3.5.5 Analysis of resting heart rate and peak heart rate
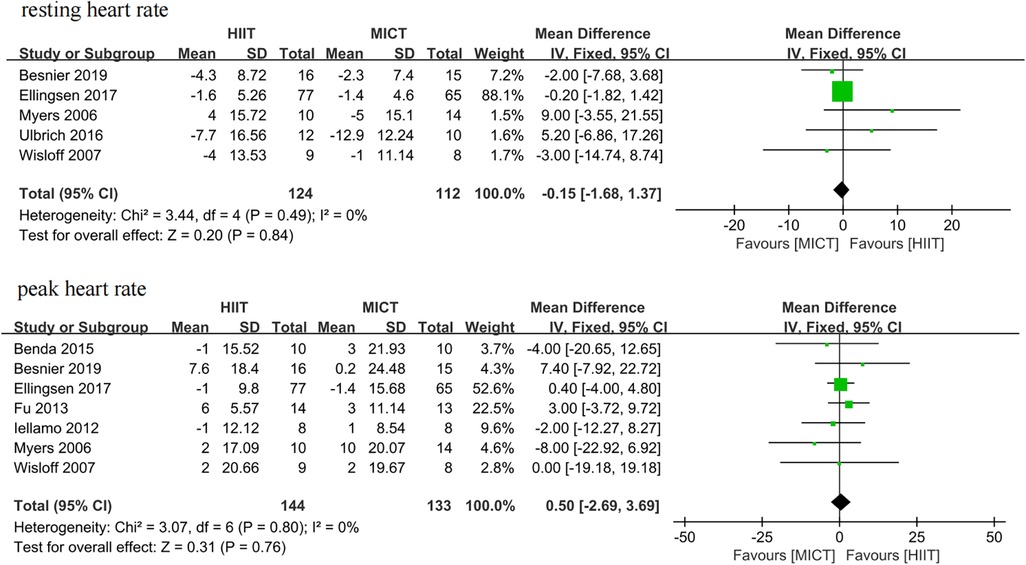
Five studies (25–27, 34, 35) and seven studies (25, 26, 28, 31, 32, 34, 35) assessed resting heart rate and peak heart rate as outcomes, respectively. The meta-analyses suggested that there was no significant difference in resting heart rate (MD = −0.15, 95% CI for −1.68 to 1.37; 236 participants; 5 studies; I2 = 0%; P = 0.84; Figure 11) and peak heart rate (MD = 0.50, 95% CI for −2.69 to 3.69; 277 participants; 7 studies; I2 = 0%; P = 0.76; Figure 11) between the HIIT and MICT groups.
4 Discussion
The objective of this systematic review and meta-analysis was to compare the effects of HIIT and MICT on aerobic and functional capacity in patients with HFrEF. The results demonstrated that HIIT was superior to MICT in terms of peak VO2, LVEF, 6MWT, and MLFHQ, while there were no significant differences in resting heart rate or peak heart rate. The findings of the current study's meta-analysis on peak VO2 are consistent with previous studies (12, 36). Additionally, when isocaloric protocols were analyzed in subgroups, high-intensity interval training remained superior in terms of peak VO2. In accordance with Okamura et al.' s previous study (36), additional meta-regression analyses demonstrated that the difference in peak VO2 was negatively correlated with BMI. However, Okamura et al.'s Meta-analysis included studies with participants with HF, including heart failure with preserved ejection fraction and HFrEF. Consequently, the strength of the current study is that all participants were exclusively HFrEF, thereby mitigating the potential influence of disease type on the outcomes. Additionally, the study demonstrated that HIIT was also significantly more favorable than MICT with respect to LVEF and MLHFQ. This finding is noteworthy as previous studies (12, 13, 36–38) did not report any significant differences between HIIT and MICT. Furthermore, the systematic review is the first to investigate the differences in 6MWT and heart rate between HIIT and MICT in patients with HFrEF.
