- Department of Cardiology, The Second Affiliated Hospital of Xi'an Jiaotong University, Shaanxi, Xi'an, China
Background: An increase in deaths has been perceived during the pandemic, which cannot be explained only by COVID-19. The actual number of deaths far exceeds the recorded data on deaths directly related to SARS-CoV-2 infection. Data from early and short-lived pandemic studies show a dramatic shift in cardiovascular mortality. Grounded in the post-pandemic era, macroscopic big data on cardiovascular mortality during the pandemic need to be further reviewed and studied, which is crucial for cardiovascular disease prevention and control.
Methods: We retrieved and collected data associated with cardiovascular disease mortality from the National Vital Statistic System from the Center for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research (CDC WONDER) platform based on the ICD-10 codes. We applied regression analysis to characterize overall cardiovascular disease mortality trends from 2010 to 2023 and built a time series model to predict mortality for 2020–2023 based on mortality data from 2010 to 2019 in order to affirm the existence of the excess deaths by evaluating observed vs. predicted mortality. We also conducted subgroup analyses by sex, age and race/ethnicity for the purpose of obtaining more specific sociodemographic information.
Results: All-cause age-standardised mortality rates (ASMRs) for CVD dramatically increased between 2019 and 2021[annual percentage change (APC) 11.27%, p < 0.01], and then decreased in the following 2021–2023(APC: −7.0%, p < 0.01). Subgroup analyses found that the ASMR change was most pronounced in Alaska Indians/Native American people (APC: 16.5% in 2019–2021, −12.5% in 2021–2023, both p < 0.01), Hispanics (APC: 12.1% in 2019–2021, −12.2% in 2021–2023, both p < 0.05) and non-Hispanic Black people (APC:11.8% in 2019–2021, −10.3% in 2021–2023, both p < 0.01)whether during the increasing or declining phase. Similarly, the ASMR change was particularly dramatic for the 25–44 age group (APC:19.8% in 2019–2021, −15.4% in 2021–2023, both p < 0.01) and males (APC: 11.5% in 2019–2021, −7.6% in 2021–2023, both p < 0.01). By the end of 2023, the proportion of COVID-related excess death remained high among the elderly (22.4%), males (42.8%) and Alaska Indians/Native American people(39.7%). In addition, we did not find the presence of excess deaths in the young (25–44) and middle-aged cohort (45–64) in 2023, while excess deaths remained persistent in the elderly.
Conclusions: All-cause ASMRs for CVD increased notably during the initial two years of the COVID-19 pandemic and then witnessed a decline in 2021–2023. The cohorts (the young, males and minorities) with the steepest rise in mortality decreased at the fastest rate instead. Previous initiatives to promote cardiovascular health were effective, but further research on cardiovascular healthcare for the elderly and racial disparities should be attached to priority considering the presence of sociodemographic differences in CVD death.
Introduction
The Covid-19 pandemic placed unprecedented stress on the world's healthcare systems, and the pandemic severely disrupted medical care for U.S. patients with cardiovascular disease (CVD) and exacerbated cardiovascular-related risk factors (1). Existing short-term studies have shown dramatic changes in mortality among U.S. patients with CVD during the pandemic (2) and the pandemic with persistent threat may have long-term effects on patients with CVD, making it particularly important to review and analyze trends in cardiovascular diseases mortality with a holistic perspective in the post-pandemic era. This initiative is bound to provide effective information to health care providers and public health departments in order to mitigate the long-term effects of this pandemic.
However, limited data are available on overall changes in CVD mortality for the long-term period after the outbreak of this pandemic, including mortality data characterized by age, sex, and race (3). We therefore queried the U.S. Centers for Disease Control and Prevention (CDC) mortality dataset to characterize and predict trends in CVD mortality in order to expand from prior studies and understand the impact of the pandemic on these trends. We also explored whether this pandemic disproportionately affected CVD mortality in different age, gender and racial subgroups.
Methods
Data retrieval
This was a retrospective study using the data obtained from the National Vital Statistics System (NVSS) dataset through the Center for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research (CDC WONDER) website. The CDC WONDER dataset is publicly available and it is relatively easy to gain access and request data from it (4). Each record in the database represents the death data of one deceased person, which includes age, sex, race and ethnicity, and cause of death (5).
Inclusion and exclusion criteria
The retrieval of death data in the database was carried out according to a variety of conditional logics, and after the preliminary research, we chose the following inclusion conditions for the study subjects. Ultimately, we chose to include data on cardiovascular disease-related deaths between January 1, 2010, and December 31, 2023, among adults aged no less than 25 years. As for the retrieval of causes of death, we opted International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes to identify the cause of death by CVD(I00-I99,diseases of the circulatory system) (6). Meanwhile, the corresponding ICD-10 code for COVID-19 was recognized as U07.1 which was classified under the codes for emergency use.
Statistical analysis
The data ultimately applied directly to the statistical analysis included year and corresponding mortality. We finally chose to extract the age-standardized mortality rates(ASMRs, per 100,000 persons) for statistical analysis which were developed using the age structure (25–85 + years) from the 2000 USA Census Standard Population and the direct standardisation method (7).
First of all, we conducted joinpoint regression analysis to determine the nationwide trend of mortality in people with CVD (8). The overall trend is composed of one or several segments divided by corresponding joinpoint(s). Then we introduced an important parameter called the annual percentage change (APC) along with Monte Carlo Permutation test to determine whether the overall trend was best depicted by one or more segments (9). The positive/negative and absolute values of APCs represent the direction and extent of the trend, respectively (10).
Next, we constructed classical time series models(Autoregressive Integrated Moving Average Model, ARIMA) based on mortality data from 2010 to 2019 to determine predicted mortality rates for 2020–2023 and compared them to observed mortality rates from the dataset (11). After the above comparative analysis, we identified and assessed the excess mortality. We also further explored the COVID-19 related portion of the excess deaths described above, which refered to the portion of the excess deaths that might be explained by COVID-19.
Additionally, We divided the study subjects into several subgroups: age (25–44, 45–64, and ≥65 years), sex (male and female), race/ethnicity (Hispanics, non-Hispanic White people, non-Hispanic Black people, non-Hispanic Asian people and non-Hispanic American Indian/Alaska Native people) (12). We expect that the subgroup analysis could provide more detailed and precise information on mortality.
COVID-related deaths are defined as the portion of the death certificate in which COVID-19 is listed as the primary cause of death. All-cause mortality is defined as mortality from any cause (whether related to cardiovascular disease or not). CVD-related mortality is mortality due to complications of cardiovascular disease as the main cause of death. As for overall analysis, we calculated mortality rates for both (13). However, as for subgruop analysis, we preferred to use all-cause mortality which leads to more holistic evaluation effectiveness (13).
