
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 16 May 2024
Sec. Cardio-Oncology
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1289663
This article is part of the Research Topic Frontiers in Cardiovascular Medicine: Rising Stars 2023 View all 28 articles
 V. Quagliariello1*
V. Quagliariello1* M. L. Canale2
M. L. Canale2 I. Bisceglia3
I. Bisceglia3 M. Iovine1
M. Iovine1 A. Paccone1
A. Paccone1 C. Maurea4
C. Maurea4 M. Scherillo5
M. Scherillo5 A. Merola6
A. Merola6 V. Giordano1
V. Giordano1 G. Palma7
G. Palma7 A. Luciano7
A. Luciano7 F. Bruzzese7
F. Bruzzese7 F. Zito Marino8
F. Zito Marino8 M. Montella8
M. Montella8 R. Franco8
R. Franco8 M. Berretta9
M. Berretta9 D. Gabrielli10
D. Gabrielli10 G. Gallucci11
G. Gallucci11 N. Maurea1
N. Maurea1
Background: Anthracycline-mediated adverse cardiovascular events are among the leading causes of morbidity and mortality in patients with cancer. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) exert multiple cardiometabolic benefits in patients with/without type 2 diabetes, chronic kidney disease, and heart failure with reduced and preserved ejection fraction. We hypothesized that the SGLT2i dapagliflozin administered before and during doxorubicin (DOXO) therapy could prevent cardiac dysfunction and reduce pro-inflammatory pathways in preclinical models.
Methods: Cardiomyocytes were exposed to DOXO alone or combined with dapagliflozin (DAPA) at 10 and 100 nM for 24 h; cell viability, iATP, and Ca++ were quantified; lipid peroxidation products (malondialdehyde and 4-hydroxy 2-hexenal), NLRP3, MyD88, and cytokines were also analyzed through selective colorimetric and enzyme-linked immunosorbent assay (ELISA) methods. Female C57Bl/6 mice were treated for 10 days with a saline solution or DOXO (2.17 mg/kg), DAPA (10 mg/kg), or DOXO combined with DAPA. Systemic levels of ferroptosis-related biomarkers, galectin-3, high-sensitivity C-reactive protein (hs-CRP), and pro-inflammatory chemokines (IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12, IL17-α, IL-18, IFN-γ, TNF-α, G-CSF, and GM-CSF) were quantified. After treatments, immunohistochemical staining of myocardial and renal p65/NF-kB was performed.
Results: DAPA exerts cytoprotective, antioxidant, and anti-inflammatory properties in human cardiomyocytes exposed to DOXO by reducing iATP and iCa++ levels, lipid peroxidation, NLRP-3, and MyD88 expression. Pro-inflammatory intracellular cytokines were also reduced. In preclinical models, DAPA prevented the reduction of radial and longitudinal strain and ejection fraction after 10 days of treatment with DOXO. A reduced myocardial expression of NLRP-3 and MyD-88 was seen in the DOXO-DAPA group compared to DOXO mice. Systemic levels of IL-1β, IL-6, TNF-α, G-CSF, and GM-CSF were significantly reduced after treatment with DAPA. Serum levels of galectine-3 and hs-CRP were strongly enhanced in the DOXO group; on the other hand, their expression was reduced in the DAPA-DOXO group. Troponin-T, B-type natriuretic peptide (BNP), and N-Terminal Pro-BNP (NT-pro-BNP) were strongly reduced in the DOXO-DAPA group, revealing cardioprotective properties of SGLT2i. Mice treated with DOXO and DAPA exhibited reduced myocardial and renal NF-kB expression.
Conclusion: The overall picture of the study encourages the use of DAPA in the primary prevention of cardiomyopathies induced by anthracyclines in patients with cancer.
Anthracyclines are associated with dose-dependent cardiotoxicity (1). Cancer patients treated with anthracyclines at 400 and 700 mg/m2 are exposed to a 5% and 48% risk of congestive heart failure, respectively (2). Mechanisms of acute and chronic anthracycline-mediated adverse events involve ferroptosis, endothelial damages, apoptosis, fibrosis, and myocardial inflammation mediated by overexpression of NF-kB mediated pathways (3, 4). Notably, short-term-induced myocardial damages of doxorubicin (DOXO) are well reported in clinical scenarios, resulting in the need for cardioprotective strategies in primary prevention in patients with cancer (5). A wide spectrum of cardioprotective drugs is proposed, including sacubitril/valsartan, beta blockers, and nutraceuticals; however, no effective risk reductions were seen in these patients (6).
Sodium-glucose cotransporter type 2 inhibitors (SGLT2i) have beneficial properties, including the improvement of systolic and diastolic functions (7), increase in calcium homeostasis, reduction of afterload and oxidative stress, improvement of mitochondrial functions in cardiomyocytes, and increase in ketone bodies, resulting in improved energy metabolism of cardiac cells, reduction of insulin and uric acid levels as well as of epicardial and visceral fat (8, 9). The most studied SGLT2is are empagliflozin (EMPA), dapagliflozin (DAPA), canagliflozin (CANA), and ertugliflozin (ERTU), which differ in their SGLT2 binding avidity, resulting in different clinical outcomes (10).
DAPA is a selective SGLT2i with multiple beneficial properties in patients with cardiovascular diseases (CVD) (10). In the DECLARE-TIMI trial, DAPA reduced cardiovascular death and hospitalization for heart failure in patients with type 2 diabetes mellitus (T2DM) (11). In the DAPA-HF TRIAL, DAPA reduced heart failure and death from cardiovascular causes in patients with heart failure and reduced ejection fraction in patients with and without T2DM (12). In the DEFINE-trial, DAPA improved heart failure-related health status and reduced natriuretic peptides in patients with heart failure with reduced ejection fraction (13). In the DELIVER trial, in patients with heart failure and preserved ejection fraction, DAPA significantly reduced cardiovascular death and urgent heart failure visits in patients with T2DM (14). A very recent trial of cancer patients with T2DM treated with anthracyclines and gliflozins reduced heart failure admissions, new cardiomyopathies, arrhythmias, and heat failure incidence (15).
The aim of the present study was to test, for the first time, whether DAPA could affect the myocardial and renal NF-κB expression, systemic levels of 12 cytokines, growth factors, troponin, and B-type natriuretic peptide NT-pro-BNP in preclinical models of short-term doxorubicin cardiotoxicity, preventing ejection fraction reduction.
To evaluate the cytoprotective effects of DAPA in human cardiomyocytes (AC16 adult human cells; Sigma Aldrich, Milan, Italy), mitochondrial dehydrogenase activity was quantified through a modified MTT [3-(4,5-dimethyldiazol-2-yl)-2,5-diphenyl tetrazolium bromide] method, known as MTS assay, according to the manufacturer's instructions (Dojindo Molecular Technologies Inc., Rockville, MD, USA). Briefly, AC16 cells were cultured in Dulbecco's Modified Eagle's Medium/Nutrient Mixture F-12 Ham (DME/F-12) supplemented with 10% fetal bovine serum (FBS; 10,000 cells/well) at 37°C in a humidified 5% CO2 atmosphere. After 24 h of appropriate growth, cells were unexposed (control) or exposed to DOXO (range 0.1–50 µM) or DAPA (10 or 100 nM) or both in combination for 24 h, in line with the literature (16). Notably, cellular DAPA doses were chosen according to the literature (close to the plasma levels of DAPA after oral administration in adults) (17–20). After treatment, cells were then washed three times with phosphate buffered solution (PBS) at pH 7.4 and then incubated with 100 μl of an MTT solution (0.5 mg/ml in cell culture medium) for 4 h at 37°C. Absorbance readings were acquired at a wavelength of 450 nm with the Tecan Infinite M200 plate-reader (Tecan Life Sciences Home, Männedorf, Switzerland) using I-control software (Tecan). Relative cell viability (%) was calculated with the following formula = [A]test/[A]control × 100, where “[A]test” is the absorbance of the test sample, and “[A]control” is the absorbance of the control cells incubated solely in culture medium (21).
DOXO-mediated cardiovascular injuries involve high intracellular calcium levels induced by intracellular Reactive Oxygen Species (iROS) (22). Intracellular Ca2+ in AC16 cells was quantified through the fluorescence dye Fluo-3 AM, according to the manufacturer's protocol. Cardiomyocytes were untreated (control) or treated with DOXO at 0.5 µM alone or combined with DAPA (10 or 100 nM) for 12 h. Notably, the DOXO concentration used in these experiments (0.5 μM) was chosen since the plasma concentration of anthracyclines in cancer patients has been reported to fluctuate in the range of 0.3–1 μM during infusion (23–25). After incubation, the cells were loaded with 5 µM Fluo-3 AM at 37°C for 30 min in the dark, and then washed three times with PBS (pH 7.4) to remove the excess dye. Fluo-3 chelated with Ca++ induces fluorescence detected by a spectrofluorometer (excitation/emission wavelengths 488 and 525 nm, respectively). Instead, intracellular adenosine-5'-triphosphate (ATP) levels were quantified through ENLITEN® ATP Assay System (Promega Italia S.r.l, Milan, Italy) according to the literature (26). Briefly, cardiomyocytes were untreated (control) or treated for 24 h, as described previously; after treatments, 100 μl of lysis/assay solution provided by the manufacturer was added to confluent cell cultures in 96-well plates. After the plates were shaken for 1 min and incubated for 10 min at 23°C, luminescence was measured in a microplate luminometer (Thermo Fisher, Milan, Italy). Data were expressed as relative units (r.u.) according to the literature (27).
Anthracyclines exert cardiotoxic effects through the induction of ferroptosis, a cell death induced by lipid peroxidation (28). AC16 cells were grown as described above; subsequently, 5,000 cells/well were seeded in a 24-well plate and allowed to grow for 24 h and exposed to DOXO (0.5 µM) or DAPA (10 or 100 nM). After centrifugation at 800 × g for 5 min, malondialdehyde (MDA) and 4-hydroxy 2-hexenal (4-HNA) were quantified though commercial kits with a spectrophotometer according to the manufacturer's protocols (Sigma Aldrich, Milan, Italy).
Cardiomyocytes were treated as described in Section 2.2; after treatment, the cells were harvested and lysed in complete lyses buffer (50 mM Tris–HCl, pH 7.4, 1 mM EDTA, 100 mM NaCl, 20 mM NaF, 3 mM Na3 VO4, 1 mM PMSF, and protease inhibitor cocktail). After centrifugation, supernatants were collected and treated to the quantification of MyD88 [Human MyD88 ELISA Kit (ab171341); Abcam, Milan, Italy] and NLRP3 [Human NLRP3 ELISA Kit (OKEH03368); Aviva Systems Biology, San Diego, CA, USA]. For the human MyD88 ELISA, the sensitivity was <10 pg/ml and the range of detection was 156–10,000 pg/ml; for the human NLRP3 ELISA assay, the sensitivity was <0.078 ng/ml and the range of detection was 0.156–10 ng/ml (29).
The expression of pro-inflammatory cytokines, such as IL-6, IL-8, and IL-1β, was performed through enzyme-linked immunosorbent assay (ELISA) methods, in line with the literature (30). Briefly, AC16 cells were treated as described in Section 2.2 for 12 h; after treatment, the cells were lysed as described in Section 2.4 and quantification of IL-1β, IL-6, and IL-8 was performed through selective ELISA kits according to the manufacturer's instructions (Sigma Aldrich, Milan, Italy).
Morphological changes and mitochondrial activity of human cardiac cells were studied through a Confocal Laser Scanning Microscope (EZ-C1-Nikon). Briefly, human cardiac cells were untreated (control) or treated with DOXO alone or combined with DAPA for 24 h. After incubation, cardiomyocytes were fixed in 4% formaldehyde (10 min) and then incubated in 1% BSA/10% normal goat serum/0.3 M glycine in 0.1% PBS-Tween20 for 1 h to permeabilize the cells and block non-specific protein–protein interactions. The cardiomyocytes were then incubated with an anti-Mitochondria antibody (113-1)—BSA and Azide free (Abcam ab92824, Milan, Italy) 5 µg/ml overnight at ±4°C. As a secondary antibody (green), a DyLight® 488 goat anti-mouse IgG (H ± L) (ab96879, Abcam, Milan, Italy) was used at a dilution of 1/250 for 1 h. Membrane staining was obtained using Concanavalin A Tetramethylrhodamine Conjugate (Invitrogen, Life Technology, Milan, Italy) at a final concentration of 100 µg/ml. Through a confocal microscope (C1-Nikon) equipped with EZ-C1 software for data acquisition and 60× oil immersion objective, intracellular mitochondria were imaged through excitation/emission at 488/518 nm and cell membrane through excitation/emission at 555/580 nm (31).