It is important to highlight that peak VO2 serves as a predictive factor for readmission and mortality in HF patients. Furthermore, this research emphasizes the importance of enhancing peak VO2 through cardiac rehabilitation (29). Chou et al. (39) conducted an investigation into the mechanisms through which HIIT promotes the enhancement of peak VO2. Their findings revealed that HIIT improves platelet mitochondrial function, and there exists a positive correlation between whole-body aerobic capacity and platelet's mitochondrial reserve capacity. Additionally, peak VO2 serves as a vital indicator of aerobic capacity. The analysis of subgroups demonstrated that the difference in peak VO2 between HIIT and MICT was associated with BMI, but not with age, duration of the study, etc. Additional meta-regression analyses indicated a negative correlation between the difference in peak VO2 among the HIIT and MICT groups and BMI, suggesting that HIIT may be more effective in enhancing aerobic exercise capacity in HFrEF patients with a lower BMI. It has been reported that over half of HF patients, particularly those with HFrEF, suffer from skeletal sarcopenia, which has been linked to increased mortality rates in this population (40, 41). A low BMI is the primary characteristic of skeletal sarcopenia (42). In their study, Abshire et al. (43) found that a decreased BMI correlated with diminished bone density and an increased risk of osteoporosis among individuals with HF. Furthermore, it has been documented (44) that HIIT exhibits superior efficacy in reducing risk factors associated with skeletal sarcopenia and mitigating the morphological and functional deterioration linked to this condition, surpassing the benefits of resistance training. Notably, compared to the MICT group, the HIIT group experienced a significant reduction in BMI and a substantial increase in peak VO2, according to the study by Tadiotto et al. (45). Based on the results of the present meta-analysis, we therefore hypothesize that HIIT is preferable to MICT for increasing peak VO2 in HFrEF patients with a low. However, the limited number of studies investigating the impact of HIIT on aerobic capacity improvement in HF patients with a low BMI necessitates further high-quality research for validation.
The present study suggested that HIIT significantly increased LVEF in patients with HfrEF. Due to the significant heterogeneity observed, we conducted a sensitivity analysis and concluded that Papathanasiou et al.'s (16) study may have been a potential source of heterogeneity. We analyzed the possible reason why the study focused solely on patients with ischemic cardiomyopathy, hypertensive HF, and a limited number of patients with idiopathic dilated cardiomyopathy, rather than encompassing the entire range of HF conditions. It is important to note that HFrEF impacts cardiac hemodynamics and leads to a decrease in ventricular contraction, accompanied by compensatory remodeling of the left ventricle, which can temporarily compensate for abnormal hemodynamics and function but ultimately result in the worsening of cardiac function (46, 47). The LVEF serves as a crucial indicator of HF, displaying a direct correlation with mortality rate (48). The result of the present study is disagreement with Araújo's research (37), which may be attributed to the inclusion of only five randomized controlled studies and the uncertain risk of bias in most of the included studies.
In patients with chronic cardiovascular disease, the 6MWT is widely used to assess functional capacity and prognosis. The test effectively replicates activities of daily living and is particularly valuable for elderly patients, whose symptoms and activity performances are typically inferior to theoretical maximal exercise capacity (49, 50). In HF patients, the 6MWT has proven to be a dependable indicator of increased hospitalization rates and mortality, with distances less than 300 meters indicating a poor prognosis (51, 52). Additionally, the 6MWT can be used to estimate peak VO2 (53). Several RCTs (24, 27, 33) have demonstrated that patients with HF in the HIIT group experienced a significant increase in the six-minute walk distance (6MWD) compared to the MICT group. This finding suggests that HIIT is more effective in improving exercise endurance than MICT, which is consistent with the present study. However, it is important to note that only three studies exploring the effect of HIIT vs. MICT on 6MWD were included in the current meta-analysis, potentially indicating reporting bias. Further studies with larger sample sizes and longer follow-up periods will be necessary to accurately quantify the effects of HIIT on 6MWD.
The Minnesota Living with Heart Failure Questionnaire (MLHFQ) is a specialized self-assessment tool used to evaluate the QoL of individuals with HF, specifically assessing their symptoms and concerns (54). Research has indicated that HF patients who performed longer distances in the 6MWT also experience notable enhancements in MLHFQ scores (55). Furthermore, it has been suggested that improvements in QoL among HF patients are closely associated with advancements in exercise capacity and clinical symptoms (56). In a study conducted by Mansueto et al. (12), there was no statistically significant difference between HIIT and MICT in terms of improving QoL for patients with HF. This may be related to the large differences in the exercise protocols (intensity and duration, etc.) of the included studies, the significant heterogeneity found, and the fact that these come from very low-quality evidence. However, previous research (24, 27) has demonstrated that patients with HFrEF who underwent HIIT still exhibited significantly higher scores on the MLHFQ compared to those in the MICT group, both post-treatment and during the follow-up period, which is consistent with the present finding. The discrepancy between our results and Okamura et al. (36) may be attributed to the fact that this study exclusively included patients with HFrEF, while Okamura et al. included patients with both HFrEF and HF with preserved ejection fraction. The MLHFQ score may have been improved more significantly in patients with HFrEF when HIIT was used.
