
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 19 February 2024
Sec. Coronary Artery Disease
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1288659
Drug-eluting stents (DES) play a crucial role in treating coronary artery disease (CAD) by preventing restenosis. These stents are coated with drug carriers that release antiproliferative drugs within the vessel. Over the past two decades, DES have been employed in clinical practice using various materials, polymers, and drug types. Despite optimizations in their design and materials to enhance biocompatibility and antithrombotic properties, evaluating their long-term efficacy and safety necessitates improved clinical follow-up and monitoring. To delineate future research directions, this study employs a bibliometric analysis approach. We comprehensively surveyed two decades' worth of literature on DES for CAD using the Web of Science Core Collection (WOSCC). Out of 5,778 articles, we meticulously screened them based on predefined inclusion and exclusion criteria. Subsequently, we conducted an in-depth analysis encompassing annual publication trends, authorship affiliations, journal affiliations, keywords, and more. Employing tools such as Excel 2021, CiteSpace 6.2R3, VOSviewer 1.6.19, and Pajek 5.17, we harnessed bibliometric methods to derive insights from this corpus. Analysis of annual publication data indicates a recent stabilisation or even a downward trend in research output in this area. The United States emerged as the leading contributor, with Columbia University and CRF at the forefront in both publication output and citation impact. The most cited document pertained to standardized definitions for clinical endpoints in coronary stent trials. Our author analysis identifies Patrick W. Serruys as the most prolific contributor, underscoring a dynamic exchange of knowledge within the field.Moreover, the dual chart overlay illustrates a close interrelation between journals in the “Medicine,” “Medical,” and “Clinical” domains and those in “Health,” “Nursing,” and “Medicine.” Frequently recurring keywords in this research landscape include DES coronary artery disease, percutaneous coronary intervention, implantation, and restenosis. This study presents a comprehensive panorama encompassing countries, research institutions, journals, keyword distributions, and contributions within the realm of DES therapy for CAD. By highlighting keywords exhibiting recent surges in frequency, we elucidate current research hotspots and frontiers, thereby furnishing novel insights to guide future researchers in this evolving field.
CAD, a cardiovascular ailment, exerts a profound global health impact. This malady stands as a principal contributor to mortality in both developed and developing nations (1–3). CAD's pathogenesis chiefly revolves around atherosclerosis development or coronary artery obstruction—a pathological sequence engendering constrained coronary blood flow. This impairment subsequently jeopardizes cardiac blood supply, potentially instigating manifestations like myocardial ischemia and angina pectoris. At its gravest, this process could culminate in myocardial infarction (4–7). The intricate etiology of CAD materializes as an intricate outcome stemming from multifaceted interactions. This intricate interplay emerges from a fusion of elements spanning lifestyle components (diet, exercise, smoking, alcohol consumption, etc.), environmental triggers (air pollution, psychological stress, etc.), and genetic predispositions (8–11). Percutaneous coronary intervention (PCI) stands as a widely employed method for treating coronary artery disease. This procedure involves the implantation of a stent within a coronary artery to alleviate stenosis or occlusion, thereby enhancing blood supply via the PCI technique (12–14). Initially, bare metal stents (BMS) marked the inception of stent utilization in clinical practice. However, this advancement was accompanied by the predicament of in-stent restenosis (ISR). The development of drug-eluting stents (DES) has been facilitated with deeper research into the pathology of ISR, improving all aspects of the stent, bringing new alloys that maintain durability and reduce strut thickness to improve deliverability, biocompatible polymers that reduce inflammatory responses and improve drug-eluting kinetics, and a new generation of drugs that predictably inhibit restenosis (15–18). These are designed to reduce endothelial hyperplasia to reduce vascular risk events after stent implantation. Bibliometric analysis serves as a robust investigative method to comprehensively understand the global distribution of countries and regions, prolific authors, research frontiers, and hotspots in the field. By scrutinizing extensive scientific data, including keyword frequencies, and more, this approach unveils intricate development nuances, emergent domains, and interconnected networks, providing clear and intuitive insights for future research trajectories (19, 20). By undertaking a meticulous bibliometric analysis, we seek to unveil the intricate fabric of pertinent countries and regions, prominent research institutions, prolific authors, recurrent keywords, and more, that compose the tapestry of this domain. Furthermore, this analysis endeavors to illuminate research hotspots and cutting-edge domains, aiming to foster innovative perspectives for the future trajectory of DES therapy for CAD.
The pertinent literature for this study was acquired from the Web of Science Core Collection (WOSCC) through comprehensive searches and downloads, culminating on July 11, 2023. The search query encompassed multiple permutations, including terms like “Drug-Eluting Stents,” “Drug Eluting Stents,” “Stents, Drug-Eluting,” and more, juxtaposed with terms such as “Coronary Artery Disease,” “Artery Disease, Coronary,” and associated variants. This formula was meticulously crafted to ensure inclusivity. The specific search query is as follows:
[TS = (Drug-Eluting Stents) OR TS = (Drug Eluting Stents) OR TS = (Stents, Drug-Eluting) OR TS = (Stents, Drug Eluting) OR TS = (Drug-Eluting Stent) OR TS = (Drug Eluting Stent) OR TS = (Stent, Drug-Eluting) OR TS = (Drug-Coated Stents) OR TS = (Drug Coated Stents) OR TS = (Stents, Drug-Coated) OR TS = (Stents, Drug Coated)] AND [TS = (Coronary Artery Disease) OR TS = (Artery Disease, Coronary) OR TS = (Artery Diseases, Coronary) OR TS = (Coronary Artery Diseases) OR TS = (Left Main Coronary Artery Disease) OR TS = (Left Main Disease) OR TS = (Left Main Diseases) OR TS = (Left Main Coronary Disease) OR TS = (Coronary Arteriosclerosis) OR TS = (Arterioscleroses, Coronary) OR TS = (Coronary Arterioscleroses) OR TS = (Atherosclerosis, Coronary) OR TS = (Atheroscleroses, Coronary) OR TS = (Coronary Atheroscleroses) OR TS = (Coronary Atherosclerosis) OR TS = (Arteriosclerosis, Coronary)].
The scope of literature encompassed the period from January 1, 2002, to July 11, 2023, enlisting records that adhered to English language criteria and were exclusively in the form of reviews and dissertations. The dataset, which spanned the specified time frame, encompassed a total of 5,778 records. These records, each encompassing comprehensive information encompassing authors, titles, sources, publication years, abstracts, keywords, DOI numbers, citation frequencies, and references cited within the respective articles, were amassed.
The resulting data were subsequently exported in plain text format, meticulously curated, and cataloged within a “download_.txt” file for further analysis and scrutiny.
Inclusion exclusion criteria:
1 Inclusion criteria
(1) Inclusion of all review and dissertation literature on DES for CAD that can be searched by WOSCC from 2002–2023;,
2 Exclusion criteria
(1) Duplicate published literature;
(2) Literature type of; conference abstracts, editorial material, conference proceedings, letters, book chapters, briefs, online at tables, revisions, retracted publications, newsletters, news, reprints, retracted content, discussions.
(3) Literature with incorrect data and for which data could not be extracted;
(4) Literature with incomplete data provided in the original text and which could not be obtained from the author(s);
(5) Literature that is not in English, has only an abstract, or for which the full text is not available.
The screening method is shown in Figure 1.
Statistical analysis of the publication data from the literature was conducted employing Excel 2021. This involved calculating key indicators like the yearly article publication count and cumulative publication count to elucidate the research field's trajectory. Furthermore, tables were generated to encapsulate the top 10 countries and top 10 authors in terms of article publication count and citation frequency, thereby facilitating a deeper comprehension of the literature's impact and collaborative dynamics.
To facilitate improved visualization and comprehensive analysis, the advanced edition of CiteSpace 6.2R3 took on responsibilities such as refining, processing, and computing data, along with generating visualization maps (21). The visualization maps, representing cited literature, adopt node size to signify project magnitude, while distinct colors denote various years. The interconnecting lines between nodes mirror collaborative efforts or co-citation associations among projects (22). Employing this approach, the dynamic interplay of node-node connections and the network of citations are observed, illuminating collaboration and citation bonds between projects. Consequently, this methodology facilitates the identification of research hotspots, discerning trends across stages, and projecting potential avenues of future development (23).
VOSviewer 1.6.19 stands as an instrumental tool catering to the visualization and analysis of scientific knowledge (24, 25). Capitalizing on its robust capabilities, this software navigates through country and region, research organization, and keyword co-occurrence analyses (26, 27). By employing this technique, we can shed light on pivotal trends and focal points within the field. This approach renders intricate structures and evolving trends within the research domain comprehensible at a glance, offering an intuitive appreciation of the field's dynamics.
Pajek 5.17, a software renowned for its network analysis and visualization capabilities. Its widespread usage extends to network science and allied disciplines, facilitating an in-depth exploration of complex network structures and attributes (28).
Furthermore, for assessment indicators, citations may take time to accumulate, which makes them not a direct indicator of research activity. For newer publications, the number of citations may not be fully representative of their impact. Focusing on the number of publications allows for a more real-time assessment of research results. So we chose number of publications as the primary indicator for assessment and added citations as a secondary indicator.