Statistical analysis was performed using the National Cancer Institute's joinpoint regression (Joinpoint Trend Analysis Software version 4.9.1.0; National Cancer Institute, Bethesda, MD) and IBM SPSS Statistics version 26.0. A 2-sided p value with the threshold of significance at 0.05 was used.
Results
Impact of COVID-19 pandemic on ASMRs for CVD
Overall analysis for all-cause ASMR
After joinpoint regression analysis, the overall trend was divided into three segments bounded by 2019 and 2021 with the average APC of 1.1% (95% CI 0.2–2.0) for 2010–2023 (Table 1). As for the trend segment analysis, we found accelerated APC from −0.2% for 2010–2019 to 11.3% (95% CI 7.8–14.8, P < 0.001) for 2019–2021 but the following declining trend with the APC of −7.0% (95% CI −10.1 to −3.8, P < 0.05) for 2021–2023 (Table 1). Similarily, we also found the observed ASMRs in 2020–2023 were all much higher than the predicted values demonstrating significant excess mortality, which was was more dramatic in 2021 and then showed a declining trend (Figure 1). Notably, more than half of the excess deaths in the first two years of the pandemic (62.6% in 2020, 50.5% in 2021) were associated with COVID-19, and this proportion moderated in 2022–2023 (29.1% in 2022, 21.5% in 2023) (Figure 1).
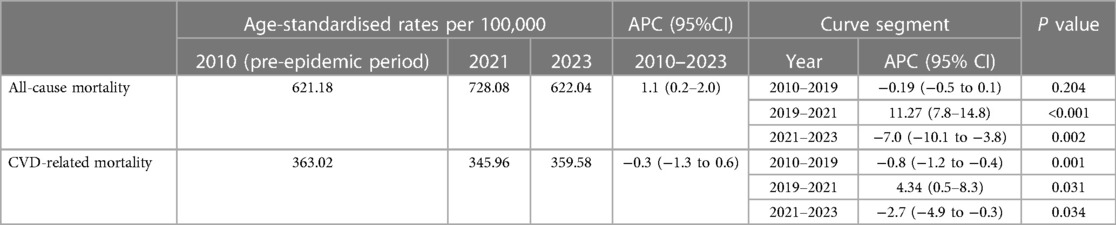
Table 1. ASMR and APC in mortality in U.S. adults with CVD, by all-cause mortality and CVD-related mortality, 2010-2023.
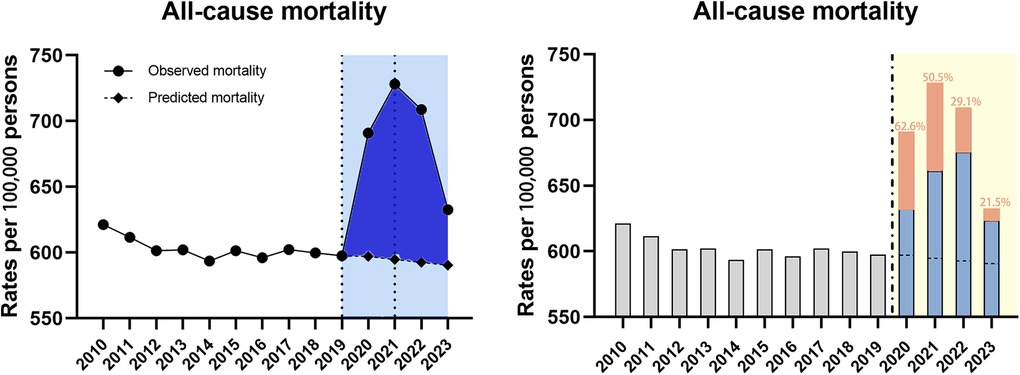
Figure 1. Temporal trends of all-cause mortality and excess deaths for CVD during the COVID-19 pandemic. Dashed horizontal line: predicted value; Blue bars: non-COVID related; Orange bars: COVID-related, with the proportion in the excess deaths.
Overall analysis for CVD-related ASMR
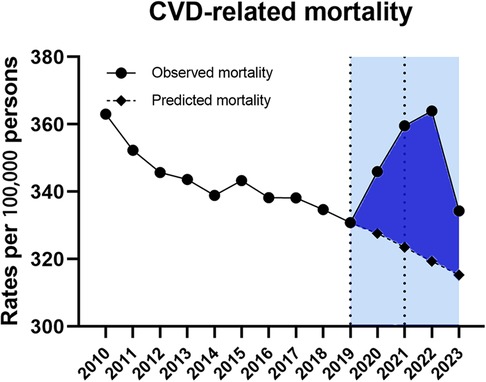
Data for CVD-related ASMRs followed the similar trend, which was also divided into three segments bounded by 2019 and 2021 (Table 1). On trend segment analysis, we found increased APC from −0.8% (95% CI −1.2–0.4) for 2010–2019 to 4.3% (95% CI 0.5–8.3) for 2019–2021 which was followed by a declining trend with the APC of −2.7% (95%CI −4.9 to −0.3, P < 0.05) for 2021–2023 (Table 1). As a result, the observed ASMRs (345.96 in 2020, 359.58 in 2021, 363.99 in 2022 and 334.31 in 2023) were much higher than the predicted values of 327.26 (95% CI 577.57–607.86) for 2020, 323.69 (95% CI 577.57–607.86) for 2021, 319.38 (95% CI 288.56–350.20) for 2022 and 315.27 (95% CI 271.54–359.01) for 2023 showing the excess death for CVD-related mortality (Figure 2).