In total, 24 female C57Bl/6 mice (aged 6–7 weeks) were purchased from ENVIGO, San Pietro al Natisone (Italy). The mice were housed six per cage and maintained on a 12-h light/dark cycle (lights on at 7.00 a.m.) in a temperature-controlled room (22°C ± 2°C) and with food and water ad libitum. Preclinical experimental protocols were in accordance with EU Directive 2010/63/EU for animal experiments, and Italian D.L.vo 26/2014 law, were approved by the Ministry of Health (authorization number 1,467/17-PR of the 13-02-2017) and the institutional ethics committees: by Organismo preposto al benessere degli animali (OPBA). After 1 week of growth, the mice were randomized for weight-adjusted treatment. The mice were divided into four experimental groups (n = 6/group): (i) 100 μl saline solution (Saline); (ii) DOXO at 2.17 mg/kg/day through intraperitoneal administration (i.p.); (iii) DAPA 10 mg/kg/day through oral gavage; and (iv) DOXO/DAPA in combination (at the same concentration of each drug tested alone). Treatments were performed according to recently published studies with the aim of assessing the cardioprotective effects of ranolazine (32) and empagliflozin (33) against DOXO-induced cardiotoxicity for 10 days (34). Low doses of anthracyclines in preclinical models were used in line with other cardioprotective outcome studies (35, 36). Moreover, this is a short-term doxorubicin treatment study that is able to detect echocardiographic changes and systemic and myocardial inflammation (33, 37) due to acute pro-inflammatory and cardiotoxicity phenomena induced by DOXO, in line with other studies by Tocchetti et al. (38) and similar studies of preclinical models of cardiotoxicity (39–41). The chosen dose of DAPA (10 mg/kg/day through oral gavage) was assessed according to several preclinical studies available in the literature (18, 42–45) as well as other preclinical studies with other SGLT2is in cardio-oncology, such as empagliflozin (34).
A non-invasive transthoracic echocardiography through a Vevo 2,100 high-resolution imaging system (40-MHz transducer; Visualsonics, Toronto, ON, Canada) was performed in line with the literature (32, 34, 46). The mice were anesthetized with tiletamine (0.09 mg/g), zolazepam (0.09 mg/g), and 0.01% atropine (0.04 ml/g). Later, the animals were sedated and placed in a supine position on a temperature-controller surgical table to maintain a rectal temperature of 37°C and continual ECG monitoring was obtained via limb electrodes. Cardiac function was evaluated at basal conditions and at 2 and 10 days of treatment. Left ventricular echocardiography was assessed in parasternal long-axis views at a frame rate of 233 Hz. Notably, we measured the strain in parasternal views because the apical view is difficult to perform in small animals (39); this method was in line with other studies for speck tracking echocardiography (STE) analyses that were performed on parasternal long-axis B-mode loops using a VisualSonics Vevo 2100 system (VisualSonics) (47, 48). Image depth, width, and gain settings were optimized to improve image quality. End-systole and end-diastole dimensions were defined as the phases corresponding to the ECG T wave, and to the R wave, respectively. M-mode LV internal dimensions, diastolic (LVID,d) and LV internal dimensions, and systolic (LVID,s) dimensions were averaged from 3–5 beats. LVID,d and LVID,s were measured from the LV M-mode at the mid-papillary muscle level. Fractional shortening percentage (% FS) was calculated as [(LVID,d − LVID,s)/LVID,d] × 100, and ejection fraction percentage (% EF) was calculated as [(EDvol − ESvol)/EDvol] × 100. The strain was expressed as percentage. The analysis started with acquired B-mode loops and were imported into the Vevo Strain software. Three consecutive cardiac cycles were selected, and the endocardium traced. Upon adequate tracing of the endocardium, an epicardial trace was added. The ST-based strain allowed for the assessment of strains specific to six myocardial segments per LV view. Internally, 10 or more points were measured for each of the six segments, resulting in a total of 48 data points. Strain and strain rate (SR) are useful in the detection of regional myocardial function. The strain was also evaluated on long-axis views as radial and longitudinal. Radial strain (RS), defined as the percent change in myocardial wall thickness, is a positive curve reflecting increasing myocardial thickness during systole and diminishing wall thickness during diastole and represents myocardial deformation toward the center of the LV cavity. Longitudinal strain (LS) detects the percent change in the length of the ventricle, typically measured from the endocardial wall in the long-axis view. The myocardial deformation rate, expressed in 1/s, was also calculated. Importantly, during echocardiography, the heart rate of the mice was carefully monitored and was similar among all experimental groups, i.e., approximately 500 bmp (range 490–510 bmp), according to the literature (49). Echocardiographic analyses were performed following the “Small Animal Echocardiography using the Vevo® 2100 Imaging System” guidelines as well as other previous studies in models of preclinical cardio-oncology (34, 50–52). The mice analyzed through echocardiography after 10 days of treatment with DOXO to measure left ventricular systolic function, heart rate, and cardiac output were previously described (34, 38, 46) and in accordance with the recommendations of the American Society of Echocardiography (53). Blood glucose determination was performed by puncture of the tail vein before and after treatments using a glucometer (Model NC).
After treatment, the hearts were fully weighed. Subsequently, a left ventricular sample was cut, fixed, and embedded in paraffin for histological studies (on left ventricular histological effects, as described in Section 2.6). The remaining heart tissue was homogenized and lysed for quantitative analyses of NLRP-3 and Myd-88. In detail, the tissue was snap-frozen in dry ice until tissue homogenization was performed in a proper lysis buffer (0.1 M PBS, pH 7.4 + 1% Triton X-100 + protease inhibitor cocktail) and processed using a high-intensity ultrasonic liquid processor (54, 55). The homogenates were centrifuged at 4°C and supernatants were used for the NLRP-3 and Myd-88 analyses through NLRP3 (Mouse NLRP3 ELISA Kit, OKEH05486; Aviva Systems Biology) and MyD88 (Mouse MyD88 ELISA Kit, OKEH03397; Aviva Systems Biology).
At the end of the treatment, blood sampling via cardiac puncture was performed to quantify the biomarkers of cardiotoxicity [Troponin-T, BNP, N-Terminal Pro-Brain Natriuretic Peptide (NT-Pro-BNP)] and the biomarkers of systemic inflammation [galectin-3 and high-sensitivity C-reactive protein (hs-CRP)]. Briefly, mouse troponin-T, BNP, and NT-Pro-BNP were quantified through the Mouse Troponin-T, cardiac muscle (TNNT2) ELISA kit (CusaBio, Houston, TX, USA), Mouse BNP ELISA Kit (A77763, Antibodies Stockholm, Sweden), and Mouse NT-Pro-BNP ELISA Kit (Abbexa, Cambridge, UK). Galectin-3 was quantified through the Galectin 3 Mouse ELISA Kit (Thermo Scientific, Milan, Italy) and hs-CRP was determined through the Mouse hs-CRP ELISA Kit (Elabscience Biotechnology Co., USA) (55).
At the end of the treatment, blood sampling via cardiac puncture was performed to quantify two biomarkers of ferroptosis, products of lipid peroxidation, MDA, and 4-HNA using commercial kits with a spectrophotometer according to the manufacturer's protocols (39) [MAK085, Sigma Aldrich, Milan, Italy, for MDA; Lipid Peroxidation (4-HNE) Assay Kit, ab238538, AbCam, Italy]. In total, 12 cytokines and growth factors (IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12, IL17-α, IFN-γ, TNF-α, G-CSF, GM-CSF) were quantified through a mouse cytokine Multiplex Assay kit (pg/ml; Qiagen, USA) (56).
Left ventricular heart samples and kidney tissue were fixed in 4% paraformaldehyde for 1 h and then kept at 4°C until paraffin embedding. Cardiac and kidney paraffin sections (with a thickness of 4 μm) were hydrated, microwaved for 8–15 min in 10 mM sodium citrate (pH 6.0) for antigen retrieval, and then probed with a rabbit antibody against murine p65/NF-kB (1:100, ab16502; Ab Cam, Milan, Italy). Immunolabeled sections were then incubated with goat anti-rabbit second antibody conjugated to horseradish peroxidase and treated with the EnVision + diaminobenzidine kit (DAB; Dako, Glostrup, Denmark) using standard protocols (57). The stained sections were analyzed by two independent observers, at least five different areas for each specimen were evaluated, and the mean was assessed. NF-kB IHC was categorized as positive or negative, as well as an overall proportion of cells (10%) with positive nuclear staining in the studied field at a magnification ×100. IHC scoring was based on the nuclear staining intensity according to the literature as follows: score 0, no nuclear staining; score 1, weak staining; score 2, moderate staining; and score 3, strong staining (58–60).
Continuous data were expressed as mean ± SD. Non-parametric tests were used both for paired and unpaired comparisons. A repeated measures ANOVA was used for all baseline to end-of-study comparisons. A p-value <0.05 was considered significant.
As described in the literature, anthracyclines exert cardiotoxic effects through lipid peroxidation, high intracellular Ca++ levels, mitochondrial damage, and myocardial inflammation mediated by NLRP-3/MyD-88/cytokine pathways (61). In line with the literature, DAPA showed cytoprotective properties in cardiomyocytes exposed to DOXO for 24 h (Figure 1A), increasing significantly their cell viability [i.e., of 20% and 38% for DAPA 10 and 100 nM, respectively, compared to only DOXO (50 µM) treated cells; p < 0.001]. Cardiac cells exposed to DOXO drastically reduced intracellular ATP levels compared to untreated cells (−64% vs. control; p < 0.001) (Figure 1B); instead, DAPA increased their content by 11% and 52% compared to DOXO groups (p < 0.001 for both). Intracellular Ca++ were significantly increased in cardiac cells exposed to DOXO (3,244.4 ± 203.3 vs. 367.6 ± 153.8 a.u.; p < 0.001) (Figure 1C); co-incubation with DAPA at 10 and 100 nM drastically reduced iCa++ levels compared to DOXO (2,123.5 ± 155.5 and 927.8 ± 234.4 vs. 3,244.4 ± 203.3 a.u., respectively; p < 0.001). Lipid peroxidation products MDA and 4-HNA (Figures 1D,E) were significantly increased in cardiomyocytes exposed to DOXO (3.35–2.96 nmol/ml vs. 0.5 nmol/ml; p < 0.001); co-incubation with DAPA reduced their intracellular levels in a concentration-dependent manner, demonstrating antioxidant properties (p < 0.001 vs. DOXO groups). Intracellular levels of NLRP-3 and Myd-88, were also drastically increased after exposure to DOXO (Figures 1F,G) (∼5.3 and 4.1 times compared to untreated cells; p < 0.001 for both). Notably, co-incubation with DAPA significantly reduced their levels (NLRP-3 levels in DAPA 100 nM were comparable to untreated cells; p < 0.001), indicating anti-inflammatory effects. Intracellular cytokine levels also changed significantly between groups (Figure 1H); in detail, IL-1β levels in DAPA 10 and 100 nM compared to the DOXO only group were 121.1 ± 17.7 and 72.2 ± 14.4 pg/mg of protein versus 177.3 ± 12.2, respectively (p < 0.001 for both); instead, IL-6 levels in DAPA 10 and 100 nM compared to the DOXO only group were 71.1 ± 13.2 and 48.8 ± 11.8 pg/mg of protein versus 103.3 ± 8.6, respectively (p < 0.001 for both); IL-8 levels in DAPA 10 and 100 nM, compared to the DOXO only group were 85.5 ± 14.3 and 42.7 ± 17.2 pg/mg of protein versus 115.2 ± 8.3, respectively (p < 0.001 for both). These results are in line with other studies on SGLT2i cardioprotective properties and indicate cytoprotective, antioxidant, and anti-inflammatory properties of DAPA in human cardiomyocytes. Confocal images clearly showed morphological changes in human cardiomyocytes exposed to DOXO (Figure 1K), with an initial loss of cell–cell interactions and lower fluorescent signal related to the cell membrane (red signals) compared to untreated cells (Figure 1I), characteristic of cellular atrophy induced by anthracyclines. Furthermore, mitochondrial staining (green signals) was significantly reduced in the DOXO group compared to the DAPA group (Figure 1J), indicating a loss of the number and functionality of mitochondria. Notably, co-incubation with DOXO and DAPA prevents the loss of cardiomyocyte morphology (Figure 1L) and prevents the reduction of mitochondrial staining, showing a high and significant green fluorescence compared to only DOXO exposed cells.