The analysis and graphing of annual and cumulative publication figures from the WOSCC literature concerning DES therapy for CAD over the past two decades are presented in Figure 2. The graphical representation unmistakably showcases a consistent year-by-year increase in the number of publications from 2002 onward. However, a significant shift in research focus occurred around 2015 due to the introduction of the clinical application of bioresorbable vascular scaffolds (BVS). This pivotal juncture spurred a redirection of attention from DES to BVS.
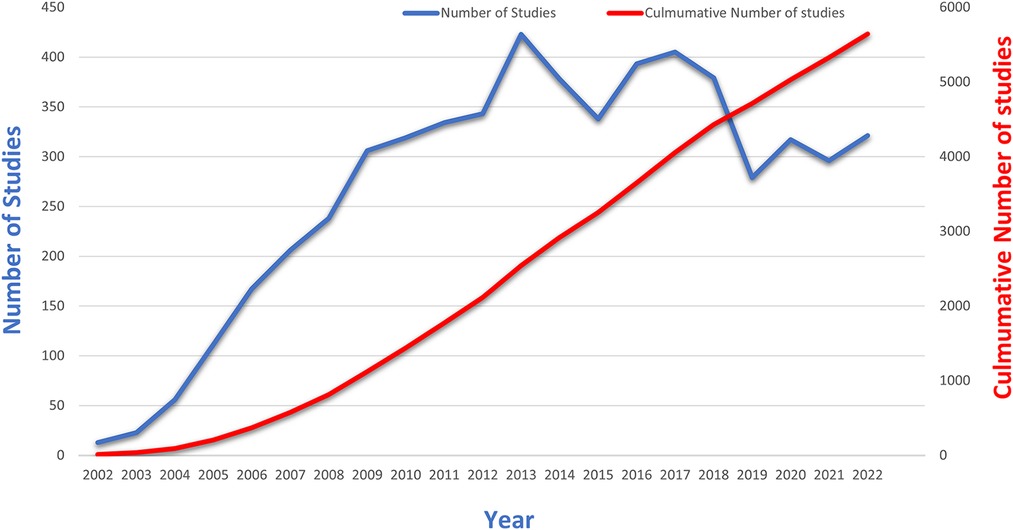
Figure 2. Trends in annual and cumulative number of publications in the WOSCC literature on CAD for DES treatment, 2002–2022.
Nevertheless, the trajectory of BVS was met with nuanced outcomes in light of accumulating clinical evidence. Specifically, the target lesion failure rates—encompassing cardiogenic death, target vessel myocardial infarction, and target lesion revascularization—as well as the risk of in-stent thrombosis demonstrated a noteworthy surge compared to metal DES (29, 30). A comprehensive meta-analysis over a 3-year span underscored that BVS markedly amplified the likelihood of in-stent thrombosis (31).
As a response to these findings, the resurgence of interest in DES was evident post-2016. However, the year 2019 marked the global onset of a novel coronavirus pandemic, significantly reshaping the landscape of medical research. Amid this paradigm shift towards pandemic-related investigations, the number of publications in the domain of DES experienced a temporary downturn. Subsequently, as pandemic-related research stabilized and the epidemic came under control, a gradual rebound in the research output related to DES was witnessed. While the situation regarding the novel coronavirus outbreak has shown signs of improvement, the field has not yet fully recovered to its previous state. This might be attributed to the decrease in stent prices, which, in turn, has resulted in reduced potential revenues for stent companies (32, 33). Along with the rapid growth in the field of structural heart disease interventions, may have diverted the attention of researchers (34, 35).
An analysis, utilizing data from WOSCC, was conducted to understand the contributions of different countries to citations. The outcomes are depicted in Figure 3. In this visual representation, the size of each circle corresponds to the number of citations, while the thickness of the lines connecting them signifies the extent of collaboration between the countries.
The top 10 countries, in terms of both publication count and citation impact, have been identified as follows: the United States, Italy, China, Germany, the Netherlands, South Korea, Japan, England, France, and Switzerland. A concise summary of these top countries can be found in Table 1.
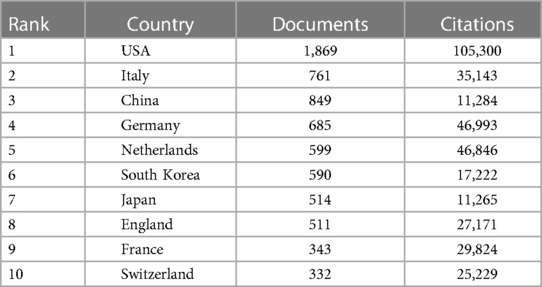
Table 1. Number of publications and citations from the top 10 countries in the WOSCC literature on CAD for DES, 2002–2023.
Notably, the United States emerges as a dominant force in this field, far surpassing other nations both in terms of publication output and citation impact. The symbiotic relationship between publication count and citation frequency underscores the United States' pivotal role in the field of DES for coronary artery disease, reinforcing its substantial influence on this critical area of research.
Utilizing VOSviewer, a visual mapping of institutional collaborations was meticulously crafted. This intricate map underwent further refinement through the application of Pajek software, culminating in the generation of Figure 4. Five predominant clusters have materialized within this collaboration network, epitomized by Columbia University, Erasmus University Medical Center, University of Ulsan, Capital Medical University, and Kyoto University.
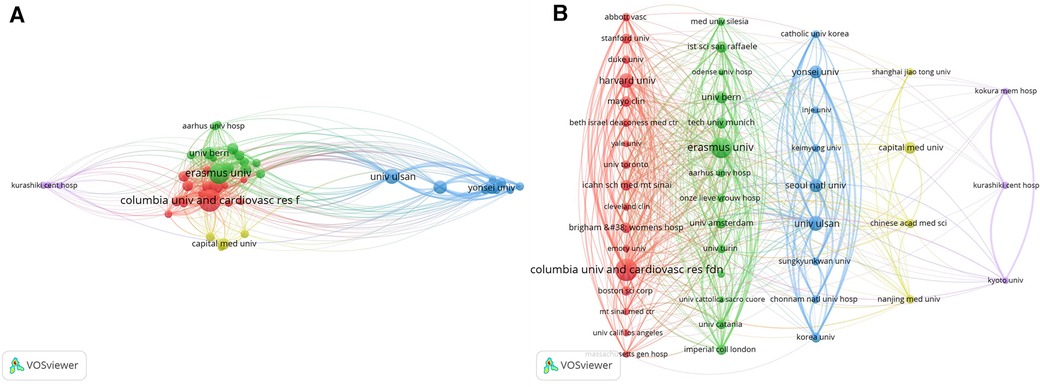
Figure 4. WOSCC collaborative network map of research institutes regarding DES treatment CAD literature, 2002–2023, (A) is derived directly from the VOSviewer 1.6.19 and (B) is processed by the Pajek 5.17.
Intriguingly, an observation surfaces: numerous institutions exhibit a lack of connectivity, implying minimal or no cooperative engagement. However, an intriguing possibility surfaces: intermediaries acting as bridges between distinct entities. This proposition seeks to foster collaboration between institutions that may not have direct connections. In essence, leveraging intermediaries to forge connections and bridge gaps between research institutions holds the potential to enhance interdisciplinary communication and foster cooperative endeavors, thereby propelling scientific advancement through synergistic efforts.
Concurrently, the tally of articles published by institutions has been meticulously compiled and is detailed in Table 2. An in-depth analysis of these statistics immediately highlights the prominent position held by Columbia University and the Cardiovascular Research Foundation (CRF) in terms of both article publications and citation frequency. This dominance firmly establishes them as central players in this domain.
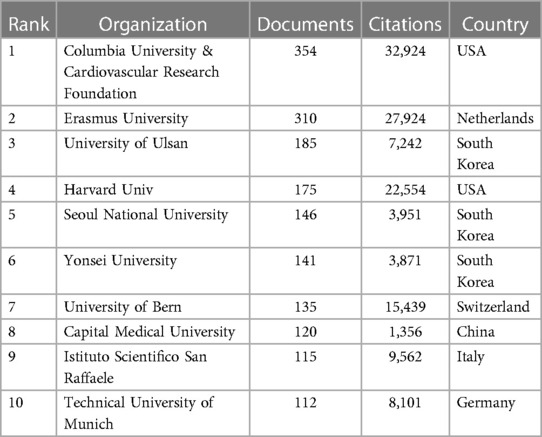
Table 2. Top 10 WOSCC research institutions in terms of number of publications on DES therapy CAD literature, 2002–2023.
Citation analysis serves as a yardstick for assessing the influence and impact of scholarly works, as illustrated in Figure 5. The top ten cited articles, replete with their titles, affiliated journals, and respective journal impact factors, provide a comprehensive snapshot of the most impactful contributions.

Figure 5. Network diagram of citation relationships in the WOSCC literature on DES treatment of CAD, 2002–2023.