Subgroup analysis for all-cause ASMR
By age group
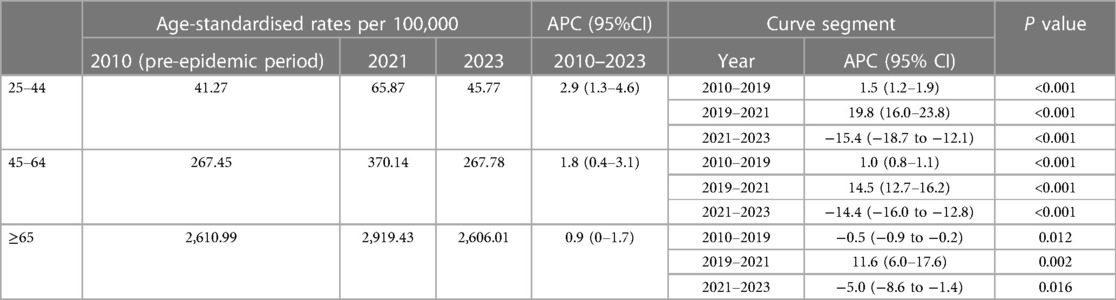
The highest ASMR was seen in the elderly group throughout the study period. All the overall trends of three subgroups were described by three segments bounded by 2019 and 2021 based on the joinpoint regression analysis (Table 2). Subgroup analysis by age found ASMR rise across the entire study period 2010–2023 among all the three groups with the average APC 2.9% for 25–44, 1.8% for 45–64 and 0.9% for ≥65 (Table 2). During the pre-epidemic period, there was a downward trend in mortality among the elderly, while the mortality in the younger and middle-aged groups steadily increased. What's more, the 2019–2021 APCs for ASMR showed significant rise in all age groups with the largest change for 25–44 (19.8%, 95% CI 16.5–23.8, P < 0.001) compared to 45–64 (14.5%, 95% CI 12.7–16.2, P < 0.001) and ≥65 (11.6%, 95% CI 6.0–17.6, P < 0.05) (Table 2). However, the increasing trend in 2019–2021 was followed by a decline in 2021–2023 in all groups with the steepest change for 25–44 (−15.4%, 95%CI −18.7 to −12.1, P < 0.001) compared to 45–64 (−14.4%, 95% CI −16.0 to −12.8, P < 0.001) and ≥65 (−5.0%, 95%CI −8.6 to −1.4, P < 0.05) (Table 2). Observed mortality rates in 2020–2022 were higher than the predicted mortality rates for all three subgroups, while we did not observe excess deaths in the young and middle-aged group in 2023(predicted mortality higher than the actual rate). Of note, the portion of excess deaths associated with COVID-19 was higher in the middle-aged and older age groups compared to the young group during the study period (Figure 3).

Figure 3. Temporal trends of all-cause mortality and excess deaths for CVD during the COVID-19 pandemic, by age. Dashed horizontal line: predicted value; Blue bars: non-COVID related; Orange bars: COVID-related, with the proportion in the excess deaths.
By sex
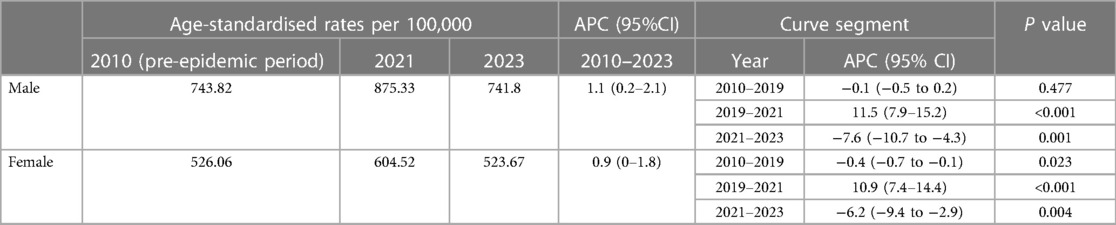
Subgroup analysis by sex found that the mortality burden was consistently higher for males than for females. Regression analysis showed that overall trend was divided into three segments bounded by 2019 and 2021 (Table 3). Significant ASMRs rise can be found during the 2019–2021 period in both subgroups (Table 3, both P < 0.001). Then the mortality rate noticed a declining trend in both groups, as evidenced by the APC of −7.6% (95% CI −10.7 to −4.3, P < 0.05) for males and −6.2% (95% CI −9.4 to −2.9, P < 0.05) for females in 2021–2023 (Table 3). As a result, the observed ASMRs were significantly higher than expected rates for both males and females showing the existence of excess deaths. Furthermore, the COVID-related component of excess deaths was larger in the male cohort during the study period (Figure 4).
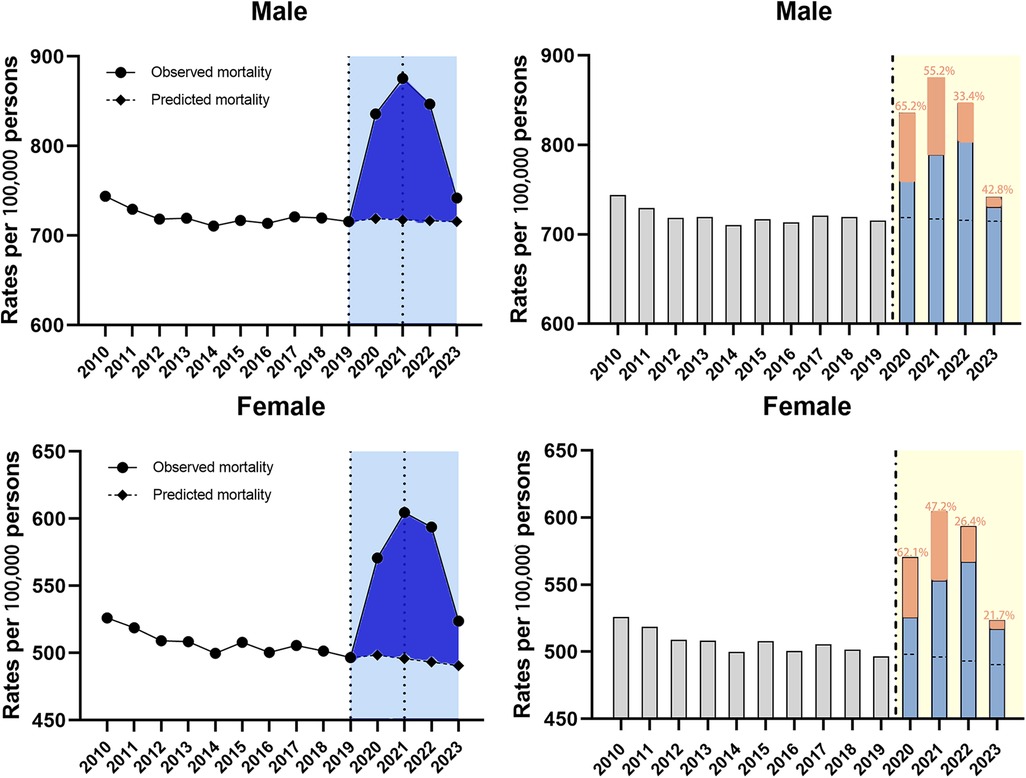
Figure 4. Temporal trends of all-cause mortality and excess deaths for CVD during the COVID-19 pandemic, by sex. Dashed horizontal line: predicted value; Blue bars: non-COVID related; Orange bars: COVID-related, with the proportion in the excess deaths.