Figure 1. DAPA exerts cardioprotective properties in human cardiomyocytes exposed to DOXO. (A) Cell viability (% of control) of cardiomyocytes exposed to DOXO (0.1, 1, and 10 µM) alone or combined to DAPA (10 or 100 nM) for 24 h. ATP levels (B) (relative units), intracellular Ca++ content (C) (fluorescence intensity), MDA (D) and 4-HNA (E) (nmol/ml), NLRP-3 (F) and MyD-88 (G) (fold of control) in human cardiomyocytes unexposed (control) or exposed for 24 h to DOXO (0.5 µM) alone or combined to DAPA (10 or 100 nM). Pro-inflammatory cytokines (H) (IL-1, IL6, and IL-8, pg of cytokine/mg of protein) in human cardiomyocytes unexposed (control) or exposed for 24 h to DOXO (0.5 µM) alone or combined to DAPA (10 or 100 nM). One-way ANOVA. Values are expressed as ±SD. ***P < 0.001; **p < 0.01; *p < 0.05; ns: not significant. Confocal scanning laser microscope (I–L) of human cardiomyocytes unexposed (I) or exposed to DAPA (J) or DOXO (K) or DOXO-DAPA (L) for 24 h. Green signals: mitochondrial staining; Red signals: cell membrane. Scale bar: 50 μM.
As reported in other studies, gliflozins did not affect serum glucose in non-diabetic mice but were able to reduce oxidative-related products both systemically and in heart tissue (62–64). In brief, DAPA-treated mice had a blood glucose of 187.6 ± 29.3 mg/dl vs. 192.8 ± 37.1 mg/dl in untreated mice (no differences were seen between groups; p = 0.71). No differences in blood glucose were seen between the DOXO and DOXO-DAPA groups (213.47 ± 41.3 mg/dl vs. 198.6 ± 32.2 mg/dl, respectively; p = 0.43) These results are in line with those of other studies (8, 65) confirming that DAPA did not significantly change blood glucose in non-diabetic mice.
The cardiac function analysis clearly shows the cardiotoxicity of DOXO even after 10 days of treatment (Table 1). Specifically, significant reductions in EF (%), FS (%), radial strain (Pk%), and longitudinal strain (Pk%) were seen compared to the controls (DOXO vs. Saline; p < 0.001). In addition, a slight but not significant increase in LV mass was seen (Table 1). Instead, the DAPA group showed preservation of cardiac function compared to the Saline group, confirming the cardiac benefits in preclinical models. On the other hand, the DOXO-DAPA group showed a significant improvement in EF (%), FS (%), radial strain (Pk%), and longitudinal strain (Pk%) versus DOXO (DOXO-DAPA vs. DOXO; p < 0.001). The representative M-mode of long-axis echocardiographic images (Figure 2) for measurements of the intraventricular septum thickness in diastole (IVSd) (mm), the thickness of the rear wall of the left ventricle (LVPWd) (mm), LVIDd (mm), and LVIDs (mm) of mice clearly indicates that DAPA (Figure 2D) improves cardiac functions during DOXO therapy compared to the DOXO only group (Figure 2B). Hearts weighed after necropsy showed a slight increase in heart weight in the DOXO groups than the Saline and DOXO groups, probably due to high inflammation and hypertrophy induced by anthracycline therapy. Notably, DAPA did not significantly reduce heart weight compared to DOXO alone.
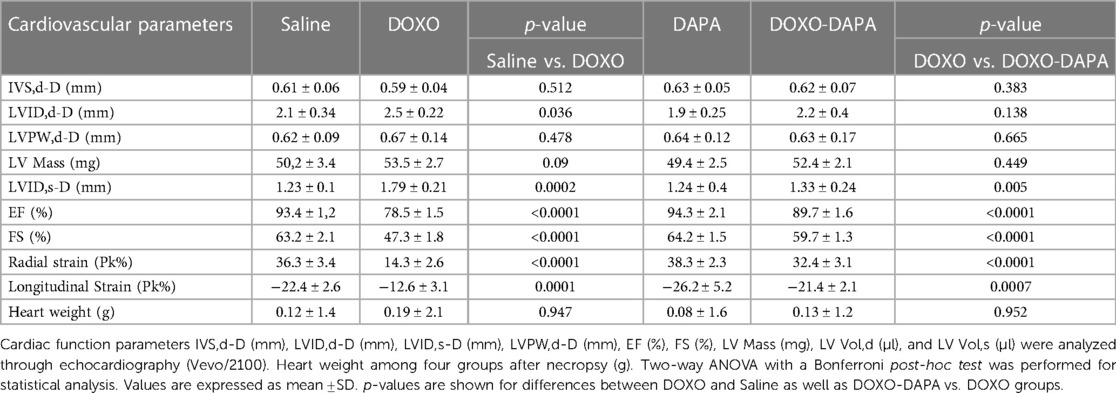
Table 1. Cardiovascular parameters of study groups, such as (saline), DOXO 2.17 mg/kg/day, DAPA 10 mg/kg/day, and DOXO-DAPA in association (n = 6 for each group).
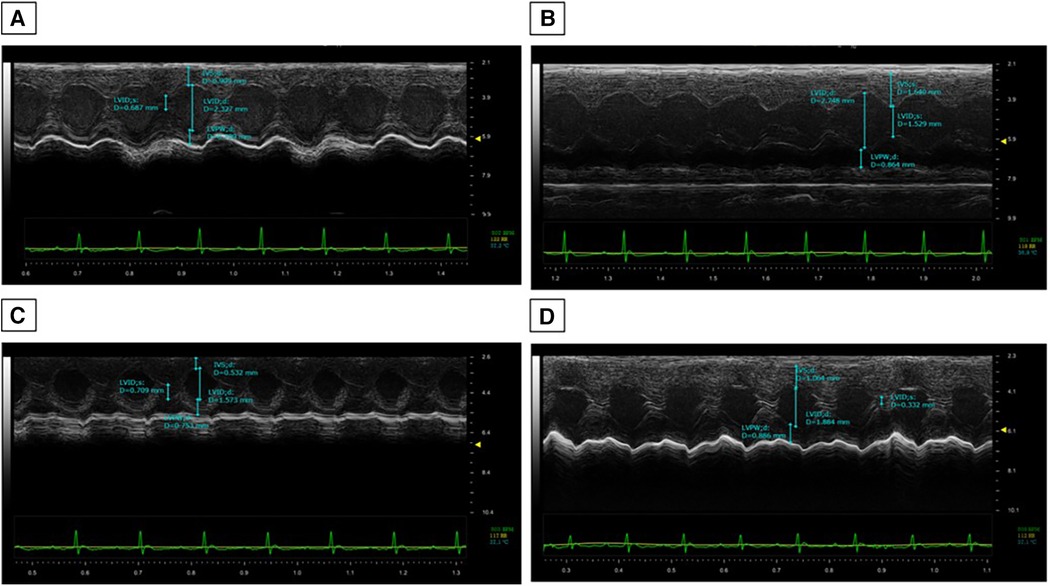
Figure 2. DAPA attenuated DOXO-induced impairment of cardiac systolic function. Echocardiography was performed on all the mice after 10 days of DOXO injection. Representative M-mode of long-axis echocardiographic images for measurements of the IVSd (mm), LVPWd (mm), LVIDd (mm), and LVIDs (mm) of Saline (A), DOXO (B), DAPA (C), and DOXO-DAPA (D) mice.
Histological analyses were performed to evaluate the anti-inflammatory effects of DAPA in preclinical models of DOXO cardiotoxicity (Figure 3). In line with the literature (4, 65), DOXO induces tissue overexpression of p65/NF-kB. It is very interesting to note that DAPA totally changed the renal and cardiac inflammatory picture, strikingly reducing the expression of p65/NF-kB, preserving the tissue microstructure of cardiomyocytes and kidney (Figure 3). In more detail, from a histological point of view, the administration of DOXO/DAPA did not show morphological alterations detectable with hematoxylin and eosin staining (Figure 3A). Cellular morphology remained essentially unchanged in terms of nucleus/cytoplasm ratio and volume of individual sarcomeres. The likely reason could be attributed to the short duration of anthracycline administration; thus further studies will be needed to assess any morphological changes upon long-term administration (as specified in the Discussion section). Instead, quantitative NF-kB staining indicates a high score (±3) of nuclear NF-kB staining in myocardial tissue in the DOXO group, indicating a pro-inflammatory effect induced by anthracycline therapy (Figure 3B); notably, the DOXO-DAPA group showed a significant reduction of nuclear NF-kB staining score compared to the DOXO group with no high score (±3) and only weak (±1) and moderate (±2) staining seen, confirming DAPA-related myocardial anti-inflammatory properties in these preclinical models (Figure 3B). A renal tissue analysis was performed as an internal control, considering that SGLT2is have mainly been used as antidiabetic drugs acting on the proximal convoluted tubule of the kidney (expressing SGLT-2), where they block the reabsorption of glucose and sodium, favoring the urinary excretion of glucose (7, 8), Subsequent studies have also demonstrated the expression of SGLT2 in cardiac tissues, broadening their clinical spectrum of action in the prevention of cardiovascular diseases (33, 34).
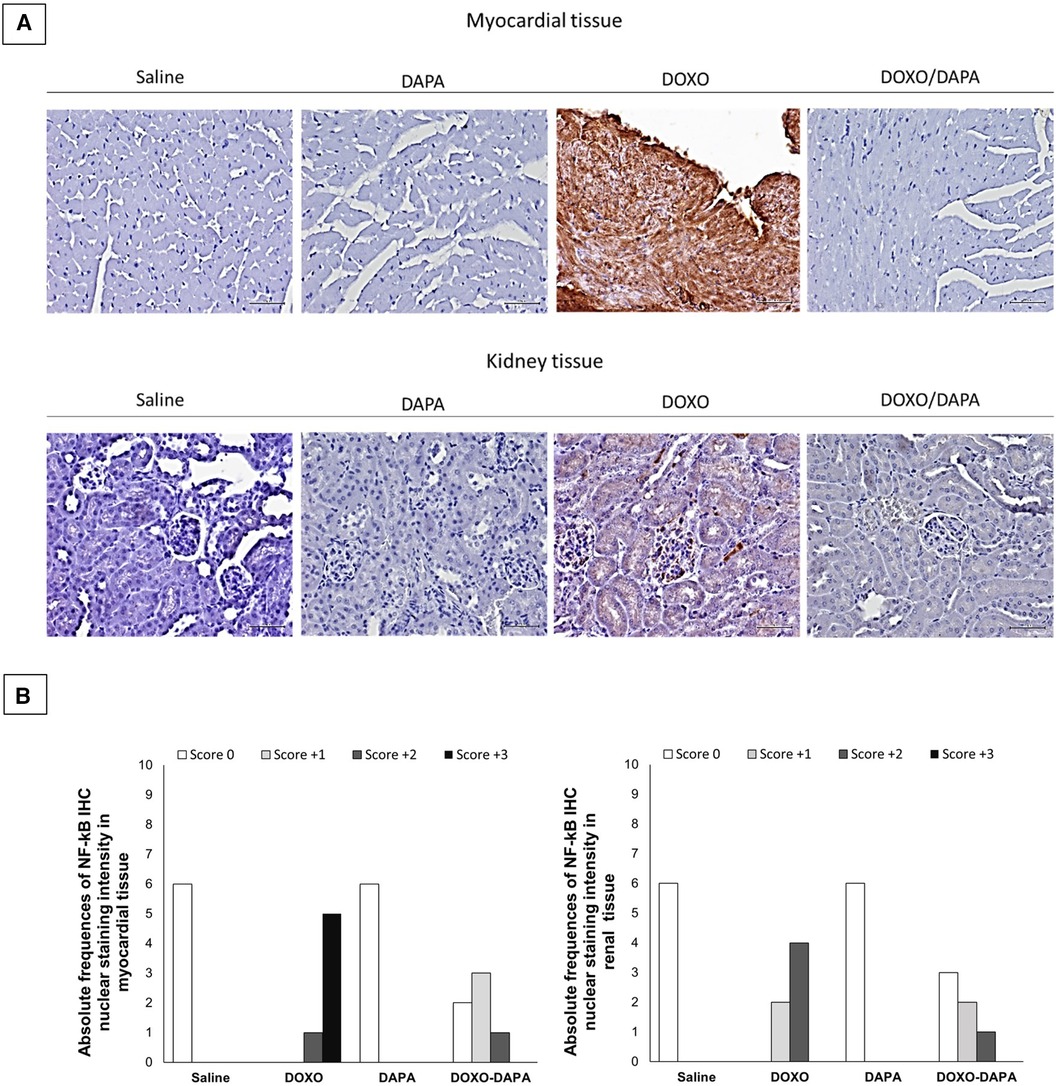
Figure 3. (A) Myocardial (up) and kidney (down) p65/NF-kB expression in mice treated with saline solution (saline), DAPA 10 mg/kg/day, DOXO 2.17 mg/kg/day. or DAPA associated to DOXO (n = 6 for each group). Scale bar: 5 µm; (B) Absolute frequencies of NF-KB IHC nuclear intensity in myocardial and renal tissues of mice treated with saline solution (Saline), DAPA 10 mg/kg/day, DOXO 2.17 mg/kg/day, or DAPA associated to DOXO. NF-kB IHC was categorized as positive or negative, as well as an overall proportion of cells (10%) with positive nuclear staining in the studied field at ×100 magnification. IHC scoring was based on the nuclear staining intensity, as follows: score 0, no nuclear staining; score 1, weak staining; score 2, moderate staining; score 3, strong staining.
