
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 10 March 2023
Sec. Cardiovascular Imaging
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.985657
This article is part of the Research Topic Current and Future Role of Artificial Intelligence in Cardiac Imaging, Volume II View all 16 articles
 Xixiang Lin1,2,†
Xixiang Lin1,2,† Feifei Yang1,†
Feifei Yang1,† Yixin Chen3,†
Yixin Chen3,† Xu Chen1,2
Xu Chen1,2 Wenjun Wang1
Wenjun Wang1 Wenxiu Li4
Wenxiu Li4 Qiushuang Wang5
Qiushuang Wang5 Liwei Zhang5
Liwei Zhang5 Xin Li6
Xin Li6 Yujiao Deng1
Yujiao Deng1 Haitao Pu3
Haitao Pu3 Xiaotian Chen3
Xiaotian Chen3 Xiao Wang1,2
Xiao Wang1,2 Dong Luo1,2
Dong Luo1,2 Peifang Zhang3
Peifang Zhang3 Daniel Burkhoff7
Daniel Burkhoff7 Kunlun He1*
Kunlun He1*
Objectives: We developed and tested a deep learning (DL) framework applicable to color Doppler echocardiography for automatic detection and quantification of atrial septal defects (ASDs).
Background: Color Doppler echocardiography is the most commonly used non-invasive imaging tool for detection of ASDs. While prior studies have used DL to detect the presence of ASDs from standard 2D echocardiographic views, no study has yet reported automatic interpretation of color Doppler videos for detection and quantification of ASD.
Methods: A total of 821 examinations from two tertiary care hospitals were collected as the training and external testing dataset. We developed DL models to automatically process color Doppler echocardiograms, including view selection, ASD detection and identification of the endpoints of the atrial septum and of the defect to quantify the size of defect and the residual rim.
Results: The view selection model achieved an average accuracy of 99% in identifying four standard views required for evaluating ASD. In the external testing dataset, the ASD detection model achieved an area under the curve (AUC) of 0.92 with 88% sensitivity and 89% specificity. The final model automatically measured the size of defect and residual rim, with the mean biases of 1.9 mm and 2.2 mm, respectively.
Conclusion: We demonstrated the feasibility of using a deep learning model for automated detection and quantification of ASD from color Doppler echocardiography. This model has the potential to improve the accuracy and efficiency of using color Doppler in clinical practice for screening and quantification of ASDs, that are required for clinical decision making.
It is estimated that in 2017, nearly 1.8 cases per 100 live births are diagnosed with congenital heart disease (CHD) worldwide (1). Atrial septal defect (ASD) is the second most common type of CHD, accounting for approximately 6%–10% of cases (2). Most patients with ASD are asymptomatic and may be identified as an incidental finding during routine echocardiographic examinations. Early detection of appropriately sized defects known to lead to problems later in life can prompt timely intervention and improve cardiovascular outcomes, avoiding substantial disability and mortality (3, 4).
Transthoracic echocardiography (TTE) with Doppler flow imaging is currently the most widely used noninvasive tool for detecting the presence of an ASD, especially in children (5). TTE cannot only be used to detect and quantify the size and shape of the septal defect, but can also be used to measure the degree and direction of shunting, changes of the size and function of the cardiac chambers and detect abnormal pressures and flows through the pulmonary circulation (6). However, accurate detection and quantification of ASD features relies on experienced, highly trained physicians which are in short supply, especially in rural areas (7). Furthermore, the low prevalence of disease and variability of image quality, number of acquired views and interpretation of TTE images causes low sensitivity and specificity of ASD detection (4), all of which hinder referral for treatment. Therefore, an effective solution for efficient, accurate and objective detection and grading of ASDs is critically needed.
Deep learning (DL) models have been applied for automated detection and assessment of cardiovascular diseases based on echocardiographic images and videos. Such models can complete a variety of tasks such as, image quality assessment, view classification, boundary segmentation, disease diagnosis and automatic quantification (8–12). However, there is no prior study investigating the effectiveness of a DL model for detecting ASD based on color Doppler images. Accordingly, we developed and validated a DL model for automated detection and quantification of ASDs (Figure 1).
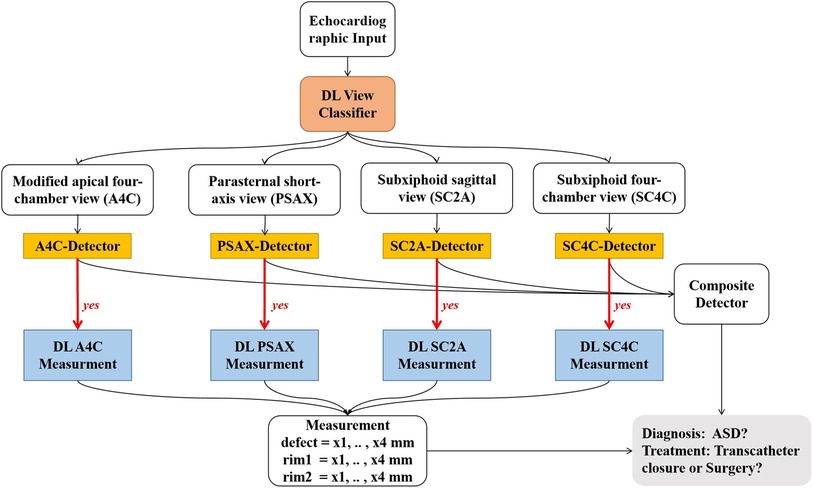
Figure 1. Work flow of the ensemble model. Step 1: raw echocardiographic videos are separated for classification of views (red box). Step 2: disease detection models use different views to detect the presence of ASD (orange rectangles). Step 3: if ASD is present (denoted by “yes”), metrics associated with severity of ASD are assessed (blue rectangles). DL, deep learning; ASD, atrial septal defect.
