
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 11 January 2024
Sec. Cardiovascular Imaging
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1288747
This article is part of the Research Topic Echocardiography in Cardiovascular Medicine View all 31 articles
 Vidhu Anand1*
Vidhu Anand1* Megan K. Covington1
Megan K. Covington1 Ushasi Saraswati1
Ushasi Saraswati1 Christopher G. Scott2
Christopher G. Scott2 Alexander T. Lee2
Alexander T. Lee2 Robert P. Frantz1
Robert P. Frantz1 Nandan S. Anavekar1
Nandan S. Anavekar1 Jeffrey B. Geske1
Jeffrey B. Geske1 Adelaide M. Arruda-Olson1
Adelaide M. Arruda-Olson1 Kyle W. Klarich1
Kyle W. Klarich1
Introduction: Apical hypertrophic cardiomyopathy (ApHCM) is a subtype of hypertrophic cardiomyopathy (HCM) that affects up to 25% of Asian patients and is not as well understood in non-Asian patients. Although ApHCM has been considered a more “benign” variant, it is associated with increased risk of atrial and ventricular arrhythmias, apical thrombi, stroke, and progressive heart failure. The occurrence of pulmonary hypertension (PH) in ApHCM, due to elevated pressures on the left side of the heart, has been documented. However, the exact prevalence of PH in ApHCM and sex differences remain uncertain.
Methods: We sought to evaluate the prevalence, risk associations, and sex differences in elevated pulmonary pressures in the largest cohort of patients with ApHCM at a single tertiary center. A total of 542 patients diagnosed with ApHCM were identified using ICD codes and clinical notes searches, confirmed by cross-referencing with cardiac MRI reports extracted through Natural Language Processing and through manual evaluation of patient charts and imaging records.
Results: In 414 patients, echocardiogram measurements of pulmonary artery systolic pressure (PASP) were obtained at the time of diagnosis. The mean age was 59.4 ± 16.6 years, with 181 (44%) being females. The mean PASP was 38 ± 12 mmHg in females vs. 33 ± 9 mmHg in males (p < 0.0001). PH as defined by a PASP value of > 36 mmHg was present in 140/414 (34%) patients, with a predominance in females [79/181 (44%)] vs. males [61/233 (26%), p < 0.0001]. Female sex, atrial fibrillation, diagnosis of congestive heart failure, and elevated filling pressures on echocardiogram remained significantly associated with PH (PASP > 36 mmHg) in multivariable modeling. PH, when present, was independently associated with mortality [hazard ratio 1.63, 95% CI (1.05–2.53), p = 0.028] and symptoms [odds ratio 2.28 (1.40, 3.71), p < 0.001].
Conclusion: PH was present in 34% of patients with ApHCM at diagnosis, with female sex predominance. PH in ApHCM was associated with symptoms and increased mortality.
Apical hypertrophic cardiomyopathy (ApHCM) is a subtype of hypertrophic cardiomyopathy (HCM) with hypertrophy localized to the left ventricular (LV) apex. The estimated prevalence rate among patients with HCM varies from 25% in the Asian population to 1%–10% in the non-Asian population (1, 2). Patients with ApHCM have a widely variable clinical presentation, ranging from being asymptomatic with a normal lifespan to being symptomatic with dyspnea, reduced exercise capacity, chest pain, atrial fibrillation, heart failure, thromboembolic events, and ventricular arrhythmias, or experiencing sudden cardiac death (2–5). The development of an apical pouch and aneurysm is known to have negative prognostic effect, predisposing to both ventricular arrhythmias and intracardiac thrombus formation (4, 6, 7).
Pulmonary hypertension (PH) is prevalent in HCM, with an estimated prevalence rate of 38% by echocardiography (8). It has been shown that older age and systolic dysfunction are independent risk factors for developing PH in HCM (9). Another case–control study found female sex, moderate or greater mitral regurgitation, and atrial fibrillation as independent risk factors for PH beyond the effect of age (10). The prevalence of PH was similar in obstructive and non-obstructive HCM in one study (8), and it was higher in obstructive and end-stage HCM in another (9). Despite the slight differences in prevalence, PH was an independent predictor of mortality in patients with both obstructive and non-obstructive HCM (8, 9, 11). A high prevalence of PH by echocardiography in ApHCM with a female predominance has been described, but the study was not designed to evaluate the risk associations and implications of PH (7). Therefore, in this study, we sought to evaluate the prevalence, sex differences, and risk associations of PH in ApHCM and to assess the prognostic implications of PH in a large cohort of patients with isolated ApHCM at a single referral center in the United States.