DOXO induces systemic inflammation in cancer patients (66, 67). We investigated the systemic anti-inflammatory effects of DAPA during DOXO therapy. In line with literature, DOXO increased serum Galectin-3, IL-1, and hs-CRP levels compared to the Saline group. DAPA is able to reduce hs-CRP, IL-1, and Galectin-3 significantly, indicating systemic anti-inflammatory effects (Figure 4).
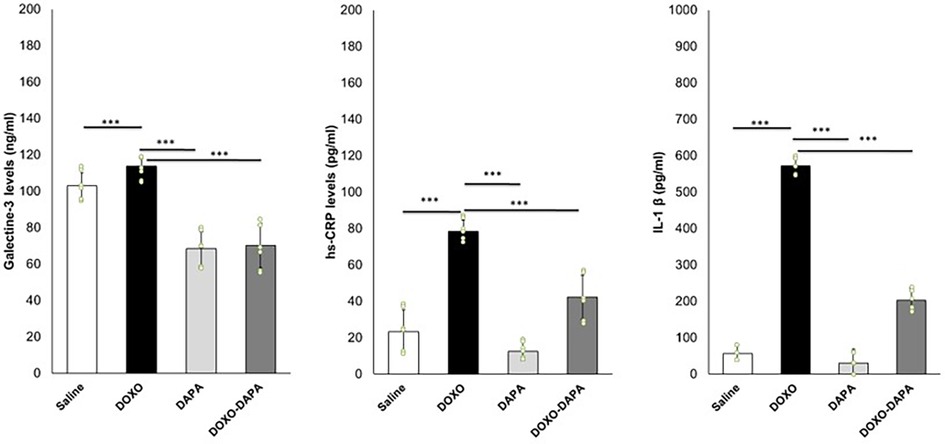
Figure 4. DAPA reduced systemic levels of galectin-3 (ng/ml), hs-CRP (pg/ml), and IL-1 (pg/ml) during treatment with DOXO. Mice were treated with saline solution (control), DOXO 2.17 mg/kg/day, DAPA 10 mg/kg/day, and DOXO-DAPA in association (n = 6 for each group). One-way ANOVA. Values are expressed as mean ±SD. ***p < 0.001; **p < 0.01; *p < 0.05; ns, not significant.
In preclinical models, it has been observed that DOXO treatment can lead to an increase in troponin and BNP, Troponin-T, and NT-pro-BNP levels (68–70). In line with the literature, short-term DOXO therapy increased the systemic levels of cardiotoxicity biomarkers compared to saline (Table 2). DAPA treatment did not significantly change the troponin and natriuretic peptide levels compared to saline, confirming no cardiac adverse events. Interestingly, in the DOXO-DAPA group, a significant reduction in Troponin-T (0.21 ± 0.05 vs. 0.46 ± 0.06; p < 0.001); BNP (128.6 ± 16.4 vs. 182.3 ± 42.1; p < 0.001); and NT-pro-BNP (1,112.7 ± 68.3 vs. 1,432.3 ± 72.1; p < 0.001) was seen, showing the cardioprotective properties of DAPA.
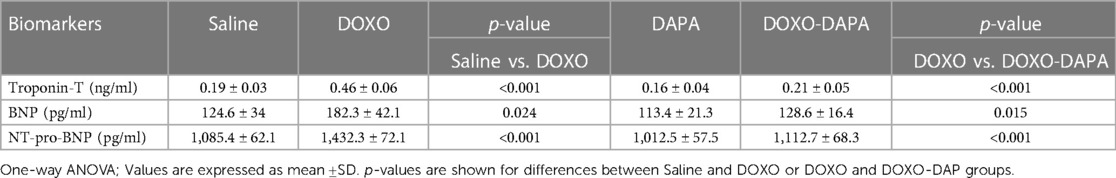
Table 2. Biomarkers of cardiotoxicity, troponin-T (ng/ml), BNP (pg/ml), NT-pro-BNP (pg/ml) quantified after 10 days of treatment with saline solution (control) DOXO 2.17 mg/kg/day, DAPA 10 mg/kg/day, and DOXO-DAPA in association (n = 6 for each group).
In recent years, there has been growing interest in understanding the involvement of NLRP3 inflammasome and MyD-88 activation in various pathological conditions, including cardiotoxicity induced by DOXO (71, 72). In line with the literature, DOXO therapy increased the myocardial levels of NLRP3 and MyD-88 compared to saline (Figure 5). A significant reduction of NLRP3 and MyD-88 were seen in the DOXO-DAPA group versus the DOXO group, demonstrating the anti-inflammatory effects of DAPA during DOXO therapy.
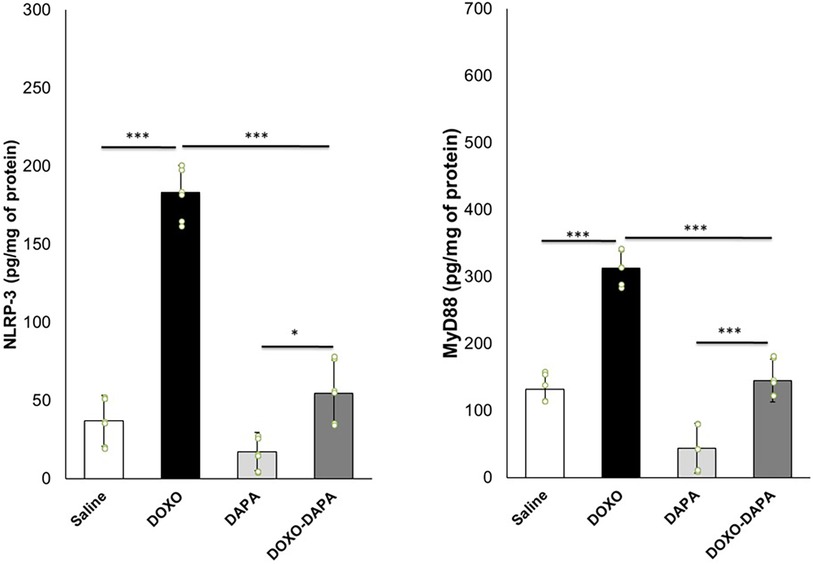
Figure 5. NLRP3 and MyD-88 expression (pg/mg of protein) in myocardial tissues of mice treated with saline solution (control), DOXO 2.17 mg/kg/day, DAPA 10 mg/kg/day, and DOXO-DAPA in association (n = 6 for each group). One-way ANOVA. Values are expressed as mean ±SD. ***p < 0.001; **p < 0.01; *p < 0.05; ns, not significant.
Emerging evidence suggests that NLRP3 inflammasome activation can induce or contribute to ferroptosis in preclinical models through the induction of cytokines able to damage mitochondria (73, 74). Lipid peroxidation products (MDA and 4-HNA) can serve as markers of ferroptosis (75, 76). During ferroptosis, the peroxidation of polyunsaturated fatty acids (PUFAs) in cellular membranes generates reactive lipid species, such as MDA and 4-HNA (77). As shown in Figure 5, myocardial levels of MDA and 4-HNA were strongly enhanced in the DOXO group compared to saline (p < 0.001). DAPA significantly reduced lipid peroxidation without DOXO and combined with DOXO, demonstrating the antioxidant and preventive properties of ferroptosis in myocardial tissues. Moreover, a pro-inflammatory cytokine profile was seen in the DOXO group (Figure 5). Instead, DAPA totally reversed the inflammatory picture induced by DOXO, reducing IL-1, IL6, TNF-a, and IL-17 levels.
Dapagliflozin is a SGLT2i primarily used for the management of type 2 diabetes mellitus (78); however, its use has expanded to the field of cardiology due to its cardiovascular benefits (79). Dapagliflozin has shown efficacy in reducing the risk of cardiovascular events and improving heart failure outcomes (80). Here are some of the key uses of dapagliflozin in cardiology: recent cardiovascular outcomes trials have demonstrated that DAPA can reduce the risk of major adverse cardiovascular events (MACE) in patients with established cardiovascular disease (81). These events include heart attack, stroke, and cardiovascular-related death. Dapagliflozin has been shown to provide cardiovascular protection in high-risk patients, including those with a history of heart disease (82).
Moreover, DAPA is able to reduce heart failure hospitalizations and improves outcomes in patients with heart failure, both with reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF) through the reduction of fluid, thus improving cardiac function (83). Notably, DAPA has also demonstrated benefits in preserving kidney function and reducing the risk of kidney disease progression in patients with or without diabetes (83, 84). This can be particularly relevant in patients with cardiovascular disease who may have concomitant renal toxicities. In brief, DAPA helps lower blood glucose levels by inhibiting SGLT2, which reduces glucose reabsorption in the kidneys and increases urinary glucose excretion; therefore, by improving glycemic control, it can have additional indirect benefits on cardiovascular health (85, 86). While DAPA is primarily indicated for the management of diabetes, some recent studies suggests that SGLT2is may exerts anticancer effects and could potentially be used as an adjunct therapy for certain types of tumors, including breast and liver tumors (87). Briefly, one of the proposed mechanisms of action for dapagliflozin in cancer is its ability to reduce glucose availability to cancer cells; considering cancer cells often exhibit increased glucose uptake compared to normal cells (depending on the type and biology of tumors), the inhibition of glucose reabsorption in the kidneys of DAPA could potentially deprive cancer cells of a key energy source (88, 89). In addition, DAPA induces euglycemic diabetic ketoacidosis (DKA), which has been shown to selectively inhibit the growth of some cancer cells, such as triple negative breast cancer and hormone-responsive breast cancer (90, 91). However, only cellular and preclinical studies are available and further research is needed to establish its clinical significance.
Anthracyclines are a class of chemotherapy drugs commonly used in the treatment of various types of cancer, including breast cancer, lymphoma, and leukemia (92). While anthracyclines have shown effectiveness in fighting cancer, they exert significant dose-related cardiotoxicity (93). Recent studies have examined the potential benefits of using SGLT2is in patients who have received anthracycline-based chemotherapy (94). These studies have shown promising results regarding the cardiac outcomes of such patients.
A recent study investigated the effects of EMPA on cardiac function in patients with breast cancer treated with anthracyclines (95). The study found that EMPA improved LVEF and reduced biomarkers of heart failure. In addition, EMPA is able to reduce the incidence of heart failure and cardiovascular death in these patients. Another recent study evaluated the cardioprotective effects of DAPA in patients with breast cancer receiving anthracycline-based chemotherapy (15, 96). Briefly, the authors concluded that DAPA preserved LVEF and reduced markers of cardiac injury compared to placebo. In that case, DAPA was also associated with a lower risk of heart failure and cardiovascular events.
These findings suggest that SGLT2is may have cardioprotective effects in patients treated with anthracyclines. The actual known mechanisms of SGLT2is related to cardioprotective agents involve the reduction of oxidative stress and promotion of sodium and water extraction, leading to reduced cardiac strain (97). From a cellular point of view, in line with the literature, our results on human cardiomyocytes demonstrated that SGLT2i DAPA exerts its cytoprotective and anti-inflammatory properties through the reduction of intracellular Ca++ levels, which are able to improve mitochondrial function in cardiomyocytes (98); moreover, DAPA is able to reduce iROS content and lipid peroxidation in cardiac cells, thus preventing ferroptosis. DAPA also exerts anti-inflammatory properties in cardiomyocytes through the reduction of NLRP-3 and Myd-88 pathways, resulting in reduced NF-kB levels and pro-inflammatory cytokines, such as IL-1β, IL-6, and IL-8 (Figure 6) (99). Interestingly, very recent findings indicate potential immune-regulating properties of SGLT2i, such as canagliflozin or empagliflozin; in line with these studies, in activated human peripheral blood mononuclear cells (hPBMC) only, a significant reduction of IL-2 secretion was seen in DAPA-exposed immune cells (Supplementary Figure S1), indicating potential immune effects of SGLT2i. These properties should be more detailed and could be of great interest in finding new immune-modulating agents in autoimmune patients or for the prevention and treatment of myocarditis, vasculitis, and endothelitis induced by viruses or immune checkpoint inhibitors (ICIs) in cancer patients (100).