Employing the impact factor (IF) as a metric, the magnitude of a journal's influence is quantified. Larger impact factors denote heightened influence. Furthermore, the Altmetric Score (AS) serves as a metric to gauge the impact of scientific articles in online media (36, 37). While it doesn't gauge the scientific quality of the article, it offers an alternative perspective on the article's impact within the community (38). This insight is explicitly articulated in Table 3. Remarkably, the top ten cited articles predominantly find their home in influential journals such as “Circulation,” “European Heart Journal,” and “New England Journal of Medicine.” This clustering within these high-impact journals underscores their profound sway within the field.
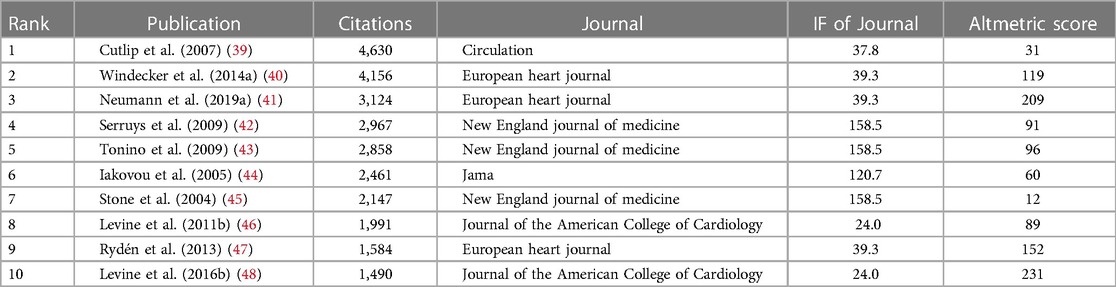
Table 3. Top 10 citations, number of citations, journal affiliation, and impact factor of WOSCC literature on DES therapy CAD, 2002–2023.
A standout article, “Clinical End Points in Coronary Stent Trials: A Case for Standardized Definitions,” emerges as the most cited. Published in “Circulation” in 2007, this seminal work spotlights the imperative for standardized clinical endpoints in coronary stent trials. The article advocates the adoption of two composite endpoints—one device-oriented, encompassing cardiac death, target-vessel myocardial infarction, and target-lesion recanalization; and another patient-oriented, comprising all-cause death, any myocardial infarction, and any recanalization. This harmonization of criteria culminates in consensus, thereby facilitating a robust framework for assessing stent safety and efficacy (39). Nevertheless, the article's AS ranks lower among the top ten articles, likely due to its early online publication when social media wasn't as prevalent.
The utilization of CiteSpace visualization software for overlay analysis of journal bi-graphs, illustrating citation interrelationships, provides a comprehensive depiction of the intricate citation landscape in the field of DES for coronary artery disease. Figure 6, generated through the “JCR Journal Map” feature and Z-Score function, portrays distinct paths represented by green and orange hues. The left side predominantly encompasses domains like “Medicine,” “Medical,” “Clinical,” “Physics,” “Materials,” and “Chemistry,” while the right side clusters around “molecular biology,” “genetics,” “health,” “nursing,” and “medicine.” Notably, the green links signify significant citation pathways, with an especially robust pathway connecting “Medicine,” “Medical,” and “Clinical” journals to “Health,” “Nursing,” and “Medicine” journals. This pathway, marked by a Z-value of 8.092809 and an F-value of 12,854, reveals a dense and influential citation network, indicating a dynamic synergy and robust information exchange between disciplines and contributing synergistically to the field's scholarly progression.
The outcomes of the comprehensive visualization and analysis of authors within this domain, executed via VOSviewer, are elegantly portrayed in Figure 7. The culmination of this analysis is encapsulated in Table 4, enumerating the top ten authors based on both publication frequency and citation counts. Merging the visual representation with the tabulated data, a clear constellation of leading authors emerges, spotlighting their contributions.The roster of esteemed authors at the helm of publication frequency encompasses luminaries such as Patrick W. Serruys, Gregg W. Stone, Seung-Jung Park, and more.
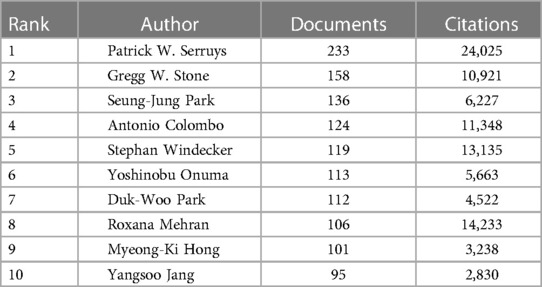
Table 4. Top 10 authors and their number of publications and citations in the field of CAD for DES therapy at WOSCC, 2002–2023.
Patrick W. Serruys stands preeminent, boasting a staggering 233 articles to his name, concurrently securing the apex position in terms of citation frequency. Currently holding positions as Professor of Interventional Medicine and Innovation at the National University of Ireland and Cardiology Honorary Professor at the National Heart and Lung Institute, Imperial College London. In addition, he is an honorary professor at the University of Erasmus and most of his works are related to the University of Erasmus. His noteworthy works, such as “A Comparison of Balloon-Expandable-Stent Implantation with Balloon Angioplasty in Patients with Coronary Artery Disease,” “Clinical End Points in Coronary Stent Trials: A Case for Standardized Definitions,” and “Late Thrombosis in Drug-Eluting Coronary Stents after Discontinuation of Antiplatelet Therapy,” showcase his substantial contributions to the field (39, 49, 50).
Patrick W. Serruys' legacy further extends to the pioneering development of DES and an array of interventional procedures and devices, including BVS and transcatheter aortic valve replacement. His profound impact encompasses innovative contributions to vascular imaging techniques like intracoronary near-infrared spectroscopy (NIRS) and optical coherence tomography, propelling the field forward (51–54). His participation in seminal clinical trials like BENESTENT, SYNTAX, and GLOBAL LEADERS has furnished pivotal evidence shaping the evaluation of stent effects and antiplatelet therapy outcomes (55–60).
The significance of keyword analysis is vividly depicted in Figures 8A,B illustrating the dynamic interplay and interdisciplinary growth of the field beyond medicine. Utilizing Pajek, Figure 8B visually organizes keywords into clusters, offering a consolidated perspective. The top 10 keywords, including “drug-eluting stents,” “coronary artery disease,” and “percutaneous coronary intervention,” are highlighted in Table 5, emphasizing a focus on understanding coronary restenosis and in-stent thrombosis post PCI. The keyword density chart in Figure 8C reveals that research predominantly centers on the triad of “drug-eluting stents,” “coronary artery disease,” and “clinical outcome,” indicating a concentration on evaluating DES clinical outcomes for CAD treatment and long-term implantation prognosis. Clinical and randomized trials emerge as pivotal methodologies, emphasizing the paramount importance of investigating coronary restenosis and stent thrombosis within this dynamic domain.
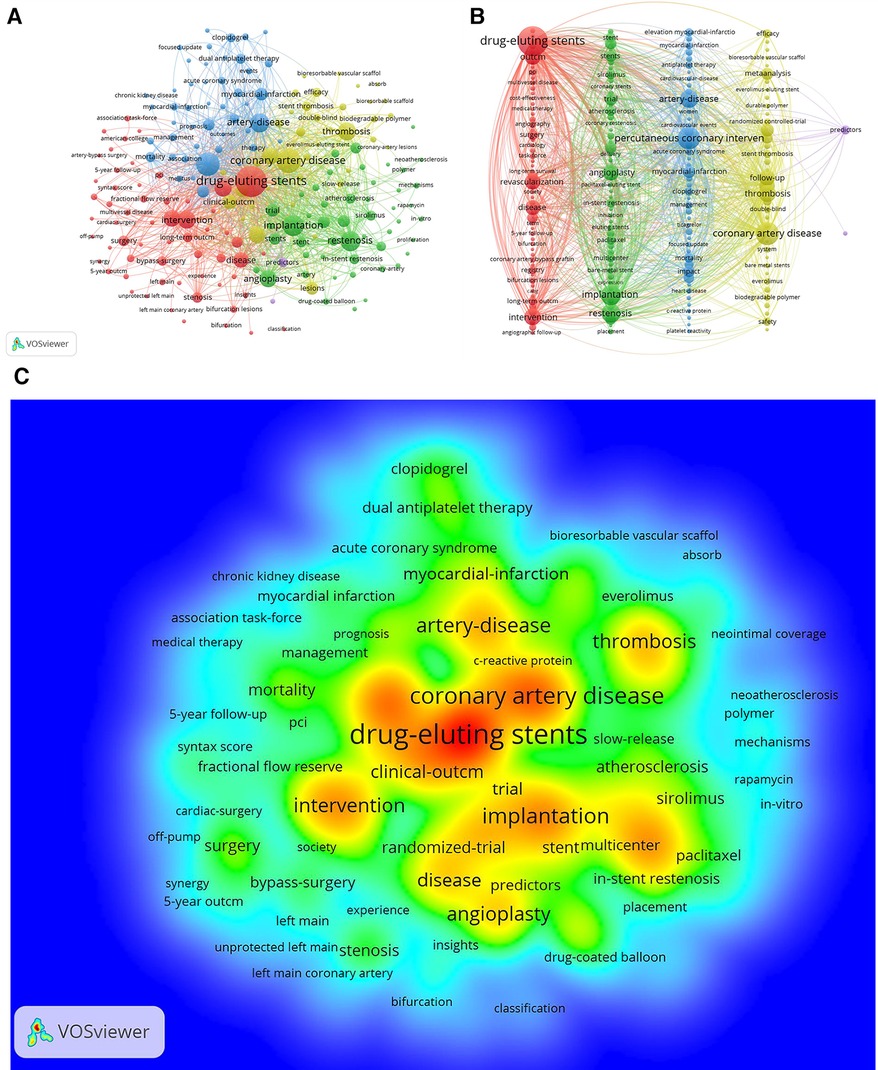
Figure 8. A,B keyword network diagram of the WOSCC literature on DES treatment of CAD, 2002–2023, figure a is derived directly from the VOSviewer 1.6.19 and figure b is processed by the Pajek 5.17. (C) Keyword density map of WOSCC literature research on DES for CAD, 2002–2023.