There is positive correlation between an elevation in resting heart rate and an increase in mortality due to cardiovascular disease (57), which also impacts the long-term prognosis of patients with HFrEF, with notably improved prognoses for individuals who experience a reduction in resting heart rate of 20 beats per minute following discharge (58). It has been demonstrated that the significance of appropriate elevations in heart rate during exercise training in maintaining sufficient cardiac output and meeting metabolic requirements in patients with HF (59). The relationship between resting heart rate, peak heart rate, peak VO2, and adverse events has been explored in previous studies (60). Besnier et al. (25) demonstrated that HIIT led to a significant decrease in resting heart rate and an increase in peak heart rate compared to the MICT group. However, Ulbrich et al. (27) reported contradictory results, showing that MICT significantly reduced resting heart rate in patients with HF, which may be related to the fact that the study was only focus on male participants and was limited to HF patients with a mean age of 53 years. According to the current study, resting heart rate and peak heart rate were not statistically different between the HIIT and MICT groups. It may be due to the fact that most studies included were conducted for less than 12 weeks, with one study (29) lasting 24 weeks. As a result, changes in heart rate may not be detected in these studies because of the short duration. For further validation, additional randomized controlled trials need to be conducted with large sample sizes and long follow-up periods.
The meta-analysis also has a few limitations, which should be mentioned: Firstly, it is important to note that the majority of RCTs included in the meta-analysis lasted less than 12 weeks, and only one study lasted 24 weeks. Therefore, it remains unclear whether HIIT is more effective than MICT in patients with HFrEF in the long term. Secondly, it is worth mentioning that the majority of HFrEF patients included in this meta-analysis were elderly men (88%), with an average age of 63 years. As a result, it is unknown whether the findings are applicable to elderly female patients with HFrEF. Finally, despite the fact that all included studies were randomized controlled trials, it was unfeasible to effectively blind both participants and outcome assessors in the exercise training intervention studies. Furthermore, to investigate isocaloric training protocols in HF patients comprehensively, more large-scale RCTs of high quality must be conducted.
5 Conclusion
The systematic review indicates that HIIT was superior to MICT in terms of Peak VO2, LVEF, 6MWT, and MLHFQ scores in patients with HFrEF. Additionally, the sub-analysis of isocaloric protocols on peak VO2 also confirmed the superiority. Moreover, HIIT may be more effective in promoting exercise capacity among HFrEF patients with a low BMI. However, we found no evidence to support significant differences in resting heart rate and peak heart rate between HIIT and MICT.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
CY: Formal Analysis, Investigation, Methodology, Resources, Software, Writing – original draft, Writing – review & editing. LZ: Investigation, Writing – review & editing. YC: Writing – original draft. MZ: Investigation, Writing – original draft. YZ: Formal Analysis, Writing – review & editing. TZ: Software, Writing – review & editing. JD: Formal Analysis, Writing – original draft. JX: Methodology, Writing – review & editing. YZ: Investigation, Writing – original draft. CW: Software, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by grants from the Hebei Provincial Department of Science and Technology, China (grant number 20377787D), the Department of Health of Hebei Province (grant number 20210816 and grant number 20160212) and the Hebei Provincial Health Planning Commission (grant number 20170509).
