
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 25 January 2024
Sec. Heart Failure and Transplantation
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1293537
Background: There is controversy in relation to commonly used drugs in heart failure (HF) and their impact on muscle function. The aim of this study was to evaluate the odds of receiving specific medications often used in clinical practice by patients with HF and sarcopenia vs. without sarcopenia.
Methods: A systematic literature search of cohort studies via databases (PubMed, Web of Science, Scopus, and Cochrane Library) was conducted from inception until March 2023. To determine if sarcopenia is linked to a higher number of specific HF-related medications, a meta-analysis using a random-effects model was used to calculate the pooled effects.
Results: Our main analyses showed no significant association of sarcopenia with administration of higher HF-related medication count vs. those without sarcopenia. Those with lower appendicular lean mass (ALM) had significantly lower odds of receiving angiotensin converting enzyme inhibitors (ACE-Is)/angiotensin receptor blockers (ARBs) (OR: 0.68, 95%CI 0.50–0.90, I2 = 12%, P < 0.01) vs. patients with higher ALM for which age could be an important confounder based on meta-regression. No statistically significant differences were found in relation to B-blockers OR: 0.84, 95%CI 0.63–1.12, I2 = 7%, P = 0.24) and loop diuretics (OR: 1.19, 95%CI 0.87–1.63, I2 = 0%, P = 0.27). Regarding handgrip strength, gait speed, and short physical performance battery, our narrative synthesis found mixed results.
Conclusion: This systematic review and meta-analysis did not find a relationship of specific medication count in sarcopenia vs. without sarcopenia in patients with HF, although increased odds of ACE-I/ARB was shown in those with higher ALM.
Systematic Review Registration: PROSPERO (CRD42023411137).
Anatomical and functional myocardial defects that impede ventricular filling or blood ejection may cause heart failure (HF). The most common cause of HF is decreased left ventricular myocardial systolic or diastolic function, but other causes include dysfunction of the valves, pericardium, or systemic conditions. HF is the most prevalent reason for hospitalisation in adults over 65, and clinically, symptoms in patients with HF are compounded by a higher prevalence of comorbidities that come with ageing (1).
It is well-established that primary sarcopenia or the loss of skeletal muscle mass and function with ageing, has a negative impact on healthspan. Secondary sarcopenia refers to the common factors outside age that could lead to losses of skeletal muscle mass and strength observed among individuals who suffer from chronic illnesses, including those with HF, contributing to increased mortality and morbidity (2). Primary and secondary sarcopenia are likely to be present together and may be additive in older people with chronic conditions, which may explain the high prevalence of this condition in patients with HF (3).
Interestingly, administration of medications has been linked to improved or impaired muscle function, depending on appropriate or inappropriate prescription, respectively. For instance, it has been suggested that angiotensin-converting enzyme inhibitors (ACE-Is) may exert positive effects on skeletal muscle in older adults, improving physical function (4) and alleviating declines in knee extension strength (5), which could be attributed to increased total insulin growth factor-1 (IGF-1) levels (6). Conversely, in a healthy older cohort (Hertfordshire Cohort Study) with a median follow-up time of 4.4 years, ACE-Is, statins, or thiazides were not associated with declines in grip strength (7), while results from the TRAIN study consisted of older people with increased cardiovascular risk also reported no significant changes in physical performance and grip strength after 6 months of fosinopril use (8). These findings may be relevant pertaining to the potential of inappropriate prescription count or duration, which may unravel potentially reduced muscle-protective responses of specific medications commonly administered in patients with HF.
The association between sarcopenia and specific drugs consumed by patients with HF has not been studied before in a systematic manner. To address this issue, the purpose of this study is to investigate observational studies in which participants with HF had sarcopenia compared to participants without sarcopenia, aiming to evaluate whether a higher prevalence of drugs commonly administered in this patient group is interlinked to sarcopenia or non-sarcopenia.
The revised 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria were followed for conducting this systematic review and meta-analysis. The protocol has been entered into PROSPERO, the Prospective Register of Systematic Reviews International Database (CRD42023411137).
From the beginning until March 2023, PubMed, Scopus, Web of Science, and Cochrane Library were searched independently by K. P. and A.A. In the supporting information (Supplementary Table S1), the complete search technique and the search phrases employed are presented. A third researcher resolved any discrepancies that arose during the literature search process (M.I.).
The following criteria were used to determine which studies should be included: (i) baseline data from observational studies (i.e., cross-sectional, longitudinal, or case-control); (ii) adults aged 50 years and above with HF; (iii) clear diagnostic criteria for sarcopenia employing data from appendicular lean mass (ALM) combined with muscle strength and/or physical function outcomes; and (iv) available data from both patients with sarcopenia and without sarcopenia. Published articles were excluded if they (i) did not assess body composition with established assessment tools; ii) included patients were under the age of 50; (iii) were reviews, letters, in vivo or in vitro experiments, commentaries, or posters; and (iv) were not published as a full text and in English.
