- 1Queensland Cardiovascular Group, Brisbane, QLD, Australia
- 2Faculty of Humanities, University of Queensland, Brisbane, QLD, Australia
Objectives: This study aimed to assess left heart remodelling changes in hypertension, excluding underlying ischaemic heart disease, utilising computed tomography coronary angiography (CTCA) and transthoracic echocardiography (TTE).
Methods: A total of 178 patients (mean age 60 ± 9 years, 53% female) were enrolled in the study: Group 1 consisted of patients with essential hypertension (n = 96, Group 1), and Group 2 served as age-matched controls (n = 82, Group 2). All participants underwent both CTCA and TTE. TTE measurements included left ventricle (LV) concentricity and function and left atrial (LA) volume and function. Using both CTCA and TTE, we measured LV diastasis volume (LVdias) and LA diastasis volume (LAdias).
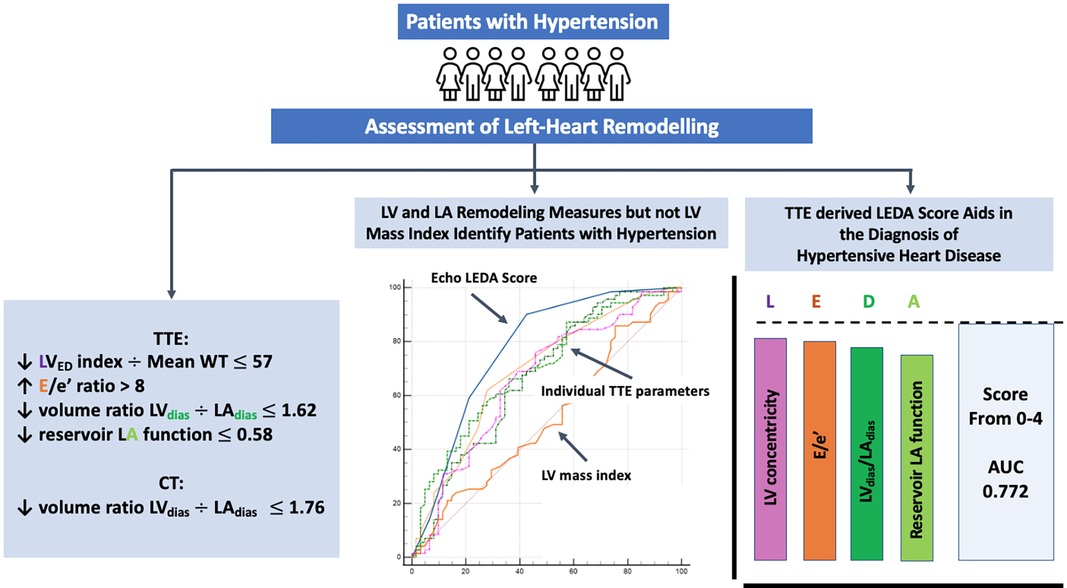
Results: LV mass index and LV mass/height2.7 were similar in both the groups. However, Group 1 had a higher prevalence of concentric LV remodelling, characterised by a larger mean LV wall thickness, increased relative wall thickness ratio, and a reduced ratio of LV end-diastolic volume (LVED) index to mean wall thickness (55 ± 14 vs. 65 ± 15, p = 0.0007). Group 1 showed higher LAdias and LA minimal volumes, while LA reservoir function was lower in Group 2. The LVdias/LAdias ratio was lower in Group 1 compared to Group 2 (TTE 1.77 ± 0.61 vs. 2.24 ± 1.24, p = 0.0025, CTCA 1.50 ± 0.23 vs. 1.69 ± 0.41, p = 0.0002). A composite score based on four combined TTE parameters, namely, LVED index/mean wall thickness ≤57, ratio of early diastolic mitral inflow to mitral annular tissue velocities (E/e’) >8, LVdias/LAdias ≤1.62, and LA reservoir function ≤0.58, yielded the highest discriminatory power (area under the curve—AUC = 0.772) for distinguishing patients with hypertensive heart disease (HHD). Collectively, we refer to these parameters as the LEDA score, with each parameter scored as one point. For LEDA scores of 0, 1, 2, 3, 4, the probability of underlying HHD was 0%, 23%, 59%, 80%, and 95%, respectively. Furthermore, a CTCA-derived LVdias/LAdias ≤1.76, considered as a single parameter, demonstrated modest accuracy in differentiating patients with HHD (AUC = 0.646).
Conclusions: The TTE LEDA score, based on four parameters, namely, LVED index/mean wall thickness, E/e’, LVdias/LAdias, and LA reservoir function, proved to be the most effective in defining left heart remodelling in hypertension.
Introduction
While there have been significant global improvements in the treatment and control of hypertension, there remains a critical need for more timely detection of this condition. This is because the cumulative effects of hypertension can ultimately lead to cardiac overload, remodelling, and heart failure (HF) (1–3). It is noteworthy that each month of active antihypertensive therapy has been associated with a one-day prolongation of life expectancy without cardiovascular death (4). Despite the well-established link between hypertension and increased all-cause mortality, the challenge lies in detecting early adaptive stages of cardiac remodelling (5–9). At present, current transthoracic echocardiogram (TTE) criteria primarily focus on assessing the effects of the maladaptive processes that occur at a stage when the left ventricular (LV) wall thickness and mass (10–13) are already increased, and LV filling has progressed to an advanced or irreversible stage, accompanied by dilatation of both the left atrium (LA) and LV (5, 8). However, it has been shown that the most favourable prognosis for patients with hypertension occurs during the adaptive stage (14, 15). Therefore, it has been proposed that an easily obtainable TTE score for the early detection of cardiac remodelling could significantly aid in treatment and improve patient prognosis. To date, there has been limited investigation into the impact of hypertension in the absence of underlying ischaemic heart disease on left heart remodelling using a combination of two-dimensional and Doppler TTE data. Our study seeks to address this gap in knowledge and assess the clinical significance of left heart remodelling indices, including LV concentricity, filling pressure, LA function, and the LV/LA volume ratio, with particular focus on the concept of atrioventricular interplay (14, 15). Moreover, our investigation includes the assessment of the diastasis LV/LA volume ratio, which we measured using both TTE and computed tomography coronary angiography (CTCA). This ratio serves as a surrogate for global (net) left heart atrioventricular adaptive remodelling in patients with hypertension. By elucidating the effects of hypertension on left heart remodelling in its early stages, we aim to contribute valuable insights that could inform more proactive clinical approaches and ultimately improve patient outcomes.
