- 1Division of Cardiovascular Medicine, Scripps Clinic, Prebys Cardiovascular Institute, La Jolla, CA, United States
- 2Department of Cardiovascular Medicine, University of Kansas Medical Center, Kansas City, KS, United States
- 3Division of Cardiology, Duke Medical Center, Durham, NC, United States
- 4Saint Luke’s Mid America Heart Inst, Kansas City, MO, United States
- 5Houston Methodist DeBakey Heart and Vascular Center, Houston Methodist Hospital, Houston, TX, United States
- 6University Hospital Health System, Cleveland, OH, United States
- 7PRISMA Health, Columbia, SC, United States
- 8Division of Cardiology, UNC Rex Healthcare, Raleigh, NC, United States
- 9Sanford Cardiovascular Institute, Sioux Falls, SD, United States
- 10Department of Heart Failure, Austin Heart PA, Austin, TX, United States
- 11Allina Health Minneapolis Heart Institute, Minneapolis, MN, United States
- 12Heart Failure Division, M Health Fairview St. Joseph’s Hospital, St. Paul, MN, United States
- 13Indiana University, Indianapolis, IN, United States
- 14Center for Cardiovascular Analytics, Research, and Data Science (CARDS), Providence St. Joseph Research Network, Portland, OR, United States
Background: Hemodynamic Frontiers in Heart Failure (HF2) is a multicenter academic research consortium comprised of 14 US institutions with mature remote monitoring programs for ambulatory patients with heart failure (HF). The consortium developed a retrospective and prospective registry of patients implanted with a wireless pulmonary artery pressure (PAP) sensor.
Goals/aims: HF2 registry collects demographic, clinical, laboratory, echocardiographic (ECHO), and hemodynamic data from patients with PAP sensors. The aims of HF2 are to advance understanding of HF and to accelerate development of novel diagnostic and therapeutic innovations.
Methods: HF2 includes adult patients implanted with a PAP sensor as per FDA indications (New York Heart Association (NYHA) Class III HF functional class with a prior hospitalization, or patients with NYHA Class II or brain natriuretic peptide (BNP) elevation without hospitalization) at a HF2 member site between 1/1/19 to present. HF2 registry is maintained at University of Kansas Medical Center (KUMC). The registry was approved by the institutional review board (IRB) at all participating institutions with required data use agreements. Institutions report data into the electronic registry database using REDCap, housed at KUMC.
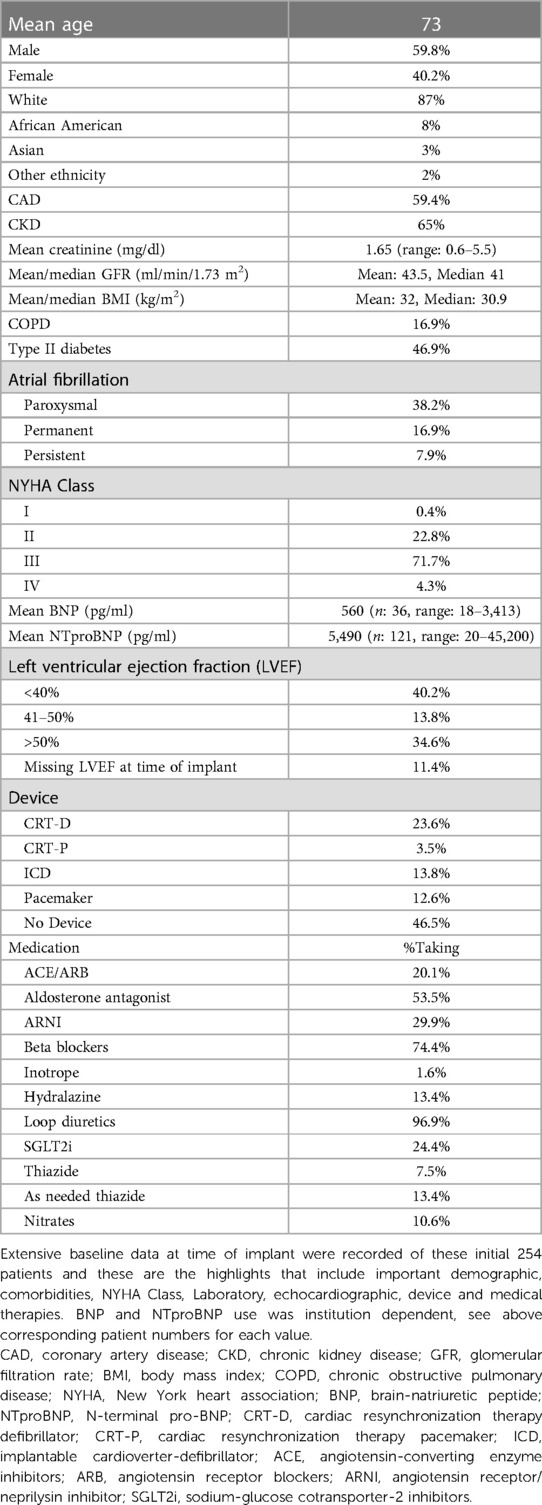
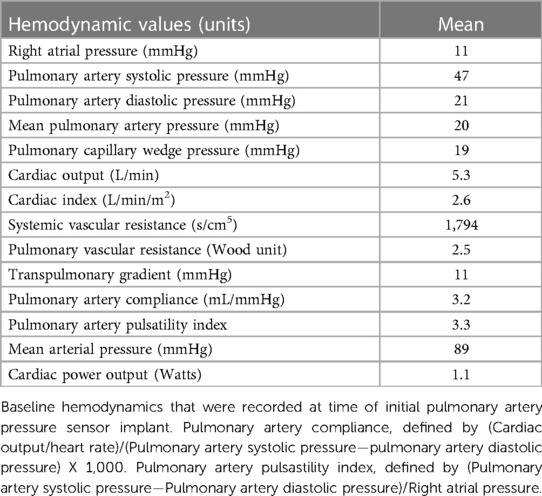