Acknowledgments
The authors particularly acknowledge CW for helping with the statistical analysis, and Dr. Feilong Zhu for correcting English language, grammar, punctuation, and phrasing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1302109/full#supplementary-material
References
1. Hitzeroth J, Mpe M, Klug E, Ranjith N, Sliwa K, Steingo L, et al. 2020 Heart Failure Society of South Africa perspective on the 2016 European society of cardiology chronic heart failure guidelines. S Afr Med J. (2020) 110:13057. doi: 10.7196/SAMJ.2020.v110i8.14681
2. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Kardiol Pol. (2016) 74:1037–147. doi: 10.5603/KP.2016.0141
3. Francis DP, Shamim W, Davies LC, Piepoli MF, Ponikowski P, Anker SD, et al. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from ve/vco2 slope and peak vo2. Eur Heart J. (2000) 21:154–61. doi: 10.1053/euhj.1999.1863
4. Coats AJS, Adamopoulos S, Meyer TE, Conway J, Sleight P. Effects of physical training in chronic heart failure. Lancet. (1990) 335:63–6. doi: 10.1016/0140-6736(90)90536-e
5. Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult. J Am Coll Cardiol. (2005) 46:1–82. doi: 10.1016/j.jacc.2005.08.022
6. Ito S, Mizoguchi T, Saeki T. Review of high-intensity interval training in cardiac rehabilitation. Internal Med. (2016) 55:2329–36. doi: 10.2169/internalmedicine.55.6068
7. Wu Q, Niu X, Zhang Y, Song J, Chi A. A comparative study of inhibition function between high-intensity interval training and moderate-intensity continuous training in healthy people: a systematic review with meta-analysis. Int J Environ Res Public Health. (2023) 20:2859. doi: 10.3390/ijerph20042859
8. Spee RF, Niemeijer VM, Schoots T, Tuinenburg A, Houthuizen P, Wijn PF, et al. High intensity interval training after cardiac resynchronization therapy: an explorative randomized controlled trial. Int J Cardiol. (2020) 299:169–74. doi: 10.1016/j.ijcard.2019.07.023
9. Van De Heyning CM, De Maeyer C, Pattyn N, Beckers PJ, Cornelissen VA, Goetschalckx K, et al. Impact of aerobic interval training and continuous training on left ventricular geometry and function: a saintex-cad substudy. Int J Cardiol. (2018) 257:193–8. doi: 10.1016/j.ijcard.2017.11.053
10. Jung ME, Bourne JE, Little JP. Where does hit fit? An examination of the affective response to high-intensity intervals in comparison to continuous moderate- and continuous vigorous-intensity exercise in the exercise intensity-affect continuum. PLoS One. (2014) 9:e114541. doi: 10.1371/journal.pone.0114541
11. Hu JY, Cai M, Shang QH, Li ZR, Lou sj. Research progress on the mechanism of improving cognitive function by high-intensity interval training. Sheng Li Xue Bao. (2021) 73:126–36. doi: 10.13294/j.aps.2020.0077
12. Gu S, Du X, Wang D, Yu Y, Guo S. Effects of high intensity interval training versus moderate intensity continuous training on exercise capacity and quality of life in patients with heart failure: a systematic review and meta-analysis. Plos One. (2023) 18:e290362. doi: 10.1371/journal.pone.0290362
13. Tucker WJ, Beaudry RI, Liang Y, Clark AM, Tomczak CR, Nelson MD, et al. Meta-analysis of exercise training on left ventricular ejection fraction in heart failure with reduced ejection fraction: a 10-year update. Prog Cardiovasc Dis. (2019) 62:163–71. doi: 10.1016/j.pcad.2018.08.006
14. Driscoll A, Srivastava P, Toia D, Gibcus J, Hare DL. A nurse-led up-titration clinic improves chronic heart failure optimization of beta-adrenergic receptor blocking therapy–a randomized controlled trial. BMC Res Notes. (2014) 7:668. doi: 10.1186/1756-0500-7-668
15. Lai P, Xue J, Xie M, Ye J, Yang N, Zhong Y, et al. High-intensity and moderate-intensity interval training in heart failure with preserved ejection fraction: a meta-analysis of randomized controlled trials. Medicine (Baltimore). (2023) 102:e33010. doi: 10.1097/MD.0000000000033010
16. Siddiqi TJ, Rashid AM, Javaid SS, Siddiqi AK, Usman MS, Hervir O, et al. High-intensity interval training versus moderate continuous training in patients with heart failure with preserved ejection fraction: a systematic review and meta-analysis. Curr Probl Cardiol. (2023) 48:8. doi: 10.1016/j.cpcardiol.2023.101720