Methods
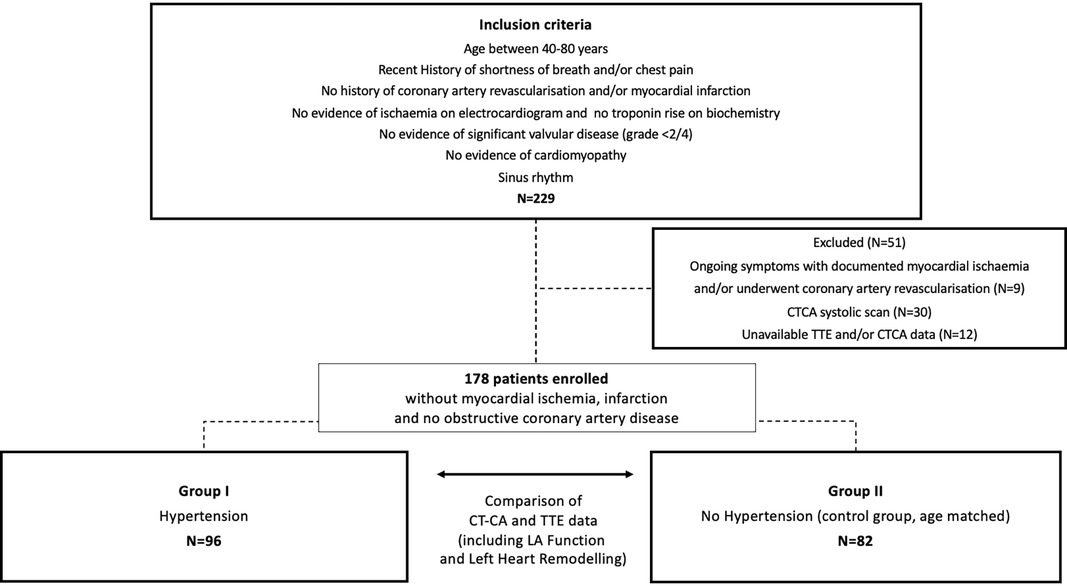
This study included patients aged 40–80 years who were referred for CTCA between February 2019 and August 2021. Patients with shortness of breath and/or chest pain were selected from our clinical consecutive referral cohort if they met the following criteria: (1) sinus rhythm, (2) absence of valvopathy ≥2/4 and/or cardiomyopathy, and (3) CTCA and TTE of diagnostic image quality. Patients with documented myocardial ischaemia and/or history of myocardial infarction or coronary artery revascularisation were excluded. The initial group consisted of 229 patients, with 51 patients excluded for the following reasons: nine of 51 patients were excluded because of subsequently documented myocardial ischaemia requiring coronary artery revascularisation. A total of 30 of the 51 patients were excluded as their CTCA was a systolic scan. A total of 12 of the 51 patients were excluded because of incomplete TTE or CTCA data (Figure 1). The final study group comprised 178 patients, divided into two groups: Group 1 consists of patients with diagnosed hypertension (n = 96) and Group 2 contains an age-matched control group that included patients who had no clinical evidence of hypertension or diabetes (n = 82). Hypertension was diagnosed according to the European Society of Cardiology guidelines (13).
Data were prospectively collected, analysed, and made available upon reasonable request to the corresponding author. This study complied with the principles of the Declaration of Helsinki. It was approved by the Uniting Care Health Human Research Ethics Committee (number 2019.29.307) of Brisbane, Australia. Informed consent was obtained from all patients.
TTE was performed using standard equipment and protocols (Philips EPIQ CVx, Philips North America Corporation, Andover, MA) or Siemens SC200 (Siemens Medical Solutions, Mountain View, CA, USA). A standard clinical imaging protocol was used for each patient. The protocol consisted of M-mode, two-dimensional, and Doppler analyses (16, 17). Apical views were optimised to avoid foreshortening of the LV and/or LA. LV volume was measured using the biplane Simpson method at end-diastole (LVED), end-systole, and diastasis (LVdias). The LA area was planimetered from the four-chamber and two-chamber views, excluding the LA appendage and pulmonary veins. LA volume was calculated using the biplane area-length method. LA volume was measured in three phases of the cardiac cycle: maximum (LAmax) at LV end-systole, diastasis (LAdias), and minimum (LAmin) at LV end-diastole. LA function parameters included (i) LA reservoir function, total emptying volume, and emptying fraction; (ii) LA conduit function, LA passive emptying volume, and emptying fraction; and (iii) LA pump function, LA active emptying volume, and emptying fraction, as previously described (18). Each measurement was averaged over the three cardiac cycles. All the patients were divided according to the recommendations of the American Society of Echocardiography/European Association of Cardiovascular Imaging for the classification of LV diastolic dysfunction (17).
CTCA was performed in a standard manner using a 256iCT scanner (Philips North America Corporation, Andover, MA, USA). The iCT had a 270-ms gantry rotation time and temporal resolution of 135 ms. The collimation scan parameters were 128 mm × 0.625 mm. An intravenous injection of iodinated contrast media (Omnipaque 350) was used to opacify the coronary arteries and, subsequently, the LA and LV. The scan was acquired during a single breath hold, using the prospective electrocardiogram-gated axial step-and-shoot/sequential technique. Only patients scanned in diastasis between 75% and 81% of the R-R interval were chosen for the analysis, with the standard acquisition occurring at 78%. The LA volume was measured by excluding the LA appendage. The LV volume was measured using an automated algorithm within the cardiac software of the Philips Intellispace Portal. The diastolic function assessment, that is, the diastolic expansion index, was measured as the ratio between diastasis LV and LA volume (LVdias/LAdias) (19).