Results: This initial data set includes 254 patients implanted from the start of 2019 until May 2023. At time of device implant, the cohort average age is 73 years old, 59.8% are male, 72% have NYHA Class III HF, 40% have left ventricular ejection fraction (LVEF) < 40%, 35% have LVEF > 50%, mean BNP is 560 pg/ml, mean N-Terminal pro-BNP (NTproBNP) is 5,490 pg/ml, mean creatinine is 1.65 mg/dl. Average baseline hemodynamics at device implant are right atrial pressure (RAP) of 11 mmHg, pulmonary artery systolic pressure (PASP) of 47 mmHg, pulmonary artery diastolic pressure (PADP) 21 mmHg, mean pulmonary artery pressure (mPAP) of 20 mmHg, pulmonary capillary wedge pressure (PCWP) of 19 mmHg, cardiac output (CO) of 5.3 L/min, and cardiac index (CI) of 2.5 L/min/m2.
Conclusion: A real-world registry of patients implanted with a PAP sensor enables long-term evaluation of hemodynamic and clinic outcomes in highly-phenotyped ambulatory HF patients, and creates a unique opportunity to validate and test novel diagnostic and therapeutic approaches to HF.
Introduction
Decompensated HF manifests through symptoms attributed to hemodynamic and neurohormonal abnormalities, leading to peripheral edema, pulmonary congestion, orthopnea, and paroxysmal nocturnal dyspnea (1). Zile et al. demonstrated that elevated filling pressures precede hospital admissions for HF and improve with therapy (2). Recent studies have shown that ambulatory PAP monitoring using the CardioMEMS© system can reduce HF hospitalizations and improve quality of life by proactively managing hemodynamic changes compared to the conventional approach of reacting to signs or symptoms of HF (3–6).
Beyond its clinical utility, ambulatory hemodynamic monitoring offers unique platform for investigation into HF. While randomized trials of PAP sensors had follow-up for 6–18 months, patients often have these devices implanted for years. Given the paucity of longitudinal hemodynamic, treatment, and clinical outcomes in ambulatory patients with HF, the HF2 registry represents a deeply and uniquely phenotyped HF population (7). Moreover, this monitored population with a documented hemodynamic history (“hemodynamic transcript”) presents an opportunity for rapid and cost-effective testing of novel diagnostic and therapeutic approaches. In the early stages of drug development for renin-angiotensin-aldosterone system (RAS) blockers, invasive inpatient hemodynamic monitoring served as a proof of concept before large randomized controlled trials were conducted (8, 9). Although these trials were costly and involved a limited number of patients, they provided crucial data that informed the design and power calculations for subsequent pivotal outcome trials. In contrast, contemporary trials could be conducted faster, more cost-effectively, and with greater safety by assessing the effect of interventions on PAP, which represents a hemodynamic surrogate tied to improved clinical outcomes (10).