17. Zhang S, Zhang J, Liang C, Li X, Meng X. High-intensity interval training for heart failure with preserved ejection fraction. Medicine (Baltimore). (2020) 99:e21062. doi: 10.1097/MD.0000000000021062
18. Vromen T, Kraal JJ, Kuiper J, Spee RF, Peek N, Kemps HM. The influence of training characteristics on the effect of aerobic exercise training in patients with chronic heart failure: a meta-regression analysis. Int J Cardiol. (2016) 208:120–7. doi: 10.1016/j.ijcard.2016.01.207
19. Gomes NM, Duraes AR, Conceicao L, Saquetto MB, Ellingsen O, Carvalho VO. High intensity interval training versus moderate intensity continuous training on exercise capacity and quality of life in patients with heart failure with reduced ejection fraction: a systematic review and meta-analysis. Int J Cardiol. (2018) 261:134–41. doi: 10.1016/j.ijcard.2018.02.076
20. Weston KS, Wisloff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med. (2014) 48:1227–34. doi: 10.1136/bjsports-2013-092576
21. Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. Grade guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. (2011) 64:1283–93. doi: 10.1016/j.jclinepi.2011.01.012
22. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
23. Hornikx M, Buys R, Cornelissen V, Deroma M, Goetschalckx K. Effectiveness of high intensity interval training supplemented with peripheral and inspiratory resistance training in chronic heart failure: a pilot study. Acta Cardiol. (2019) 75:339–47. doi: 10.1080/00015385.2019.1591676
24. Papathanasiou JV, Petrov I, Tokmakova MP, Dimitrova DD, Spasov L, Dzhafer NS, et al. Group-based cardiac rehabilitation interventions. A challenge for physical and rehabilitation medicine physicians: a randomized controlled trial. Eur J Phys Rehabil Med. (2020) 56:479–88. doi: 10.23736/S1973-9087.20.06013-X
25. Besnier F, Labrunée M, Richard L, Faggianelli F, Kerros H, Soukarié L, et al. Short-term effects of a 3-week interval training program on heart rate variability in chronic heart failure. A randomised controlled trial. Ann Phys Rehabil Med. (2019) 62:321–8. doi: 10.1016/j.rehab.2019.06.013
26. Ellingsen Ø, Halle M, Conraads V, Støylen A, Dalen H, Delagardelle C, et al. High-intensity interval training in patients with heart failure with reduced ejection fraction. Circulation. (2017) 135:839–49. doi: 10.1161/CIRCULATIONAHA.116.022924
27. Ulbrich AZ, Angarten VG, Schmitt Netto A, Sties SW, Bündchen DC, Mara LSD, et al. Comparative effects of high intensity interval training versus moderate intensity continuous training on quality of life in patients with heart failure: study protocol for a randomized controlled trial. Clin Trials Regul Sci Cardiol. (2016) 13:21–8. doi: 10.1016/j.ctrsc.2015.11.005
28. Benda NMM, Seeger JPH, Stevens GGCF, Hijmans-Kersten BTP, van Dijk APJ, Bellersen L, et al. Effects of high-intensity interval training versus continuous training on physical fitness, cardiovascular function and quality of life in heart failure patients. PLoS One. (2015) 10:e0141256. doi: 10.1371/journal.pone.0141256
29. Koufaki P, Mercer TH, George KP, Nolan J. Low-volume high-intensity interval training vs continuous aerobic cycling in patients with chronic heart failure: a pragmatic randomised clinical trial of feasibility and effectiveness. J Rehabil Med. (2014) 46:348–56. doi: 10.2340/16501977-1278
30. Iellamo F, Caminiti G, Sposato B, Vitale C, Massaro M, Rosano G, et al. Effect of high-intensity interval training versus moderate continuous training on 24-h blood pressure profile and insulin resistance in patients with chronic heart failure. Intern Emerg Med. (2014) 9:547–52. doi: 10.1007/s11739-013-0980-4
31. Iellamo F, Manzi V, Caminiti G, Vitale C, Castagna C, Massaro M, et al. Matched dose interval and continuous exercise training induce similar cardiorespiratory and metabolic adaptations in patients with heart failure. Int J Cardiol. (2013) 167:2561–5. doi: 10.1016/j.ijcard.2012.06.057
32. Fu T, Wang C, Lin P, Hsu C, Cherng W, Huang S, et al. Aerobic interval training improves oxygen uptake efficiency by enhancing cerebral and muscular hemodynamics in patients with heart failure. Int J Cardiol. (2013) 167:41–50. doi: 10.1016/j.ijcard.2011.11.086