Statistical analysis
Continuous variables are expressed as mean ± standard deviation, and categorical variables are expressed as numbers and percentages. The two-sample t-test, χ2 test, Fisher exact test, or non-parametric Wilcoxon/Kruskal–Wallis rank-sum test was used when appropriate for comparison between Group 1 and Group 2. Pearson's correlation coefficient was used to estimate the degree of association between two quantitative variables.
For predefined TTE and CTCA measurements, logistic regression analysis with the DeLong method was used to assess the area under the receiver operating characteristic curve and Youden index J point with a cut-off value to differentiate patients from Groups 1 and 2. Sensitivity and specificity were calculated in a standard manner. Multivariate analysis of variance (MANOVA) was performed to determine the difference in complicated and uncomplicated subgroups of hypertension compared with the age-matched group. A multivariate correlation coefficient or likelihood ratio was used to determine the relationship between echo score and clinical parameters. For all analyses, p-values <0.05 were considered significant. All analyses were performed using JMP 15 (SAS Institute, Cary, NC, USA) and MedCalc Version 20.109 (Oostende, Belgium).
Results
Patient characteristics are presented in Table 1.
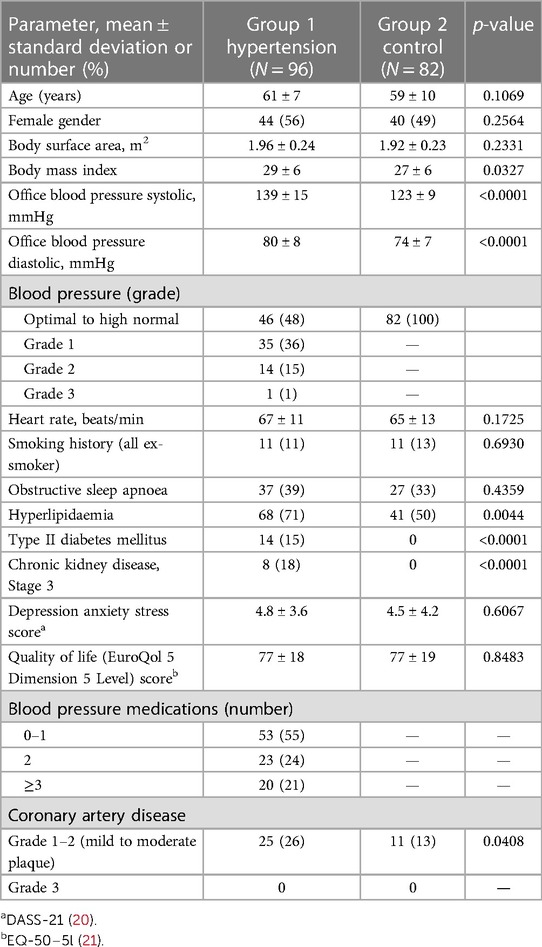
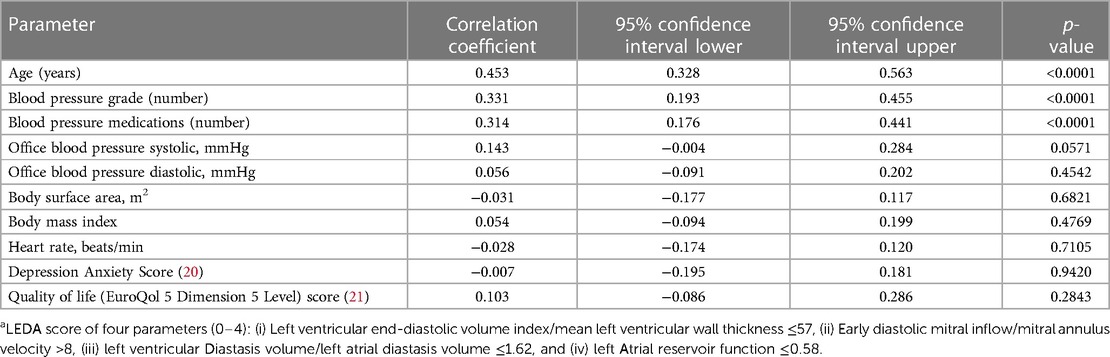
Group 1 had a higher body mass index, higher rates of hyperlipidaemia, Stage 3 chronic kidney disease, and often mild-to-moderate coronary artery plaques. Complicated hypertension with diabetes or Stage 3 chronic kidney disease was observed in 28 (29%) patients. The depression anxiety stress score (20) and quality of life score (21) were similar in both the groups.