This study involved algorithm development and initial testing based on a retrospective data set, and final testing from a prospective, real-world data set of consecutively acquired echocardiographic studies. 396 TTE examinations obtained between July 2020 and April 2021 from Anzhen hospital served as our training dataset. A total of 425 consecutively obtained examinations between May 2020 and Dec 2020 from the Chinese PLA General Hospital were collected as the external testing set, which including 48 ASD cases and 377 cases without ASD. The age of all cases in both training and testing datasets was less than 18. ASD diagnostic criteria were based on the 2015 ASE guideline (6), as detailed below. The ground truth for the presences of an ASD was based on the diagnosis present in the electronic medical record and echocardiographic clinical report which were provided by experienced echocardiography readers and reviewed by cardiologists who authorized the final reports. Other hemodynamically significant cardiac lesions (such as tetralogy of fallot and valvular heart disease) were excluded.
Each echocardiographic study was acquired through standard methods. Four standard views were suggested by ASE guideline for detection and quantification of ASDs (6): (1) the modified apical four-chamber view (A4C); (2) the parasternal short-axis view (PSAX); (3) the subxiphoid sagittal view (SC2A); and (4) the subxiphoid four-chamber view (SC4C). These images were acquired from a diverse array of echocardiography machine manufacturers and models including Phillips iE-elite and 7C with transducer S5-1 and X5-1 (Phillips, Andover, MA, USA), Vivid E95 (General Electric, Fairfield, CT, USA), Mindray M9cv with transducer SP5-1s (Mindray, Shenzhen, Guangdong, China), Siemens SC2000 with transducer 4V1c (Siemens, Munich, Germany). All images were downloaded and stored with a standard Digital Imaging and Communication in Medicine (DICOM) format according to the instructions from each manufacturer.
We labeled 3,404 images to develop a method to classify 29 standard views, and then selected the 4 views required for detection and quantification of ASD detailed above. View selection was performed using a Xception Net neural network model according to methods that were similar to those described previously (8, 10, 13).
We selected 792 videos inclusive of the four standard color Doppler views required for segmentation from among the ASD cases. However, not every case had all four standard views, restricted by the limitation of retrospective data and the improper body position during examination. The atrial septum and margins of the defects were annotated with the LabelMe (Figure 2). In the modified apical four-chamber and subxiphoid four-chamber views, we labelled the atrial septum from atrioventricular valve to the roof of the atria (boundaries indicated by the dots in Figure 2). In the parasternal short-axis view, we labelled the atrial septum from aortic adventitia to the roof of the atria. In the subxiphoid sagittal view, we labelled the atrial septum from the bottom to the roof of the atria. We labelled the defect based on the width of the shunt jet detected on color Doppler flow images and the anechoic area of atrial septum in each view.
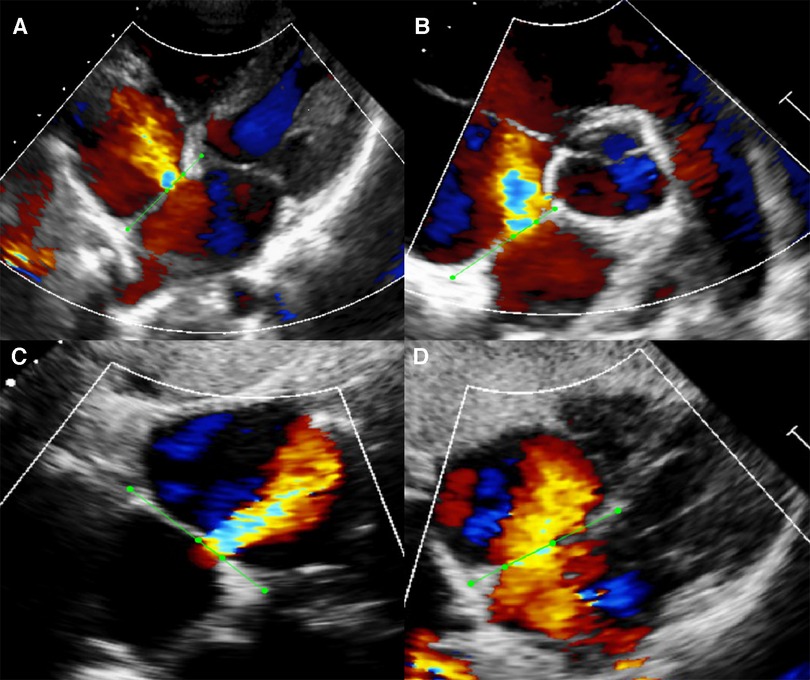
Figure 2. Example of manual segmentation. Green dots were manually labeled as the endpoints of atrial septum and defect with the open-source program LabelMe. (A) Modified apical four-chamber view (A4C). (B) Parasternal short-axis view (PSAX). (C) Subxiphoid sagittal view (SC2A). (D) Subxiphoid four-chamber view (SC4C).
For the ASD detection task, videos were labelled as either ASD or normal based on the electronic medical record and echocardiographic clinical report. Each frame was resized to 240 × 320 pixels from 600 × 800 DICOM-formatted images. The pixel value was normalized to between 0 and 1. No clipping or interpolation operations were performed on frame numbers; therefore, the number of frames used for the analysis differed from video to video. To effectively increase the number of videos for training, we employed affine transformations including RandomShift (10%), RandomScale (10%) and RandomRotation (20°). The batch size was set to 1 because of the difference of frame number. Finally, we adopted the Adam optimizer with a weight decay of 1e-5. The learning rate was set to 3e-5. All models were trained on an Nvidia Tesla P100 GPU.