Keyword emergence analysis is a bibliometric-driven methodology designed to unearth keywords that have experienced a noteworthy surge in frequency within a specific timeframe within a given research domain. Leveraging the Citespace system, keyword emergence analysis gauges the intensity and duration of keyword emergence, utilizing diverse metrics. This analysis is then translated into a comprehensive keyword emergence map and corresponding tabulated results, culminating in the identification of the 25 most prominent emergent keywords, as portrayed in Figures 9, 10. This visual and tabular synthesis offers researchers a more intuitive grasp of the trajectory of a particular subject's development and the trajectory of its evolution.
This scrutinized information acts as a compass, deftly directing scholars toward emerging research trajectories and unfolding themes. Delving into Figure 10, it becomes evident that the earliest and most robust keyword is “balloon angioplasty,” while the keywords that continue to surge into 2023 encompass “5-year outcome,” “Everolimus-eluting stents(EES),” “duration,” “dual antiplatelet therapy,” “focus,” and others, such as “antiplatelet therapy,” “focused update,” “segment elevation,” “PCI,” and “drug-coated balloon.”
As shown in Figure 9, the keyword time zone graph can gather the nodes (keywords) in the same time zone at a similar time, which can show how the related research hotspots evolve gradually over time, and this form can clearly show the development of the related research.
The analysis of the annual publication trend within the realm of DES for CAD reveals a upswing in recent years. However, it will take time to determine whether the previous number of postings can be fully restored. Delving into the contribution by countries, it becomes evident that the United States leads the pack in terms of publication volume. When considering institutions, Columbia University and CRF emerges as the frontrunner with the highest count of articles and citations. Notably, the most cited work stems from a paper titled “Clinical end points in coronary stent trials: a case for standardized definitions,” underscoring its influential impact.
Intriguingly, a scrutiny of the dual-map overlay analysis of journals brings forth a robust interaction between those situated in the domains of “Medicine,” “Medical,” and “Clinical,” harmoniously collaborating with counterparts in the spheres of “Health,” “Nursing,” and “Medicine.” This synergy collectively propels the progression of DES for CAD treatment. The examination of prolific authors paints a clear portrait, with Patrick W. Serruys emerging as the predominant figure both in the quantity of publications and the magnitude of citations within the top author cohort.
Examining Figure 10, it is evident that the earliest keyword to emerge was “balloon angioplasty” (angioplasty), marked by the most intense outbreak, which persisted for a span of seven years from 2002 to 2008. In its initial stages, plain old balloon angioplasty (POBA) utilizing balloon catheters demonstrated short-term success. However, long-term prognoses fell short of expectations due to a significant incidence of restenosis. This predicament catalyzed the development of novel techniques and mechanical aids, colloquially referred to as stents, designed to sustain vessel patency post POBA (16). The first-generation solution came in the form of BMS, which served as pioneering interventional therapy stents. BMS managed to mitigate the incidence of restenosis, yet a persistent 20% of cases exhibited restenosis after six months, necessitating recurrent procedures. To address these limitations, DES were conceptualized, leveraging localized drug release to suppress neointimal hyperplasia (16, 61, 62). The advent of DES led to a substantial reduction in restenosis frequency, surpassing the safety and efficacy of radiation therapy or systemic drug administration.
The earliest DES iterations were constructed using stainless steel, featuring a metal substrate, a polymer for controlled antiproliferative drug release, and the antiproliferative drug itself. Subsequent generations ushered in enhancements: thinner struts and refined drug coatings in the second generation, transitioning from sirolimus to everolimus. The third generation introduced ultrathin struts and advanced biodegradable polymer coating technology (63–65). Each progressive DES generation has witnessed an unceasing evolution of technology and design, all with the collective aspiration of achieving heightened long-term safety and efficacy (66).
The keywords that have shown sustained prominence in 2023 include: “5 year outcome,” “Everolimus-eluting stents,” “duration,” “dual antiplatelet therapy,” “focused update,” and more. To enhance analysis, we have categorized similar keywords into the following themes.
DES have played a significant role in the management of ST segment elevation myocardial infarction (STEMI), a severe cardiovascular condition often caused by the abrupt blockage of coronary arteries, leading to inadequate blood supply to the heart muscle and subsequent ischemic necrosis. PCI has emerged as the preferred treatment approach for STEMI, offering rapid vessel dilation, restoration of blood flow, reduction of myocardial injury, and potential reduction in mortality and recurrent ischemia rates (41, 67, 68). This technique replaced the conventional pharmacologic therapy and introduced stent-based interventions to the treatment landscape (69–72). The past two decades have witnessed continuous evolution within the realm of DES. The transition from paclitaxel to rapamycin derivatives (such as zotarolimus and everolimus) exemplifies this progression. Furthermore, there have been iterative updates in stent platforms, transitioning from stainless steel to cobalt-chromium (CoCr) and platinum-chromium (PtCr) alloys. Concurrently, novel technologies, ranging from angiography to endovascular imaging, have emerged (63, 73–77). Drug-eluting stent coating technologies have also evolved from permanent polymer coatings to bioabsorbable coatings to polymer-free coatings (63, 78, 79). These advancements have been complemented by insights from pertinent clinical investigations.
The transition from POBA to the use of BMS marked a significant advancement in the field. The introduction of the first-generation DES further revolutionized the approach to treatment. While the initial DES proved effective in reducing adverse events and short-term repeat revascularization compared to BMS, late adverse events such as in-stent thrombosis, reinfarction, and target vessel revascularization (TVR) persisted (80–83).
However, the evolution to second- and third-generation DES has yielded improved outcomes. These advanced DES technologies have led to reductions in the occurrence of adverse events such as TVR and ISR, ultimately enhancing the overall prognosis of patients with STEMI (84–88). The continuous refinement and development of DES have contributed to enhancing patient outcomes and have solidified their pivotal role in the management of ST segment elevation myocardial infarction (89). The assessment of prognostic outcomes between percutaneous coronary intervention with drug-eluting stents (PCI-DES) and coronary artery bypass graft surgery, traditional options for CAD management, reveals comparable long-term results, with no significant differences observed over at least a five-year period (90–92). This finding underscores the importance of offering patients treatment options that yield similar long-term outcomes, allowing for more personalized decision-making in the management of CAD (93).
The evolving landscape of stent technology and PCI procedures holds promise for advancing individualized treatment strategies in coronary artery disease, with tailored surgical protocols and stent selection aiming to optimize outcomes and shape the future of CAD treatment paradigms.
Dual antiplatelet therapy (DAPT) plays a crucial role in reducing the risk of thrombosis following DES placement in patients undergoing PCI. This therapeutic approach involves the simultaneous use of two antiplatelet agents, typically aspirin and a P2Y12 receptor blocker like clopidogrel or ticagrelor (94). The duration of this antiplatelet therapy, referred to as “Duration,” is a critical factor that requires careful consideration.
In the context of PCI, DAPT is indispensable for preventing atherothrombotic complications, ensuring the long-term success of the procedure (94–97). However, determining the optimal duration of DAPT for patients with PCI-DES remains a complex challenge (98). Insufficient duration of antiplatelet therapy can heighten the risk of thrombus reocclusion, while excessively prolonged therapy can increase the likelihood of bleeding complications. Therefore, identifying the ideal duration of DAPT is pivotal for achieving favorable outcomes in patients with DES (99–101).
To aid in decision-making regarding DAPT duration post-PCI, the PRECISE-DAPT score was introduced as a five-point scoring system designed to assess the risk of bleeding associated with dual antiplatelet regimens (102). This scoring model assists clinicians in determining the appropriate duration of DAPT following PCI. For patients with acute coronary syndromes who have undergone PCI-DES, a 12-month DAPT regimen is commonly recommended as the standard of care. In contrast, for individuals with chronic coronary artery disease, a treatment approach involving 6 months of DAPT followed by a transition to single antiplatelet therapy (SAPT), typically aspirin, is often advised (97, 103, 104). This tailored approach takes into account the specific clinical characteristics of each patient population, ensuring optimal balance between efficacy and safety in antiplatelet therapy management.