Comparison of two-dimensional echocardiographic data
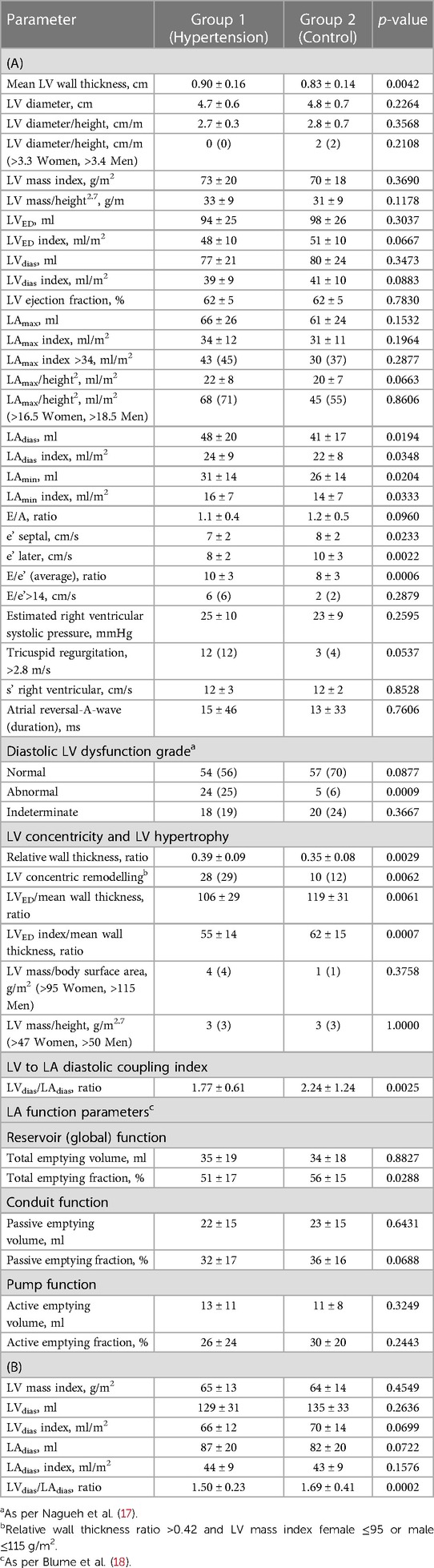
As shown in Table 2: Patients in Group 1 had increased mean LV wall thickness (mm) (0.90 ± 0.16 vs. 0.83 ± 0.14, p = 0.0042) with no differences in LV diameter, LVED, or LVdias. The ratios of LVED to mean wall thickness and LVED index to mean wall thickness were lower in Group 1 than in Group 2 (106 ± 29 vs. 119 ± 31, p = 0.0061; and 55 ± 14 vs. 62 ± 15, p = 0.0007, respectively). The LV relative wall thickness ratio was higher in Group 1 (0.39 ± 0.09 vs. 0.35 ± 0.08, p = 0.0029). Concentric LV remodelling was more frequent in Group 1 than in Group 2 [28 (29%) vs. 10 (12%), p = 0.0062].
There were no differences between the groups for the following measurements: LV mass index, LV mass/height2.7, and LV ejection fraction.
The analysis of the LA showed that in Group 1, the LA was bigger for the following measurements: LAdias (ml) (48 ± 20 vs. 41 ± 17, p = 0.0194), LAdias index (ml/m2) (24 ± 9 vs. 22 ± 8, p = 0.0348), LAmin (ml) (31 ± 14 vs. 26 ± 14, p = 0.0204), and LAmin index (ml/m2) (16 ± 7 vs. 14 ± 7, p = 0.0333). LAmax as the absolute value or indexed (by either body surface area or height2.7) did not differ between Groups 1 and 2. The LVdias/LAdias (ml) was lower in Group 1 compared to Group 2 (1.77 ± 0.61 vs. 2.24 ± 1.24, p = 0.0025). Among all the studied LA function parameters, only the LA reservoir function measured by LA total emptying fraction (%) was lower in Group 1 compared to Group 2 (51 ± 0.17 vs. 56 ± 15, p = 0.0288).
Comparison of Doppler data
Table 2: Group 1 had lower e’ values (cm/s) for both the septum (7 ± 2 vs. 8 ± 2, p = 0.0233) and lateral wall (8 ± 2 vs. 10 ± 3, p = 0.0022). The mean value of the ratio of early diastolic mitral inflow to mitral annular tissue velocities (E/e’) averaged for both the septum and lateral wall was higher in Group 1 (10 ± 3 vs. 8 ± 3, p = 0.0006).
Comparison of combined criteria for LV diastolic dysfunction
Table 2, LV diastolic dysfunction was more common in Group 1 than in Group 2 [24 (25%) vs. 5 (6%), p = 0.0009]. However, a similar percentage of patients in Groups 1 and 2 had normal or indeterminate diastolic function.
CT data
Table 2: As noted in the TTE data, there was no difference between the groups in terms of the LV mass and LV mass index. There was no difference in LVdias or LAdias between the groups, whether considering the absolute value or the indexed body surface area. The ratio of LVdias/LAdias was lower in Group 1 than in Group 2 (1.50 ± 0.23 vs. 1.69 ± 0.41, p = 0.0002, respectively).
There was a good correlation between TTE LVdias/LAdias and CT LVdias/LAdias [for the whole group: r = 0.559, confidence interval (CI) 0.448–0.652, p < 0.0001; for Group 1: r = 0.431, CI, 0.252–0.581, p < 0.0001; for Group 2: r = 0.560, CI, 0.391–0.693, p < 0.0001].
Both TTE and CTCA parameters were analysed using receiver operating characteristics to identify patients with hypertensive heart disease (HHD), see Table 3. We followed the original concept of classifying symptoms into two categories: disease and no disease. Predictive modelling for CTCA data showed that the LVdias/LAdias ratio ≤1.76 had discriminatory power to differentiate patients with HHD with an area under the curve (AUC) of 0.646 (p = 0.0006). The LVdias/LAdias ratio ≤ of 1.76 had a high sensitivity of 91% but low specificity of 36%.