Against this backdrop, we have established a consortium of experienced CardioMEMS© centers to create a comprehensive retrospective and prospective registry of patients implanted with a sensor. The HF2 registry capture extensive demographic, laboratory, ECHO and treatment information, hemodynamic data from the CardioMEMS© device, and clinical events. The purpose of this manuscript is to provide a detailed description of the dataset and present information on the first 254 patients enrolled.
Methods
Study design and participants
The HF2 registry includes data from participating institutions on consecutive adult patients implanted with CardioMEMS© as per FDA indications (NYHA Class III HF diagnosis with a prior hospitalization or patients with NYHA Class II or BNP elevation without hospitalization).
This registry was approved by the KUMC IRB (IRB study 00147383) and by local IRBs at participating institutions. KUMC and all participating institutions have signed an appropriate data use agreement. Participating institutions reported the data into the electronic registry database, which is built and maintained using REDCap data capture tools and housed at KUMC (11). Patient demographic data, blood test results, hemodynamic data from device implant, hemodynamic data from the CardioMEMS©, ECHO data, electrocardiogram (ECG) data, and clinical outcomes (HF hospitalizations, all-cause hospitalizations, emergency department (ED) visits, intravenous (IV) diuretic administration in the clinic, device-related complications, device recalibrations after initial implant, heart transplant, left ventricular assist device (LVAD), and death) are captured.
HF2 has a Registry scientific steering committee that is the governing body and serve as the gate keepers of the HF2 Working Group's intellectual property, vetting project proposals in which the HF2 Registry will be queried for research purposes to ensure the projects meet the objectives of the Registry as well as the Mission Statement of the HF2 Working Group. There is also a publication committee which reviews and prioritizes research proposals based on the study design and analysis plan and subsequently manages how Registry publications are organized (see Figure 1).
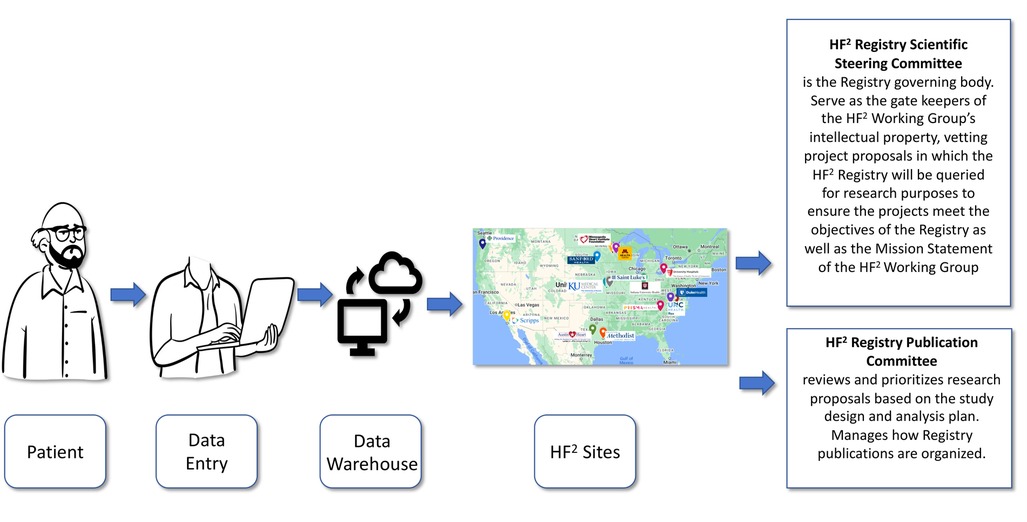
Figure 1. Workflow of the HF2 registry. The flow of data in the HF2 Registry from patient, to data entry, to the central Data warehouse, via individual institutions across the United States and overseen by both a scientific steering committee and a publication committee.
Statistical analysis
Statistical analysis was done using IBM SPSS software version 29.0. Descriptive statistics were used to calculate frequencies, mean & median for the data variables used which include age, race, gender, comorbidity conditions, laboratory values, ECHO, NYHA class, implanted devices, medications and right heart catheterization data.
Results
This initial report includes 254 patients implanted from the beginning of 2019 until May 2023 at 12 participating sites across the United States. See Table 1 for baseline demographic, comorbidity, laboratory, echocardiographic, and medication data. See Table 2 for initial hemodynamic measurements at the time of implant.