Data on the first author, publication date, country of origin, study design, participant age, left ventricular ejection fraction (LVEF) rate, number of participants, gender, reported comorbidities, assessment tool for ALM, sarcopenia definition, and type and number of HF-related medications were all extracted independently by two authors (K. P. and A.A.).
Two independent reviewers used Newcastle–Ottawa Scale (NOS) tool to assess the risk of bias of the included studies (A.A., and S.S.). The NOS is divided into three domains: selection (4 items), comparability (1 item), and result (3 items). When a study fulfils the methodological expected standard, each item in the selection and outcome domains receives one star, with a maximum of two stars awarded for the comparability domain. Studies with a star rating from 0 to 5 have a high risk of bias, 6 to 7 a moderate risk, and 8 to 9 a low risk of bias (9).
To determine the odds ratio (OR) relating to the use of specific medications, quantitative data were handled as dichotomous measurements, and changes in outcomes from patients with and without sarcopenia were compared between groups. The inverse-variance approach and the random-effects model were used to determine statistical significance.
The overlap of their 95% confidence intervals (95% CI) and measures of Cochran's Q (Chi-square test) and I2 were used to analyse the statistical heterogeneity of outcome data across various studies. Low heterogeneity was defined as I2 of 30% to 49%, moderate heterogeneity as I2 of 50%–74%, and high heterogeneity as I2 of 75% and above. Sensitivity analyses that discounted the impact of sarcopenia definition that did not assess ALM and an increased risk of bias of the included cohort studies were carried out to assess the robustness of reported statistical results. The meta-analysis was synthesized using Review Manager (RevMan 5.4.1) software and a P value of <0.05 was considered statistically significant.
Meta-regressions were performed using a random-effects model to assess unexplained variance among studies with significant heterogeneity. Individual factors included age, LVEF (%), and body mass index (BMI), using STATA/MP 13.0.
The initial literature search provided 591 publications. Following the exclusion of duplicates and abstracts, 38 full texts were identified as eligible for inclusion in the systematic review and meta-analysis. Of these 38 studies, six studies were dismissed due to inadequate data on listed medications, five studies because they used identical cohorts relevant to ones included in our study, two studies due to insufficient details pertinent to ALM and handgrip strength, one study used psoas muscle index as definition of sarcopenia, one study used an inappropriate equation/non-established body composition assessment tool for ALM measurements, and one study included patients with non-severe or no sarcopenia. In total, 22 studies (10–31) were included in the systematic review and meta-analysis exploring the association of different HF-related medications with sarcopenia vs. without sarcopenia in cohorts with patients with HF (Figure 1). Characteristics of the included studies are summarised in Table 1.

Table 1. Study and participant characteristics of the included studies in the systematic review and meta-analysis.
Ten studies assessed the prevalence of different HF-related medications in patients with sarcopenia (12, 13, 16, 18, 20, 23, 24, 26, 27, 31), five studies in patients with low ALM (11, 14, 17, 25, 29), two studies with low handgrip strength (21, 30), four studies with low gait speed (10, 15, 22, 28), and one study with low short physical performance battery (SPPB) scores (19). Detailed characteristics of the included studies are outlined in Table 1.
To define sarcopenia, two studies used the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) criteria (13, 27), two studies used the EWGSOP1 criteria (20, 24), four studies used the Asian Working Group for Sarcopenia 2014 criteria (18, 23, 26, 31), one study used the Japanese Geriatrics Society criteria (16), and one study used the Ishii index (12).
Our main analysis showed no significant association of sarcopenia with angiotensin converting enzyme inhibitors (ACE-Is)/angiotensin receptor blockers (ARBs) use vs. no sarcopenia (OR: 0.78, 95%CI 0.54–1.13, I2 = 45%, P = 0.19) (Figure 2). Likewise, no differences were found in relation to B-blocker use (OR: 0.95, 95%CI 0.57–1.57, I2 = 61%, P = 0.83) (Figure 3), loop diuretics (OR: 1.09, 95%CI 0.73–1.63, I2 = 47%, P = 0.68) (Figure 4), and statins (Sarcopenia, n = 334; No sarcopenia, n = 640; OR: 0.80, 95%CI 0.48–1.34, I2 = 61%, P = 0.40) (Figure 5).
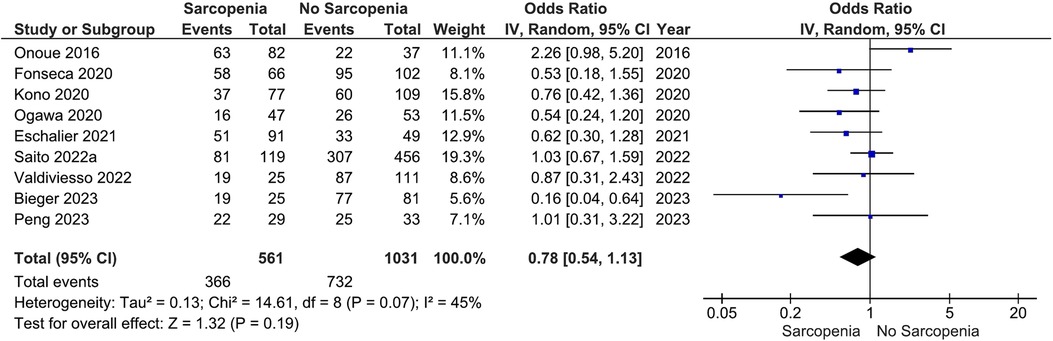
Figure 2. Association of ACE-I/ARB administration in patients with HF and sarcopenia versus without sarcopenia.