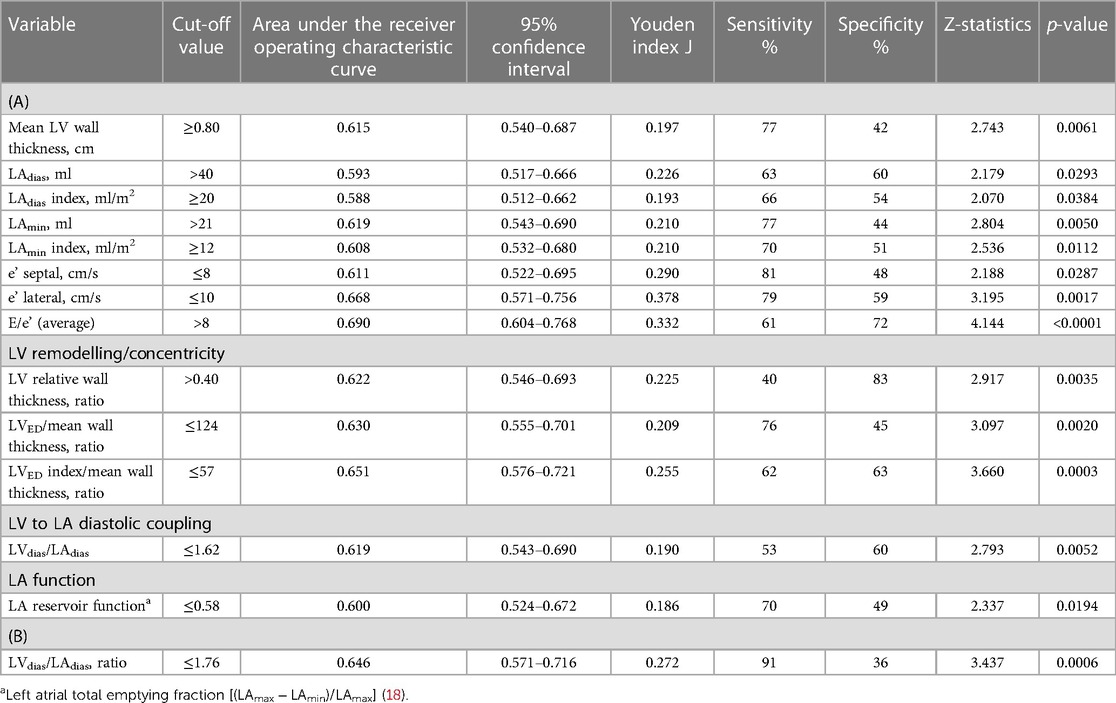
Table 3. Cut-off values and power analysis of (A) echocardiographic- and (B) computed tomography–derived parameters for diagnosis of hypertensive heart disease.
Predictive modelling for TTE data includes the parameters listed in Table 3. Doppler-derived E/e’>8, LV concentricity/remodelling measured by the ratio of LVED index/mean wall thickness ≤57, ratio of LVdias/LAdias ≤1.62, and LA reservoir function ≤0.58 had a high discriminatory power to diagnose HHD (AUC: 0.690, 0.651, 0.619, and 0.600, p < 0.0001, p = 0.0003, p = 0.0052, and p = 0.0194, respectively). These four TTE-derived parameters were chosen for their overall accuracy in predicting HHD. They were grouped to obtain an overall simplified diagnostic TTE (LEDA) score. This method provided the highest discriminatory power with an AUC of 0.772 and overpowered single TTE measurements as well as the LV mass index (Figure 2). The LEDA score was graded accordingly, where 0 was the minimum and 4 was the maximum score obtained if each of the four parameters scored one point. The description of each parameter with their corresponding cut-off values and the probability of underlying HHD based on the LEDA score are shown in Table 4. A LEDA echo score of 0 indicated a 0% probability of the presence of HHD, but a score of 4 indicated a 95% probability of underlying HHD. Logistic analysis confirmed that the proposed echo score allowed for the differentiation of complicated from uncomplicated patients with early-stage hypertension (ΔAUC = 0.120, p = 0.0244) (Figure 3). LEDA was positively correlated with the severity of underlying hypertension and patient age (Table 5). Furthermore, LEDA was found to be closely linked to the presence of type 2 diabetes (likelihood ratio: 16, p = 0.0036) and Stage 3 chronic kidney disease (likelihood ratio: 12, p = 0.0190). A borderline but not significant relationship was observed between LEDA and obstructive sleep apnoea (likelihood ratio: 9, p = 0.0556), as well as mild to moderate coronary artery disease (likelihood ratio: 9, p = 0.0525). LEDA score did not exhibit a significant association with smoking history (likelihood ratio: 2, p = 0.7895) or underlying hyperlipidaemia (likelihood ratio: 7, p = 0.1423).
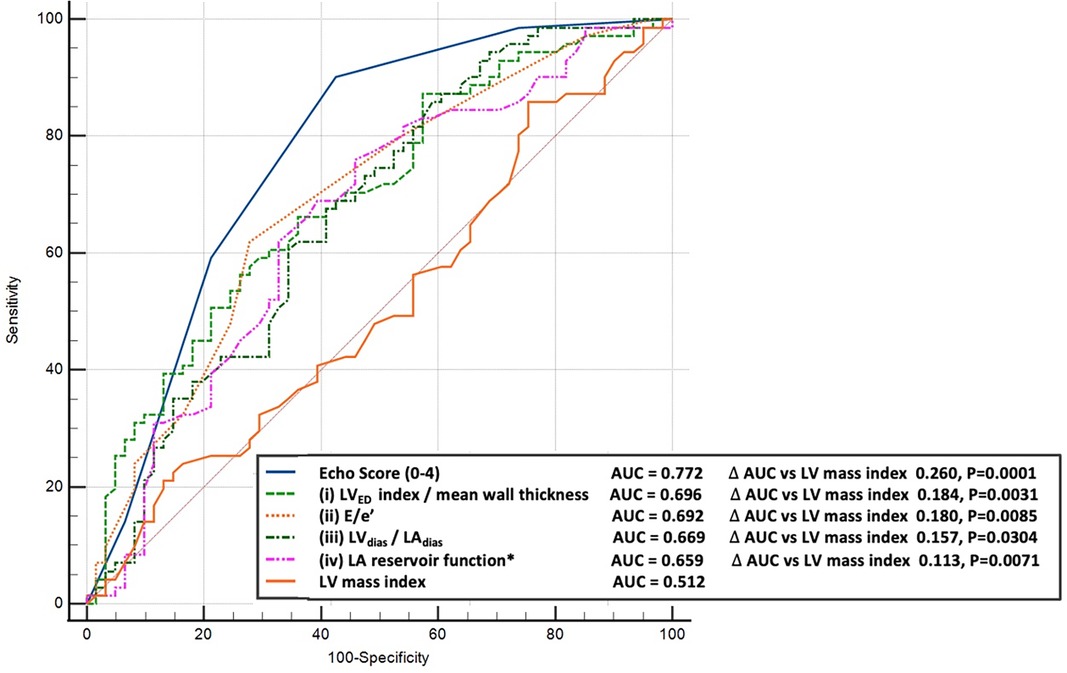
Figure 2. Comparison between the receiver operating characteristic curves for diagnosis of hypertension. Top-echo score (blue line), middle-selected individual echocardiographic parameters (dotted coloured lines) compared to left ventricular mass index (bottom brown line).