By race/ethnicity
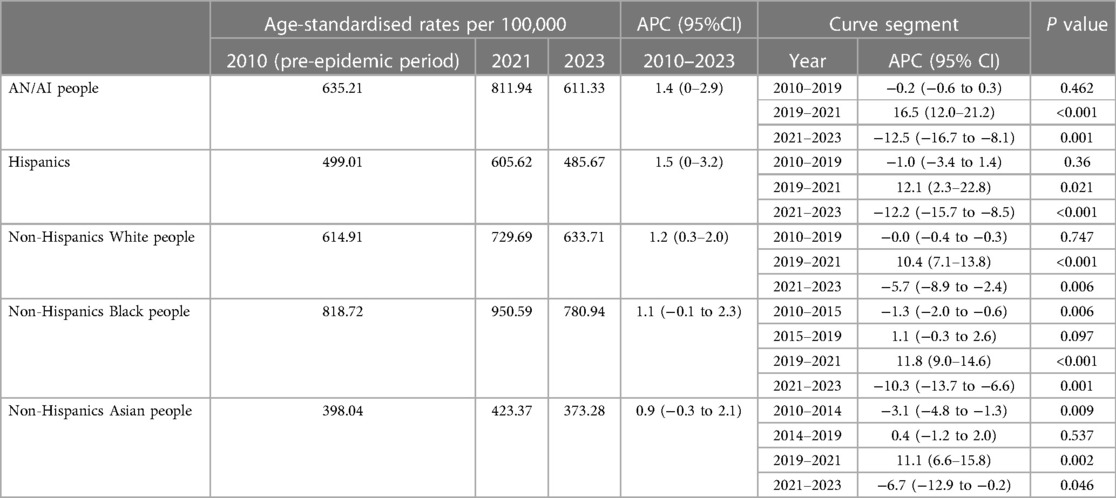
During the entire study period, mortality trends for several racial subgroups were divided into three or four segments based on the regression analysis (Table 4). Besides, the most dramatic APC rise during the 2019–2021 period was in American Indians/Alaska Native people (16.5%, 95% CI 12.0–21.2, p < 0.001) followed by non-Hispanic Black people(11.8%, 95% CI 9.0–14.6, p < 0.001), and non-Hispanic Asian people (11.1%, 95% CI 6.6–15.8, p < 0.05), with the shortest rise during the same period in non-Hispanic White people (10.4%, 95% CI 7.1–13.8, p < 0.001) (Table 4). Meanwhile, the regression analysis found the declining trends during 2021–2023 among all the subgroups, with the most significant change of APC for American Indians/Alaska Native people (−12.5%, 95% CI −16.7 to −8.1, p < 0.05) (Table 4). For all racial subgroups, the observed mortality was higher than the predicted mortality showing the existence of excess deaths (Figure 5). Moreover, the highest proportion of COVID-related component of excess deaths during 2020–2021 was found among the Hispanics (70.6% in 2020, 68.5% in 2021) and AN/AI people (68.8% in 2020, 50.1% in 2021), and this proportion remained relatively high among AN/AI people (39.7%) and non-Hispanic Black people (33.7%) by the end of 2023 (Figure 5).
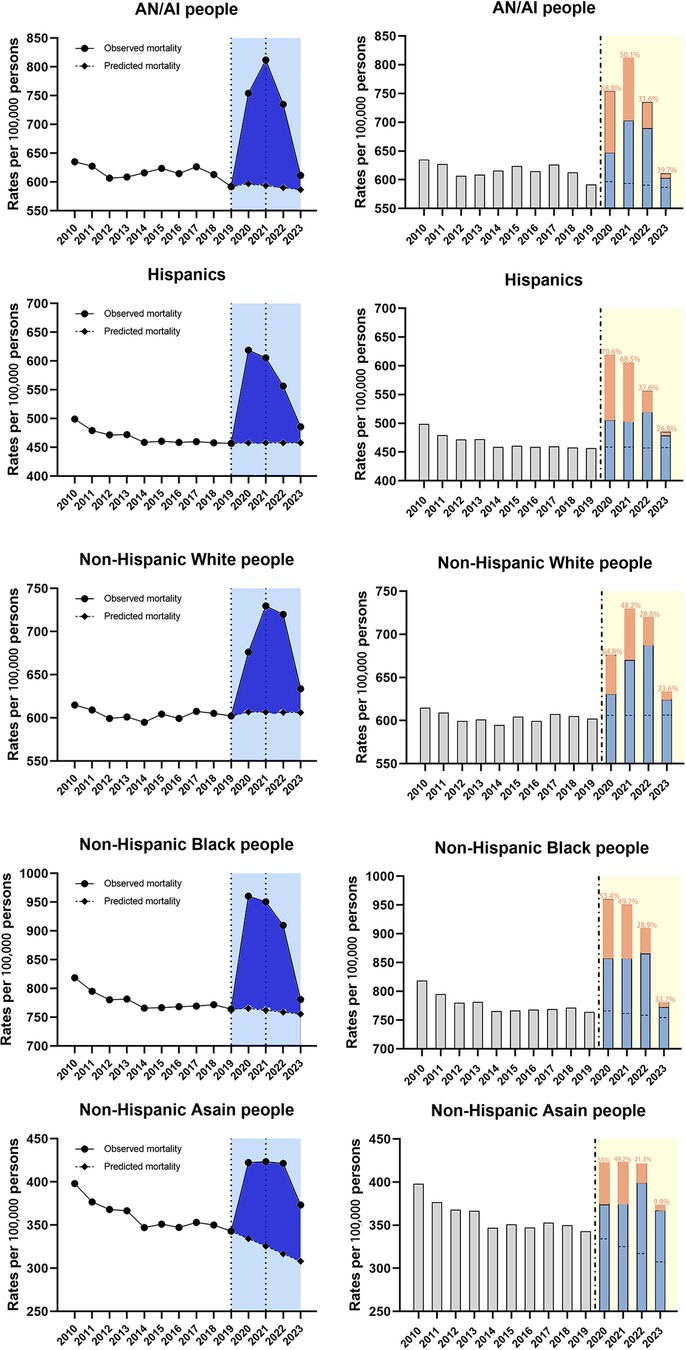
Figure 5. Temporal trends of all-cause mortality and excess deaths for CVD during the COVID-19 pandemic, by race/ethnicity. Dashed horizontal line: predicted value; Blue bars: non-COVID related; Orange bars: COVID-related, with the proportion in the excess deaths.