33. Freyssin C, Verkindt C, Prieur F, Benaich P, Maunier S, Blanc P. Cardiac rehabilitation in chronic heart failure: effect of an 8-week, high-intensity interval training versus continuous training. Arch Phys Med Rehabil. (2012) 93:1359–64. doi: 10.1016/j.apmr.2012.03.007
34. Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients—a randomized study. Circulation. (2007) 115:3086–94. doi: 10.1161/CIRCULATIONAHA.106.675041
35. Myers J, Hadley D, Oswald U, Bruner K, Kottman W, Hsu L, et al. Effects of exercise training on heart rate recovery in patients with chronic heart failure. Am Heart J. (2007) 153:1056–63. doi: 10.1016/j.ahj.2007.02.038
36. Okamura M, Shimizu M, Yamamoto S, Nishie K, Konishi M. High-intensity interval training versus moderate-intensity continuous training in patients with heart failure: a systematic review and meta-analysis. Heart Fail Rev. (2023) 28:1113–28. doi: 10.1007/s10741-023-10316-3
37. Araújo BTS, Leite JC, Fuzari HKB, Pereira De Souza RJ, Remígio MI, Dornelas De Andrade A, et al. Influence of high-intensity interval training versus continuous training on functional capacity in individuals with heart failure. J Cardiopulm Rehabil Prev. (2019) 39:293–8. doi: 10.1097/HCR.0000000000000424
38. Xie B, Yan X, Cai X, Li J. Effects of high-intensity interval training on aerobic capacity in cardiac patients: a systematic review with meta-analysis. Biomed Res Int. (2017) 2017:1–16. doi: 10.1155/2017/5420840
39. Chou CH, Fu TC, Tsai HH, Hsu CC, Wang CH, Wang JS. High-intensity interval training enhances mitochondrial bioenergetics of platelets in patients with heart failure. Int J Cardiol. (2019) 274:214–20. doi: 10.1016/j.ijcard.2018.07.104
40. Konishi M, Akiyama E, Matsuzawa Y, Sato R, Kikuchi S, Nakahashi H, et al. Prognostic impact of muscle and fat mass in patients with heart failure. J Cachexia Sarcopenia Muscle. (2021) 12:568–76. doi: 10.1002/jcsm.12702
41. Konishi M, Kagiyama N, Kamiya K, Saito H, Saito K, Ogasahara Y, et al. Impact of sarcopenia on prognosis in patients with heart failure with reduced and preserved ejection fraction. Eur J Prev Cardiol. (2021) 28:1022–9. doi: 10.1093/eurjpc/zwaa117
42. von Haehling S, Ebner N, Dos Santos MR, Springer J, Anker SD. Muscle wasting and cachexia in heart failure: mechanisms and therapies. Nat Rev Cardiol. (2017) 14:323–41. doi: 10.1038/nrcardio.2017.51
43. Abshire DA, Moser DK, Clasey JL, Chung ML, Pressler SJ, Dunbar SB, et al. Body composition and bone mineral density in patients with heart failure. West J Nurs Res. (2017) 39:582–99. doi: 10.1177/0193945916658885
44. Bruseghini P, Calabria E, Tam E, Milanese C, Oliboni E, Pezzato A, et al. Effects of eight weeks of aerobic interval training and of isoinertial resistance training on risk factors of cardiometabolic diseases and exercise capacity in healthy elderly subjects. Oncotarget. (2015) 6:16998–7015. doi: 10.18632/oncotarget.4031
45. Tadiotto MC, Corazza PRP, Menezes-Junior FJD, Moraes-Junior FBD, Tozo TAA, Purim KSM, et al. Effects and individual response of continuous and interval training on adiponectin concentration, cardiometabolic risk factors, and physical fitness in overweight adolescents. Eur J Pediatr. (2023) 182:2881–9. doi: 10.1007/s00431-023-04974-6
46. Bloom MW, Greenberg B, Jaarsma T, Januzzi JL, Lam CSP, Maggioni AP, et al. Heart failure with reduced ejection fraction. Nat Rev Dis Primers. (2017) 3:17058. doi: 10.1038/nrdp.2017.58
47. Aimo A, Gaggin HK, Barison A, Emdin M, Januzzi JL. Imaging, biomarker, and clinical predictors of cardiac remodeling in heart failure with reduced ejection fraction. JACC Heart Fail. (2019) 7:782–94. doi: 10.1016/j.jchf.2019.06.004
48. Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol. (2003) 42:736–42. doi: 10.1016/S0735-1097(03)00789-7
49. Papathanasiou JV, Ilieva E, Marinov B. Six-minute walk test: an effective and necessary tool in modern cardiac rehabilitation. Hellenic J. Cardiol. (2013) 54:126–30. doi: 10.1016/j.rehab.2014.03.1071
50. Papathanasiou J, Petrov I, Ferreira A, Lopes AJ. Cardiac Rehabilitation. In: Papathanasiou J, Panayotov KE 2nd editors. Essentials of Physical and Rehabilitation Medicine for Undergraduate Medical Students. Ruse, BG: Avangard (2023). p. 427–38.