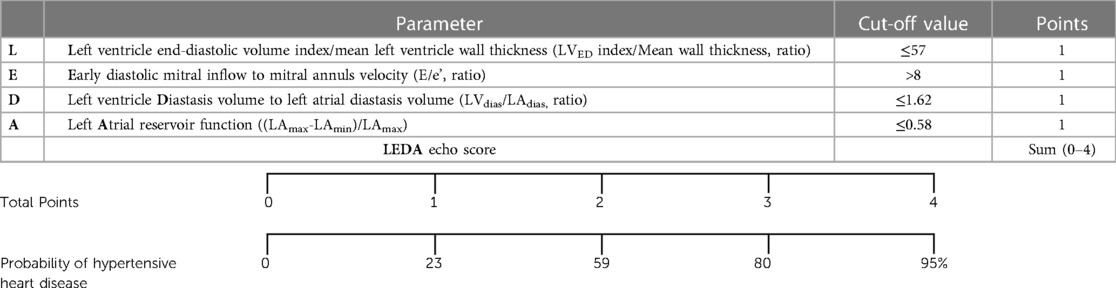
Table 4. Echo score (LEDA) of left heart remodelling indices characteristics for hypertensive heart disease. Top – transthoracic echocardiographic parameters with cut off value and one point for each measurement. Bottom – total score with corresponding probability of underlying hypertension.
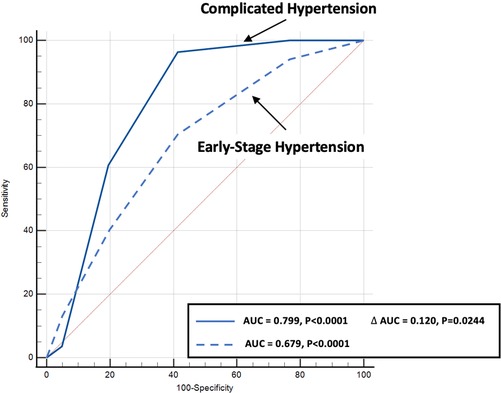
Figure 3. Comparison of echo score receiver operating characteristic curves for the diagnosis of complicated (solid blue line) as compared to early-stage hypertension (dotted blue line) patients.
Discussion
The cumulative effects of hypertension on cardiac remodelling and vascular aging are well recognised (22). Therefore, the detection and monitoring of HHD are important in clinical practice, with the overarching goal of improving diagnosis rates and long-term prognoses.
Hypertension is commonly associated with an increase in LV wall thickness, leading to LV hypertrophy. This phenomenon signifies the maladaptive response to chronic pressure overload, eventually resulting in contractile dysfunction (10–14, 23). LV hypertrophy and LV mass are widely used measures for assessing established features of HHD and are familiar to clinicians (24). Understanding the sequence of adaptive changes in left heart anatomy and function, which ultimately culminate in alterations in LV geometry, filling pressure, and the development of LV hypertrophy, remains a topic of interest (25, 26).
In this study, we prospectively analysed changes in left heart remodelling in patients with hypertension, excluding those with underlying myocardial ischaemia or obstructive coronary disease. We used TTE-derived parameters, including LV concentricity, filling pressure, and LA function. In addition, we expanded our observations by introducing a parameter obtained from both TTE and CTCA, specifically measured during diastasis: the left heart chamber volume ratio (LVdias/LAdias).
LA volume and function
Larger LA volumes were noted in diastasis (LAdias) and end-diastole (LAmin), but not in systole at LAmax. This pattern of LA dilatation in mid to late diastole, rather than in late systole, has been documented previously, and is associated with increasing LV filling pressure, consequent elevation in LA pressure, and, finally, augmented LA wall stress (26, 27). Simultaneously, we observed a reduction in LA reservoir function. These findings support the notion that the reduction in LA reservoir function occurs at an earlier stage, while LA systolic dysfunction (pump function) emerges later in the progression of HHD, often coinciding with HF symptoms. This systolic dysfunction is often secondary to LV structural remodelling and LA afterload mismatch (28, 29). LA diastolic filling, specifically reservoir filling, depends on both LA relaxation and compliance, with the latter being a determinant of stroke volume and an important determinant of LA function (29). A recent study has demonstrated that reduced LA reservoir function is associated with increased mortality in Patients with HF (30).
Volume ratio analysis
The value of the ratio of LA to LV diameter, as a single TTE non-invasive marker of LV compliance, has been investigated previously and has been observed to be ≥1.0 in patients with hypertension and diabetes (31). In addition, the ratio of LA to LV end-diastolic volume, measured using magnetic resonance imaging, has been shown to be a strong predictor of HF incidence, atrial fibrillation, and coronary disease mortality (32), while CTCA volumetric data obtained from retrospective acquisition was applied to evaluate diastolic dysfunction and to predict HF (33).
In the current study, we incorporated our previous concept (19) by analysing the ratio of LV to LA volume measured in diastasis, obtained from both TTE and CTCA examinations. Notably, even in the absence of LV dilatation, LV hypertrophy defined by either LV mass index or LV mass/height2.7, and with no changes in LAmax, we observed a reduction in the LVdias/LAdias ratio in patients with hypertension. Power analysis revealed that both TTE- and CTCA-derived LVdias/LAdias ratios accurately distinguished patients with HHD, with cut-off values of ≤1.62 and ≤1.76, respectively.