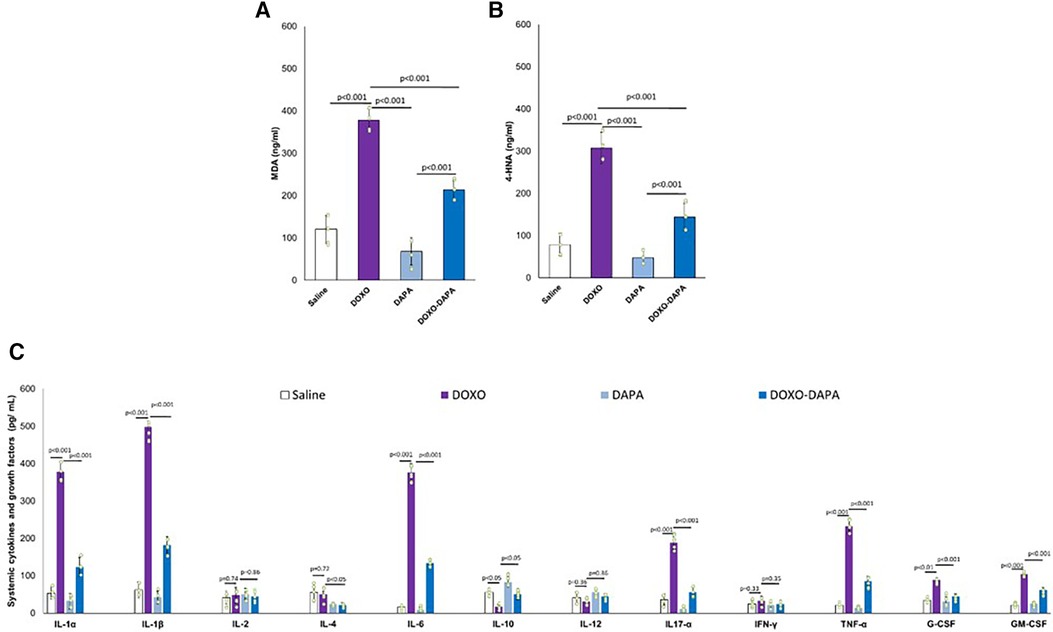
Figure 6. Schematic representation of DAPA-related cytoprotective properties in cardiomyocytes. SGLT2i reduces both glucose and sodium intake leading to intracellular hyponatremia in the cardiomyocyte. Lower Na+ levels leads to a reduced function of sodium-calcium exchanger, resulting in low levels of intracellular calcium. Preserving excess intracellular calcium improves the mitochondrial functions of cardiomyocyte, optimizing ATP production. Furthermore, DAPA has antioxidant effects, counteracting lipid peroxidation and the intracellular concentration of iROS, reducing ferroptosis and the consequent release of cardiac troponins. Moreover, DAPA reduces the expression of MyD-88 and NLRP3 in the cardiomyocyte, counteracting the synthesis of pro-inflammatory and cardiotoxic cytokines through NF-kB pathways.
Moreover, in this study, for the first time, the different beneficial effects of DAPA were analyzed in preclinical models of anthracycline-induced cardiotoxicity. In line with other studies, DAPA demonstrated both systemic and cardio-renal anti-inflammatory effects. Recently, Gongora et al. (15) performed a retrospective study to test the preventive properties of cardiac dysfunctions and overall safety of SGLT2i in more than 3,000 cancer patients with T2DM treated with anthracyclines. The primary cardiac outcome was a composite of cardiac events [heart failure incidence, heart failure admissions, new cardiomyopathy (>10% decline in ejection fraction to <53%) and clinically significant arrhythmias]; the primary safety outcome was overall mortality. There were 20 cardiac events over a median follow-up period of 1.5 years. The incidence of cardiac events was lower among case patients in comparison to control participants (3% vs. 20%; p = 0.025). Patients treated with SGLT2is patients also experienced lower overall mortality when compared with control participants (9% vs. 43%; p < 0.001) and a lower composite of sepsis and neutropenic fever (16% vs. 40%; p = 0.013). This study demonstrated, for the first time, the abilities of SGLT2i in the prevention of cardiac dysfunctions in cancer patients with no relevant toxicities (15). Another more recent observational study (96) concluded that dapagliflozin is well-tolerated and associated with high compliance in patients with advanced, inoperable pancreatic ductal adenocarcinoma, significantly reducing some cancer-associated biomarkers (96). Systemic inflammation, also known as systemic inflammatory response syndrome (SIRS), can occur in cancer patients treated with doxorubicin (101). Cancer patients treated with DOXO experienced high levels of CRP, erythrocyte sedimentation rate, IL-6, and IL-1β that may contribute to additional complications, including organ dysfunction or failure (102, 103). DAPA significantly reduced the biomarkers of inflammation and of heart failure, including troponins and NT-pro-BNP, confirming systemic anti-inflammatory and cardioprotective properties. Myocardial analysis showed that DAPA reduced NLRP3 and Myd-88 expression in heart tissue. NLRP3 inflammasome and Myd-88 activation have been implicated in several diseases, including cancer and cardiomyopathies (Figure 7). Both induce cardiomyocyte death and exacerbate myocardial injury by promoting inflammation and fibrosis through IL-1β and IL-18, which activates macrophages and immune cells in heart tissue (104).
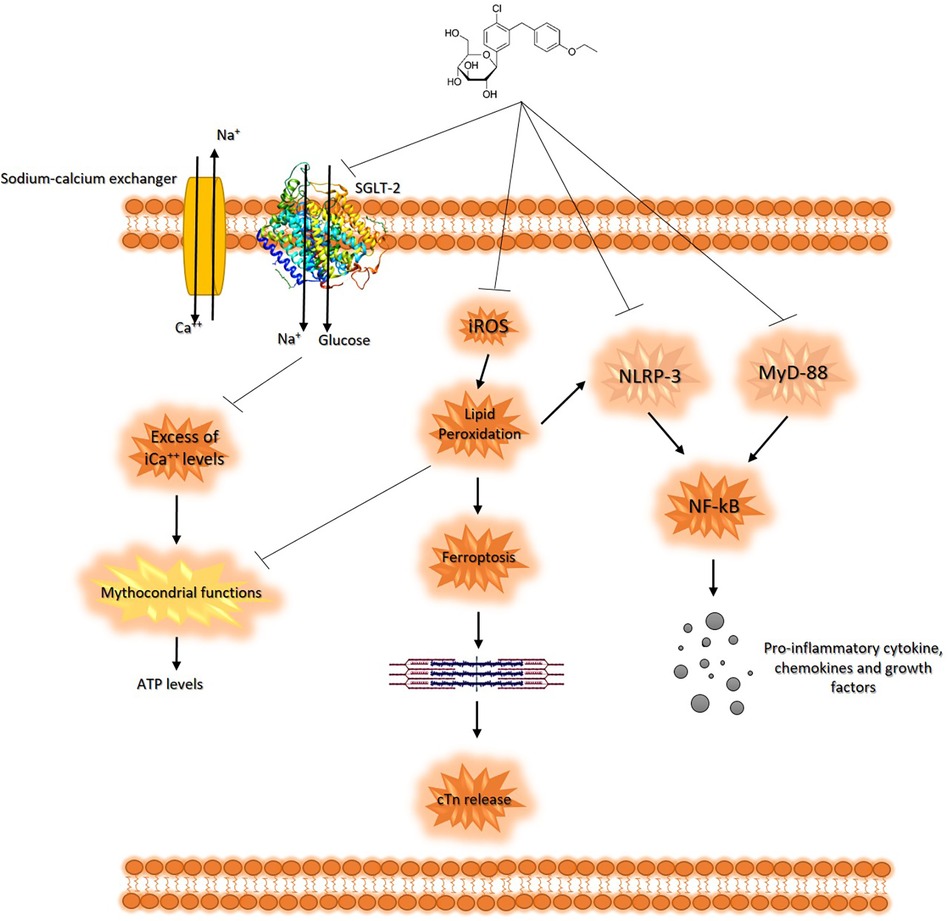
Figure 7. MDA and 4-HNA (A,B) systemic levels, and systemic cytokines (C) in mice treated with saline solution (control), DOXO 2.17 mg/kg/day, DAPA 10 mg/kg/day, and DOXO-DAPA in association (n = 6 for each group). One-way ANOVA. Values are expressed as mean ±SD.
Moreover, activation of NF-κB has been implicated in the inflammatory response and development of cardiac injuries (105); DOXO increases myocardial reactive oxygen species that can activate NF-κB signaling. Once activated, NF-κB translocates into the nucleus and promotes the expression of various pro-inflammatory genes, including cytokines, chemokines, and adhesion molecules involved in heart failure and fibrosis (106). Overall, NF-κB activation plays a significant role in doxorubicin-induced cardiotoxicity by mediating the inflammatory response and modulating cell survival pathways (107). To the best of our knowledge, this is the earliest evidence that DAPA is able to suppress NF-Kb expression in myocardial and renal tissue through IHC methods in preclinical models of DOXO cardiotoxicity. The overall picture of the study (Figure 8) summarizes the potential systemic and cardio-renal benefits of DAPA in preclinical models of cardio-oncology.
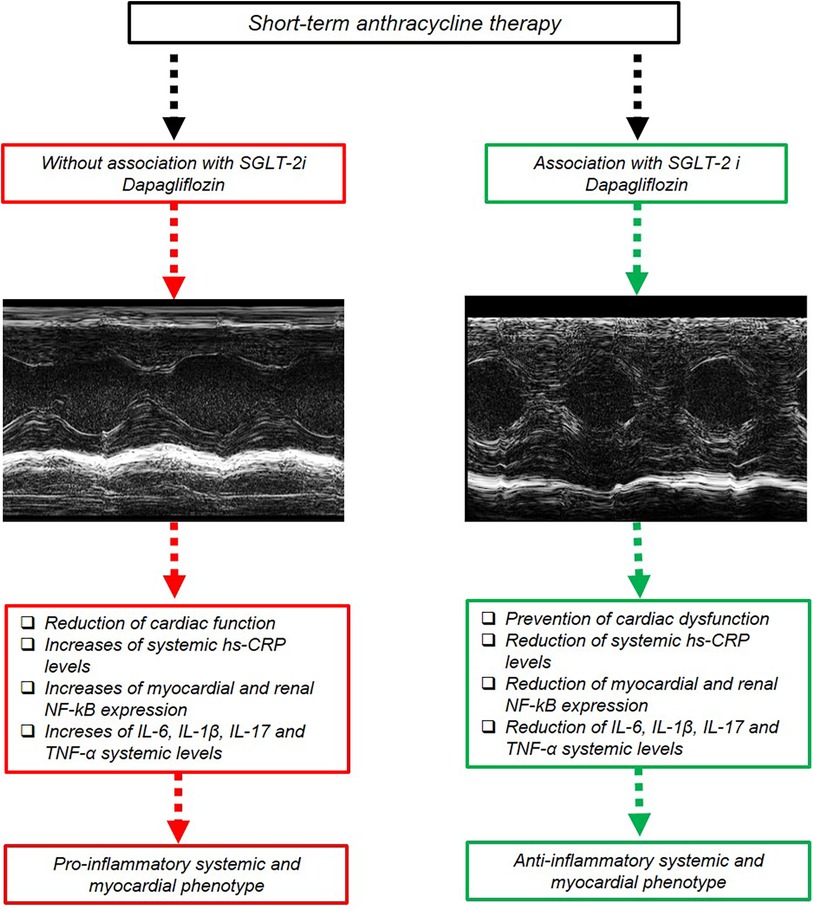
Figure 8. Schematic representation of DAPA-mediated cardioprotective and anti-inflammatory effect in preclinical models. Short-term DOXO therapy reduced systolic cardiac function; increased systemic hs-CRP, IL-6, IL-1β, IL-17, and TNF-α levels; and increased myocardial and kidney NF-kB expression. DAPA attenuated DOXO-induced phenotype through inhibition of NLRP-3 and Myd-88 pathway, resulting in preservation of cardiac function and reduced systemic levels of hs-CRP, IL-6, IL-1β, IL-17, and TNF-α.