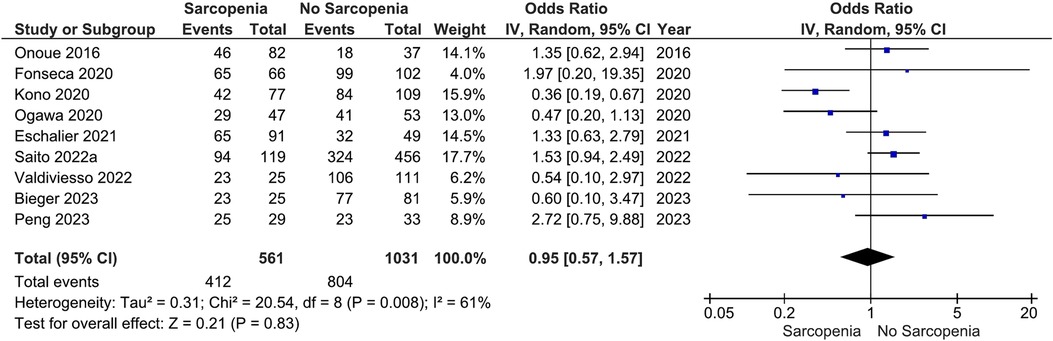
Figure 3. Association of B-blocker administration in patients with HF and sarcopenia versus without sarcopenia.
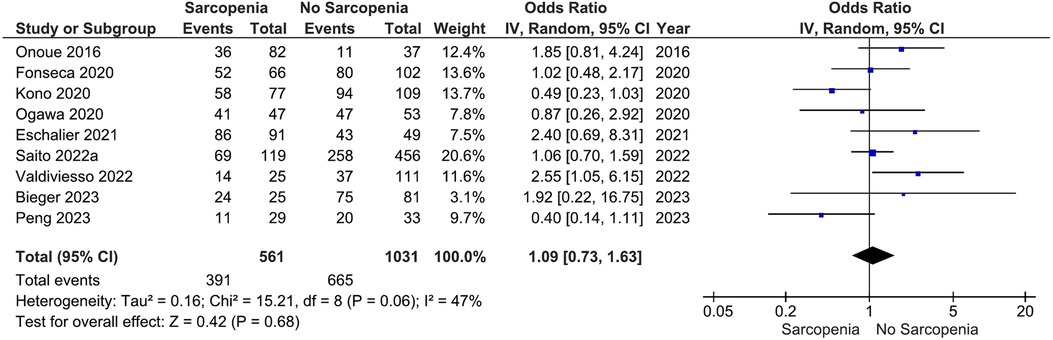
Figure 4. Association of loop diuretic administration in patients with HF and sarcopenia versus without sarcopenia.
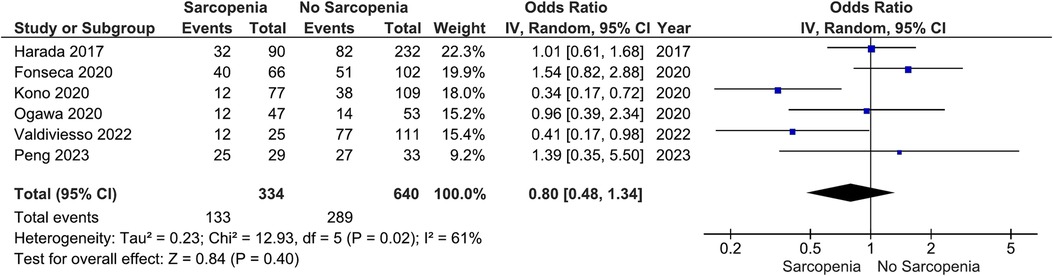
Figure 5. Association of statin administration in patients with HF and sarcopenia versus without sarcopenia.
Sensitivity analyses based on impartial sarcopenia definition did not reveal any significant differences (ACE-I/ARB; OR: 0.65, 95%CI 0.42–1.02, I2 = 41%, P = 0.06 (Figure S1); B-blockers; OR: 1.19, 95%CI 0.73–1.95, I2 = 35%, P = 0.49 (Figure S2); Loop diuretics; OR: 1.00, 95%CI 0.70–1.43, I2 = 9%, P = 0.98 (Figure S3); Statins; OR: 1.16, 95%CI 0.82–1.65, I2 = 0%, P = 0.39 (Figure S4)) nor by excluding studies with high risk of bias (ACE-I/ARB; OR: 0.81, 95%CI 0.38–1.73, I2 = 75%, P = 0.58 (Figure S5); B-blockers; OR: 1.39; 95%CI 0.98–1.98, I2 = 0%, P = 0.07 (Figure S6); Loop diuretics; OR: 1.26, 95%CI 0.89–1.79, I2 = 0%, P = 0.19 (Figure S7)). No sensitivity analysis was conducted regarding statins given that all studies were scored as high risk of bias.