The potential advantage of LVdias/LAdias is that it is a simple and easy to obtain parameter available from most standard TTEs and, as examined in this study, is valuable when using both TTE and CTCA. Whether this method is applicable to other cardiac imaging techniques remains to be determined.
Combined TTE scoring index
We introduced a novel TTE-derived score, the LEDA score, comprising four parameters to aid in the identification of HHD: (1) LVED/index/mean wall thickness ≤57, (2) E/e’ ratio by Doppler >8, (3) LVdias/LAdias ≤1.62, and (4) LA reservoir function ≤0.58. The concept of combining these four parameters surpassed the diagnostic utility of individual LA or LV TTE parameter, as well as the LV mass index (Figure 2). Each parameter, if present, contributes one point to the score, allowing for a maximum total score of four for an individual patient. A score of four yielded a 95% probability of the presence of HHD. LEDA was positively correlated with the severity of underlying hypertension and patient age, and was also found to be closely linked to the presence of type 2 diabetes and Stage 3 chronic kidney disease. There was only a borderline correlation with obstructive sleep apnoea and mild to moderate coronary artery disease. There was no correlation between LEDA and body mass index.
Conclusions
This study offers critical insights into the complex dynamics of left heart remodelling in hypertensive patients. By identifying novel parameters, LVdias/LAdias, and introducing the LEDA score, we aim to improve the diagnostic accuracy of hypertension-related cardiac changes. These findings hold promise for enhancing clinical practice and ultimately improving the long-term prognosis of patients with hypertension. More data are needed to validate the LEDA score and age-related changes. One of the potential advantages of LVdias/LAdias lies in its simplicity and ease of measurement. It is accessible through most standard TTE examinations, making it readily available in routine clinical practice. Furthermore, our study demonstrated its value when obtained through both TTE and CTCA assessments, suggesting its versatility in different imaging modalities.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AL: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing, Validation, Visualization. VP: Data curation, Formal Analysis, Investigation, Visualization, Writing – review & editing. CB: Data curation, Investigation, Project administration, Software, Validation, Writing – review & editing. HH: Investigation, Validation, Writing – review & editing. JM: Data curation, Formal Analysis, Project administration, Writing – review & editing. SA: Data curation, Formal Analysis, Investigation, Writing – review & editing. RS: Investigation, Methodology, Writing – review & editing. PP: Conceptualization, Data curation, Formal Analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article.
The project was supported by Wesley Research Institute Clinical Research Grant #2020-23.
Acknowledgments
The authors would like to thank Robert Ware and Emily Young from Griffith University, Queensland, for their critical comments and statistical advice. The authors also thank Andrzej Lange for his comments and suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AUC, area under the curve; CI, confidence interval; CT, computed tomography; HF, heart failure; LA, left atrial/atrium; LAdias, left atrial diastasis volume; LAmax, left atrial maximum volume; LAmin, left atrial minimal volume; LV, left ventricle/ventricular; LVdias, left ventricular diastasis volume; LVED, left ventricular end-diastolic volume; TTE, transthoracic echocardiogram; E/e’, ratio of early diastolic mitral inflow to mitral annular tissue velocity; HHD, hypertensive heart disease
References
1. Messarli FH, Rimoldi SF, Bangalore S. The transition from hypertension to heart failure. Contemporary update. J Am Coll Cardiol HF. (2017) 8:544–51. doi: 10.1016/j.jchf.2017.04.012
2. Reges O, Ning H, Wilkins JT, Wu CO, Tian X, Domanski MJ, et al. Association of cumulative systolic blood pressure with long-term risk of cardiovascular disease and healthy longevity. Findings from the lifetime risk pooling project cohorts. Hypertension. (2021) 77:347–56. doi: 10.1161/HYPERTENSIONAHA.120.15650
3. Tsioufis C, Georgiopoulos G, Oikonomou D, Thomopoulos C, Katsiki N, Kasiakogias A, et al. Hypertension and heart failure with preserved ejection fraction: connecting the dots. Curr Vasc Pharmacol. (2017) 16:15–22. doi: 10.2174/1570161115666170414120532
4. Kostis JB, Cabrera J, Cheng JQ, Cosgrove NM, Deng Y, Pressel SL, et al. Association between chlorthalidone treatment of systolic hypertension and long-term survival. JAMA. (2011) 306:2588–93. doi: 10.1001/jama.2011.1821
5. Bang CN, Gerdts E, Aurigemma GP, Boman K, de Simone G, Dahlöf B, et al. Four-group classification of left ventricular hypertrophy based on ventricular concentricity and dilatation identifies a low-risk subset of eccentric hypertrophy in hypertensive heart. Circ Cardiovasc Imaging. (2014) 7:422–9. doi: 10.1161/CIRCIMAGING.113.001275
6. González A, Ravassa S, López B, Moreno MU, Beaumont MJ, José GS, et al. Myocardial remodelling in hypertension. Toward a new view of hypertensive heart disease. Hypertension. (2018) 72:549–58. doi: 10.1161/HYPERTENSIONAHA.118.11125
7. Kockskämper J, Pluteanu F. Left atrial myocardium in arterial hypertension. Cells. (2022) 11:3157. doi: 10.3390/cells11193157
8. Santos M, Shah AM. Alterations in cardiac structure and function in hypertension. Curr Hypertens Rep. (2014) 16:428. doi: 10.1007/s11906-014-0428-x