The present study has some limitations. First, this is a preliminary indication that DAPA could prevent cardiac dysfunctions and decrease biomarkers of cardiotoxicity in preclinical models of short-term-induced cardiomyopathies; however, a detailed mechanistic study of DAPA-related cardioprotection should be carried out, through the use of selective inhibitors of intracellular pathways potentially involved in beneficial properties of DAPA (i.e., through the use of NLRP-3 and MyD-88 selective inhibitors). Second, DOXO-induced cardiotoxicity also occurs many years after chemotherapy (108), especially in young women with breast cancer. Therefore, the long-term effects of DAPA in preclinical models exposed to anthracyclines should be performed; however, acute, short-term, cardiac, and endothelial biochemical changes are frequently seen in these patients and are clinically relevant. On the other hand, we studied the early effects of DAPA on the myocardial metabolism of preclinical models without assessing insulin levels, homeostatic model assessment (HOMA)-index, and ketogenic bodies (SGLT2is increase acetate and butyrate systemic levels that could affect myocardial metabolism) (109). Moreover, this study focalized the cardiovascular benefits only in female preclinical models, in line with other similar studies in cardio-oncology (34, 37–39). Anthracycline-induced cardiotoxicity is frequently seen in female breast cancer patients; therefore, a preclinical female model to mimic the clinical condition that we frequently observe in cardio-oncology was used, i.e., women with breast cancer treated with anthracyclines who develop cardiomyopathies. However, subsequent studies will be performed also in male mouse models to evaluate the impact of sex difference (110) in DAPA cardioprotection.
Currently, there is a need for cardioprotective strategies in cancer patients treated with doxorubicin, considering its relevant cardiotoxicity (111). The cardiovascular benefits (e.g., HHF and cardiovascular death) of SGLT2is are different and the mechanisms are partially elucidated. Recent clinical evidence of SGLT2is in cancer patients with T2DM indicate that gliflozins could reduce cardiovascular mortality, MACE, and hospitalization for heart failure. The data in the present study recommend the use of DAPA in the primary prevention of anthracycline-induced cardiotoxicities in cancer patients without diabetes, consequently reducing the discontinuation of therapies, hospitalizations for cardiovascular diseases, and the index of relevant cardiotoxic events.
The present study highlights the mechanisms of DAPA-mediated cardio-renal benefits in preclinical models of anthracycline toxicity. We provide new insight into the cardiovascular benefits of DAPA, as our data show that DAPA induced an anti-inflammatory systemic phenotype during DOXO therapy, reducing NF-kB expression in myocardial and kidney tissue. The overall picture of the study encourages the use of DAPA in non-diabetic cancer patients treated with anthracyclines to prevent adverse cardiac events. Further studies are warranted to investigate interconnected pathophysiological mechanisms of DAPA-induced cardioprotection.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession numbers can be found here: https://zenodo.org/record/8119945.
This animal study was approved by EU Directive 2010/63/EU for animal experiments, and Italian D.L.vo 26/2014 law; the Ministry of Health (authorization number 1467/17-PR of the 13-02-2017); and institutional ethics committees: Organismo preposto al benessere degli animali (OPBA). The study was conducted in accordance with the local legislation and institutional requirements.
VQ: Writing – original draft, Conceptualization. MC: Writing – review & editing, Investigation. IB: Writing – review & editing, Data curation. MI: Writing – review & editing, Software. AP: Writing – review & editing, Data curation. CM: Writing – review & editing, Supervision. MS: Writing – review & editing, Supervision. AM: Writing – review & editing, Data curation. VG: Writing – review & editing, Formal Analysis. GP: Writing – review & editing, Methodology. AL: Writing – review & editing, Methodology. FB: Writing – review & editing, Supervision. FZ: Writing – review & editing, Supervision, Methodology. MM: Writing – review & editing, Methodology, Data curation. RF: Writing – review & editing, Supervision, Investigation. MB: Writing – review & editing, Visualization, Validation. DG: Writing – review & editing, Supervision, Investigation. GG: Writing – review & editing, Validation, Software. NM: Writing – review & editing, Visualization, Validation, Funding acquisition.
The authors declare financial support was received for the research, authorship, and/or publication of this article.
This work was funded by a Ricerca Corrente grant, “Cardiotossicità da chemioterapie, targeted therapies e immunoterapie, diagnosi precoce e cardioprotezione. Ricerca preclinica e clinica” Linea 1/6, funded by Ministero della Salute (Italy).
We thank Dr. Trocino of Istituto Nazionale Tumori-IRCCS-Fondazione G. Pascale of Naples for bibliographic assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1289663/full#supplementary-material
1. Cardinale D, Iacopo F, Cipolla CM. Cardiotoxicity of anthracyclines. Front Cardiovasc Med. (2020) 7:26. doi: 10.3389/fcvm.2020.00026
2. Agunbiade TA, Zaghlol RY, Barac A. Heart failure in relation to anthracyclines and other chemotherapies. Methodist Debakey Cardiovasc J. (2019) 15(4):243–9. doi: 10.14797/mdcj-15-4-243
3. Bian X, McAllister-Lucas LM, Shao F, Schumacher KR, Feng Z, Porter AG, et al. NF-kappa B activation mediates doxorubicin-induced cell death in N-type neuroblastoma cells. J Biol Chem. (2001) 276(52):48921–9. doi: 10.1074/jbc.M108674200
4. Wang S, Kotamraju S, Konorev E, Kalivendi S, Joseph J, Kalyanaraman B. Activation of nuclear factor-kappaB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: the role of hydrogen peroxide. Biochem J. (2002) 367(Pt 3):729–40. doi: 10.1042/BJ20020752
5. Kamphuis JAM, Linschoten M, Cramer MJ, Doevendans PA, Asselbergs FW, Teske AJ. Early- and late anthracycline-induced cardiac dysfunction: echocardiographic characterization and response to heart failure therapy. Cardiooncology. (2020) 6:23. doi: 10.1186/s40959-020-00079-3
6. Kourek C, Touloupaki M, Rempakos A, Loritis K, Tsougkos E, Paraskevaidis I, et al. Cardioprotective strategies from cardiotoxicity in cancer patients: a comprehensive review. J Cardiovasc Dev Dis. (2022) 9(8):259. doi: 10.3390/jcdd9080259
7. Butler J, Usman MS, Khan MS, Greene SJ, Friede T, Vaduganathan M, et al. Efficacy and safety of SGLT2 inhibitors in heart failure: systematic review and meta-analysis. ESC Heart Fail. (2020) 7(6):3298–309. Erratum in: ESC Heart Fail. (2021) 8(3):2362. doi: 10.1002/ehf2.13169.33586910
8. Salvatore T, Galiero R, Caturano A, Rinaldi L, Di Martino A, Albanese G, et al. An overview of the cardiorenal protective mechanisms of SGLT2 inhibitors. Int J Mol Sci. (2022) 23:3651. doi: 10.3390/ijms23073651
9. Udell JA, Jones WS, Petrie MC, Harrington J, Anker SD, Bhatt DL, et al. Sodium glucose cotransporter-2 inhibition for acute myocardial infarction: JACC review topic of the week. J Am Coll Cardiol. (2022) 79(20):2058–68. doi: 10.1016/j.jacc.2022.03.353
10. Usman MS, Siddiqi TJ, Anker SD, Bakris GL, Bhatt DL, Filippatos G, et al. Effect of SGLT2 inhibitors on cardiovascular outcomes across various patient populations. J Am Coll Cardiol. (2023) 81(25):2377–87. doi: 10.1016/j.jacc.2023.04.034
11. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2019) 380(4):347–57. doi: 10.1056/NEJMoa1812389
12. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381(21):1995–2008. doi: 10.1056/NEJMoa1911303
13. Nassif ME, Windsor SL, Tang F, Khariton Y, Husain M, Inzucchi SE, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF trial. Circulation. (2019) 140(18):1463–76. doi: 10.1161/CIRCULATIONAHA.119.042929
14. Solomon SD, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, et al. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial. Eur J Heart Fail. (2021) 23(7):1217–25. doi: 10.1002/ejhf.2249
15. Gongora CA, Drobni ZD, Quinaglia Araujo Costa Silva T, Zafar A, Gong J, Zlotoff DA, et al. Sodium-glucose co-transporter-2 inhibitors and cardiac outcomes among patients treated with anthracyclines. JACC Heart Fail. (2022) 10(8):559–67. doi: 10.1016/j.jchf.2022.03.006
16. Hsieh PL, Chu PM, Cheng HC, Huang YT, Chou WC, Tsai KL, et al. Dapagliflozin mitigates doxorubicin-caused myocardium damage by regulating AKT-mediated oxidative stress, cardiac remodeling, and inflammation. Int J Mol Sci. (2022) 23(17):10146. doi: 10.3390/ijms231710146
17. Nugrahaningrum DA, Marcelina O, Liu C, Wu S, Kasim V. Dapagliflozin promotes neovascularization by improving paracrine function of skeletal muscle cells in diabetic hindlimb ischemia mice through PHD2/HIF-1α axis. Front Pharmacol. (2020) 11:1104. doi: 10.3389/fphar.2020.01104
18. Chiba Y, Yamada T, Tsukita S, Takahashi K, Munakata Y, Shirai Y, et al. Dapagliflozin, a sodium-glucose co-transporter 2 inhibitor, acutely reduces energy expenditure in BAT via neural signals in mice. PLoS One. (2016) 11(3):e0150756. doi: 10.1371/journal.pone.0150756
19. Åstrand A, Wingren C, Benjamin A, Tregoning JS, Garnett JP, Groves H, et al. Dapagliflozin-lowered blood glucose reduces respiratory Pseudomonas aeruginosa infection in diabetic mice. Br J Pharmacol. (2017) 174(9):836–47. doi: 10.1111/bph.13741
20. Wu W, Zhang Z, Jing D, Huang X, Ren D, Shao Z, et al. SGLT2 inhibitor activates the STING/IRF3/IFN-β pathway and induces immune infiltration in osteosarcoma. Cell Death Dis. (2022) 13(6):523. doi: 10.1038/s41419-022-04980-w
21. Quagliariello V, Gennari A, Jain SA, Rosso F, Iaffaioli RV, Barbarisi A, et al. Double-responsive hyaluronic acid-based prodrugs for efficient tumour targeting. Mater Sci Eng C Mater Biol Appl. (2021) 131:112475. doi: 10.1016/j.msec.2021.112475
22. Boutagy NE, Wu J, Cai Z, Zhang W, Booth CJ, Kyriakides TC, et al. In vivo reactive oxygen species detection with a novel positron emission tomography tracer, 18F-DHMT, allows for early detection of anthracycline-induced cardiotoxicity in rodents. JACC Basic Transl Sci. (2018) 3(3):378–90. doi: 10.1016/j.jacbts.2018.02.003
23. Bosman M, Krüger DN, Favere K, Wesley CD, Neutel CHG, Van Asbroeck B, et al. Doxorubicin impairs smooth muscle cell contraction: novel insights in vascular toxicity. Int J Mol Sci. (2021) 22(23):12812. doi: 10.3390/ijms222312812
24. Murata T, Yamawaki H, Yoshimoto R, Hori M, Sato K, Ozaki H, et al. Chronic effect of doxorubicin on vascular endothelium assessed by organ culture study. Life Sci. (2001) 69:2685–95. doi: 10.1016/S0024-3205(01)01352-2