Our main analysis found significantly lower odds of ACE-I/ARB (OR: 0.68, 95%CI 0.50–0.90, I2 = 12%, P < 0.01) (Figure 6) in patients with lower vs. higher ALM. No statistically significant differences were found in relation to B-blockers (OR: 0.84, 95%CI 0.63–1.12, I2 = 7%, P = 0.24) (Figure 7) and loop diuretics (OR: 1.19, 95%CI 0.87–1.63, I2 = 0%, P = 0.27) (Figure 8). A higher prevalence between statins and low ALM was found in one study (11), however, it was not considered significant (P = 0.33).

Figure 6. Association of ACE-I/ARB administration in patients with HF and higher ALM versus low ALM.
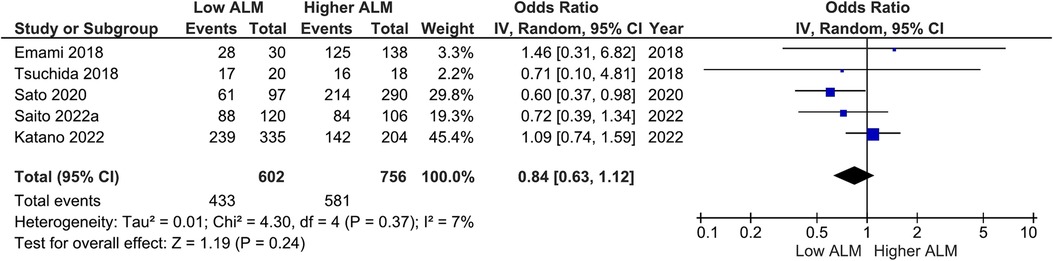
Figure 7. Association of B-blocker administration in patients with HF and higher ALM versus low ALM.
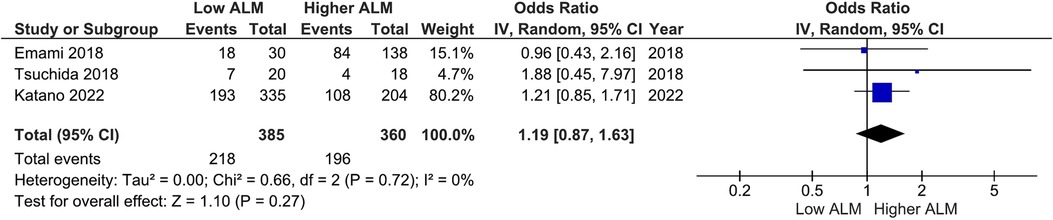
Figure 8. Association of loop diuretics administration in patients with HF and higher ALM versus low ALM.
Sensitivity analyses based on exclusion of studies with increased risk of bias did not alter the findings of the main analyses [ACE-I/ARB; OR: 0.66, 95%CI 0.44–0.99, I2 = 53%, P = 0.04 (Figure S8); B-blockers; OR: 0.81, 95%CI 0.55–1.19, I2 = 47%, P = 0.27 (Figure S9)].
In one study, no significant differences were found between low and higher handgrip strength index groups, and B-blockers (30). A higher % of patients with low handgrip strength was receiving statins (63% vs. 43%) and loop diuretics (88% vs. 82%), while those with higher handgrip strength were administered a greater proportion of ACE-I/ARBs (54% vs. 44%) (21).
In the Kitasato cohort from the study by Ozawa et al. (2021), no differences were found between slow gait and faster gait groups in relation to ACE-I/ARB, B-blocker, MRA, and loop diuretics (15). The slow gait group however in the FRAGILE-HF cohort had a lower prevalence of B-blocker administration vs. the non-slow group (67.3% vs. 74.9%, P < 0.01). Those with increased gait speed also exhibited higher prevalence of B-blocker administration vs. the slowest tertile group (65.9% vs. 49.6%, P = 0.04) with no differences related to ACE-I/ARBs (22). No changes among gait speed quartiles regarding ACE-I/ARBs, and B-blockers were observed, however, those with slow walking speed exhibited a higher prevalence of loop diuretics vs. faster groups (Quartile I: 26.6% vs. Quartile IV: 10.4%, P < 0.05). Similarly, those with slower walking speed were in a greater proportion in receiving statins (Quartile I: 50.3% vs. Quartile IV: 60.1%, P < 0.05) (10). Finally, Matsuzawa et al. (2013) found a higher prevalence of ACE-I/ARBs in the fastest tertile vs. the slowest tertile group (89.7% vs. 77.2%, P < 0.05), although no changes were highlighted in terms of B-blockers (57.4% vs. 51.9%, P > 0.05).