The mean age of patients is 73 years old, and 40.2% are female. Most patients are White (87%), with 8% African American, 3% Asian, and 2% other ethnicity. There is a high prevalence of coronary artery disease (CAD) (59.4%), atrial fibrillation (AF) (63%), chronic kidney disease (CKD) (65%), Chronic Obstructive Pulmonary Disease (COPD) (16.9%), and diabetes mellitus type II (DMII) (46.9%).
Most patients have NYHA Class III symptoms (71.7%), and the remaining have predominantly NYHA II symptoms (22.8%). Multiple lab parameters, including a complete metabolic panel, natriuretic peptides, hemoglobin, and hemoglobin A1c were recorded into the database. The cohort, on average has elevated BNP and NTproBNP levels, 560 pg/ml and 5,490 pg/ml, respectively. At time of implant, 40.2% of patients had an LVEF <40%, 13.8% of patients had an LVEF of 41%–50%, 34.6% of patients have an LVEF of >50%. Regarding device therapy, 23.6% of patients have a Cardiac Resynchronization Therapy Defibrillator (CRT-D), 3.5% have a Cardiac Resynchronization Therapy Pacemaker (CRT-P) device, 13.8% have an implantable cardioverter-defibrillator (ICD), and 46.5% have no device. 20.1% of patients are on angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), 29.9% of patients are on angiotensin receptor/neprilysin inhibitor (ARNI), 74.4% of patients are on beta blockers (BB), 53.5% of patients are on aldosterone antagonists, 24.4% of patients are on sodium-glucose cotransporter-2 inhibitors (SGLT2i), 96.9% of patients are on some form of loop diuretic and 1.6% of patients are on inotrope therapy.
Mean hemodynamic values at the time of implant are listed in Table 2. Right-sided pressures were elevated with a mean RAP of 11 mmHg, there was mildly elevated left-sided pressures with a mean PCWP of 19 mmHg, and elevated pulmonary pressures with PASP/PADP of 47/21 mmHg and mPAP of 30 mmHg. CO and CI by indirect Fick were normal, 5.3 L/min and 2.6 L/min/m2, respectively. Systemic vascular resistance (SVR) mean was 1,794 s/cm5, pulmonary vascular resistance (PVR) mean was 2.5 Wood unit with a mean transpulmonary gradient (TPG) of 11mmHg. Pulmonary artery compliance, defined as (CO/heart rate)/(PASP—PADP) X 1,000, mean was 3.2 ml/mmHg. The mean pulmonary artery pulsastility index, defined as (PASP—PADP)/RAP, was 3.3.
Discussion
HF patients present with a wide array of comorbidities, devices, and ECHO and hemodynamic profiles and are treated with an increasingly complex therapeutic regimen. Through this registry of patients with the CardioMEMS© device, our aim is to better understand this population of patients through the collection of relevant patient-level demographic, clinical, laboratory, echocardiographic and hemodynamic data. The creation of this registry will help facilitate future HF research by providing a platform to answer a variety of questions and test various therapeutic interventions (see Figure 2, Central Illustration).
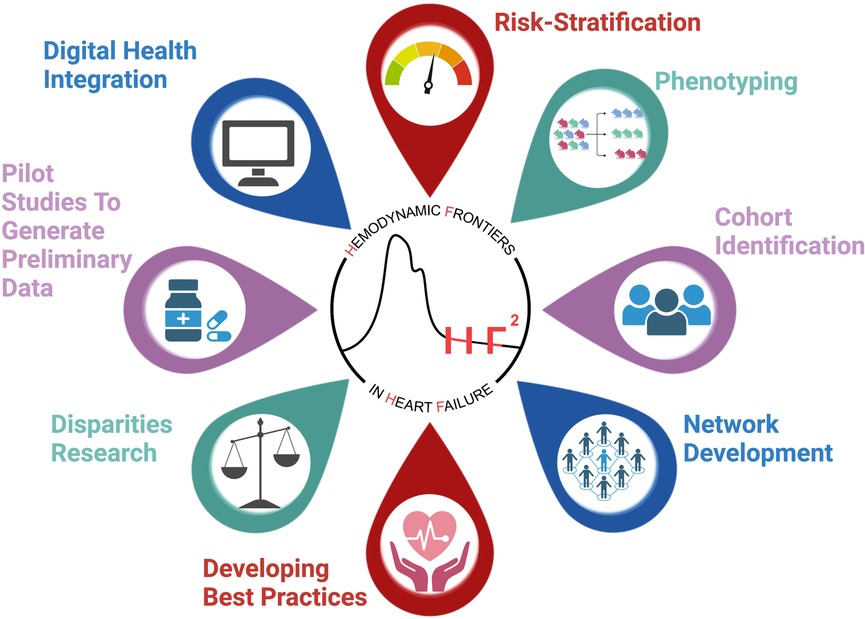
Figure 2. Central illustration demonstrating the utility of the HF2 registry as a test bed for multiple applications.