This study was approved by the institutional review board and deemed exempt. All patients provided consent to the use of their data for research. A total of 542 patients diagnosed with ApHCM were identified by using ICD-9/10 codes and a search of text contained within clinical notes. The diagnosis was further confirmed by cross-referencing with cardiac MRI reports extracted through Natural Language Processing, as well as through manual review of patient charts, echocardiograms, and cardiac MRIs from January 1999 to May 2018. ApHCM was defined on imaging as the presence of apical wall thickness measuring ≥15 mm or ≥13 mm in individuals with a positive family history or positive genetic mutation. Patients with other patterns of LV hypertrophy without clear apical predominance (reverse curve, sigmoid, neutral septum), infiltrative cardiomyopathies including amyloidosis, Fabry disease, or secondary causes of LV hypertrophy including hypertensive heart disease were excluded. Patients with eosinophilic heart disease/eosinophilic myocarditis were also excluded after conducting a careful review of their cardiac MRI and pathology results when available. While patients with hypertensive heart disease were excluded, those with ApHCM and a concomitant diagnosis of hypertension were not excluded (12). All included patients underwent a comprehensive transthoracic echocardiography (TTE), and the initial study that diagnosed ApHCM was used for analysis. Baseline characteristics recorded at the time of index TTE, including demographic data, diagnosis of congestive heart failure and comorbidities determined from ICD codes, and Charlson comorbidity index, were extracted from the electronic medical records. The New York Heart Association (NYHA) functional class and the presence of symptoms (NYHA functional classes II–IV) were manually abstracted by review of charts. The vital status was retrieved using the Mayo Clinic records. Patients not known to be deceased were censored at the date of the last follow-up.
Echocardiographic assessment was reported by Level 3 trained echocardiographer, and data included LV linear dimensions and ejection fraction, mitral inflow early/ late diastolic velocity (E/A), mitral annular early tissue Doppler velocity (e'), medial E/e' (surrogate of elevated filling pressures), left atrial volume index, pulmonary artery systolic pressure (PASP), estimated right atrial pressure, RV size, and RV systolic function. Pulmonary hypertension was defined as none (PASP < 36 mmHg), mild-to-moderate (PASP 36–59 mmHg), and severe (PASP ≥ 60 mmHg). The PASP was evaluated from the highest (or average of five cardiac cycles in patients with atrial fibrillation or significant respiratory variation) and most complete signal of tricuspid regurgitation from the RV inflow, parasternal short axis, and apical views using modified Bernoulli equation as 4v2 + estimated right atrial pressure, where v is the velocity of the tricuspid regurgitation jet in m/s. The right atrial pressure was estimated based on the size and collapse of the inferior vena cava as follows: 5 mmHg when it displayed both normal size and collapse, 10 mmHg when it was either enlarged or had reduced collapse, 15 mmHg when it was both enlarged and had reduced collapse, and 20 mmHg when it was enlarged with no collapse. The RV was considered enlarged if its size exceeded mild enlargement, and its function was categorized as reduced if it was more than mildly reduced. RV size and function assessments, as specified in the echocardiographic reports, primarily included qualitative evaluations and, when available, quantitative measures (available in less than 50% of the patients).
The primary outcome was the prevalence of PH in patients with ApHCM, sex differences in the same, and impact of PH on all-cause mortality. The secondary outcomes were the risk associations of PH and the impact of PH on symptoms.
Data are presented as frequencies and percentages for categorical variables, and as mean with standard deviation (SD) for approximately normally distributed continuous variables or median and quartiles for those that were not. Chi-square test was used to compare the categorical variables and t-test or Kruskal–Wallis for continuous variables, as appropriate. The survival curves were constructed using the Kaplan–Meier method, and the groups of PH patients were compared using the log-rank test. Cox proportional hazards regression was used to examine the association between PH and all-cause mortality after adjusting for other factors noted to be significant in the univariate analysis. These results are presented as hazard ratios (HR) with 95% confidence intervals (CI). Multivariable models were created using backward selection starting from those variables that were significant in the univariate analyses. Logistic regression was used to examine the factors associated with PH and to assess the association between PH and symptoms, and these results are presented in terms of odds ratios (OR) and 95% CI. The model assumptions were checked graphically to ensure that no violations were noted. The risk for mortality by PASP was illustrated graphically after fitting the PASP using penalized smoothing splines, and Youden's J index was used to identify the optimal cut point for this association. All analyses were performed using SAS version 9.4 (SAS Institute, Inc. Cary, NC, USA), and a p-value of < 0.05 was considered statistically significant.