Only one study was included in this systematic review pertinent to SPPB scores (19). Those with a higher score (≥7) had a significantly higher prevalence of B-blockers (76.8% vs. 68.9%, P < 0.01), ACE-I/ARBs (71.6% vs. 61.4%, P < 0.01), and direct oral anticoagulants (35% vs. 29%), but not in relation to MRAs (9.2% vs. 6.7%), digoxin (3.4% vs. 1.6%), and warfarin (24% vs. 23%). Interestingly, those with a higher score had a lower prevalence of loop diuretic use (86% vs. 92%, P < 0.01).
The increased heterogeneity displayed for the prevalence of higher number of HF-related medications in patients with vs. without sarcopenia was further investigated through meta-regression analyses, using age, LVEF, and BMI as covariates. It was found that age, LVEF, and BMI were significant moderators of B-blockers, BMI of loop diuretics, and LVEF of statins, in patients with sarcopenia vs. without sarcopenia (Table S2). In addition, age was a significant moderator of ACE-I/ARB and B-blocker count in patients with lower vs. higher ALM (Table S3).
The overall quality of the included studies was considered moderate. In particular, four studies had a low risk of bias (15, 17, 18, 30), 10 studies had a moderate risk (10, 12, 14, 19–22, 27–29), while eight studies had a high risk of bias (11, 13, 16, 23–26, 31). A detailed description of the risk of bias is shown in Table S4.
In this systematic review and meta-analysis, we found no differences in specific drug administration prevalence in subjects with HF and sarcopenia vs. without sarcopenia. When we attempted to evaluate the impact of individual sarcopenia components, our analysis revealed significantly higher odds of ACE-I/ARB administration in patients with higher vs. lower ALM. In relation to handgrip strength, gait speed, and SPPB status, our narrative synthesis found mixed results that do not allow the extrapolation of conclusions, confidently. It is worth noting that age was a significant moderator of ACE-I/ARB count, which could explain, in part, our statistically significant findings.
Cross-sectional studies have shown a positive link among ACE-I/ARB usage, ALM, and muscle function (32, 33), while others have not observed such relationship (34). Likewise, longitudinal and clinical studies have failed to report positive outcomes in relation to muscle strength and physical performance (7, 8). A recent study showed that losartan could enhance the effects of exercise on muscle mass and muscle cross-sectional area in mice (35), however, in community-dwelling older adults and older subjects with chronic obstructive pulmonary disease (COPD), ACE-Is did not show benefits in response to an exercise programme (36, 37). Currently, research is lacking in patients with HF in order to show how ACE-I/ARBs could be connected to greater ALM.
Recently, an observational study linked the combination of ARBs and statin with higher ALM in patients with cardiovascular disease (38), however, research around the impact of statins on skeletal muscle is controversial. Mechanistic studies conducted in rats have shown that statins could induce acute muscle damage (39), although in mdx mice with Duchenne muscular dystrophy, no signs of inflammation, fibrosis, and angiogenesis reflecting muscle injury were observed (40). Furthermore, dmd/mdx mice treated with simvastatin have displayed decreased CYBB/NOX2-mediated oxidative stress and higher autophagy that corresponded with reduced muscle damage and inflammation, and increased muscle force production (41). Conversely, in C2C12 mice myotubes, simvastatin administration led to overexpression of myostatin in skeletal muscle (42), while in human myotubes, it was linked with impaired adenosine diphosphate (ADP)-stimulated maximal mitochondrial respiratory capacity and mitochondrial oxidative stress (43). Although some mechanistic evidence primarily from animal and cell models indicate a negative response of skeletal muscle to statin administration, these findings are currently unknown in humans and particularly patients with HF. These results also confirm our non-significant association of statin administration count in sarcopenia vs. no sarcopenia. Nevertheless, considering the various cardiovascular benefits of statins, future research unravelling its impact on skeletal muscle may be critical.
This is the first study attempting to quantify the relationship between sarcopenia and its parameters in patients with HF with specific drug administration. One of the limitations of this study is the possibility of reverse causation pertinent to those with higher ALM to be receiving more ACE-I/ARBs on the actual impact of these medications in promoting better muscle health. The nature of this cross-sectional study is unable or provide definitive answers and considering the limited research around this area in patients with HF, accurate conclusions cannot be extrapolated. In addition, some studies used different definitions of sarcopenia alongside different body composition assessment tools which could explain, in part, the moderate heterogeneity among studies in our analyses. Furthermore, considering that the majority of studies were conducted in Japan, our findings do not represent the general patient with HF and sarcopenia and lower ALM. Angiotensin receptor neprilysin inhibitor and sodium-glucose co-transporter-2 (SGLT2) inhibitors are relatively new medications in use for HF and there is therefore a relative paucity of studies that have looked at the relationship of sarcopenia and use of these medications. Lastly, it is worth noting that there is a likelihood of inflation in the number of listed medications, which could misrepresent their status, given the inaccuracies that may occur due to faulty coding of drug prescriptions and/or incorrect tabulations performed electronically.