Tailoring antiplatelet therapy adjustment strategies for patients based on their individual risks and clinical characteristics is a critical aspect of managing patients with DES. Various factors such as clinical features, risk factors, type of surgery, and platelet function tests can guide treatment choices and adjustments to strike a balance between the risk of thrombosis or ischemia and the risk of bleeding (105, 106). The two primary strategies for antiplatelet therapy adjustment are “step-down,” aimed at mitigating bleeding risks by transitioning to a less potent agent, and “step-up,” designed to address thrombotic concerns by intensifying therapy through switching to a more potent agent, increasing the dose, or adding a second antiplatelet agent (75, 105, 107–111).
Clinicians face the crucial task of balancing potential benefits and risks in selecting therapeutic strategies for DES treatment, with ongoing research needed to refine late treatment approaches, including the optimal timing and transition between dual and single antiplatelet therapy, to enhance patient outcomes and safety.
The emphasis on the keyword “5 year outcome” indicates that a significant focus of research in the field of DES for CAD is centered around assessing the long-term efficacy and prognosis of these stents. The evaluation of sustained benefits and prevention of late adverse events over a period of at least 5 years is a critical aspect of improving coronary stenting, particularly for first-generation DES implantations (112).
The surge in interest in “Everolimus-eluting stents” highlights a focus on this second-generation drug-eluting stent, utilizing everolimus, categorized as an immunosuppressive drug and belonging to the class of mTOR (mammalian target protein of rapamycin) inhibitors, to counteract vascular endothelial cell proliferation and reduce the risk of ISR (113–115). Everolimus-eluting stents offer diverse designs and materials, facilitating comparisons with other stent types in long-term prognosis studies (85, 116–118). The popularity and versatility of EES in research and clinical practice contribute to their prominence and comprehensive understanding in the field of DES for CAD treatment.
The burst of keywords related to long-term outcomes of DES underscores the significance of understanding the lasting effects of therapeutic interventions. This burst reflects the medical community's recognition of the importance of assessing not only short-term benefits but also the durability of treatment effects over an extended period.
The predicted future research directions and hotspots in the field of DES for CAD are indeed aligned with the evolving needs and advancements in medical research and practice. Ongoing technological innovations introduced into clinical practice hold the promise of enhancing the performance and therapeutic effectiveness of coronary stents, while also providing new insights into the long-term outcomes of coronary interventions.
Optimal update of stent medications: With the integration of new technologies into stent platform development, including nanotechnology and 3D printing, significant advancements are on the horizon. Nanotechnology, for instance, offers the potential to enhance stent coatings and drug delivery systems, ultimately improving stent surface biocompatibility and drug release control. This advancement aims to reduce toxicity and enhance treatment efficacy, with the ultimate goal of decreasing the occurrence of ISR and late-stage vascular risk events (119, 120). On the other hand, 3D printing technology provides the opportunity for individualized treatment options, crafting stents that match the patient's specific vascular characteristics (121).
Optimal update of stent medications: Stent implantation often causes damage to the vascular endothelium. When the process of vascular reendothelialization is not smooth, it can lead to in-stent thrombus formation and restenosis (122, 123). The primary objective of optimizing stent medications is to prevent vascular events by safeguarding endothelial cells and mitigating the adverse impacts on vascular smooth muscle cells and inflammatory cells. This can be achieved through two general approaches: renewing drug combinations and developing novel targeted drugs. A combination of antiproliferative, anti-inflammatory, antithrombotic, and immunosuppressive agents can lead to improved therapeutic outcomes (124). As an illustration, a combination of sirolimus and an antioxidant, which is currently under development, has shown promising results in promoting rapid vascular endothelial cell recovery in an animal model (125). When it comes to the development of novel targeted drugs, this is driven by the fact that current mTOR drugs, like sirolimus and everolimus, lack differential effects on smooth muscle cells and endothelial cells (126, 127). Conversely, microRNA-based cell selection therapy specifically targets vascular smooth muscle cells and inflammatory cells, while safeguarding endothelial cells, leading to a decreased risk of restenosis (127). The development of such targeted drugs represents a future trend.
Additionally, there's a vision of a potential future where smart stents can monitor restenosis and adapt treatment. For instance, these smart stents could measure blood flow using a small ultrasound transducer and adjust the rate of drug release based on the patient's needs to prevent restenosis (128).
In summary, these forthcoming innovations hold the promise of enhancing the safety and effectiveness of coronary interventions.
Publication volume and citation impact among academic institution. The interdisciplinary nature of DES research is evident through its interaction with journals spanning “Medicine”, “Medical”, and “Clinical” domains, reflecting the collaborative effort to advance healthcare outcomes.
The most cited publication, “Clinical end points in coronary stent trials: a case for standardized definitions,” underscores the importance of standardized criteria in advancing research and clinical practice. Patrick W. Serruys emerges as a prominent author in this field, contributing significantly to its advancement.
Keywords analysis highlights the core themes that underpin the DES research landscape, focusing on critical areas such as DES themselves, coronary artery disease, percutaneous coronary intervention, stent implantation, and restenosis. Keyword burst analysis emphasizes the substantial progress achieved in ST-segment elevation myocardial infarction and long-term outcomes. However, the iterative evolution of stent technology necessitates continued trial data and follow-up to comprehensively assess short- and long-term effectiveness. Looking ahead, the ongoing optimization and updates of stent platforms and medications may usher in new changes, and the introduction of novel stent types promises safer and more effective treatments for patients.
ZY: Software, Writing – original draft, Writing – review & editing. JX: Software, Visualization, Writing – original draft. YD: Data curation, Writing – original draft. XL: Investigation, Writing – original draft. WC: Formal Analysis, Writing – review & editing. YT: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Science and Technology Program of Jiangxi Provincial Health Commission: General Science and Technology Program (202210056); Science and Technology Program of Jiangxi Provincial Health Commission: General Science and Technology Program (202211009); Jiangxi Provincial Tumor Hospital “Outstanding Young Talent Program” (2021EYS05).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. (2015) 372:1333–41. doi: 10.1056/NEJMoa1406656
2. Okrainec K, Banerjee DK, Eisenberg MJ. Coronary artery disease in the developing world. Am Heart J. (2004) 148:7–15. doi: 10.1016/j.ahj.2003.11.027
3. McCullough PA. Coronary artery disease. Clin J Am Soc Nephrol. (2007) 2:611–6. doi: 10.2215/CJN.03871106
4. Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. (2005) 111:3481–8. doi: 10.1161/CIRCULATIONAHA.105.537878
5. Mendis S, Puska P, Norrving B. World heart federation, world stroke organization. Global Atlas on Cardiovascular Disease Prevention and Control. Geneva: World Health Organization in collaboration with the World Heart Federation and the World Stroke Organization (2011).
6. Tiong AY, Brieger D. Inflammation and coronary artery disease. Am Heart J. (2005) 150:11–8. doi: 10.1016/j.ahj.2004.12.019
7. Santos-Gallego CG, Picatoste B, Badimón JJ. Pathophysiology of acute coronary syndrome. Curr Atheroscler Rep. (2014) 16:1–9. doi: 10.1007/s11883-014-0401-9
8. Malakar AK, Choudhury D, Halder B, Paul P, Uddin A, Chakraborty S. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. (2019) 234:16812–23. doi: 10.1002/jcp.28350
9. Sayols-Baixeras S, Lluís-Ganella C, Lucas G, Elosua R. Pathogenesis of coronary artery disease: focus on genetic risk factors and identification of genetic variants. Appl Clin Genet. (2014) 7:15–32. doi: 10.2147/TACG.S35301
10. Lett HS, Blumenthal JA, Babyak MA, Sherwood A, Strauman T, Robins C, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. (2004) 66:305–15.15184688
11. Gensini G, Comeglio M, Colella A. Classical risk factors and emerging elements in the risk profile for coronary artery disease. Eur Heart J. (1998) 19:A53–61. PMID: 9519344.9519344
12. Schmidt T, Abbott JD. Coronary stents: history, design, and construction. J Clin Med. (2018) 7:126. doi: 10.3390/jcm7060126
13. Bhatt DL. Percutaneous coronary intervention in 2018. Jama. (2018) 319:2127–8. doi: 10.1001/jama.2018.5281
14. Khan SQ, Ludman PF. Percutaneous coronary intervention. Medicine (Baltimore). (2022) 50:437–44. doi: 10.1016/j.mpmed.2022.04.008
15. Hong S-J, Hong M-K. Drug-eluting stents for the treatment of coronary artery disease: a review of recent advances. Expert Opin Drug Delivery. (2022) 19:269–80. doi: 10.1080/17425247.2022.2044784
16. Doostzadeh J, Clark LN, Bezenek S, Pierson W, Sood PR, Sudhir K. Recent progress in percutaneous coronary intervention: evolution of the drug-eluting stents, focus on the XIENCE V drug-eluting stent. Coron Artery Dis. (2010) 21:46–56. doi: 10.1097/MCA.0b013e328333f550
17. Polimeni A, Sorrentino S, Spaccarotella C, Mongiardo A, Sabatino J, De Rosa S, et al. Stent thrombosis after percutaneous coronary intervention: from bare-metal to the last generation of drug-eluting stents. Cardiol Clin. (2020) 38:639–47. doi: 10.1016/j.ccl.2020.07.008
18. Katz G, Harchandani B, Shah B. Drug-eluting stents: the past, present, and future. Curr Atheroscler Rep. (2015) 17:1–11. doi: 10.1007/s11883-014-0485-2
19. Donthu N, Kumar S, Mukherjee D, Pandey N, Lim WM. How to conduct a bibliometric analysis: an overview and guidelines. J Bus Res. (2021) 133:285–96. doi: 10.1016/j.jbusres.2021.04.070
20. Boyack KW, Klavans R. Co-citation analysis, bibliographic coupling, and direct citation: which citation approach represents the research front most accurately? J Am Soc Inf Sci Technol. (2010) 61:2389–404. doi: 10.1002/asi.21419
21. Chen C. Citespace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol. (2006) 57:359–77. doi: 10.1002/asi.20317
23. Chen C. Citespace: A Practical Guide for Mapping Scientific Literature. Hauppauge, NY, USA: Nova Science Publishers (2016).