9. Rønningen PS, Berge T, Gankås Solberg M, Enger S, Pervez MO, Orstad EB, et al. Impact of blood pressure in the early 40s on left atrial volumes in the mid-60s: data from ACE 1950 study. J Am Heart Assoc. (2022) 11:e023738. doi: 10.1161/JAHA.121.023738
10. Drazner MH. The progression of hypertensive heart disease. Circulation. (2011) 123:327–34. doi: 10.1161/CIRCULATIONAHA.108.845792
11. Aronow WS. Hypertension and left ventricular hypertrophy. Ann Trans Med. (2017) 5:310. doi: 10.21037/atm.2017.06.14
12. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. (2020) 75:1334–57. doi: 10.1161/HYPERTENSIONAHA.120.15026
13. Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G, Januszewicz A, et al. 2023 ESH guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the European Society of Hypertension endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. (2023) 41. doi: 10.1097/HJH.0000000000003480
14. Avraham S, Abu-Sharki S, Shofti R, Haas T, Korin B, Kalfon R, et al. Early cardiac remodelling promotes tumor growth and metastasis. Circulation. (2020) 142:670–83. doi: 10.1161/CIRCULATIONAHA.120.046471
15. Zakeri R, Moulay G, Chai Q, Ogut O, Hussain S, Takahama H, et al. Left atrial remodelling and atrioventricular coupling in a canine model of early heart failure with preserved ejection fraction. Circ Heart Fail. (2016) 9:e003238. doi: 10.1161/CIRCHEARTFAILURE.115.003238
16. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. (2015) 16:233–71. doi: 10.1093/ehjci/jev014
17. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2016) 29:277–314. doi: 10.1016/j.echo.2016.01.011
18. Blume GG, McLeod CJ, Barnes ME, Seward JB, Pellikka PA, Bastiansen PM. Tsang TSM left atrial function: physiology, assessment, and clinical implications. Eur J Echocardiogr. (2011) 12:421–30. doi: 10.1093/ejechocard/jeq175
19. Lange A, Huntress H, Steindl J, Palka P. Incremental role of CT coronary angiography in the assessment of left ventricular diastolic function. Open Heart. (2021) 8:e001566. doi: 10.1136/openhrt-2020-001566
20. Henry JD, Crawford JR. The short-form version of the depression anxiety stress scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br J Clin Psychol. (2005) 44:227–39. doi: 10.1348/014466505X29657
21. Hernandez G, Garin O, Pardo Y, Vilgaut G, Pont A, Suárez M, et al. Validity of the EQ-5D-5l and reference norms for the Spanish population. Qual Life Res. (2018) 27:2337–48. doi: 10.1007/s11136-018-1877-5
22. Wang Y, Wang J, Zheng X-W, Du M-F, Zhang X, Chu C, et al. Early-life cardiovascular risk factor trajectories and vascular aging in midlife: a 30 year prospective cohort study. Hypertension. (2023) 80:1057–66. doi: 10.1161/HYPERTENSIONAHA.122.20518
23. Bombelli M, Vanoli J, Facchetti R, Maloberti A, Cuspidi C, Grassi G, et al. Impact of the increase in left ventricular mass on the risk of long-term cardiovascular mortality: a prospective cohort study. Hypertension. (2023) 80:1321–30. doi: 10.1161/HYPERTENSIONAHA.122.19988
24. Marwick TH, Gillebert TC, Aurigemma G, Chirinos J, Derumeaux G, Galderisi M, et al. Recommendations on the use of echocardiography in adult hypertension: a report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). J Am Soc Echocardiogr. (2015) 28:727–54. doi: 10.1016/j.echo.2015.05.002
25. Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. (2019) 40:3297–317. doi: 10.1093/eurheartj/ehz641
26. Baltabaeva A, Marciniak M, Bijnens B, Parsai C, Moggridge J, Antonios TF, et al. How to detect early left atrial remodelling and dysfunction in mild-to-moderate hypertension. J Hypertens. (2009) 27:2086–93. doi: 10.1097/HJH.0b013e32832f4f3d
27. Eshoo S, Ross DL, Thomas L. Impact of mild hypertension on left atrial size and function. Circ Cardiovasc Imaging. (2009) 2:93–9. doi: 10.1161/CIRCIMAGING.108.793190
28. Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. (2006) 47:2357–63. doi: 10.1016/j.jacc.2006.02.048
29. Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. (2015) 8:295–303. doi: 10.1161/CIRCHEARTFAILURE.114.001667
30. Barbier P, Solomon SB, Schiller NB, Glantz SA. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation. (1999) 100:427–36. doi: 10.1161/01.CIR.100.4.427
31. Spevack DM, Blum L, Malhotra D, Nazari R, Ostfeld RJ, Doddamani S, et al. Ratio of left atrial to left ventricular size: an anatomical marker of the diastolic left ventricular pressure-volume relationship. Echocardiography. (2008) 25:366–73. doi: 10.1111/j.1540-8175.2007.00619.x
32. Pezel T, Venkatesh BA, De Vasconcellos HD, Kato Y, Shabani M, Xie E, et al. Left atrioventricular coupling index as a prognostic marker of cardiovascular events: the MESA study. Hypertension. (2021) 78:661–71. doi: 10.1161/HYPERTENSIONAHA.121.17339
Keywords: hypertension, left atrial function, left ventricular hypertrophy, left heart, echocardiography, diastasis, left ventricular-to-left atrial ratio, computed tomography
Citation: Lange A, Palka V, Bian C, Huntress H, Morgan J, Allwood S, Swann R and Palka P (2023) Left heart remodelling in hypertensive patients: a comprehensive echocardiography and computed tomography study. Front. Cardiovasc. Med. 10:1295537. doi: 10.3389/fcvm.2023.1295537
Received: 16 September 2023; Accepted: 3 November 2023;
Published: 24 November 2023.
Edited by:
Costantino Mancusi, Federico II University Hospital, ItalyReviewed by:
Ramiro Sánchez, Fundacion Favaloro Hospital Universitario, ArgentinaAudrey Adji, Victor Chang Cardiac Research Institute, Australia
© 2023 Lange, Palka, Bian, Huntress, Morgan, Allwood, Swann and Palka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aleksandra Lange YWxla3NhbmRyYWxAcWNnLmNvbS5hdQ==
†ORCID Aleksandra Lange orcid.org/0000-0003-3556-1214 Viktoria Palka orcid.org/0000-0003-0130-9440
 Aleksandra Lange
Aleksandra Lange Viktoria Palka1,2,†
Viktoria Palka1,2,† Harry Huntress
Harry Huntress Przemysław Palka
Przemysław Palka