This systematic review and meta-analysis found no link between number of specific drug administration in patients with HF and sarcopenia vs. without sarcopenia, although increased odds of ACE-I/ARB prescription was found in those with higher ALM. The emergence of inappropriate prescription is a critical phenomenon in medicine, impacting patient healthcare and potentially musculoskeletal health. Future research in patients with HF could clarify whether specific medications are linked to muscle-protective or impairing properties and identify potential inappropriate medications.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
KP: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing – original draft. AA: Validation, Writing – original draft. SS: Validation, Writing – original draft. MI: Supervision, Writing – review & editing. RS: Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors would like to thank Dunhill Medical Trust for supporting this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1293537/full#supplementary-material
Supplementary Figure S1
Association of ACE-I/ARB administration in patients with HF and sarcopenia versus without sarcopenia according to sarcopenia definition.
Supplementary Figure S2
Association of B-blocker administration in patients with HF and sarcopenia versus without sarcopenia according to sarcopenia definition.
Supplementary Figure S3
Association of loop diuretic administration in patients with HF and sarcopenia versus without sarcopenia according to sarcopenia definition.
Supplementary Figure S4
Association of statin administration in patients with HF and sarcopenia versus without sarcopenia according to sarcopenia definition.
Supplementary Figure S5
Association of ACE-I/ARB administration in patients with HF and sarcopenia versus without sarcopenia based on risk of bias.
Supplementary Figure S6
Association of B-blockers administration in patients with HF and sarcopenia versus without sarcopenia based on risk of bias.
Supplementary Figure S7
Association of loop diuretic administration in patients with HF and sarcopenia versus without sarcopenia based on risk of bias.
Supplementary Figure S8
Association of ACE-I/ARB administration in patients with HF and higher ALM versus low ALM based on risk of bias.
Supplementary Figure S9
Association of B-blockers administration in patients with HF and higher ALM versus low ALM based on risk of bias.
1. Rodgers JL, Jones J, Bolleddu SI, Vanthenapalli S, Rodgers LE, Shah K, et al. Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis. (2019) 6(2):19. doi: 10.3390/jcdd6020019
2. Attaway A, Bellar A, Dieye F, Wajda D, Welch N, Dasarathy S. Clinical impact of compound sarcopenia in hospitalized older adult patients with heart failure. J Am Geriatr Soc. (2021) 69(7):1815–25. doi: 10.1111/jgs.17108
3. Chen R, Xu J, Wang Y, Jiang B, Xu X, Lan Y, et al. Prevalence of sarcopenia and its association with clinical outcomes in heart failure: an updated meta-analysis and systematic review. Clin Cardiol. (2023) 46(3):260–8. doi: 10.1002/clc.23970
4. Buford TW, Manini TM, Hsu FC, Cesari M, Anton SD, Nayfield S, et al. Angiotensin-converting enzyme inhibitor use by older adults is associated with greater functional responses to exercise. J Am Geriatr Soc. (2012) 60(7):1244–52. doi: 10.1111/j.1532-5415.2012.04045.x
5. Onder G, Penninx BW, Balkrishnan R, Fried LP, Chaves PH, Williamson J, et al. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. (2002) 359(9310):926–30. doi: 10.1016/S0140-6736(02)08024-8
6. Giovannini S, Cesari M, Marzetti E, Leeuwenburgh C, Maggio M, Pahor M. Effects of ACE-inhibition on IGF-1 and IGFBP-3 concentrations in older adults with high cardiovascular risk profile. J Nutr Health Aging. (2010) 14:457–60. doi: 10.1007/s12603-010-0036-7
7. Witham MD, Syddall HE, Dennison E, Cooper C, McMurdo ME, Sayer AA. ACE Inhibitors, statins and thiazides: no association with change in grip strength among community dwelling older men and women from the hertfordshire cohort study. Age Ageing. (2014) 43(5):661–6. doi: 10.1093/ageing/afu008
8. Cesari M, Pedone C, Incalzi RA, Pahor M. ACE-inhibition and physical function: results from the trial of angiotensin-converting enzyme inhibition and novel cardiovascular risk factors (TRAIN) study. J Am Med Dir Assoc. (2010) 11(1):26–32. doi: 10.1016/j.jamda.2009.09.014
9. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford: Medicine (2000).