The ASD detection network architecture was shown in Figure 3A. The model was based on the ResNet architecture with modifications (14). First, we used a frame-based max pool to fuse blood flow information in each frame. Second, we used Atrous Spatial Pyramid Pooling (ASPP) to increase the visual field of the convolution feature extractor. ASPP consisted of a global average pool layer and four convolution layers with dilation coefficients of 1, 4, 8 and 12 respectively. Third, we used GroupNorm to replace BatchNorm since the batch size was 1. The loss function was binary cross-entropy. Finally, the model could provide the ASD probability of each frame in the video. Therefore, the frame with the highest probability would be selected as the keyframe of model diagnosis. We have made our code available at GitHub (15).
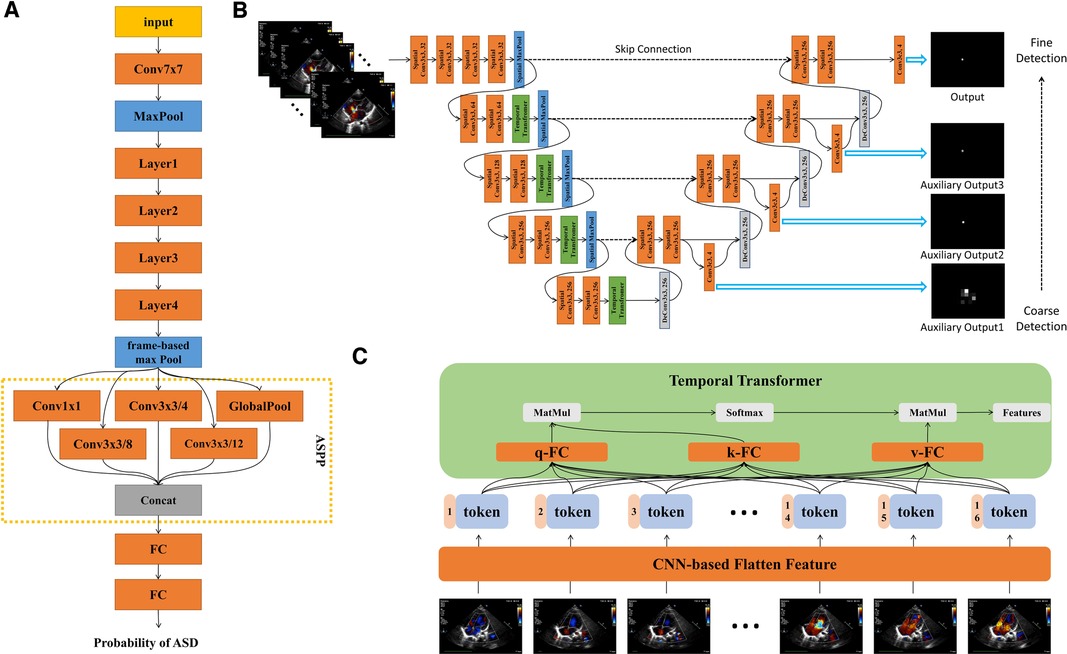
Figure 3. Detailed description of model architecture. (A) ASD detection neural network architecture, based on Resnet-18, combined with ASPP to increase visual field. The output of the model is the probability of ASD between 0 and 1. (B) ASD quantification neural network architecture, combining spatial convolution and temporal transformer, had three auxiliary outputs and one output from coarse to fine. Each auxiliary output is added to the feature map as an additional feature. (C) Structure diagram of temproal transformer. Each frame is encoded into a feature token by CNN. Self-attention could realize the long-distance dependence between frames. q, query; k, key; v, value.
For the quantification tasks, each frame was labelled with 4 points, two of which were the edges of the ASD, and the other two were the ends of the septum. Each frame was resized to 240 × 320 pixels. The pixel value was normalized to between 0 and 1. For the training stage, we randomly clipped 16 consecutive frames as an input from the video. Because of the large number of training epochs, we believed that the model had fully learned the entire information for each training sample. For validation and testing stage, each video was clipped into multiple segments. According to the majority voting principle, we made the prediction from those of segments. Each prediction took into account all the video information and did not receive the impact of randomness. We adopted the same affine transformation described above. In this case, the batch size was set to 2. We adopted Adam optimizer with a weight decay of 1e-5. The learning rate was set to 3e-5. All models were trained on an Nvidia Tesla P100 GPU.
The quantification network architecture was shown in Figure 3B. The networks architecture adopted UNet-style design (16). There were two dilemmas in using deep learning to fulfil the ASD quantification task.
Firstly, measuring the length of ASD was defined as a segmentation task, so we also adopted the structure of 3D-UNET (16). However, the performance was far less than expected. Segmenting the area of atrial septal defect was not a routine segmentation task. The common segmentation task assumes a segmentation boundary in the image, but the atrial septal defect was a disappearing region. Experienced doctors need to annotate the region based on sequent images. Therefore, we defined the task of quantifying ASD as key point detection since each key point exists in the image. We continued 3D-Unet-style and applied it to the task of key point detection.