Discussion
In this study, we updated the evaluation on the impact of the COVID-19 pandemic on mortality in CVD patients by characterising the temporal trends in cardiovascular disease mortality from 2010 to 2023. First, the regression analysis showed a striking increase in both all-cause mortality and CVD-related mortality among CVD patients during 2019–2021, which was followed by a downward trend in mortality during 2021–2023. The mortality surge even reversed pre-pandemic trends in nearly all subgroups, most of which were not contributed by direct effect of COVID-19. Second, relevant APCs showed that the shift in mortality trends (whether increasing or subsequent declining trends) was more significant in the youngest group (25–44), males and minorities, respectively. Third, excess mortality was observed in all subgroups, which declined from 2021 but persisted in the vast majority of subgroups (except the young and middle-aged groups) until 2023. The percentage of COVID-related portion of excess deaths was in generally decreasing trend, but it remained high among the elderly, males and some minorities.
The increased CVD mortality caused by COVID-19 pandemic was well documented, which was reported from the initial phase of the pandemic to following one or two years. In the early months of the pandemic, studies showed a significant increase in deaths from CVD compared to the same period in 2019 (14). Subsequently, other studies comparing cardiovascular mortality rates before and after the pandemic found significant increase in mortality and excess deaths despite 2 years since the onset of the pandemic (6). A recent study demonstrated elevated cardiovascular deaths persisted until early 2022 by characterizing trends in excess CVD deaths (15). Our study expands from prior work by analyzing recent provisional data from the online database covering a longer time span, describing trends in mortality, comparing observed to predicted mortality rates, assessing the excess deaths and further stratifying by age, sex and race/ethnicity.
The increased excess CVD deaths during the pandemic was attributed to a combination of effects, whether direct or indirect factors. In one hand, SARS-CoV2 infection is closely associated with acute cardiovascular complications such as myocarditis, acute coronary syndrome and microvascular thrombosis, and may accelerate or activate the development of existing subclinical disease, which undoubtedly leads to myocardial injury in healthy people or patients with cardiovascular diseases (16). Survivors of SARS-CoV-2 infection are at increased long-term risk for various chronic cardiovascular diseases, with reports estimating a relative increase of approximately 55% in the risk of major adverse cardiovascular events over the next year (17). In addition, post-COVID-19 cardiac syndrome had been reported among COVID-19 survivors (18). SARS-CoV-2 targets multiple cells expressing ACE-2, which would lead to corresponding dysfunction and alteration of the cardiometabolic environment such as new onset diabetes and chronic kidney disease (19). In another hand, the indirect effects of the pandemic are far-reaching and cannot be ignored. In the initial phase of the epidemic, emergency medical care was usually directed towards the patients with acute infection, and non-essential medical services were suspended (20). Multinational studies have shown that all phases of health care for patients with cardiovascular disease (outpatient visits, admissions, diagnosis, and treatment) are affected to varying degrees during a pandemic, mainly in terms of decreased outpatient activity, fewer hospital admissions, delayed visits, worse admissions, and reduced and delayed diagnostic and treatment procedures (21). Besides, lockdown measures also brought about barriers and obstacles for seeking medical services. Relevant studies analyzed and summarized cardiovascular-related risk factors in the general population during the pandemic, showing a decrease in physical activity and an increase in sedentary behavior in all age groups during the epidemic lockdown (22). What's more, the increase in alcohol and tobacco intake and the deterioration in dietary structure also raised concerns (23). Such aggravated risk factors also induced adverse cardiovascular outcomes attributing to the increased mortality.
As time went on reports indicated that increased mortality could not be explained by COVID-19 alone and concerns arose about the effects of the pandemic on patients with non-communicable chronic diseases such as cardiovascular diseases (24). Similarly, our findings suggested that the non-COVID-directly related component also accounted for a certain proportion of excess deaths. Therefore, strategies and initiatives emerged to promote cardiovascular health during the pandemic. Professional cardiovascular associations and organizations issued several guidelines and consensus covering a wide range of topics such as emergency life support, diagnosis, treatment and cardiac care of CVD during the epidemic, and advice on rehabilitation of the cardiovascular sequelae of COVID-19 (25–27). Healthcare resource allocation was optimized and medical services for CVD were improved as well. By the end of 2020, emergency medical services in U.S. had recovered to some extent (28). A worldwide study coordinated by The International Atomic Energy Agency (IAEA) showed a 4% increase in cardiovascular testing in the U.S. in April 2021 compared with pre-pandemic baseline volumes (29). Application of telemedicine visits provided additional opportunities for cardiovascular health monitoring (30). Besides, one year into the pandemic, COVID-19 drug therapy and vaccines were approved (31, 32). These factors mentioned above may help to explain the declined trend in mortality from 2021 to 2023 based on the regression analysis in our study.
Furthermore, we also focused on the demographic comparisons. Regression analysis showed that the change of mortality was more dramatic in the younger cohort (25–44), with an APC of 19.8% in 2019–2021, almost twice that of the older cohort (≥65). The younger adults experienced the greatest relative increase in mortality during the initial period. Similar reports can be found in published literature as well. A retrospective study identified age related differences in CVD mortality trends over the first year of the pandemic in USA showing that adults aged 55 and younger experienced the largest increases in mortality (assessed by APC as well) vs. the older groups (33). Similarly, another study found the APC in deaths over the initial period during the pandemic in USA was largest for adults aged 25–44 years (34). There may be several explanations for these findings. First, younger cohorts may be more susceptible to pandemic indirect factors, as another study found that mortality trends in this age group were statistically associated with the strength of the intervention (35). Second, reduced physical activity during the intervention is perhaps more common in the young, who have to work from home compared to the elderly, which explains their sedentary lifestyles (36). However, sedentary lifestyles have a negative impact on cardiovascular outcomes. Third, adults in U.S. who are under Medicare age (generally under 65) have unequal access to health insurance, which may affect the access to health care and thus lead to an increase in mortality. Meanwhile, the elderly with cardiovascular diseases may actively seek outpatient care, whereas healthier young people may delay seeking care (37). In addition, the elderly still experienced the greatest absolute mortality and excess deaths remained persistent even in 2023 compared to the other two groups. Our findings imply that the government or health authorities may prudently reconsider about restrictions in the early stage of the pandemic, as well as health education for the young group. Meanwhile, health insurance policies should be altered in due course. However, considering the persistence of excess deaths among the elderly recently, concerns about long-term impacts of COVID-19 on these patient populations should be attached to higher priorities.