10. Chiaranda G, Bernardi E, Codecà L, Conconi F, Myers J, Terranova F, et al. Treadmill walking speed and survival prediction in men with cardiovascular disease: a 10-year follow-up study. BMJ Open. (2013) 3(10):e003446. doi: 10.1136/bmjopen-2013-003446
11. Tsuchida K, Fujihara Y, Hiroki J, Hakamata T, Sakai R, Nishida K, et al. Significance of sarcopenia evaluation in acute decompensated heart failure skeletal muscle mass index versus fat-free mass index. Int Heart J. (2018) 59(1):143–8. doi: 10.1536/ihj.17-057
12. Onoue Y, Izumiya Y, Hanatani S, Tanaka T, Yamamura S, Kimura Y, et al. A simple sarcopenia screening test predicts future adverse events in patients with heart failure. Int J Cardiol. (2016) 215:301–6. doi: 10.1016/j.ijcard.2016.04.128
13. Valdiviesso R, Sousa-Santos AR, Azevedo LF, Moreira E, Amaral TF, Silva-Cardoso J, et al. Statins are associated with reduced likelihood of sarcopenia in a sample of heart failure outpatients: a cross-sectional study. BMC Cardiovasc Disord. (2022) 22(1):1–11. doi: 10.1186/s12872-022-02804-5
14. Sato R, Akiyama E, Konishi M, Matsuzawa Y, Suzuki H, Kawashima C, et al. Decreased appendicular skeletal muscle mass is associated with poor outcomes after ST-segment elevation myocardial infarction. J Atheroscler Thromb. (2020) 27(12):1278–87. doi: 10.5551/jat.52282
15. Ozawa T, Yamashita M, Seino S, Kamiya K, Kagiyama N, Konishi M, et al. Standardized gait speed ratio in elderly patients with heart failure. ESC Heart Failure. (2021) 8(5):3557–65. doi: 10.1002/ehf2.13392
16. Kono Y, Izawa H, Aoyagi Y, Ishikawa A, Sugiura T, Mori E, et al. The difference in determinant factor of six-minute walking distance between sarcopenic and non-sarcopenic elderly patients with heart failure. J Cardiol. (2020) 75(1):42–6. doi: 10.1016/j.jjcc.2019.07.002
17. Saito H, Matsue Y, Maeda D, Kasai T, Kagiyama N, Endo Y, et al. Prognostic values of muscle mass assessed by dual-energy X-ray absorptiometry and bioelectrical impedance analysis in older patients with heart failure. Geriatr Gerontol Int. (2022) 22(8):610–5. doi: 10.1111/ggi.14424
18. Saito H, Matsue Y, Kamiya K, Kagiyama N, Maeda D, Endo Y, et al. Sarcopenic obesity is associated with impaired physical function and mortality in older patients with heart failure: insight from FRAGILE-HF. BMC Geriatr. (2022) 22(1):556. doi: 10.1186/s12877-022-03168-3
19. Kitai T, Shimogai T, Tang WW, Iwata K, Xanthopoulos A, Otsuka S, et al. Short physical performance battery vs. 6-minute walking test in hospitalized elderly patients with heart failure. Eur Heart J Open. (2021) 1(1):oeab006. doi: 10.1093/ehjopen/oeab006
20. Eschalier R, Massoullié G, Boirie Y, Blanquet M, Mulliez A, Tartière P-L, et al. Sarcopenia in patients after an episode of acute decompensated heart failure: an underdiagnosed problem with serious impact. Clin Nutr. (2021) 40(6):4490–9. doi: 10.1016/j.clnu.2020.12.033
21. Chung CJ, Wu C, Jones M, Kato TS, Dam TT, Givens RC, et al. Reduced handgrip strength as a marker of frailty predicts clinical outcomes in patients with heart failure undergoing ventricular assist device placement. J Card Fail. (2014) 20(5):310–5. doi: 10.1016/j.cardfail.2014.02.008
22. Pulignano G, Del Sindaco D, Di Lenarda A, Alunni G, Senni M, Tarantini L, et al. Incremental value of gait speed in predicting prognosis of older adults with heart failure: insights from the IMAGE-HF study. JACC: Heart Fail. (2016) 4(4):289–98. doi: 10.1016/j.jchf.2015.12.017
23. Harada H, Kai H, Niiyama H, Nishiyama Y, Katoh A, Yoshida N, et al. Effectiveness of cardiac rehabilitation for prevention and treatment of sarcopenia in patients with cardiovascular disease-a retrospective cross-sectional analysis. J Nutr Health Aging. (2017) 21:449–56. doi: 10.1007/s12603-016-0743-9
24. Fonseca G, Dos Santos MR, de Souza FR, Takayama L, Rodrigues Pereira RM, Negrão CE, et al. Discriminating sarcopenia in overweight/obese male patients with heart failure: the influence of body mass index. ESC Heart Fail. (2020) 7(1):85–92. doi: 10.1002/ehf2.12545
25. Emami A, Saitoh M, Valentova M, Sandek A, Evertz R, Ebner N, et al. Comparison of sarcopenia and cachexia in men with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur J Heart Fail. (2018) 20(11):1580–7. doi: 10.1002/ejhf.1304
26. Peng J, Gong H, Lyu X, Liu Y, Li S, Tan S, et al. Characteristics of the fecal microbiome and metabolome in older patients with heart failure and sarcopenia. Front Cell Infect Microbiol. (2023) 13:1127041. doi: 10.3389/fcimb.2023.1127041
27. Bieger P, Sangali TD, Ribeiro ÉCT, Schweigert Perry ID, Souza GC. Association of phase angle values and sarcopenia in older patients with heart failure. Nutr Clin Pract. (2023) 38(3):672–85. doi: 10.1002/ncp.10956