In many ways, the 254 patients included in this report reflect real world HF patients that are often excluded from clinical trials. Our patient population is comparable to those in major preceding CardioMEMS© trials as well as recent pivotal HF trials, though with some notable differences. Our patients are older than patients enrolled in contemporary device HF trials (3–5, 12, 13). With 40% women, our cohort is more balanced for gender than the above trials, though similar to the EMPEROR trial (45% women) (14). In our cohort patients were predominantly White, 87%, though similar to the heart failure with preserved ejection fraction (HFpEF) group of GUIDE-HF which had 89% white patients (4, 13). The cohort predominantly includes patients with NYHA class III symptoms, with elevated baseline BNP or NTproBNP levels, as is comparable to GUIDE-HF, and suggests advanced disease and a high event rate (4, 13). There are almost equal portions of patients with HFpEF and a smaller portion of patients with mid-range ejection fraction (41–50%), also comparable to GUIDE-HF (4, 13). Our patients frequently have CAD, CKD, DMII, and AF, as is typical in hospitalized HF patients. However, 63% of our cohort have AF, which is higher than the aforementioned trials (3–5, 12–14). Also, our patients more frequently have CKD and lower GFR, which is more typical of real word HF patients. Just over half of patients had some type of device, either ICD or CRT. About 40% of patients had heart failure with reduced ejection fraction (HFrEF), and a large portion of patients were on appropriate guideline-directed medical therapy (GDMT) with ACE/ARB, ARNI, aldosterone antagonists, BB. Although only 24.4% of patients were on SGLT2i, the time of implant for a large portion of patients was prior to practice changing trials using these medications. Nearly the entire cohort, 97% of patients, were on loop diuretics of varying types, either furosemide, torsemide, or bumetanide. Baseline hemodynamic profiles at the time of implant further exemplify the heterogenous nature of HF patients. Patients, on average, had elevated RAP (11 mmHg) and PCWP (19 mmHg), with a mixture of pre, post, and mixed pulmonary hypertension, though patients did on average have normal CO. In our cohort, PASP was 47 mmHG, PADP was 21 mmHg and mPAP was 30 mmHg, similar to GUIDE-HF, with 45 mmHg, 19 mmHg, and 29 mmHg respectively (4, 13).
Having coalesced easily accessible and extensive data in our registry on this population of patients with the CardioMEMS© device, we will be able to better understand this group of patients through both retrospective research studies on the existing data and by collecting prospective data for future research studies. The registry formed by the ADHERE-HF group demonstrated the importance of following patients over time, and they were able to provide insights into the clinical characteristics, patterns of care, and outcomes of HF patients, while subsequently developing tools for the improvement of quality of care for HF patients (15).
Prior work by Lam et al, demonstrated the importance of pulmonary hypertension (PH) in patients with HFpEF (16). One of the first population-based estimates showed that PH was highly prevalent, often severe in HFpEF patients, and an adverse prognostic factor in HFpEF, independent of age (16). In addition, they proved that there was a pre-capillary component to these patients with HFpEF and PH, outside of the pulmonary venous congestion alone, suggesting a potential role in the treatment of pulmonary artery hypertension in the treatment of HFpEF (16). The majority of the registry patients have PH which could provide a convenient way to assess the reversibility of this process in left heart disease.
An extremely important goal of the registry will be to serve as a test bed for early HF therapies. Notably, two such cohorts with sacubitril/valsartan, and one with dapagliflozin, have taken these guideline directed medications and shown the ability to effectively decrease pulmonary pressures after a period of 4–6 weeks (10, 17, 18). As a consortium of HF cardiologists around the country, we will be able to combine ideas and hypothesis and rapidly test them utilizing the registry (6, 19, 20). With a test bed of HF patients, we can also enroll them in prospective studies to test a variety of drugs, approved or unapproved, and monitor their responses outside of large retrospective clinical trials. We can generate fast crossover trials to evaluate hemodynamic and cardiorenal effects of new therapies and compounds in HF that provide important data in early drug and device development in a period of months rather than years. This should speed product development and thus reduce costs.