The second point was how to make the neural network perform similarly to the senior physician. We have made two improvements: we have added different scales of auxiliary loss so that the model was from coarse to fine for key point identification; we found that the temporal convolution was unsuitable for the ASD key point detection task in echocardiography and therefore replaced it with a temporal transformer to overcome the long-range dependence dilemma of the frame dimension. The dependency dilemma can be described as follows: the key point detection of unclear video frames depends on the information obtained from the previous and next video frames. Such unclear video frames were present in human pose detection (17), for example, in the form of occlusion or overlap. However, this occlusion or overlap exists for a short period of time, usually no more than 3 consecutive frames. In echocardiography, most of the frames were not clear enough. Therefore, an experienced sonographer will prioritize the key points in clear video frames and then identify key points in other frames that were not clear enough. Convolution was a natural local attention mechanism and was not global, so using convolution to extract features will suffer long-range forgetting dilemma. As a simple example, suppose we use a 3 × 3 × 3 convolution kernel for 3D convolution and we want the features of frame i to fuse the features of frame j. If , then the features of i and j were ready for fusion in the 1st convolutional layer. If , then the features of i and j need to go to the 3rd convolutional layer before they can be fused with each other. If the distance between frame i and frame j was long, the long-range forgetting dilemma will occur. In order to allow the features of clear video frames to be efficiently propagated throughout the whole video, we use the temporal transformer module. The structure of temporal transformer was shown in Figure 3C. Firstly, CNN-based extractor extracted the feature from each frame. In each temporal transformer module, the feature was transferred to three parts, query (q), key (k) and value (v). We calculated the correlation between q and k, which was called self-attention. We had 16 frames (tokens) so that the correlation map was 16 × 16. This map represented the correlation between any two frames. Finally, we used the correlation map processed by softmax as the weight, and sum the value. For the whole model, we only downsampled in the spatial dimension, and did not downsample in the temporal dimension so that for each temporal transformer block, the token number was always 16.
In addition, we have made the following adjustments: considering the cost of computation and time, we used the 2D spatial convolution and temporal transformer to replace the 3D convolution. Second, we used GroupNorm to replace BatchNorm since the batch size was small. Video (W means width, H means height and F means frame) goes through convolutional layers for feature extraction in W and H spatial dimensions. Then the W and H dimensions were merged into token T so feature map can be writen as or . The token of each frame was spliced with the corresponding position code, and then can be used as the input of the temporal transformer. The self-attention mechanism follows the design of ViT (18). The model can be divided into 4 stages, each containing a spatial downsampling layer, spatial convolution layers and a temporal transformer module.
The model provided an index of the “confidence” with which the septal length was estimated. Confidence was calculated as the percent of “stable frames” contained in the entire video. A frame was designated as “stable” if the absolute difference of the AI-predicted septal length from that of the prior frame divided by the average length of the 2 frames was less than 0.5. The model only calculated defect size and septal length based on the stable frames. Specifically, septal length was calculated as the average value of the lengths on all stable frames. ASD defect size was calculated the largest value among all stable frames. Accuracy of measurements of atrial septal lengths and defect sizes were compared to those made by expert echocardiographers using Bland & Altman analysis.
Analyses were performed using algorithms written in Python 3.6 from the libraries of Numpy, Pandas, and Scikit-learn. Continuous variables were expressed as mean ± standard deviation, median and interquartile range, or counts and percentage, as appropriate. Comparisons of reports and machine algorithm performances were performed using one-way analysis of variance (ANOVA), followed by the least significant difference (LSD) t-test. Results were regarded as statistically significant when P < 0.05. The models were assessed according to the area under the receiver operating characteristic (ROC) curves which plotted sensitivity vs. 1-specificity derived from the model's prediction confidence score. All calculations were performed by using IBM SPSS version 23.0.
A total of 821 patients with transthoracic echocardiographic examinations were included. The clinical and echocardiographic characteristics of included cases were summarized in Table 1. In the training dataset, patients with ASD had a median age of 3 years (IQR: 1, 10), 34.3% were male, and EF had a mean value of 70.0 ± 5.0. In the external testing dataset, patients with ASD had a median age of 1 years (IQR: 0, 9), 52.0% were male, and ejection fraction (EF) had a mean value of 64.7 ± 5.3.
As summarized in Supplementary Figure S1, the deep-learning model identified four standard color Doppler views with an average accuracy of 0.99, including apical four-chamber view (0.97), parasternal short-axis view (0.99), subxiphoid frontal view (0.99) and subxiphoid sagittal view (1.0).
For each echo-Doppler video, the ASD detection model provided a probability level for the presence of an ASD; the frame with the highest probability was tagged as the keyframe of the video (examples shown in Supplementary Figure S2). The ROCs for the detection of an ASD in each of the 4 views for the external validation dataset were shown in Figure 4. The AUROC for ASD detection ranged from 0.901 to 0.956 for the individual views. The final diagnosis was made by the composite classifier model, which had an AUROC = 0.92. Youden's Index was used to evaluate model performance, which yielded sensitivities of 87.8% and specificities of 89.4% (Figure 4 and Table 2).
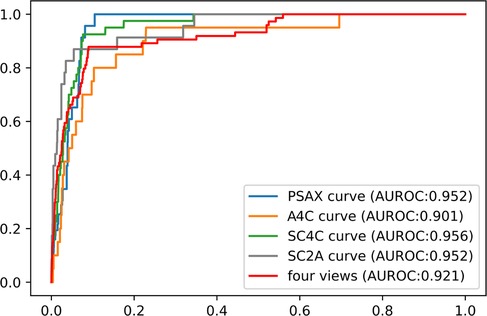
Figure 4. The performance of ASD detection model in the external dataset. The performance of composite classifier model (red curve) had an AUROC of 0.92. Abbreviations as in Figure 1.
Examples of segmentation model outputs were shown in the still image of Figure 5. As shown, the blue dots show where the DL model identified the ends of the atrial septum, while the orange dots show the model-identified edges of the ASD. Examples of frame-by-frame segmentation throughout entire videos, along with the model-derived measurements of the defect size and rim lengths compared to those measured by the expert physicians were shown in the videos provided in with online supplement material. These videos show results obtained from different echocardiographic views and different image qualities.