24. Van Eck NJ, Waltman L. Text mining and visualization using VOSviewer. arXiv [preprint]. arXiv:1109.2058 (2011). Available online at: https://arxiv.org/abs/1109.2058
25. Van Eck N, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
26. Van Eck NJ, Waltman L. Citation-based clustering of publications using CitNetExplorer and VOSviewer. Scientometrics. (2017) 111:1053–70. doi: 10.1007/s11192-017-2300-7
27. Arruda H, Silva ER, Lessa M, Proença Jr D, Bartholo R. VOSviewer and bibliometrix. J Med Library Assoc. (2022) 110:392. doi: 10.5195/jmla.2022.1434
28. Mrvar A, Batagelj V. Analysis and visualization of large networks with program package pajek. Complex Adapt Syst Model. (2016) 4:1–8. doi: 10.1186/s40294-016-0017-8
29. Bangalore S, Toklu B, Bhatt DL. Outcomes with bioabsorbable vascular scaffolds versus everolimus eluting stents: insights from randomized trials. Int J Cardiol. (2016) 212:214–22. doi: 10.1016/j.ijcard.2016.03.070
30. Stone GW, Gao R, Kimura T, Kereiakes DJ, Ellis SG, Onuma Y, et al. 1-year outcomes with the absorb bioresorbable scaffold in patients with coronary artery disease: a patient-level, pooled meta-analysis. Lancet. (2016) 387:1277–89. doi: 10.1016/S0140-6736(15)01039-9
31. Ali ZA, Gao R, Kimura T, Onuma Y, Kereiakes DJ, Ellis SG, et al. Three-year outcomes with the absorb bioresorbable scaffold: individual-patient-data meta-analysis from the ABSORB randomized trials. Circulation. (2018) 137:464–79. doi: 10.1161/CIRCULATIONAHA.117.031843
32. Ya SJ, Ting Y, Zhiyong L. When State Becomes the Only Buyer: Effects of National Volume-Based Procurement of Cardiac Stents in China (2023).
33. Hashimoto S, Motozawa Y, Mano T. Mechanisms that affect reimbursement prices for medical devices in Japan, the world’s third largest medical device market: a scoping review. Int J Healthc Manag. (2023):1–8. doi: 10.1080/20479700.2023.2250612
34. Wagener M, Boeddinghaus J, Gaemperli O, Räber L, Nietlispach F, Meier P, et al. Trends in coronary and structural heart interventions in Switzerland over the last 16 years and impact of COVID-19: insights from the national Swiss PCI survey. J Clin Med. (2022) 11:7459. doi: 10.3390/jcm11247459
35. Tabata N, Sinning J-M, Kaikita K, Tsujita K, Nickenig G, Werner N. Current status and future perspective of structural heart disease intervention. J Cardiol. (2019) 74:1–12. doi: 10.1016/j.jjcc.2019.02.022
36. Trueger NS, Thoma B, Hsu CH, Sullivan D, Peters L, Lin M. The altmetric score: a new measure for article-level dissemination and impact. Ann Emerg Med. (2015) 66:549–53. doi: 10.1016/j.annemergmed.2015.04.022
37. Araujo AC, Vanin AA, Nascimento DP, Gonzalez GZ, Costa LOP. What are the variables associated with altmetric scores? Syst Rev. (2021) 10:1–9. doi: 10.1186/s13643-021-01735-0
38. Sener H, Polat OA. Altmetric analysis of the most-cited 100 articles on the retina published between 2010 and 2020. Retina. (2022) 42:283–9. doi: 10.1097/IAE.0000000000003318
39. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es G-A, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. (2007) 115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313
40. Windecker S, Kolh P, Alfonso F, Collet J, Cremer J, Falk V, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European society of cardiology (ESC) and the European association for cardio-thoracic surgery (EACTS) developed with the special contribution of the European association of percutaneous cardiovascular interventions (EAPCI). Eur Heart J. (2014) 35:2541–619. doi: 10.1093/eurheartj/ehu278
41. Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. (2019) 40:87–165. doi: 10.1093/eurheartj/ehy394
42. Serruys PW, Morice M-C, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. (2009) 360:961–72. doi: 10.1056/NEJMoa0804626
43. Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, vant Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. (2009) 360:213–24. doi: 10.1056/NEJMoa0807611
44. Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. Jama. (2005) 293:2126–30. doi: 10.1001/jama.293.17.2126
45. Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. (2004) 350:221–31. doi: 10.1056/NEJMoa032441
46. Glenn ERB, Levine N, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American college of cardiology foundation/American heart association task force on practice guidelines and the society for cardiovascular angiography and interventions. J Am Coll Cardiol. (2011) 58(24):e44–e122. doi: 10.1016/j.jacc.2011.08.007
47. Authors/Task Force Members; Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force on diabetes, pre-diabetes, and cardiovascular diseases of the European society of cardiology (ESC) and developed in collaboration with the European association for the study of diabetes (EASD). Eur Heart J. (2013) 34:3035–87. doi: 10.1093/eurheartj/eht108
48. Glenn ERB, Levine N, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. (2016) 68(10):1082–115. doi: 10.1016/j.jacc.2016.03.513
49. Serruys PW, De Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. N Engl J Med. (1994) 331:489–95. doi: 10.1056/NEJM199408253310801
50. McFadden EP, Stabile E, Regar E, Cheneau E, Ong AT, Kinnaird T, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. (2004) 364:1519–21. doi: 10.1016/S0140-6736(04)17275-9
51. Serruys P. Featuring: Dr Patrick Serruys. Eur Cardiol Rev. (2018) 13:80. doi: 10.15420/ecr.13:2:CM1
52. Kappetein AP, Head SJ, Généreux P, Piazza N, Van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research consortium-2 consensus document. J Am Coll Cardiol. (2012) 60:1438–54. doi: 10.1016/j.jacc.2012.09.001
53. Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the valve academic research consortium. J Am Coll Cardiol. (2011) 57:253–69. doi: 10.1016/j.jacc.2010.12.005
54. Serruys PW, Kutryk MJ, Ong AT. Coronary-artery stents. N Engl J Med. (2006) 354:483–95. doi: 10.1056/NEJMra051091
55. Head SJ, Farooq V, Serruys PW, Kappetein AP. The SYNTAX score and its clinical implications. Heart. (2014) 100:169–77. doi: 10.1136/heartjnl-2012-302482
56. G. Leaders, a clinical study comparing two forms of anti-platelet therapy after stent Implantation. ClinicalTrials. gov (2015). Available online at: https://clinicaltrials.gov/ct2/show/NCT01813435 (accessed March 10, 2017).