Overall mortality levels were higher for male patients than for females, with a slightly more obvious increasing trend in mortality for males than for females over the 2019–2021 period. Our findings parallel other studies that reported higher mortality in males (38, 39), and some factors may help explain this phenomenon. First of all, higher rates of CVD comorbidity in males may be associated with higher mortality rates, which is mentioned in published papers (40, 41). Next, sexual disparities in immune-inflammatory responses may affect the relation between CVD and COVID-19 risk and therefore lead to the higher mortality in males (42). Relevant studies have shown that estrogen and the X chromosome in women play a vital role in developing a more powerful response to infectious diseases and greater immune-mediated tissue repair (40). In addition, CVD and COVID-19-related inflammation/immune dysregulation might combine to increase risk associated with both diseases (43). Our findings on gender differences in mortality may encourage researchers to consider gender as a variable in research on the relationship between COVID-19 and CVD.
Moreover, our findings also demonstrated the diverse impact of the pandemic on different racial/ethnic groups. Non-Hispanic Black people mortality rates topped the list, while American Indian/Alaska Native people and non-Hispanic White people mortality rates were closer and in the middle of the pack. Hispanics had the forth highest death rate, what's more, non-Hispanic Asian people maintained the lowest level of mortality during the pandemic. In terms of the mortality trends, American Indian/Alaska Native people, Hispanics and non-Hispanic Black people had the top three steepest upward trend during pandemic according to the APCs in 2019–2021. However, Non-Hispanic white people, who are demographically predominant, had the slowest change in mortality trend. Racial disparities in cardiovascular disease mortality during the pandemic are notably discouraging. One rational explanation for this phenomenon concentrates on a higher prevalence of CVD comorbidities in minority populations, and avoidance of medical care may have greater impact on these populations (44). However, it may only play a small role compared to racism and health inequities. A study of the relationship between CVD mortality and socio-demographic factors during the COVID-19 pandemic, using the Socio-Vulnerability Index (SVI), further revealed this phenomenon (45). SVI refers to the sum of economic, demographic, and social factors that affect a community's risk exposure and ability to respond to hazardous events such as natural disasters (46). This study confirms the large overlap between black people communities and areas with high SVI indices and the significant correlation between SVI and CVD mortality is enhanced during the epidemic (46). The lower utilization of telemedicine by minorities and lower socioeconomic groups during the epidemic may also have increased the risk of adverse cardiovascular events (47). In addition, one recent meta-analysis found that the risk of infection remained elevated among ethnic minority groups beacuse of poor ventilation in homes and workplaces (48). That is, structural and social factors, such as housing and job insecurity or high poverty rates, were already contributing to the increased burden of CVD in specific communities prior to the pandemic, and the COVID-19 pandemic has largely exacerbated these social disadvantages (49). Recent study pointed that the previous barriers to vaccine readiness among minorities had been basically addressed (50). Nevertheless, racial disparities in healthcare access persisted with minority populations and sociodemographic disparities in telemedicine use was increasing (51, 52). The declined trend of mortality in 2021–2023 can be attributed to combined forces such as policies shift, immunity and re-distribution of healthcare resources for CVD, however, further assessment of these disparities and the development of strategies cannot be ignored.
The strength of our study was that we used the regression analysis and predictive models to demonstrate changes in mortality trends and predict mortality rates during the pandemic separately. We also devided the study population into subgroups in order to provide more detailed demographic characteristics. The study also had some limitations. We did not focus on non-CVD-related deaths and data for specific cardiovascular diseases were not included in our study. The death dataset we used is more readily available and easier to retrieve and organize data. In addition, the dataset has a broader coverage and offers a variety of categorical search options, providing more detailed information on deaths while minimizing selection bias. However, such large database may incorrectly code the cause of death, especially in the early stage of the pandemic. In addition, the database lack important clinically relevant information such as nursing procedures, surgical treatments, etc.
Conclusions
Our study provided more detailed information on mortality trends for CVD during the pandemic in U.S. The mortality significantly increased in the initial two years of the pandemic, which was followed by a decline in 2022–2023. Further subgroup analyses found that mortality trends were more dramatic in the young, male and minority populations. By the end of 2023, excess death remained persistent in nearly all groups except the young and middle-aged cohorts. Considering the necessity for the research on long-term impacts of COVID-19, further research on cardiovascular healthcare for the elderly and racial disparities should continue to be emphasized. We are also committed to providing policymakers and healthcare executives with information to optimize resource allocation in the post-epidemic era in order to improve the resilience to similar events in the future.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://wonder.cdc.gov/mcd.html.
Author contributions
SS: Writing – original draft, Data curation, Formal Analysis, Methodology, Software. CG: Writing – review & editing, Methodology. RW: Supervision, Writing – review & editing. HZ: Writing – review & editing, Supervision. QL: Writing – review & editing, Methodology. J-hD: Writing – review & editing. F-sG: Writing – review & editing. JW: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by grants from Shaanxi Provincial Science and Technology Department (No. 2020ZDLSF02-09).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mak IL, Wan EYF, Wong TKT, Lee WWJ, Chan EWY, Choi EPH, et al. The spill-over impact of the novel coronavirus-19 pandemic on medical care and disease outcomes in non-communicable diseases: a narrative review. Public Health Rev. (2022) 43:1604121. doi: 10.3389/phrs.2022.1604121