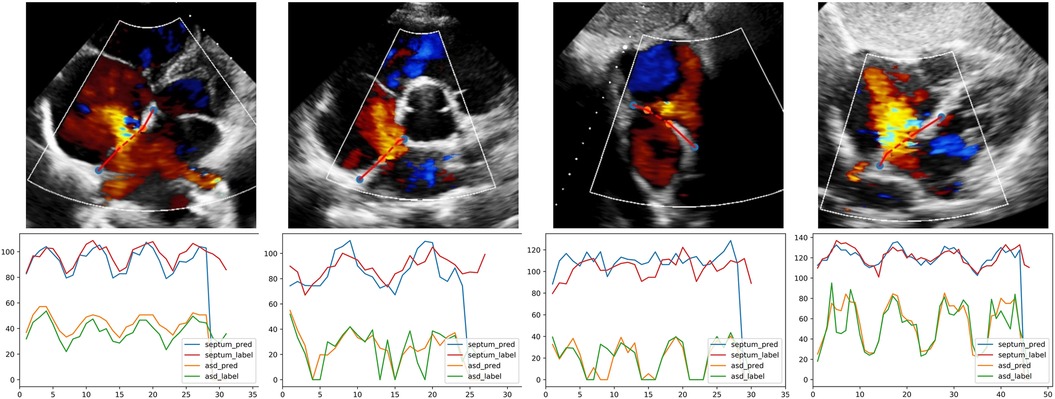
Figure 5. The output of quantification model in different views. The first row, one example test image of patients with ASD is shown respectively in A4C, PSAX, SC2A and SC4C view; orange and blue dots are the endpoints of defect and septum predicted by DL model. The second row, corresponding curves showing the variation of defect and septum in the video. Abbreviations as in Figure 1.
As detailed in Methods, the model provided an index of the “confidence” with which the septal length was estimated. Examples of results with different confidence levels were shown in Figure 6. The quantification model had greater performance in videos with higher confidence values; the relationships between the absolute difference between AI- and expert-determined septal length and ASD lengths as a function of the confidence values were shown in Supplementary Figure S3.
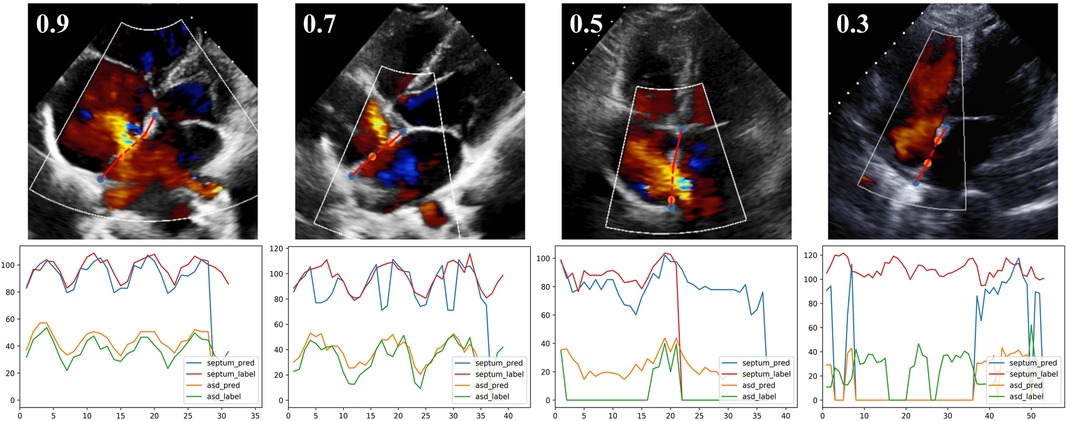
Figure 6. The output of quantification model with different confidence levels. The first row, one example test image of A4C view is shown respectively with the confidence of 0.9, 0.7, 0.5 and 0.3. The second row, corresponding curves showing the variation of defect and septum in the video.
Results of the Bland & Altman analysis comparing values provided by the AI algorithm and experts' measurements were summarized in Figure 7. The mean bias for the measurement of defect size and septum length were 1.9 and 2.2 mm. We also recruited three experts to measure defect size and septum length in the test dataset. As shown in Supplementary Figure S4, the mean biases of defect size were respectively 1.5 mm, 2.3 mm, 0.3 mm, and the mean biases of septum length were respectively 0.8 mm, 2.1 mm, 1.2 mm. Despite the fact that inter-expert variability was lower than the AI model bias, the difference was insignificant. Therefore, we believed that the bias of algorithm is comparable to that encountered in current clinical practice.
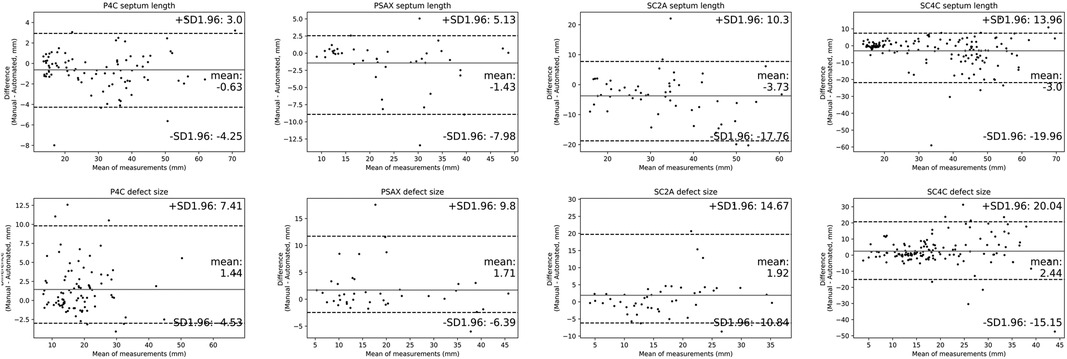
Figure 7. Comparisons of quantitative metrics derived from the deep learning (DL) algorithm and physician based on bland and altman analysis. Bland-Altman plots compare automated and manual measurements for septum length and defect size in A4C, PSAX, SC2A and SC4C view. Abbreviations as in Figure 1.