25. Young RC, Ozols RF, Myers CE. The anthracycline antineoplastic drugs. N Engl J Med. (1981) 305:139–53. doi: 10.1056/NEJM198107163050305
26. Deng W, Leu HB, Chen Y, Chen YH, Epperson CM, Juang C, et al. Protein kinase B (PKB/AKT1) formed signaling complexes with mitochondrial proteins and prevented glycolytic energy dysfunction in cultured cardiomyocytes during ischemia-reperfusion injury. Endocrinology. (2014) 155(5):1618–28. doi: 10.1210/en.2013-1817
27. Boudina S, Han YH, Pei S, Tidwell TJ, Henrie B, Tuinei J, et al. UCP3 regulates cardiac efficiency and mitochondrial coupling in high fat-fed mice but not in leptin-deficient mice. Diabetes. (2012) 61(12):3260–9. doi: 10.2337/db12-0063
28. Zhang G, Yuan C, Su X, Zhang J, Gokulnath P, Vulugundam G, et al. Relevance of ferroptosis to cardiotoxicity caused by anthracyclines: mechanisms to target treatments. Front Cardiovasc Med. (2022) 9:896792. doi: 10.3389/fcvm.2022.896792
29. Novac MB, Boldeanu L, Rotaru LT, Dijmărescu AL, Şerbănescu MS, Radu L, et al. The perioperative effect of anesthetic drugs on the immune response in total intravenous anesthesia in patients undergoing minimally invasive gynecological surgery. Rom J Morphol Embryol. (2021) 62(4):961–9. doi: 10.47162/RJME.62.4.08
30. Quagliariello V, Berretta M, Buccolo S, Iovine M, Paccone A, Cavalcanti E, et al. Polydatin reduces cardiotoxicity and enhances the anticancer effects of sunitinib by decreasing pro-oxidative stress, pro-inflammatory cytokines, and NLRP3 inflammasome expression. Front Oncol. (2021) 11:680758. doi: 10.3389/fonc.2021.680758
31. Liu L, Chen Y, Zhang Q, Li C. Silencing of KCNA1 suppresses the cervical cancer development via mitochondria damage. Channels (Austin). (2019) 13(1):321–30. doi: 10.1080/19336950.2019.1648627
32. Riccio G, Antonucci S, Coppola C, D'Avino C, Piscopo G, Fiore D, et al. Ranolazine attenuates trastuzumab-induced heart dysfunction by modulating ROS production. Front Physiol. (2018) 9:38. doi: 10.3389/fphys.2018.00038
33. Sabatino J, De Rosa S, Tammè L, Iaconetti C, Sorrentino S, Polimeni A, et al. Empagliflozin prevents doxorubicin-induced myocardial dysfunction. Cardiovasc Diabetol. (2020) 19(1):66. doi: 10.1186/s12933-020-01040-5
34. Quagliariello V, De Laurentiis M, Rea D, Barbieri A, Monti MG, Carbone A, et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc Diabetol. (2021) 20(1):150. doi: 10.1186/s12933-021-01346-y
35. Desai VG, Vijay V, Han T, Moland CL, Phanavanh B, Lee T, et al. Doxorubicin-induced delayed-onset subclinical cardiotoxicity in mice. J Appl Toxicol. (2022) 42(5):778–92. doi: 10.1002/jat.4256
36. Pecoraro M, Rodríguez-Sinovas A, Marzocco S, Ciccarelli M, Iaccarino G, Pinto A, et al. Cardiotoxic effects of short-term doxorubicin administration: involvement of Connexin 43 in calcium impairment. Int J Mol Sci. (2017) 18(10):2121. doi: 10.3390/ijms18102121
37. Kuno A, Hosoda R, Tsukamoto M, Sato T, Sakuragi H, Ajima N, et al. SIRT1 in the cardiomyocyte counteracts doxorubicin-induced cardiotoxicity via regulating histone H2AX. Cardiovasc Res. (2023) 118(17):3360–73. doi: 10.1093/cvr/cvac026
38. Tocchetti CG, Carpi A, Coppola C, Quintavalle C, Rea D, Campesan M, et al. Ranolazine protects from doxorubicin-induced oxidative stress and cardiac dysfunction. Eur J Heart Fail. (2014) 16(4):358–66. doi: 10.1002/ejhf.50
39. Fedele C, Riccio G, Coppola C, Barbieri A, Monti MG, Arra C, et al. Comparison of preclinical cardiotoxic effects of different ErbB2 inhibitors. Breast Cancer Res Treat. (2012) 133(2):511–21. doi: 10.1007/s10549-011-1783-9
40. Toldo S, Goehe RW, Lotrionte M, Mezzaroma E, Sumner ET, Biondi-Zoccai GG, et al. Comparative cardiac toxicity of anthracyclines in vitro and in vivo in the mouse. PLoS One. (2013) 8(3):e58421. doi: 10.1371/journal.pone.0058421
41. Toldo S, Bogaard HJ, Van Tassell BW, Mezzaroma E, Seropian IM, Robati R, et al. Right ventricular dysfunction following acute myocardial infarction in the absence of pulmonary hypertension in the mouse. PLoS One. (2011) 6(3):e18102. doi: 10.1371/journal.pone.0018102
42. Alshnbari AS, Millar SA, O’Sullivan SE, Idris I. Effect of sodium-glucose cotransporter-2 inhibitors on endothelial function: a systematic review of preclinical studies. Diabetes Ther. (2020) 11(9):1947–63. doi: 10.1007/s13300-020-00885-z
43. Chi PJ, Lee CJ, Hsieh YJ, Lu CW, Hsu BG. Dapagliflozin ameliorates lipopolysaccharide related acute kidney injury in mice with streptozotocin-induced diabetes mellitus. Int J Med Sci. (2022) 19(4):729–39. doi: 10.7150/ijms.69031
44. Oe Y, Kim YC, Sidorenko VS, Zhang H, Kanoo S, Lopez N, et al. SGLT2 inhibitor dapagliflozin protects the kidney in a murine model of Balkan nephropathy. Am J Physiol Renal Physiol. (2024) 326(2):F227–40. doi: 10.1152/ajprenal.00228.2023
45. Nikolaou PE, Mylonas N, Makridakis M, Makrecka-Kuka M, Iliou A, Zerikiotis S, et al. Cardioprotection by selective SGLT-2 inhibitors in a non-diabetic mouse model of myocardial ischemia/reperfusion injury: a class or a drug effect? Basic Res Cardiol. (2022) 117(1):27. doi: 10.1007/s00395-022-00934-7
46. Riccio G, Antonucci S, Coppola C, D’Avino C, Piscopo G, Fiore D, et al. Ranolazine attenuates trastuzumab-induced heart dysfunction by modulating ROS production. Front Physiol. (2018) 9:38. doi: 10.3389/fphys.2018.00038
47. de Lucia C, Wallner M, Eaton DM, Zhao H, Houser SR, Koch WJ. Echocardiographic strain analysis for the early detection of left ventricular systolic/diastolic dysfunction and dyssynchrony in a mouse model of physiological aging. J Gerontol A Biol Sci Med Sci. (2019) 74(4):455–61. doi: 10.1093/gerona/gly139
48. Donner DG, Kiriazis H, Du XJ, Marwick TH, McMullen JR. Improving the quality of preclinical research echocardiography: observations, training, and guidelines for measurement. Am J Physiol Heart Circ Physiol. (2018) 315(1):H58–70. doi: 10.1152/ajpheart.00157.2018
49. Rutledge C, Cater G, McMahon B, Guo L, Nouraie SM, Wu Y, et al. Commercial 4-dimensional echocardiography for murine heart volumetric evaluation after myocardial infarction. Cardiovasc Ultrasound. (2020) 18(1):9. doi: 10.1186/s12947-020-00191-5
50. Tee N, Gu Y, Shim W. Comparative myocardial deformation in 3 myocardial layers in mice by speckle tracking echocardiography. Biomed Res Int. (2015) 2015:148501. doi: 10.1155/2015/148501
51. Coppola C, Riccio G, Barbieri A, Monti MG, Piscopo G, Rea D, et al. Antineoplastic-related cardiotoxicity, morphofunctional aspects in a murine model: contribution of the new tool 2D-speckle tracking. Onco Targets Ther. (2016) 9:6785–94. doi: 10.2147/OTT.S106528
52. Kohut A, Patel N, Singh H. Comprehensive echocardiographic assessment of the right ventricle in murine models. J Cardiovasc Ultrasound. (2016) 24(3):229–38. doi: 10.4250/jcu.2016.24.3.229
53. Gardin JM, Adams DB, Douglas PS, Feigenbaum H, Forst DH, Fraser AG, et al. Recommendations for a standardized report for adult transthoracic echocardiography: a report from the American Society of Echocardiography’s Nomenclature and Standards Committee and Task Force for a Standardized Echocardiography Report. J Am Soc Echocardiogr. (2002) 15(3):275–90. doi: 10.1067/mje.2002.121536
54. Pistner A, Belmonte S, Coulthard T, Blaxall B. Murine echocardiography and ultrasound imaging. J Vis Exp. (2010) 42:2100. doi: 10.3791/2100
55. Quagliariello V, Passariello M, Di Mauro A, Cipullo C, Paccone A, Barbieri A, et al. Corrigendum: immune checkpoint inhibitor therapy increases systemic SDF-1, cardiac DAMPs Fibronectin-EDA, S100/Calgranulin, galectine-3, and NLRP3-MyD88-chemokine pathways. Front Cardiovasc Med. (2023) 10:1129873. Erratum in: Front Cardiovasc Med. (2022) 9:930797. doi: 10.3389/fcvm.2023.1129873
56. Brindle E, Fujita M, Shofer J, O'Connor KA. Serum, plasma, and dried blood spot high-sensitivity C-reactive protein enzyme immunoassay for population research. J Immunol Methods. (2010) 362(1–2):112–20. doi: 10.1016/j.jim.2010.09.014
57. Quagliariello V, Passariello M, Rea D, Barbieri A, Iovine M, Bonelli A, et al. Evidences of CTLA-4 and PD-1 blocking agents-induced cardiotoxicity in cellular and preclinical models. J Pers Med. (2020) 10(4):179. doi: 10.3390/jpm10040179
58. Al-Mutairi MS, Habashy HO. Nuclear factor-κB clinical significance in breast cancer: an immunohistochemical study. Med Princ Pract. (2023) 32(1):33–9. doi: 10.1159/0005278289
59. Jenkins GJ, Mikhail J, Alhamdani A, Brown TH, Caplin S, Manson JM, et al. Immunohistochemical study of nuclear factor-kappaB activity and interleukin-8 abundance in oesophageal adenocarcinoma; a useful strategy for monitoring these biomarkers. J Clin Pathol. (2007) 60(11):1232–7. doi: 10.1136/jcp.2006.043976
60. Cortellino S, Quagliariello V, Delfanti G, Blaževitš O, Chiodoni C, Maurea N, et al. Fasting mimicking diet in mice delays cancer growth and reduces immunotherapy-associated cardiovascular and systemic side effects. Nat Commun. (2023) 14(1):5529. doi: 10.1038/s41467-023-41066-3
61. Kabel AM, Salama SA, Adwas AA, Estfanous RS. Targeting oxidative stress, NLRP3 inflammasome, and autophagy by fraxetin to combat doxorubicin-induced cardiotoxicity. Pharmaceuticals (Basel). (2021) 14(11):1188. doi: 10.3390/ph14111188
62. He L, Wong CK, Cheung KK, Yau HC, Fu A, Zhao H-L, et al. Anti-inflammatory effects of exendin-4, a glucagon-like peptide-1 analog, on human peripheral lymphocytes in patients with type 2 diabetes. J Diabetes Investig. (2013) 4:382–92. doi: 10.1111/jdi.12063
63. Yao Y, Tao R, Wang X, Wang Y, Mao Y, Zhou LF. B7-H1 is correlated with malignancy-grade gliomas but is not expressed exclusively on tumor stem-like cells. Neuro Oncol. (2009) 11(6):757–66. doi: 10.1215/15228517-2009-014
64. Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. (2017) 60:215–25. doi: 10.1007/s00125-016-4157-3
65. Terami N, Ogawa D, Tachibana H, Hatanaka T, Wada J, Nakatsuka A, et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS One. (2014) 9(6):e100777. doi: 10.1371/journal.pone.0100777
66. Reis-Mendes A, Padrão AI, Duarte JA, Gonçalves-Monteiro S, Duarte-Araújo M, Remião F, et al. Role of inflammation and redox status on doxorubicin-induced cardiotoxicity in infant and adult CD-1 male mice. Biomolecules. (2021) 11(11):1725. doi: 10.3390/biom11111725
67. Wang L, Chen Q, Qi H, Wang C, Wang C, Zhang J, et al. Doxorubicin-induced systemic inflammation is driven by upregulation of toll-like receptor TLR4 and endotoxin leakage. Cancer Res. (2016) 76(22):6631–42. doi: 10.1158/0008-5472.CAN-15-3034
68. Todorova VK, Wei JY, Makhoul I. Subclinical doxorubicin-induced cardiotoxicity update: role of neutrophils and endothelium. Am J Cancer Res. (2021) 11(9):4070–91.34659877
69. Zeiss CJ, Gatti DM, Toro-Salazar O, Davis C, Lutz CM, Spinale F, et al. Doxorubicin-induced cardiotoxicity in collaborative cross (CC) mice recapitulates individual cardiotoxicity in humans. G3. (2019) 9(8):2637–46. doi: 10.1534/g3.119.400232