2. Mountantonakis SE, Makker P, Saleh M, Coleman KM, Husk G, Jauhar R, et al. Increased inpatient mortality for cardiovascular patients during the first wave of the COVID-19 epidemic in New York. J Am Heart Assoc. (2021) 10(16):e020255. doi: 10.1161/JAHA.120.020255
3. Rossen LM, Ahmad FB, Anderson RN, Branum AM, Du C, Krumholz HM, et al. Disparities in excess mortality associated with COVID-19—united States, 2020. MMWR Morb Mortal Wkly Rep. (2021) 70(33):1114–9. Published August 20, 2021. doi: 10.15585/mmwr.mm7033a2
5. Khan SU, Javed Z, Lone AN, Dani SS, Amin Z, Al-Kindi SG, et al. Social vulnerability and premature cardiovascular mortality among US counties, 2014 to 2018. Circulation. (2021) 144(16):1272–9. doi: 10.1161/CIRCULATIONAHA.121.054516
6. Janus SE, Makhlouf M, Chahine N, Motairek I, Al-Kindi SG. Examining disparities and excess cardiovascular mortality before and during the COVID-19 pandemic. Mayo Clin Proc. (2022) 97(12):2206–14. doi: 10.1016/j.mayocp.2022.07.008
7. Gao X, Lv F, He X, Zhao Y, Liu Y, Zu J, et al. Impact of the COVID-19 pandemic on liver disease-related mortality rates in the United States. J Hepatol. (2023) 78(1):16–27. doi: 10.1016/j.jhep.2022.07.028
8. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. (2000) 19(3):335–51. doi: 10.1002/(SICI)1097-0258(20000215)19:3%3C335::AID-SIM336%3E3.0.CO;2-Z
9. Desai MM, Cacciamani GE, Gill K, Zhang J, Liu L, Abreu A, et al. Trends in incidence of metastatic prostate cancer in the US. JAMA Netw Open. (2022) 5(3):e222246. doi: 10.1001/jamanetworkopen.2022.2246
10. Zakaria D, Shaw A. The impact of multiple primary rules on cancer statistics in Canada, 1992 to 2012. J Registry Manag. (2018) 45(1):8–20.30183692
11. Ilu SY, Prasad R. Improved autoregressive integrated moving average model for COVID-19 prediction by using statistical significance and clustering techniques. Heliyon. (2023) 9(2):e13483. doi: 10.1016/j.heliyon.2023.e13483
12. Jani C, Mouchati C, Abdallah N, Mariano M, Jani R, Salciccioli JD, et al. Trends in prostate cancer mortality in the United States of America, by state and race, from 1999 to 2019: estimates from the centers for disease control WONDER database. Prostate Cancer Prostatic Dis. (2022) 26(3):552–62. doi: 10.1038/s41391-022-00628-0
13. Bergman J, Ballin M, Nordström A, Nordström P. Risk factors for COVID-19 diagnosis, hospitalization, and subsequent all-cause mortality in Sweden: a nationwide study. Eur J Epidemiol. (2021) 36(3):287–98. doi: 10.1007/s10654-021-00732-w
14. Wadhera RK, Shen C, Gondi S, Chen S, Kazi DS, Yeh RW. Cardiovascular deaths during the COVID-19 pandemic in the United States. J Am Coll Cardiol. (2021) 77(2):159–69. doi: 10.1016/j.jacc.2020.10.055
15. Han L, Shi H, Li Y, Qi H, Wang Y, Gu J, et al. Excess deaths of gastrointestinal, liver, and pancreatic diseases during the COVID-19 pandemic in the United States. Int J Public Health. (2023) 68:1606305. Published August 15, 2023. doi: 10.3389/ijph.2023.1606305
16. Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID19 pandemic. J Am Coll Cardiol. (2020) 75:2352–71. doi: 10.1016/j.jacc.2020.03.031
17. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. (2022) 28:583–90. doi: 10.1038/s41591-022-01689-3
18. Raman B, Bluemke DA, Lüscher TF, Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J. (2022) 43(11):1157–72. doi: 10.1093/eurheartj/ehac031
19. Copur S, Berkkan M, Basile C, Tuttle K, Kanbay M. Post-acute COVID-19 syndrome and kidney diseases: what do we know? J Nephrol. (2022) 35:795–805. doi: 10.1007/s40620-022-01296-y
20. Josephson RA, Gillombardo CB. Cardiovascular services in COVID-19—impact of the pandemic and lessons learned. Prog Cardiovasc Dis. (2023) 76:12–9. doi: 10.1016/j.pcad.2023.01.005
21. Yasmin F, Shujauddin SM, Naeem A, Jabeen A, Shah SMI, Ochani RK, et al. Exploring the impact of the COVID-19 pandemic on provision of cardiology services: a scoping review. Rev Cardiovasc Med. (2021) 22(1):83–95. doi: 10.31083/j.rcm.2021.01.241
22. Freiberg A, Schubert M, Romero Starke K, Hegewald J, Seidler A. A rapid review on the influence of COVID-19 lockdown and quarantine measures on modifiable cardiovascular risk factors in the general population. Int J Environ Res Public Health. (2021) 18(16):8567. doi: 10.3390/ijerph18168567
23. Niedzwiedz CL, Green MJ, Benzeval M, Campbell D, Craig P, Demou E, et al. Mental health and health behaviours before and during the initial phase of the COVID-19 lockdown: longitudinal analyses of the UK household longitudinal study. J Epidemiol Community Health. (2021) 75(3):224–31. doi: 10.1136/jech-2020-215060
24. Nadarajah R, Gale CP. Collateral cardiovascular damage during the COVID-19 pandemic. Nat Rev Cardiol. (2022) 19(2):81–2. doi: 10.1038/s41569-021-00661-x
25. ESC Guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: part 2-care pathways, treatment, and follow-up. Cardiovasc Res. (2022) 118(7):1618–66. doi: 10.1093/cvr/cvab343
26. Writing Committee, Gluckman TJ, Bhave NM, Allen LA, Chung EH, Spatz ES, Ammirati E, et al. 2022 ACC expert consensus decision pathway on cardiovascular sequelae of COVID-19 in adults: myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection, and return to play: a report of the American college of cardiology solution set oversight committee. J Am Coll Cardiol. (2022) 79(17):1717–56. doi: 10.1016/j.jacc.2022.02.003
27. Wyckoff MH, Singletary EM, Soar J, Olasveengen TM, Greif R, Liley HG, et al. 2021 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations: summary from the basic life support; advanced life support; neonatal life support; education, implementation, and teams; first aid task forces; and the COVID-19 working group [published correction appears in circulation. 2022 mar;145(9):e760]. Circulation. (2022) 145(9):e645–721. doi: 10.1161/CIR.0000000000001017
28. Handberry M, Bull-Otterson L, Dai M, Mann NC, Chaney E, Ratto J, et al. Changes in emergency medical services before and during the COVID-19 pandemic in the United States, January 2018-December 2020. Clin Infect Dis. (2021) 73(Suppl 1):S84–91. doi: 10.1093/cid/ciab373
29. Hirschfeld CB, Dorbala S, Shaw LJ, Villines TC, Choi AD, Better N, et al. Cardiovascular testing in the United States during the COVID-19 pandemic: volume recovery and worldwide comparison. Radiol Cardiothorac Imaging. (2023) 5(5):e220288. doi: 10.1148/ryct.220288
30. Xu H, Granger BB, Drake CD, Peterson ED, Dupre ME. Effectiveness of telemedicine visits in reducing 30-day readmissions among patients with heart failure during the COVID-19 pandemic. J Am Heart Assoc. (2022) 11(7):e023935. doi: 10.1161/JAHA.121.023935