Applying these automatic measurements to the indications and contraindications detailed in the 2015 ASE guidelines, we used the model to predict whether a given patient should be referred for transcatheter intervention (6). The results of the prediction were compared with the recommendations provided by an expert physician, who applied his own manual measurements to the guideline recommendations. The accuracy of model to predict the expert's conclusion was 85.4% (Supplementary Table S1).
Echocardiography is the primary method for confirming the diagnosis of an ASD, for defining its anatomic and physiological characteristics and for deciding upon the need for and approach to treatment. However, accurate interpretation of echocardiograms for each of these purposes is in many respects subjective and time-consuming, requiring highly skilled clinicians which are not readily available in all hospitals. With the advantages of objectivity, efficiency, accuracy and consistency, deep learning (DL) models have been shown to be helpful in interpreting medical images in many fields of medicine (19–21), including echocardiography (8–12). However, ours is the first study to employed DL model for accurate detection and quantification of ASD through automated interpretation of color Doppler videos.
As in most DL models applied to echocardiography, the first step in our pipeline was echocardiographic view classification (22–24). However, newly introduced in our study is a classification model that includes color Doppler views. This model automatically selected the guideline-recommended echocardiographic views required for the detection and quantification of ASD with a high degree of accuracy.
The next step was implementation of a DL model to detect the presence of an ASD based on interpretation of the color Doppler views. This model also proved to be accurate, with high levels of sensitivity and specificity for disease detection. Similar degrees of detection accuracy were reproduced in all four echo-Doppler views examined and the AUC of the composite classifier model reached to 0.92. In addition, to address the “black box” problem and improve the interpretability, our model also automatically identified the key frame which can be provided to the clinician as a reference for final diagnosis and manual verification. Accordingly, the model has the potential to be used as a screening tool to aid doctors in identifying patients with an ASD, particularly in geographies where access to expert clinicians is limited.
Following view selection and disease detection, the final step was automated quantification of ASD size and the length of the residual rim; these are critical for determining the need for, and choice of treatment: transcatheter intervention or cardiothoracic surgery. To make these measurements, the quantification model automatically located the endpoints of the atrial septum and of the defect. In order to ensure the stability and reliability of automated quantification, the model generated an index of “confidence” with which the septal length was estimated. Naturally, the quantification model had greater performance in videos with higher confidence values. The performance of the algorithm was assessed by the bias of measurement of defect size and septum length, which provided a quantitative index of the degree of concordance between the DL model and expert physicians. Values of bias achieved by the model were low. Because the model explicitly detected and displayed the location of endpoints of the septum and defect, physicians can readily verify the accuracy of the DL algorithm on a case-by-case basis. All these features are illustrated in the videos provided on the online supplemental material.
Finally, we note that whereas the metrics of defect and rim size are helpful for deciding between use of a transcatheter or surgical intervention, such decisions are not made based on these metrics alone. According to society guidelines (6) such decisions should be made based on additional metrics and other imaging approaches, such as transesophageal echocardiography and three-dimensional imaging. While this tool has potential utility in areas where access to expert physicians is limited, the method was trained and validated on images acquired by experts. So its translation to resource-limited environments might require additional adaptations to give real-time feedback on image quality if datasets are acquired by individuals with limited specialization in echocardiography of congenital defects.
Recent studies have shown remarkable performance of deep learning models in diagnosing ASDs (25–28). Wang et al. proposed an end-to-end framework which automatically analyzed multi-view echocardiograms and selected keyframes for disease diagnosis. As a result, the framework differentiated ASD, VSD and normal cases with an accuracy of 92.1% (25). Rima et al. used fetal screening ultrasound to train a DL model for these tasks, including view selection, segmentation and complex congenital heart disease detection. In the test of 4,108 fetal sonograms, the model achieved an AUC of 0.99 in distinguishing normal from abnormal hearts, which was comparable to expert clinicians' performance (26). Zhao et al. developed a variant of U-Net architecture to segment the structure of the atrial septum in magnetic resonance images of pre- and post-occlusion ASD patients, with mean Dice index of 0.81 (27). Mori et al. proposed a DL model that used electrocardiograms (ECGs) to detect the presence of ASDs. This model outperformed 12 pediatric cardiologists in diagnosing ASD from ECG interpretations, with an accuracy of 0.89 (28). However, ours is the first study to detect the presence of an ASD based on multiple color Doppler views and to automatically identify the margins of the atrial septum and the margins of the ASD in order to provide quantitative measurements of ASD size and rim size. These represent significant advances since quantification of these anatomic features of an ASD are critical for determining treatment. Specifically, according to the 2015 ASE guideline (6), echocardiography provides important information for deciding on whether or not to treat an ASD and whether the defect is most suitably treated by transcatheter or surgical techniques. In this regard, studies have shown that ASD diameter measured directly at surgery is most accurately estimated by color flow Doppler echocardiography, while significant errors can arise if measurements are estimated from standard 2D echocardiograms alone (29).