Limitations
The cohort of patients that remain in the database are those that are still alive, so future prospective research on this group of patients may be skewed by survivor bias. Patients with advanced CKD are limited in this data set given their inability to respond to diuretics and thus lower likelihood to be implanted with the CardioMEMS© device.
Conclusions
Presented here is the first cohort of HF patients implanted with the CardioMEMS© device in our multisite registry. A real-world registry of patients with extended hemodynamic monitoring can evaluate long-term outcomes in HF patients, provide data in unique patient groups, and provide an opportunity to evaluate diagnostic and therapeutic HF approaches in rapid turnaround cross over trials.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by University of Kansas Medical Center (KUMC). The registry was approved by the institutional review board (IRB) at all participating institutions with required data use agreements. Institutions report data into the electronic registry database using REDCap, housed at KUMC. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. TJ: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. EM: Conceptualization, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Visualization, Writing – original draft. MF: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AS: Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. MM: Data curation, Writing – original draft. HS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AB: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft. RK: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – original draft. MR: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft. PM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft. EV: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft. AG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft. OJ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft. KB: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft. MB: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft. TB: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft. MG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft. JA: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
TH received honoraria from Johnson and Johnson, United Therapeutics, Boehringer Ingelheim, Bayer, Lily as well as research grant support from Johnson and Johnson. JA is a consultant for Abiomed and Abbott, and is on the speaker's bureau for Abiomed, Abbott, Ancora. MF is a speaker for Abbott. AS disclosures include consulting for or receiving research funding from Abbott, Boston Scientific, Medtronic, Biotronik, Impulse Dynamics, Acorai, General Prognostics, Edwards Lifesciences, Story Health, Bayer, CSL Vifor. HS is a consultant for Endotronix and a speaker for Impulse Dynamics. AB is a consultant for Abbott. PM is a consultant for Abbott, AstraZeneca, Janssen, Daxor, and CVRx. OJ is a speaker for Abbott. KB is on the speaker's bureau for Abbott, Pfizer, and Novartis and is a consultant for Abbott and Vectorius. MB is a consultant for Abbott and Rhythm Management Group. TAB is a consultant for Abbott.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey Jr DE, et al. 2013 ACCF/AHA guideline for the management of heart failure. J Am Coll Cardiol. (2013) 62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019
2. Zil MR, Bennett TD, St John Sutton M, Cho TK, Adamson PB, et al. Transition from chronic compensated to acute decompensated heart failure. Circulation. (2008) 118(14):1433–41. doi: 10.1161/CIRCULATIONAHA.108.783910
3. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. (2011) 377(9766):658–66. doi: 10.1016/S0140-6736(11)60101-3
4. Lindenfeld J, Zile MR, Desai AS, Bhatt K, Ducharme A, Horstmanshof D. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomised controlled trial. Lancet. (2021) 398(10304):991–1001. doi: 10.1016/S0140-6736(21)01754-2
5. Brugts JJ, Radhoe SP, Clephas PRD, Aydin D, van Gent MWF, Szymanski MK. Remote haemodynamic monitoring of pulmonary artery pressures in patients with chronic heart failure (MONITOR-HF): a randomised clinical trial. Lancet. (2023) 401(10394):2113–23. doi: 10.1016/S0140-6736(23)00923-6