32. United States Food and Drug Administration. FDA Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine (2020).
33. Kobo O, Abramov D, Fudim M, Sharma G, Bang V, Deshpande A, et al. Has the first year of the COVID-19 pandemic reversed the trends in CV mortality between 1999 and 2019 in the United States? Eur Heart J Qual Care Clin Outcomes. (2023) 9(4):367–76. doi: 10.1093/ehjqcco/qcac080
34. Rossen LM, Branum AM, Ahmad FB, Sutton P, Anderson RN. Excess deaths associated with COVID-19, by age and race and ethnicity—United States, January 26-October 3, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69(42):1522–7. Published October 23, 2020. doi: 10.15585/mmwr.mm6942e2
35. Lee WE, Woo Park S, Weinberger DM, Olson D, Simonsen L, Grenfell BT, et al. Direct and indirect mortality impacts of the COVID-19 pandemic in the United States, March 1, 2020 to January 1, 2022. Elife. (2023) 12:e77562. Published February 22, 2023. doi: 10.7554/eLife.77562
36. van Bakel BMA, Bakker EA, de Vries F, Thijssen DHJ, Eijsvogels TMH. Changes in physical activity and sedentary behaviour in cardiovascular disease patients during the COVID-19 lockdown. Int J Environ Res Public Health. (2021) 18(22):11929. Published November 13, 2021. doi: 10.3390/ijerph182211929
37. Čelutkienė J, Čerlinskaitė-Bajorė K, Bajoras V, Višinskienė R, Lizaitis M, Budrys P, et al. Collateral effect of the COVID-19 pandemic on cardiology service provision and cardiovascular mortality in a population-based study: COVID-COR-LT. Clin Res Cardiol. (2022) 111(10):1130–46. doi: 10.1007/s00392-022-02033-y
38. Butt JH, Fosbøl EL, Gerds TA, Andersson C, Kragholm K, Biering-Sørensen T, et al. All-cause mortality and location of death in patients with established cardiovascular disease before, during, and after the COVID-19 lockdown: a Danish nationwide cohort study. Eur Heart J. (2021) 42(15):1516–23. doi: 10.1093/eurheartj/ehab028
39. Aggarwal NR, Patel HN, Mehta LS, Sanghani RM, Lundberg GP, Lewis SJ, et al. Sex differences in ischemic heart disease: advances, obstacles, and next steps. Circ Cardiovasc Qual Outcomes. (2018) 11(2):e004437. doi: 10.1161/CIRCOUTCOMES.117.004437
40. Bienvenu LA, Noonan J, Wang X, Peter K. Higher mortality of COVID-19 in males: sex differences in immune response and cardiovascular comorbidities. Cardiovasc Res. (2020) 116(14):2197–206. doi: 10.1093/cvr/cvaa284
41. Pastor-Barriuso R, Pérez-Gómez B, Hernán MA, Pérez-Olmeda M, Yotti R, Oteo-Iglesias J, et al. Infection fatality risk for SARS-CoV-2 in community dwelling population of Spain: nationwide seroepidemiological study. Br Med J. (2020) 371:m4509. Published November 27, 2020. doi: 10.1136/bmj.m4509
42. Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. (2020) 588(7837):315–20. doi: 10.1038/s41586-020-2700-3
43. Gao C, Cai Y, Zhang K, Zhou L, Zhang Y, Zhang X, et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J. (2020) 41(22):2058–66. doi: 10.1093/eurheartj/ehaa433
44. Acosta AM, Garg S, Pham H, Whitaker M, Anglin O, O'Halloran A, et al. Racial and ethnic disparities in rates of COVID-19-associated hospitalization, intensive care unit admission, and in-hospital death in the United States from march 2020 to February 2021. JAMA Netw Open. (2021) 4(10):e2130479. doi: 10.1001/jamanetworkopen.2021.30479
45. Kim YJ, Kim S, An J, Volgman AS, Nazir NT. Impact of the COVID-19 pandemic on cardiovascular disease mortality in a major metropolitan area. Am Heart J Plus. (2022) 18:100173. doi: 10.1016/j.ahjo.2022.100173
46. Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry, CDC/ATSDR Social Vulnerability Index. Available online at: Available at: https://www.atsdr.cdc.gov/placeandhealth/svi/index.html (cited December 5, 2021).
47. Eberly LA, Kallan MJ, Julien HM, Haynes N, Khatana SAM, Nathan AS, et al. Patient characteristics associated with telemedicine access for primary and specialty ambulatory care during the COVID-19 pandemic [published correction appears in JAMA Netw Open. 2021 feb 1;4(2):e211913]. JAMA Netw Open. (2020) 3(12):e2031640. Published December 1, 2020. doi: 10.1001/jamanetworkopen.2020.31640
48. Irizar P, Pan D, Kapadia D, Bécares L, Sze S, Taylor H, et al. Ethnic inequalities in COVID-19 infection, hospitalisation, intensive care admission, and death: a global systematic review and meta-analysis of over 200 million study participants. EClinicalMedicine. (2023) 57:101877. doi: 10.1016/j.eclinm.2023.101877
49. Nordhues HC, Bhagra A, Stroud NN, Vencill JA, Kuhle CL. COVID-19 Gender disparities and mitigation recommendations: a narrative review. Mayo Clin Proc. (2021) 96(7):1907–20. doi: 10.1016/j.mayocp.2021.04.009
50. Kranzler EC, Ihongbe TO, Marshall MC, Denison B, Dahlen H, Hoffman B, et al. Racial and ethnic differences in COVID-19 vaccine readiness among adults in the United States, January 2021–April 2023. Vaccine. (2024) S0264-410X(23):01537-2. doi: 10.1016/j.vaccine.2023.12.078 [Epub ahead of print].38182461
51. Freelander L, Rickless DS, Anderson C, Curriero F, Rockhill S, Mirsajedin A, et al. The impact of COVID-19 on healthcare coverage and access in racial and ethnic minority populations in the United States. Geospat Health. (2023) 18(2). doi: 10.4081/gh.2023.1222
Keywords: COVID- 19, cardiovascular diseases, mortality, predictive analysis, disparity
Citation: Song S, Guo C, Wu R, Zhao H, Li Q, Dou J-h, Guo F-s and Wei J (2024) Impact of the COVID-19 pandemic on cardiovascular mortality and contrast analysis within subgroups. Front. Cardiovasc. Med. 11:1279890. doi: 10.3389/fcvm.2024.1279890
Received: 18 August 2023; Accepted: 18 January 2024;
Published: 7 February 2024.
Edited by:
Reza Lashgari, Shahid Beheshti University, IranReviewed by:
Frank Annie, Charleston Area Medical Center, United StatesAlan P. Pan, Houston Methodist Hospital, United States
© 2024 Song, Guo, Wu, Zhao, Li, Dou, Guo and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Wei d2VpamlueGp0dUAxMjYuY29t
 Shoufang Song
Shoufang Song Chen Guo
Chen Guo Jia-hao Dou
Jia-hao Dou Jin Wei
Jin Wei