The results of our study need to be considered within the context of several limitations. First, all of the images were acquired by transthoracic echocardiography (TTE) rather than transesophageal echocardiography (TEE), as most of the included population were children who cannot tolerate TEE examination. Additionally, patients did not undergo cardiac computed tomography or magnetic resonance imaging, which can provide more detail information of ASD anatomy. Second, the training and testing dataset is based on images obtained from children. Despite the low prevalence, the algorithm performed very well to identify and quantify the sizes of these ASDs. This indicates that the absolute size of the heart does not influence accuracy of the model since the images are ultimately scaled to the same pixel dimensions with adequate special resolution. Third, limited by the retrospective nature, the study included a relatively small number of patients. Although our model achieved good performance in the external test set, testing of the model in a prospective multi-center cohort is warranted. Finally, the “black box” problem of our DL algorithm poses an inherent impediment to acceptance into clinical practice because of the opaqueness on how diagnoses are made. To overcome this limitation, we implemented an algorithm which provided keyframe selected by the DL model and identified the endpoints of defect and the septum on the images. This is intended to promote physician confidence in the model-based diagnoses and measurements. Even then, it is emphasized that the algorithm is intended to assistant, not replace, physician decision making.
We developed and validated a novel deep learning model applicable to color Doppler echocardiography for automatic detection and quantification of atrial septal defect and rim sizes. This model has the potential to improve the accuracy and efficiency of color Doppler echocardiographic screening and quantification of ASDs.
Echocardiography is the most commonly used non-invasive imaging tool for detection and quantification of atrial septal defects. Manual evaluations of echocardiographic videos required highly skilled clinical experts and is a time-consuming process.
Algorithms based on deep learning approaches have the potential to automate and increase efficiency of the clinical workflow for detecting atrial septal defects and measuring the size of defect and the residual rim.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Committee (EC) approvals were obtained from each hospital for the use of deidentified echocardiographic and patient demographic data and the study was registered with the Chinese clinical trial registry (ChiCTR2000030278).
KH, XL and FY designed this study, analyzed and interpreted the patient data, drafted the manuscript, and guaranteed that all aspects of the work was investigated and resolved. DB and KH critically revised the important intellectual content of the manuscript. XC, WW, WL, QW, LZ, XL, YD, XW and DL collected, analysed and interpreted the data. YC, XC, HP and PZ designed the network architecture, and performed the data preparation and analysis. All authors contributed to the article and approved the submitted version.
FY received support from National Natural Science Foundation of China (82202265). KH received support from Ministry of Industry and Information Technology of China (2020-0103-3-1).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.985657/full#supplementary-material.
1. Zimmerman MS, Smith AGC, Sable CA, Echko MM, Wilner LB, Olsen HE, et al. Global, regional, and national burden of congenital heart disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Child Adolesc Health. (2020) 4(3):185–200. doi: 10.1016/s2352-4642(19)30402-x
2. Roberson DA, Cui W, Patel D, Tsang W, Sugeng L, Weinert L, et al. Three-Dimensional transesophageal echocardiography of atrial septal defect: a qualitative and quantitative anatomic study. J Am Soc Echocardiogr. (2011) 24(6):600–10. doi: 10.1016/j.echo.2011.02.008
3. Baumgartner H, De Backer J, Babu-Narayan SV, Budts W, Chessa M, Diller G-P, et al. 2020 Esc guidelines for the management of adult congenital heart disease. Eur Heart J. (2021) 42(6):563–645. doi: 10.1093/eurheartj/ehaa554
4. Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, et al. Diagnosis and treatment of fetal cardiac disease. Circulation. (2014) 129(21):2183–242. doi: 10.1161/01.cir.0000437597.44550.5d
5. Bartakian S, El-Said HG, Printz B, Moore JW. Prospective randomized trial of transthoracic echocardiography versus transesophageal echocardiography for assessment and guidance of transcatheter closure of atrial septal defects in children using the amplatzer septal occluder. JACC Cardiovasc Interv. (2013) 6(9):974–80. doi: 10.1016/j.jcin.2013.05.007
6. Silvestry FE, Cohen MS, Armsby LB, Burkule NJ, Fleishman CE, Hijazi ZM, et al. Guidelines for the echocardiographic assessment of atrial septal defect and patent foramen Ovale: from the American society of echocardiography and society for cardiac angiography and interventions. J Am Soc Echocardiogr. (2015) 28(8):910–58. doi: 10.1016/j.echo.2015.05.015
7. unçalp Ö, Pena-Rosas JP, Lawrie T, Bucagu M, Oladapo OT, Portela A, et al. Who recommendations on antenatal care for a positive pregnancy experience-going beyond survival. BJOG. (2017) 124(6):860–2. doi: 10.1111/1471-0528.14599
8. Zhang J, Gajjala S, Agrawal P, Tison GH, Hallock LA, Beussink-Nelson L, et al. Fully automated echocardiogram interpretation in clinical practice. Circulation. (2018) 138(16):1623–35. doi: 10.1161/CIRCULATIONAHA.118.034338
9. Kusunose K, Abe T, Haga A, Fukuda D, Yamada H, Harada M, et al. A deep learning approach for assessment of regional wall motion abnormality from echocardiographic images. JACC Cardiovasc Imaging. (2020) 13(2 Pt 1):374–81. doi: 10.1016/j.jcmg.2019.02.024
10. Huang MS, Wang CS, Chiang JH, Liu PY, Tsai WC. Automated recognition of regional wall motion abnormalities through deep neural network interpretation of transthoracic echocardiography. Circulation. (2020) 142(16):1510–20. doi: 10.1161/CIRCULATIONAHA.120.047530
11. Ouyang D, He B, Ghorbani A, Yuan N, Ebinger J, Langlotz CP, et al. Video-Based ai for beat-to-beat assessment of cardiac function. Nature. (2020) 580(7802):252–6. doi: 10.1038/s41586-020-2145-8
12. Yang F, Chen X, Lin X, Chen X, Wang W, Liu B, et al. Automated analysis of Doppler echocardiographic videos as a screening tool for valvular heart diseases. JACC Cardiovasc Imaging. (2022) 15(4):551–63. doi: 10.1016/j.jcmg.2021.08.015
13. Chollet F. Xception: deep learning with depthwise separable convolutions. 2017 IEEE conference on computer vision and pattern recognition (CVPR) (2017).