57. Macaya C, Serruys PW, Ruygrok P, Suryapranata H, Mast G, Klugmann S, et al. Continued benefit of coronary stenting versus balloon angioplasty: one-year clinical follow-up of benestent trial. J Am Coll Cardiol. (1996) 27:255–61. doi: 10.1016/0735-1097(95)00473-4
58. Serruys PW, van Hout B, Bonnier H, Legrand V, Garcia E, Macaya C, et al. Randomised comparison of implantation of heparin-coated stents with balloon angioplasty in selected patients with coronary artery disease (benestent II). Lancet. (1998) 352:673–81. doi: 10.1016/S0140-6736(97)11128-X
59. Masuda S, Muramatsu T, Ishibashi Y, Kozuma K, Tanabe K, Nakatani S, et al. Reduced-dose prasugrel monotherapy without aspirin after PCI with the SYNERGY stent in east Asian patients presenting with chronic coronary syndromes or non-ST-elevation acute coronary syndromes: rationale and design of the ASET Japan pilot study. AsiaIntervention. (2023) 9:39. doi: 10.4244/AIJ-D-22-00033
60. Kogame N, Modolo R, Tomaniak M, Cavalcante R, de Martino F, Tinoco J, et al. Prasugrel monotherapy after PCI with the SYNERGY stent in patients with chronic stable angina or stabilised acute coronary syndromes: rationale and design of the ASET pilot study. Eurointervention. (2019) 15:e547–50. doi: 10.4244/EIJ-D-19-00131
61. Simard T, Hibbert B, Ramirez FD, Froeschl M, Chen Y-X, O'Brien ER. The evolution of coronary stents: a brief review. Can J Cardiol. (2014) 30:35–45. doi: 10.1016/j.cjca.2013.09.012
62. Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. (2003) 349:1315–23. doi: 10.1056/NEJMoa035071
63. Hassan S, Ali MN, Ghafoor B. Evolutionary perspective of drug eluting stents: from thick polymer to polymer free approach. J Cardiothorac Surg. (2022) 17:1–20. doi: 10.1186/s13019-022-01812-y
64. McKavanagh P, Zawadowski G, Ahmed N, Kutryk M. The evolution of coronary stents. Expert Rev Cardiovasc Ther. (2018) 16:219–28. doi: 10.1080/14779072.2018.1435274
65. Foerst J, Vorpahl M, Engelhardt M, Koehler T, Tiroch K, Wessely R. Evolution of coronary stents: from bare-metal stents to fully biodegradable, drug-eluting stents. Comb Prod Ther. (2013) 3:9–24. doi: 10.1007/s13556-013-0005-7
66. Koźlik M, Harpula J, Chuchra PJ, Nowak M, Wojakowski W, Gąsior P. Drug-eluting stents: technical and clinical progress. Biomimetics. (2023) 8:72. doi: 10.3390/biomimetics8010072
67. Members WC, Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 79:e21–e129. doi: 10.1016/S0735-1097(22)01012-9
68. de Lemos JA, Ettinger SM. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol. (2013) 61:e78–140. doi: 10.1016/j.jacc.2012.11.019
69. Bavry AA, Kumbhani DJ, Quiroz R, Ramchandani SR, Kenchaiah S, Antman EM. Invasive therapy along with glycoprotein IIb/IIIa inhibitors and intracoronary stents improves survival in non–ST-segment elevation acute coronary syndromes: a meta-analysis and review of the literature. Am J Cardiol. (2004) 93:830–5. doi: 10.1016/j.amjcard.2003.12.019
70. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. (2003) 361:13–20. doi: 10.1016/S0140-6736(03)12113-7
71. Mehta SR, Cannon CP, Fox KA, Wallentin L, Boden WE, Spacek R, et al. Routine vs selective invasive strategies in patients with acute coronary syndromes: a collaborative meta-analysis of randomized trials. JAMA. (2005) 293:2908–17. doi: 10.1001/jama.293.23.2908
72. Armstrong PW, Gershlick AH, Goldstein P, Wilcox R, Danays T, Lambert Y, et al. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med. (2013) 368:1379–87. doi: 10.1056/NEJMoa1301092
73. Niu Y, Bai N, Ma Y, Zhong P-Y, Shang Y-S, Wang Z-L. Efficacy of intravascular imaging-guided drug-eluting stent implantation: a systematic review and meta-analysis of randomized clinical trials. BMC Cardiovasc Disord. (2022) 22:327. doi: 10.1186/s12872-022-02772-w
74. Nicolas J, Pivato CA, Chiarito M, Beerkens F, Cao D, Mehran R. Evolution of drug-eluting coronary stents: a back-and-forth journey from the bench to bedside. Cardiovasc Res. (2023) 119:631–46. doi: 10.1093/cvr/cvac105
75. Kim H-S, Kang J, Hwang D, Han J-K, Yang H-M, Kang H-J, et al. Prasugrel-based de-escalation of dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (HOST-REDUCE-POLYTECH-ACS): an open-label, multicentre, non-inferiority randomised trial. Lancet. (2020) 396:1079–89. doi: 10.1016/S0140-6736(20)31791-8
76. Mihatov N, Kirtane AJ, Stoler RC, Feldman RL, Neumann F-J, Boutis LS, et al. Ischemic risk matters-risk stratification in high bleeding risk (HBR) patients implanted with the synergy stent: a post-hoc analysis of the evolve short DAPT study. J Am Coll Cardiol. (2023) 81:784. doi: 10.1016/S0735-1097(23)01228-7
77. Kirtane AJ, Stoler R, Feldman R, Neumann F-J, Boutis L, Tahirkheli N, et al. Primary results of the EVOLVE short DAPT study: evaluation of 3-month dual antiplatelet therapy in high bleeding risk patients treated with a bioabsorbable polymer-coated everolimus-eluting stent. Circ Cardiovasc Interv. (2021) 14:e010144. doi: 10.1161/CIRCINTERVENTIONS.120.010144
78. Ishaque N, Naseer N, Abbas MA, Javed F, Mushtaq S, Ahmad NM, et al. Optimize PLA/EVA polymers blend compositional coating for next generation biodegradable drug-eluting stents. Polymers (Basel). (2022) 14:3547. doi: 10.3390/polym14173547
79. Park J-K, Kim D-G, Bae IH, Lim KS, Jeong MH, Choi C, et al. Blood-compatible and biodegradable polymer-coated drug-eluting stent. Macromol Res. (2015) 23:237–44. doi: 10.1007/s13233-015-3023-3
80. Morice M-C, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. (2002) 346:1773–80. doi: 10.1056/NEJMoa012843
81. Mauri L, Silbaugh TS, Garg P, Wolf RE, Zelevinsky K, Lovett A, et al. Drug-eluting or bare-metal stents for acute myocardial infarction. N Engl J Med. (2008) 359:1330–42. doi: 10.1056/NEJMoa0801485
82. Mauri L, Silbaugh T, Garg P. Drug-eluting or bare-metal stents for acute myocardial infarction. J Vasc Surg. (2009) 49:1357. doi: 10.1016/j.jvs.2009.03.018
83. Kalesan B, Pilgrim T, Heinimann K, Räber L, Stefanini GG, Valgimigli M, et al. Comparison of drug-eluting stents with bare metal stents in patients with ST-segment elevation myocardial infarction. Eur Heart J. (2012) 33:977–87. doi: 10.1093/eurheartj/ehs036
84. Bangalore S, Kumar S, Fusaro M, Amoroso N, Attubato MJ, Feit F, et al. Short-and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation. (2012) 125:2873–91. doi: 10.1161/CIRCULATIONAHA.112.097014
85. Gada H, Kirtane AJ, Newman W, Sanz M, Hermiller JB, Mahaffey KW, et al. 5-year results of a randomized comparison of XIENCE V everolimus-eluting and TAXUS paclitaxel-eluting stents: final results from the SPIRIT III trial (clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC: Cardiovascular Interventions. (2013) 6:1263–6. doi: 10.1016/j.jcin.2013.07.009
86. Palmerini T, Benedetto U, Biondi-Zoccai G, Della Riva D, Bacchi-Reggiani L, Smits PC, et al. Long-term safety of drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. (2015) 65:2496–507. doi: 10.1016/j.jacc.2015.04.017
87. Navarese EP, Kowalewski M, Kandzari D, Lansky A, Górny B, Kołtowski Ł, et al. First-generation versus second-generation drug-eluting stents in current clinical practice: updated evidence from a comprehensive meta-analysis of randomised clinical trials comprising 31 379 patients. Arch Dis Child. (2014) 1:e000064. doi: 10.1136/openhrt-2014-000064
88. Park S-J, Ahn J-M, Kim Y-H, Park D-W, Yun S-C, Lee J-Y, et al. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med. (2015) 372:1204–12. doi: 10.1056/NEJMoa1415447
90. Stone GW, Sabik JF, Serruys PW, Simonton CA, Généreux P, Puskas J, et al. Everolimus-eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med. (2016) 375:2223–35. doi: 10.1056/NEJMoa1610227
91. Palmerini T, Serruys P, Kappetein AP, Genereux P, Della Riva D, Reggiani LB, et al. Clinical outcomes with percutaneous coronary revascularization vs coronary artery bypass grafting surgery in patients with unprotected left main coronary artery disease: a meta-analysis of 6 randomized trials and 4,686 patients. Am Heart J. (2017) 190:54–63. doi: 10.1016/j.ahj.2017.05.005
92. Stone GW, Kappetein AP, Sabik JF, Pocock SJ, Morice M-C, Puskas J, et al. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med. (2019) 381:1820–30. doi: 10.1056/NEJMoa1909406
93. Lee JM, Choi KH, Song YB, Lee J-Y, Lee S-J, Lee SY, et al. Intravascular imaging–guided or angiography-guided complex PCI. N Engl J Med. (2023) 388:1668–79. doi: 10.1056/NEJMoa2216607
94. Valgimigli M, Bueno H, Byrne RA, Collet J-P, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European society of cardiology (ESC) and of the European association for cardio-thoracic surgery (EACTS). Eur Heart J. (2018) 39:213–60. doi: 10.1093/eurheartj/ehx419