51. Imamura T, Narang N, Ushijima R, Sobajima M, Fukuda N, Ueno H, et al. Prognostic impact of baseline six-minute walk distance following trans-catheter aortic valve replacement. J Clin Med. (2023) 12:2504. doi: 10.3390/jcm12072504
52. Daullxhiu I, Haliti E, Poniku A, Ahmeti A, Hyseni V, Olloni R, et al. Predictors of exercise capacity in patients with chronic heart failure. J Cardiovasc Med (Hagerstown). (2011) 12:223–5. doi: 10.2459/JCM.0b013e328343e950
53. Ingle L, Goode K, Rigby ASR, Cleland JGF, Clark AL. Predicting peak oxygen uptake from 6-min walk test performance in male patients with left ventricular systolic dysfunction. Eur J Heart Fail. (2006) 8:198–202. doi: 10.1016/j.ejheart.2005.07.008
54. Cong J, Zhu Y, Du J, Lin L, He Y, Zhang Q, et al. Mapping the Minnesota living with heart failure questionnaire (mlhfq) to sf-6dv2 in Chinese patients with heart failure. Health Qual Life Outcomes. (2022) 20:98. doi: 10.1186/s12955-022-02004-x
55. Napier R, McNulty SE, Eton DT, Redfield MM, AbouEzzeddine O, Dunlay SM. Comparing measures to assess health-related quality of life in heart failure with preserved ejection fraction. JACC Heart Fail. (2018) 6:552–60. doi: 10.1016/j.jchf.2018.02.006
56. Giannuzzi P, Temporelli PL, Corrà U, Tavazzi L. Antiremodeling effect of long-term exercise training in patients with stable chronic heart failure. Circulation. (2003) 108:554–9. doi: 10.1161/01.CIR.0000081780.38477.FA
57. Kannel WB, Kannel C, Paffenbarger RS, Cupples LA. Heart rate and cardiovascular mortality: the Framingham study. Am Heart J. (1987) 113:1489–94. doi: 10.1016/0002-8703(87)90666-1
58. Tsai M, Lin S, Kao Y, Lin H, Lin M, Peng J, et al. Optimal heart rate control improves long-term prognosis of decompensated heart failure with reduced ejection fraction. Medicina (B Aires). (2023) 59:348. doi: 10.3390/medicina59020348
59. Nguyen UC, Verzaal NJ, van Nieuwenhoven FA, Vernooy K, Prinzen FW. Pathobiology of cardiac dyssynchrony and resynchronization therapy. Europace. (2018) 20:1898–909. doi: 10.1093/europace/euy035
Keywords: heart failure with reduced ejection fraction, high-intensity interval training, moderate-intensity continuous training, exercise capacity, functional capacity
Citation: Yang C, Zhang L, Cheng Y, Zhang M, Zhao Y, Zhang T, Dong J, Xing J, Zhen Y and Wang C (2024) High intensity interval training vs. moderate intensity continuous training on aerobic capacity and functional capacity in patients with heart failure: a systematic review and meta-analysis. Front. Cardiovasc. Med. 11:1302109. doi: 10.3389/fcvm.2024.1302109
Received: 26 September 2023; Accepted: 7 February 2024;
Published: 21 February 2024.
Edited by:
Matteo Cameli, University of Siena, ItalyReviewed by:
Maurizio Volterrani, IRCCS San Raffaele, ItalyJannis Papathanasiou, Medical University Sofia, Bulgaria
© 2024 Yang, Zhang, Cheng, Zhang, Zhao, Zhang, Dong, Xing, Zhen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Xing 785196405@qq.com Yuzhi Zhen yuzhi96230@sina.com Cuihua Wang 58303056@hebmu.edu.cn
†These authors have contributed equally to this work
Abbreviations HIIT, high-intensity interval training; MICT, moderate-intensity continuous training; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; Peak VO2, peak oxygen uptake; LVEF, left ventricular ejection fraction; 6MWT, six-minute walk test; MLHFQ, minnesota living with heart failure questionnaire.
 Changran Yang
Changran Yang