70. Kittiwarawut A, Vorasettakarnkij Y, Tanasanvimon S, Manasnayakorn S, Sriuranpong V. Serum NT-proBNP in the early detection of doxorubicin-induced cardiac dysfunction. Asia Pac J Clin Oncol. (2013) 9(2):155–61. doi: 10.1111/j.1743-7563.2012.01588.x
71. Maayah ZH, Takahara S, Dyck JRB. The beneficial effects of reducing NLRP3 inflammasome activation in the cardiotoxicity and the anti-cancer effects of doxorubicin. Arch Toxicol. (2021) 95(1):1–9. doi: 10.1007/s00204-020-02876-2
72. Alzokaky AA, Al-Karmalawy AA, Saleh MA, Abdo W, Farage AE, Belal A, et al. Metformin ameliorates doxorubicin-induced cardiotoxicity targeting HMGB1/TLR4/NLRP3 signaling pathway in mice. Life Sci. (2023) 316:121390. doi: 10.1016/j.lfs.2023.121390
73. Sun Z, Lu W, Lin N, Lin H, Zhang J, Ni T, et al. Dihydromyricetin alleviates doxorubicin-induced cardiotoxicity by inhibiting NLRP3 inflammasome through activation of SIRT1. Biochem Pharmacol. (2020) 175:113888. doi: 10.1016/j.bcp.2020.113888
74. Mauro AG, Bonaventura A, Vecchié A, Mezzaroma E, Carbone S, Narayan P, et al. The role of NLRP3 inflammasome in pericarditis: potential for therapeutic approaches. JACC Basic Transl Sci. (2021) 6(2):137–50. doi: 10.1016/j.jacbts.2020.11.016
75. Li X, Liang J, Qu L, Liu S, Qin A, Liu H, et al. Exploring the role of ferroptosis in the doxorubicin-induced chronic cardiotoxicity using a murine model. Chem Biol Interact. (2022) 363:110008. doi: 10.1016/j.cbi.2022.110008
76. Jing W, Guo X, Qin F, Li Y, Wang G, Bi Y, et al. G-CSF shifts erythropoiesis from bone marrow into spleen in the setting of systemic inflammation. Life Sci Alliance. (2020) 4(1):e202000737. doi: 10.26508/lsa.202000737
77. Mortensen MS, Ruiz J, Watts JL. Polyunsaturated fatty acids drive lipid peroxidation during ferroptosis. Cells. (2023) 12(5):804. doi: 10.3390/cells12050804
78. Brown A, Gandy S, Mordi IR, McCrimmon R, Ramkumar PG, Houston JG, et al. Dapagliflozin improves left ventricular myocardial longitudinal function in patients with type 2 diabetes. JACC Cardiovasc Imaging. (2021) 14(2):503–4. doi: 10.1016/j.jcmg.2020.07.025
79. Vaduganathan M, Claggett BL, Jhund P, de Boer RA, Hernandez AF, Inzucchi SE, et al. Estimated long-term benefit of dapagliflozin in patients with heart failure. J Am Coll Cardiol. (2022) 80(19):1775–84. doi: 10.1016/j.jacc.2022.08.745
80. Zheng XD, Qu Q, Jiang XY, Wang ZY, Tang C, Sun JY. Effects of dapagliflozin on cardiovascular events, death, and safety outcomes in patients with heart failure: a meta-analysis. Am J Cardiovasc Drugs. (2021) 21(3):321–30. doi: 10.1007/s40256-020-00441-x
81. Zhai M, Du X, Liu C, Xu H. The effects of dapagliflozin in patients with heart failure complicated with type 2 diabetes: a meta-analysis of placebo-controlled randomized trials. Front Clin Diabetes Healthc. (2021) 2:703937. doi: 10.3389/fcdhc.2021.703937
82. Cefalu WT, Leiter LA, de Bruin TW, Gause-Nilsson I, Sugg J, Parikh SJ. Dapagliflozin’s effects on glycemia and cardiovascular risk factors in high-risk patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. Diabetes Care. (2015) 38(7):1218–27. doi: 10.2337/dc14-0315
83. Jhund PS, Claggett BL, Talebi A, Butt JH, Gasparyan SB, Wei LJ, et al. Effect of dapagliflozin on total heart failure events in patients with heart failure with mildly reduced or preserved ejection fraction: a prespecified analysis of the DELIVER trial. JAMA Cardiol. (2023) 8(6):554–63. doi: 10.1001/jamacardio.2023.0711
84. Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. (2020) 383(15):1436–46. doi: 10.1056/NEJMoa2024816
85. Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci. (2020) 5(6):632–44. doi: 10.1016/j.jacbts.2020.02.004
86. Xie Y, Wei Y, Li D, Pu J, Ding H, Zhang X. Mechanisms of SGLT2 inhibitors in heart failure and their clinical value. J Cardiovasc Pharmacol. (2023) 81(1):4–14. doi: 10.1097/FJC.0000000000001380
87. Lau KTK, Ng L, Wong JWH, Loong HHF, Chan WWL, Lee CH, et al. Repurposing sodium-glucose co-transporter 2 inhibitors (SGLT2i) for cancer treatment—a review. Rev Endocr Metab Disord. (2021) 22(4):1121–36. doi: 10.1007/s11154-021-09675-9
88. Kuang H, Liao L, Chen H, Kang Q, Shu X, Wang Y. Therapeutic effect of sodium glucose co-transporter 2 inhibitor dapagliflozin on renal cell carcinoma. Med Sci Monit. (2017) 23:3737–45. doi: 10.12659/msm.902530
89. Zhou J, Zhu J, Yu SJ, Ma HL, Chen J, Ding XF, et al. Sodium-glucose co-transporter-2 (SGLT-2) inhibition reduces glucose uptake to induce breast cancer cell growth arrest through AMPK/mTOR pathway. Biomed Pharmacother. (2020) 132:110821. doi: 10.1016/j.biopha.2020.110821
90. Dutka M, Bobiński R, Francuz T, Garczorz W, Zimmer K, Ilczak T, et al. SGLT-2 inhibitors in cancer treatment-mechanisms of action and emerging new perspectives. Cancers (Basel). (2022) 14(23):5811. doi: 10.3390/cancers14235811
91. Nasiri AR, Rodrigues MR, Li Z, Leitner BP, Perry RJ. SGLT2 inhibition slows tumor growth in mice by reversing hyperinsulinemia. Cancer Metab. (2019) 7:10. doi: 10.1186/s40170-019-0203-1
92. de Gregorio A, Janni W, Friedl TWP, Nitz U, Rack B, Schneeweiss A, et al. The impact of anthracyclines in intermediate and high-risk HER2-negative early breast cancer—a pooled analysis of the randomised clinical trials PlanB and SUCCESS C. Br J Cancer. (2022) 126(12):1715–24. doi: 10.1038/s41416-021-01690-6
93. Menna P, Paz OG, Chello M, Covino E, Salvatorelli E, Minotti G. Anthracycline cardiotoxicity. Expert Opin Drug Saf. (2012) 11(Suppl 1):S21–36. doi: 10.1517/14740338.2011.589834
94. Abdel-Qadir H, Carrasco R, Austin PC, Chen Y, Zhou L, Fang J, et al. The association of sodium-glucose cotransporter 2 inhibitors with cardiovascular outcomes in anthracycline-treated patients with cancer. JACC CardioOncol. (2023) 5(3):318–28. doi: 10.1016/j.jaccao.2023.03.011
95. Daniele AJ, Gregorietti V, Costa D, Lopez-Fernandez T. Use of emgliflozine in cardiotoxicity treatment. EMPACARD-treatment registry. Six-months follow-up. Eur Heart J. (2022) 43(Supplement_2):ehac544.2590. doi: 10.1093/eurheartj/ehac544.2590
96. Park LK, Lim KH, Volkman J, Abdiannia M, Johnston H, Nigogosyan Z, et al. Safety, tolerability, and effectiveness of the sodium-glucose cotransporter 2 inhibitor (SGLT2i) dapagliflozin in combination with standard chemotherapy for patients with advanced, inoperable pancreatic adenocarcinoma: a phase 1b observational study. Cancer Metab. (2023) 11(1):6. doi: 10.1186/s40170-023-00306-2
97. Lahnwong S, Chattipakorn SC, Chattipakorn N. Potential mechanisms responsible for cardioprotective effects of sodium-glucose co-transporter 2 inhibitors. Cardiovasc Diabetol. (2018) 17(1):101. doi: 10.1186/s12933-018-0745-5
98. He L, Li Y, Zhang D, Song H, Xu D, Song Z. Dapagliflozin improves endothelial cell dysfunction by regulating mitochondrial production via the SIRT1/PGC-1α pathway in obese mice. Biochem Biophys Res Commun. (2022) 615:123–30. doi: 10.1016/j.bbrc.2022.05.022
99. Mauro AG, Mezzaroma E, Toldo S, Melendez GC, Franco RL, Lesnefsky EJ, et al. NLRP3-mediated Inflammation in cardio-oncology: sterile yet harmful. Transl Res. (2023) 252:9–20. doi: 10.1016/j.trsl.2022.08.004
100. Moslehi J, Salem JE. Immune checkpoint inhibitor myocarditis treatment strategies and future directions. JACC CardioOncol. (2022) 4(5):704–7. doi: 10.1016/j.jaccao.2022.11.005
101. Syukri A, Hatta M, Amir M, Rohman MS, Mappangara I, Kaelan C, et al. Doxorubicin induced immune abnormalities and inflammatory responses via HMGB1, HIF1-α and VEGF pathway in progressive of cardiovascular damage. Ann Med Surg. (2022) 76:103501. doi: 10.1016/j.amsu.2022.103501
102. Sauter KA, Wood LJ, Wong J, Iordanov M, Magun BE. Doxorubicin and daunorubicin induce processing and release of interleukin-1β through activation of the NLRP3 inflammasome. Cancer Biol Ther. (2011) 11(12):1008–16. doi: 10.4161/cbt.11.12.15540
103. Xiao H, Wang X, Li S, Liu Y, Cui Y, Deng X. Advances in biomarkers for detecting early cancer treatment-related cardiac dysfunction. Front Cardiovasc Med. (2021) 8:753313. doi: 10.3389/fcvm.2021.753313
104. Fujimura K, Karasawa T, Komada T, Yamada N, Mizushina Y, Baatarjav C, et al. NLRP3 inflammasome-driven IL-1β and IL-18 contribute to lipopolysaccharide-induced septic cardiomyopathy. J Mol Cell Cardiol. (2023) 180:58–68. doi: 10.1016/j.yjmcc.2023.05.003
105. Liu Y, Xu Y, Yao Y, Cao Y, Chen G, Cai Y, et al. I-κB kinase-ε deficiency improves doxorubicin-induced dilated cardiomyopathy by inhibiting the NF-κB pathway. Front Physiol. (2022) 13:934899. doi: 10.3389/fphys.2022.934899
106. Fiordelisi A, Iaccarino G, Morisco C, Coscioni E, Sorriento D. NFkappab is a key player in the crosstalk between inflammation and cardiovascular diseases. Int J Mol Sci. (2019) 20(7):1599. doi: 10.3390/ijms20071599
107. Zhang DX, Ma DY, Yao ZQ, Fu CY, Shi YX, Wang QL, et al. ERK1/2/p53 and NF-κB dependent-PUMA activation involves in doxorubicin-induced cardiomyocyte apoptosis. Eur Rev Med Pharmacol Sci. (2016) 20(11):2435–42.27338072
108. Rawat PS, Jaiswal A, Khurana A, Bhatti JS, Navik U. Doxorubicin-induced cardiotoxicity: an update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed Pharmacother. (2021) 139:111708. doi: 10.1016/j.biopha.2021.111708
109. Iqbal I, Hamid M, Khan MAA, Kainat A, Tariq S. Dapagliflozin-induced late-onset euglycemic diabetic ketoacidosis. Cureus. (2019) 11(11):e6089. doi: 10.7759/cureus.6089
110. Appiah D, Mai M, Parmar K. A prospective population-based study of cardiovascular disease mortality following treatment for breast cancer among men in the United States, 2000–2019. Curr Oncol. (2022) 30(1):284–97. doi: 10.3390/curroncol30010023
Keywords: cancer, dapagliflozin, cardio-oncology, cardioprotection, doxorubicin, NF-kB
Citation: Quagliariello V, Canale ML, Bisceglia I, Iovine M, Paccone A, Maurea C, Scherillo M, Merola A, Giordano V, Palma G, Luciano A, Bruzzese F, Zito Marino F, Montella M, Franco R, Berretta M, Gabrielli D, Gallucci G and Maurea N (2024) Sodium-glucose cotransporter 2 inhibitor dapagliflozin prevents ejection fraction reduction, reduces myocardial and renal NF-κB expression and systemic pro-inflammatory biomarkers in models of short-term doxorubicin cardiotoxicity. Front. Cardiovasc. Med. 11:1289663. doi: 10.3389/fcvm.2024.1289663
Received: 6 September 2023; Accepted: 9 April 2024;
Published: 16 May 2024.
Edited by:
Aaron L. Sverdlov, The University of Newcastle, AustraliaReviewed by:
Alessandra Cuomo, University of Naples Federico II, Italy© 2024 Quagliariello, Canale, Bisceglia, Iovine, Paccone, Maurea, Scherillo, Merola, Giordano, Palma, Luciano, Bruzzese, Zito Marino, Montella, Franco, Berretta, Gabrielli, Gallucci and Maurea. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: V. Quagliariello, cXVhZ2xpYXJpZWxsby5lbnpvQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.