28. Matsuzawa Y, Konishi M, Akiyama E, Suzuki H, Nakayama N, Kiyokuni M, et al. Association between gait speed as a measure of frailty and risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol. (2013) 61(19):1964–72. doi: 10.1016/j.jacc.2013.02.020
29. Katano S, Honma S, Nagaoka R, Numazawa R, Yamano K, Fujisawa Y, et al. Anthropometric parameters-derived estimation of muscle mass predicts all-cause mortality in heart failure patients. ESC Heart Fail. (2022) 9(6):4358–65. doi: 10.1002/ehf2.14121
30. Castillo-Martínez L, Rodríguez-García WD, González-Islas DG, Orea-Tejeda A, Lozada-Mellado M, Rodríguez-Silverio J, et al. Abnormal fluid distribution and low handgrip strength index as predictors of mortality in Mexican patients with chronic heart failure. Nutrition. (2020) 72:110699. doi: 10.1016/j.nut.2019.110699
31. Ogawa A, Shimizu K, Nakagami T, Maruoka H, Shirai K. Physical function and cardio-ankle vascular index in elderly heart failure patients. Int Heart J. (2020) 61(4):769–75. doi: 10.1536/ihj.20-058
32. Bea JW, Wassertheil-Smoller S, Wertheim BC, Klimentidis Y, Chen Z, Zaslavsky O, et al. Associations between ACE-inhibitors, angiotensin receptor blockers, and lean body mass in community dwelling older women. J Aging Res. (2018) 2018:8491092. doi: 10.1155/2018/8491092
33. Lin Y-L, Chen S-Y, Lai Y-H, Wang C-H, Kuo C-H, Liou H-H, et al. Angiotensin II receptor blockade is associated with preserved muscle strength in chronic hemodialysis patients. BMC Nephrol. (2019) 20:1–7. doi: 10.1186/s12882-018-1181-1
34. Spira D, Walston J, Buchmann N, Nikolov J, Demuth I, Steinhagen-Thiessen E, et al. Angiotensin-converting enzyme inhibitors and parameters of sarcopenia: relation to muscle mass, strength and function: data from the Berlin aging study-II (BASE-II). Drugs Aging. (2016) 33:829–37. doi: 10.1007/s40266-016-0396-8
35. Lin C-H, Chang P-C, Chu P-H, Chuang Y-F, Huang R-C, Chen C-N. Effects of losartan and exercise on muscle mass and exercise endurance of old mice. Exp Gerontol. (2022) 165:111869. doi: 10.1016/j.exger.2022.111869
36. Curtis KJ, Meyrick VM, Mehta B, Haji GS, Li K, Montgomery H, et al. Angiotensin-converting enzyme inhibition as an adjunct to pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2016) 194(11):1349–57. doi: 10.1164/rccm.201601-0094OC
37. Sumukadas D, Band M, Miller S, Cvoro V, Witham M, Struthers A, et al. Do ACE inhibitors improve the response to exercise training in functionally impaired older adults? A randomized controlled trial. J Gerontol A Biol Sci Med Sci. (2014) 69(6):736–43. doi: 10.1093/gerona/glt142
38. Harada H, Nishiyama Y, Niiyama H, Katoh A, Kai H. Angiotensin II receptor blocker and statin combination therapy associated with higher skeletal muscle index in patients with cardiovascular disease: a retrospective study. J Clin Pharm Ther. (2022) 47(1):89–96. doi: 10.1111/jcpt.13540
39. Wei J, Huan Y, Heng Z, Zhao C, Jia L, Yu Y, et al. Dynamic urine proteome changes in a rat model of simvastatin-induced skeletal muscle injury. J Proteomics. (2022) 254:104477. doi: 10.1016/j.jprot.2021.104477
40. Mucha O, Podkalicka P, Kaziród K, Samborowska E, Dulak J, Łoboda A. Simvastatin does not alleviate muscle pathology in a mouse model of duchenne muscular dystrophy. Skelet Muscle. (2021) 11(1):1–16. doi: 10.1186/s13395-021-00276-3
41. Whitehead NP. Enhanced autophagy as a potential mechanism for the improved physiological function by simvastatin in muscular dystrophy. Autophagy. (2016) 12(4):705–6. doi: 10.1080/15548627.2016.1144005
42. Wang L, Zheng Z-G, Meng L, Zhu L, Li P, Chen J, et al. Statins induce skeletal muscle atrophy via GGPP depletion-dependent myostatin overexpression in skeletal muscle and brown adipose tissue. Cell Biol Toxicol. (2021) 37:441–60. doi: 10.1007/s10565-020-09558-w
Keywords: heart failure, sarcopenia, medications, skeletal muscle, drugs
Citation: Saied S, Prokopidis K, Adenaya A, Isanejad M and Sankaranarayanan R (2024) Is sarcopenia an associated factor of increased administration of specific medications in patients with heart failure? A systematic review and meta-analysis. Front. Cardiovasc. Med. 11:1293537. doi: 10.3389/fcvm.2024.1293537
Received: 13 September 2023; Accepted: 5 January 2024;
Published: 25 January 2024.
Edited by:
Vittorio Palmieri, San Sebastiano & Sant’Anna National Hospital, ItalyReviewed by:
Kornanong Yuenyongchaiwat, Thammasat University, Thailand© 2024 Saied, Prokopidis, Adenaya, Isanejad and Sankaranarayanan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Konstantinos Prokopidis ay5wcm9rb3BpZGlzQGxpdmVycG9vbC5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.