6. Abraham J, McCann PJ, Guglin ME, Bhimaraj A, Benjamin TAS, Robinson MR. Management of the patient with heart failure and an implantable pulmonary artery hemodynamic sensor. Curr Cardiovasc Risk Rep. (2020) 14(9):12. doi: 10.1007/s12170-020-00646-4
7. Heywood JT, Zalawadiya S, Bourge RC, Costanzo MR, Desai AS, Rathman LD. Sustained reduction in pulmonary artery pressures and hospitalizations during 2 years of ambulatory monitoring. J Card Fail. (2023) 29(1):56–66. doi: 10.1016/j.cardfail.2022.10.422
8. de Graeff PA, Kingma JH, Viersma JW, Wesseling H, Lie KI. Acute and chronic effects of ramipril and captopril in congestive heart failure. Int J Cardiol. (1989) 23(1):59–67. doi: 10.1016/0167-5273(89)90330-6
9. Dickstein K, Søyland E, Gundersen T, Abrahamsen AM, Kjekshus J. Acute and chronic hemodynamic effects of enalapril (MK-421) in congestive heart failure. Int J Cardiol. (1984) 6(4):445–56. doi: 10.1016/0167-5273(84)90324-3
10. Mullens W, Martens P, Forouzan O, Dauw J, Vercammen J, Luwel E. Effects of dapagliflozin on congestion assessed by remote pulmonary artery pressure monitoring. ESC Heart Fail. (2020) 7(5):2071–3. doi: 10.1002/ehf2.12850
11. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42(2):377–81. doi: 10.1016/j.jbi.2008.08.010
12. McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. (2014) 371(11):993–1004. doi: 10.1056/NEJMoa1409077
13. Zile MR, Mehra MR, Ducharme A, Sears SF, Desai AS, Maisel A. Hemodynamically-guided management of heart failure across the ejection fraction spectrum. JACC Heart Fail. (2022) 10(12):931–44. doi: 10.1016/j.jchf.2022.08.012
14. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385(16):1451–61. doi: 10.1056/NEJMoa2107038
15. Fonarow GC, ADHERE Scientific Advisory Committee. The acute decompensated heart failure national registry (ADHERE): opportunities to improve care of patients hospitalized with acute decompensated heart failure. Rev Cardiovasc Med. (2003) 4 (Suppl 7):S21–30. PMID: 14668697
16. Lam CSP, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction. J Am Coll Cardiol. (2009) 53(13):1119–26. doi: 10.1016/j.jacc.2008.11.051
17. Codina P, Domingo M, Barceló E, Gastelurrutia P, Casquete D, Vila J. Sacubitril/valsartan affects pulmonary arterial pressure in heart failure with preserved ejection fraction and pulmonary hypertension. ESC Heart Fail. (2022) 9(4):2170–80. doi: 10.1002/ehf2.13952
18. Desai AS, Heywood JT, Rathman L, Abraham WT, Adamson P, Brett ME. Early reduction in ambulatory pulmonary artery pressures after initiation of sacubitril/valsartan. Circ Heart Fail. (2021) 14(7):e008212. doi: 10.1161/CIRCHEARTFAILURE.120.008212
19. Khedraki R, Abraham J, Jonsson O, Bhatt K, Omar HR, Bennett M. Impact of exercise on pulmonary artery pressure in patients with heart failure using an ambulatory pulmonary artery pressure monitor. Front Cardiovasc Med. (2023) 10:1–6. doi: 10.3389/fcvm.2023.1077365
Keywords: pulmonary artery pressure, pulmonary hypertension, heart failure, hemodynamics, remote monitoring, CardioMEMS
Citation: Heywood JT, Munshi K, Jordan T, Muse E, Fudim M, Sauer AJ, McDermott M, Shah H, Bhimaraj A, Khedraki R, Robinson MR, McCann P, Volz E, Guha A, Jonsson O, Bhatt KA, Bennett MK, Benjamin TA, Guglin M and Abraham J (2023) Multicenter registry and test bed for extended outpatient hemodynamic monitoring: the hemodynamic frontiers in heart failure (HF2) initiative. Front. Cardiovasc. Med. 10:1321415. doi: 10.3389/fcvm.2023.1321415
Received: 14 October 2023; Accepted: 13 November 2023;
Published: 29 November 2023.
Edited by:
Michael Henein, Umeå University, SwedenReviewed by:
Juan Aranda, Jr., University of Florida, United StatesGarrie Haas, The Ohio State University, United States
© 2023 Heywood, Munshi, Jordan, Muse, Fudim, Sauer, McDermott, Shah, Bhimaraj, Khedraki, Robinson, McCann, Volz, Guha, Jonsson, Bhatt, Bennett, Benjamin, Guglin and Abraham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Timothy Jordan Sm9yZGFuLnRpbW90aHlAc2NyaXBwc2hlYWx0aC5vcmc=
 J. Thomas Heywood1
J. Thomas Heywood1 Kartik Munshi
Kartik Munshi Timothy Jordan
Timothy Jordan Marat Fudim
Marat Fudim Arvind Bhimaraj
Arvind Bhimaraj Orvar Jonsson
Orvar Jonsson Jacob Abraham
Jacob Abraham