14. Li X, Ding L, Li W, Fang C editors. Fpga accelerates deep residual learning for image recognition. 2017 IEEE 2nd information technology, networking, electronic and automation control conference (ITNEC) (2017).
16. Iek Z, Abdulkadir A, Lienkamp SS, Brox T, Ronneberger OJS. Cham. 3d U-Net: Learning Dense Volumetric Segmentation from Sparse Annotation (2016).
17. Zhe C, Simon T, Wei SE, Sheikh Y editors. Realtime multi-person 2d pose estimation using part affinity fields. 2017 IEEE conference on computer vision and pattern recognition (CVPR) (2017).
18. Dosovitskiy A, Beyer L, Kolesnikov A, Weissenborn D, Zhai X, Unterthiner T, et al. An image is worth 16 × 16 Words: transformers for image recognition at scale. International conference on learning representations (2021).
19. Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal Fundus photographs. Jama. (2016) 316(22):2402–10. doi: 10.1001/jama.2016.17216
20. Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, et al. Dermatologist-Level classification of skin cancer with deep neural networks. Nature. (2017) 542(7639):115–8. doi: 10.1038/nature21056
21. Chilamkurthy S, Ghosh R, Tanamala S, Biviji M, Campeau NG, Venugopal VK, et al. Deep learning algorithms for detection of critical findings in head ct scans: a retrospective study. Lancet. (2018) 392(10162):2388–96. doi: 10.1016/s0140-6736(18)31645-3
22. Østvik A, Smistad E, Aase SA, Haugen BO, Lovstakken L. Real-Time standard view classification in transthoracic echocardiography using convolutional neural networks. Ultrasound Med Biol. (2019) 45(2):374–84. doi: 10.1016/j.ultrasmedbio.2018.07.024
23. Madani A, Arnaout R, Mofrad M, Arnaout R. Fast and accurate view classification of echocardiograms using deep learning. NPJ Digit Med. (2018) 1:91–7. doi: 10.1038/s41746-017-0013-1
24. Kusunose K, Haga A, Inoue M, Fukuda D, Yamada H, Sata M. Clinically feasible and accurate view classification of echocardiographic images using deep learning. Biomolecules. (2020) 10(5):665. doi: 10.3390/biom10050665
25. Wang J, Liu X, Wang F, Zheng L, Gao F, Zhang H, et al. Automated interpretation of congenital heart disease from multi-view echocardiograms. Med Image Anal. (2021) 69:101942. doi: 10.1016/j.media.2020.101942
26. Arnaout R, Curran L, Zhao Y, Levine JC, Chinn E, Moon-Grady AJ. An ensemble of neural networks provides expert-level prenatal detection of complex congenital heart disease. Nat Med. (2021) 27(5):882–91. doi: 10.1038/s41591-021-01342-5
27. Zhao M, Wei Y, Lu Y, Wong KKL. A novel U-net approach to segment the cardiac chamber in magnetic resonance images with ghost artifacts. Comput Methods Programs Biomed. (2020) 196:105623. doi: 10.1016/j.cmpb.2020.105623
28. Mori H, Inai K, Sugiyama H, Muragaki Y. Diagnosing atrial septal defect from electrocardiogram with deep learning. Pediatr Cardiol. (2021) 42(6):1379–87. doi: 10.1007/s00246-021-02622-0
Keywords: artificial intelligence, deep learning, echocardiography, atrial septal defects, congenital heart disease
Citation: Lin X, Yang F, Chen Y, Chen X, Wang W, Li W, Wang Q, Zhang L, Li X, Deng Y, Pu H, Chen X, Wang X, Luo D, Zhang P, Burkhoff D and He K (2023) Echocardiography-based AI for detection and quantification of atrial septal defect. Front. Cardiovasc. Med. 10:985657. doi: 10.3389/fcvm.2023.985657
Received: 4 July 2022; Accepted: 21 February 2023;
Published: 10 March 2023.
Edited by:
Steffen Erhard Petersen, Queen Mary University of London, United KingdomReviewed by:
Duraisamy Balaguru, Harvard Medical School, United States© 2023 Lin, Yang, Chen, Chen, Wang, Li, Wang, Zhang, Li, Deng, Pu, Chen, Wang, Luo, Zhang, Burkhoff and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
Specialty Section: This article was submitted to Cardiovascular Imaging, a section of the journal Frontiers in Cardiovascular Medicine
*Correspondence: Kunlun He a3VubHVuaGVAcGxhZ2gub3Jn
†These authors have contributed equally to this work
Abbreviations ASD, Atrial septal defect; AUC, Area under the curve; A4C, Modified apical four-chamber view; CDI, Color Doppler imaging; CHD, Congenital heart disease; CNN, Convolutional neural networks; DL, Deep learning; PSAX, Parasternal short-axis view; ROC, Receiver operating characteristic; SC2A, Subxiphoid sagittal view; SC4C, Subxiphoid four-chamber view; TTE, Transthoracic echocardiography.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.