95. Bergmeijer TO, Janssen PW, Schipper JC, Qaderdan K, Ishak M, Ruitenbeek RS, et al. CYP2C19 genotype–guided antiplatelet therapy in ST-segment elevation myocardial infarction patients—rationale and design of the patient outcome after primary PCI (POPular) genetics study. Am Heart J. (2014) 168:16–22.e1. doi: 10.1016/j.ahj.2014.03.006
96. Rollini F, Franchi F, Angiolillo DJ. Switching P2Y12-receptor inhibitors in patients with coronary artery disease. Nat Rev Cardiol. (2016) 13:11–27. doi: 10.1038/nrcardio.2015.113
97. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. (2016) 134:e123–55. doi: 10.1161/CIR.0000000000000404
98. Wilson SJ, Newby DE, Dawson D, Irving J, Berry C. Duration of dual antiplatelet therapy in acute coronary syndrome. Heart. (2017) 103:573–80. doi: 10.1136/heartjnl-2016-309871
99. Costa F, Van Klaveren D, Feres F, James S, Räber L, Pilgrim T, et al. Dual antiplatelet therapy duration based on ischemic and bleeding risks after coronary stenting. J Am Coll Cardiol. (2019) 73:741–54. doi: 10.1016/j.jacc.2018.11.048
100. Steg PG, Huber K, Andreotti F, Arnesen H, Atar D, Badimon L, et al. Bleeding in acute coronary syndromes and percutaneous coronary interventions: position paper by the working group on thrombosis of the European society of cardiology. Eur Heart J. (2011) 32:1854–64. doi: 10.1093/eurheartj/ehr204
101. Vranckx P, Valgimigli M, Jüni P, Hamm C, Steg PG, Heg D, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. (2018) 392:940–9. doi: 10.1016/S0140-6736(18)31858-0
102. Costa F, Van Klaveren D, James S, Heg D, Räber L, Feres F, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. (2017) 389:1025–34. doi: 10.1016/S0140-6736(17)30397-5
103. Kimura K, Kimura T, Ishihara M, Nakagawa Y, Nakao K, Miyauchi K, et al. JCS 2018 guideline on diagnosis and treatment of acute coronary syndrome. Circ J. (2019) 83:1085–196. doi: 10.1253/circj.CJ-19-0133
104. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes: the task force for the diagnosis and management of chronic coronary syndromes of the European society of cardiology (ESC). Eur Heart J. (2020) 41:407–77. doi: 10.1093/eurheartj/ehz425
105. Capodanno D, Mehran R, Krucoff MW, Baber U, Bhatt DL, Capranzano P, et al. Defining strategies of modulation of antiplatelet therapy in patients with coronary artery disease: a consensus document from the academic research consortium. Circulation. (2023) 147:1933–44. doi: 10.1161/CIRCULATIONAHA.123.064473
106. Lip GY, Collet J-P, Haude M, Byrne R, Chung EH, Fauchier L, et al. 2018 Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: a joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Ep Europace. (2019) 21:192–3. doi: 10.1093/europace/euy174
107. Hwang D, Lim Y-H, Park KW, Chun KJ, Han J-K, Yang H-M, et al. Prasugrel dose de-escalation therapy after complex percutaneous coronary intervention in patients with acute coronary syndrome: a post hoc analysis from the HOST-REDUCE-POLYTECH-ACS trial. JAMA Cardiol. (2022) 7:418–26. doi: 10.1001/jamacardio.2022.0052
108. Kim CJ, Park M-W, Kim MC, Choo E-H, Hwang B-H, Lee KY, et al. Unguided de-escalation from ticagrelor to clopidogrel in stabilised patients with acute myocardial infarction undergoing percutaneous coronary intervention (TALOS-AMI): an investigator-initiated, open-label, multicentre, non-inferiority, randomised trial. Lancet. (2021) 398:1305–16. doi: 10.1016/S0140-6736(21)01445-8
109. Kim B-K, Hong S-J, Cho Y-H, Yun KH, Kim YH, Suh Y, et al. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA. (2020) 323:2407–16. doi: 10.1001/jama.2020.7580
110. Jourdi G, Marquis-Gravel G, Martin A-C, Lordkipanidze M, Godier A, Gaussem P. Antiplatelet therapy in atherothrombotic diseases: similarities and differences across guidelines. Front Pharmacol. (2022) 13:878416. doi: 10.3389/fphar.2022.878416
111. Collet J-P, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2021) 42:1289–367. doi: 10.1093/eurheartj/ehaa575
112. Kimura T, Morimoto T, Nakagawa Y, Kawai K, Miyazaki S, Muramatsu T, et al. Very late stent thrombosis and late target lesion revascularization after sirolimus-eluting stent implantation: five-year outcome of the j-cypher registry. Circulation. (2012) 125:584–91. doi: 10.1161/CIRCULATIONAHA.111.046599
113. MacKeigan JP, Krueger DA. Differentiating the mTOR inhibitors everolimus and sirolimus in the treatment of tuberous sclerosis complex. Neuro-oncology. (2015) 17:1550–9. doi: 10.1093/neuonc/nov152
114. Nashan B. The role of certican (everolimus, rad) in the many pathways of chronic rejection. Transplant Proc. (2001) 33:3215–20. doi: 10.1016/S0041-1345(01)02369-7
115. Kawakami R, Hao H, Imanaka T, Shibuya M, Ueda Y, Tsujimoto M, et al. Initial pathological responses of second-generation everolimus-eluting stents implantation in Japanese coronary arteries: comparison with first-generation sirolimus-eluting stents. J Cardiol. (2018) 71:452–7. doi: 10.1016/j.jjcc.2017.11.009
116. Kobayashi T, Sotomi Y, Suzuki S, Suwannasom P, Nakatani S, Morino Y, et al. Five-year clinical efficacy and safety of contemporary thin-strut biodegradable polymer versus durable polymer drug-eluting stents: a systematic review and meta-analysis of 9 randomized controlled trials. Cardiovasc Intervention Ther. (2020) 35:250–8. doi: 10.1007/s12928-019-00613-w
117. El-Hayek G, Bangalore S, Casso Dominguez A, Devireddy C, Jaber W, Kumar G, et al. Meta-analysis of randomized clinical trials comparing biodegradable polymer drug-eluting stent to second-generation durable polymer drug-eluting stents. JACC Cardiovasc Interv. (2017) 10:462–73. doi: 10.1016/j.jcin.2016.12.002
118. Otsuka F, Vorpahl M, Nakano M, Foerst J, Newell JB, Sakakura K, et al. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus-and paclitaxel-eluting stents in humans. Circulation. (2014) 129:211–23. doi: 10.1161/CIRCULATIONAHA.113.001790
119. Trepanier CM, Burke-Kleinman J, Strauss BH, Santerre JP, Bendeck MP. Less is more: developments in nanotechnology for antirestenosis therapies. Arterioscler, Thromb Vasc Biol. (2023) 43:1096–110. doi: 10.1161/ATVBAHA.123.318450
120. Cherian AM, Nair SV, Maniyal V, Menon D. Surface engineering at the nanoscale: a way forward to improve coronary stent efficacy. APL Bioeng. (2021) 5:1–28. doi: 10.1063/5.0037298
121. Jiang W, Zhao W, Zhou T, Wang L, Qiu T. A review on manufacturing and post-processing technology of vascular stents. Micromachines (Basel). (2022) 13:140. doi: 10.3390/mi13010140
122. Cornelissen A, Vogt FJ. The effects of stenting on coronary endothelium from a molecular biological view: time for improvement? J Cell Mol Med. (2019) 23:39–46. doi: 10.1111/jcmm.13936
123. Condello F, Spaccarotella C, Sorrentino S, Indolfi C, Stefanini GG, Polimeni A. Stent thrombosis and restenosis with contemporary drug-eluting stents: predictors and current evidence. J Clin Med. (2023) 12:1238. doi: 10.3390/jcm12031238
124. Adhami M, Martin NK, Maguire C, Courtenay AJ, Donnelly RF, Domínguez-Robles J, et al. Drug loaded implantable devices to treat cardiovascular disease. Expert Opin Drug Delivery. (2023) 20:507–22. doi: 10.1080/17425247.2023.2190580
125. Wang R, Lu J, Yin J, Chen H, Liu H, Xu F, et al. A TEMPOL and rapamycin loaded nanofiber-covered stent favors endothelialization and mitigates neointimal hyperplasia and local inflammation. Bioactive Materials. (2023) 19:666–77. doi: 10.1016/j.bioactmat.2022.04.033
126. Frismantiene A, Philippova M, Erne P, Resink TJ. Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity. Cell Signal. (2018) 52:48–64. doi: 10.1016/j.cellsig.2018.08.019
127. Canfield J, Totary-Jain H. 40 Years of percutaneous coronary intervention: history and future directions. J Pers Med. (2018) 8:33. doi: 10.3390/jpm8040033
Keywords: drug-eluting stents, coronary artery disease, bibliometric analysis, percutaneous coronary intervention, outcome, dual antiplatelet therapy
Citation: Zeng Y, Xu J, Deng Y, Li XChen W and Tang Y (2024) Drug-eluting stents for coronary artery disease in the perspective of bibliometric analysis. Front. Cardiovasc. Med. 11:1288659. doi: 10.3389/fcvm.2024.1288659
Received: 4 September 2023; Accepted: 30 January 2024;
Published: 19 February 2024.
Edited by:
Tommaso Gori, University Medical Centre, Johannes Gutenberg University Mainz, GermanyReviewed by:
Robert-Jan Van Geuns, Radboud University Medical Centre, Netherlands© 2024 Zeng, Xu, Deng, Li, Chen and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen Chen MTU2MTQ5NjE5QHFxLmNvbQ== Yu Tang eXV0YW5nZG9jdG9yQDEyNi5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.