The study cohort consisted of 414/542 (76%) patients who had a sufficient tricuspid regurgitation Doppler signal for estimating PASP. The mean age was 59.4 ± 16.6 years, with 181 (44%) being females. Among the patients, 184 (47%) had hypertension, 41 (10%) had diabetes, 39 (10%) had a diagnosis of coronary artery disease, and 21 (5%) had prior myocardial infarction (Table 1). In terms of history of ventricular arrhythmias, 34 (9%) patients had ventricular tachycardia, seven (2%) had ventricular fibrillation, and three (1%) had prior cardiac arrest. The baseline characteristics are presented in Table 1. Pulmonary hypertension was present in 140 (34%) patients, with a higher prevalence in females (n = 79/181, 44%) than males (n = 61/233, 26%), p < 0.001 (Figure 1). Severe PH was present in 10 (2%) patients with a higher prevalence in females than males [seven (4%) vs. three (1%), p = 0.001]. The baseline characteristics of males and females are presented separately in Supplementary Table S1. The univariate factors associated with PH were found to be age, female sex, moderate or greater MR, larger left atrial volume index, higher medial E/e', atrial fibrillation, diagnosis of congestive heart failure, and Charlson comorbidity index. MR etiology included annular dilatation (atrial functional MR) in 11 patients, mitral valve prolapse in one patient, and annular dilatation along with significant mitral annulus calcification in one patient. In the multivariable model, female sex [OR 1.88 (1.17, 3.04), p = 0.009], medial E/e' > 15 [OR 2.06 (1.22, 3.49), p = 0.007], atrial fibrillation [OR 2.09 (1.09, 4.01), p = 0.026], and a diagnosis of congestive heart failure [OR 2.48 (1.28, 4.81), p = 0.007] remained independently associated with PH (Table 2). In another model, when left atrial volume index was substituted for medial E/e’, it was also observed to have a statistically significant correlation (Supplementary Table S2).
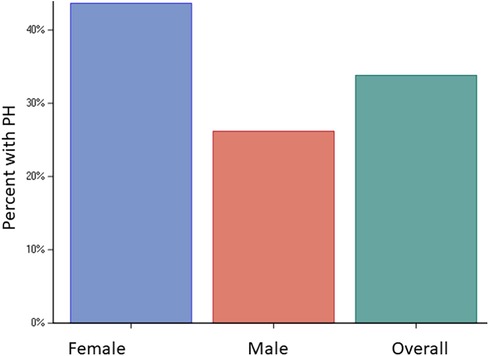
Figure 1. Prevalence of PH. Bar graphs showing prevalence of PH in the entire cohort (34%) and in females (44%) vs. males (26%), p < 0.001.
Mortality was assessed over a median of 4.4 years (IQR: 0.1–10.7). The spline curves analysis showed that the risk of death started to rise continuously after the PASP reached a level between 35 and 36 mmHg for the whole cohort (Figure 2), and in males and females separately (Supplementary Figures S1A and B). Therefore, PASP of 36 mmHg was used as a cutoff, and PH (PASP > 36 mmHg) was associated with higher all-cause mortality in the univariate and multivariable analysis [HR for PH 1.63, 95% CI (1.05–2.53), p = 0.028] (Table 3 and Figure 3). Other independent factors associated with mortality were female sex [HR 1.88, 95% CI (1.20, 2.96), p = 0.006] and Charlson comorbidity index [HR 1.10, 95% CI (1.03, 1.18), p = 0.005] (Table 3). The correlation between PH and higher mortality was observed in both males and females (Supplementary Figures S2A, B). However, after adjusting for other factors, this correlation became non-significant (p = 0.08) for females.
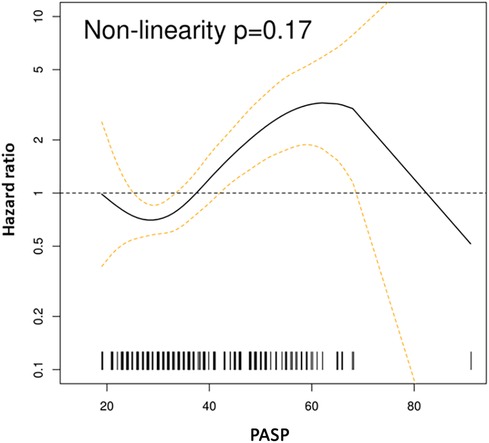
Figure 2. Spline curves for PASP cutoff. Spline curves demonstrate mortality risk across the range of measured PASP in the whole cohort. The risk increased for PASP > 35–36 mmHg.
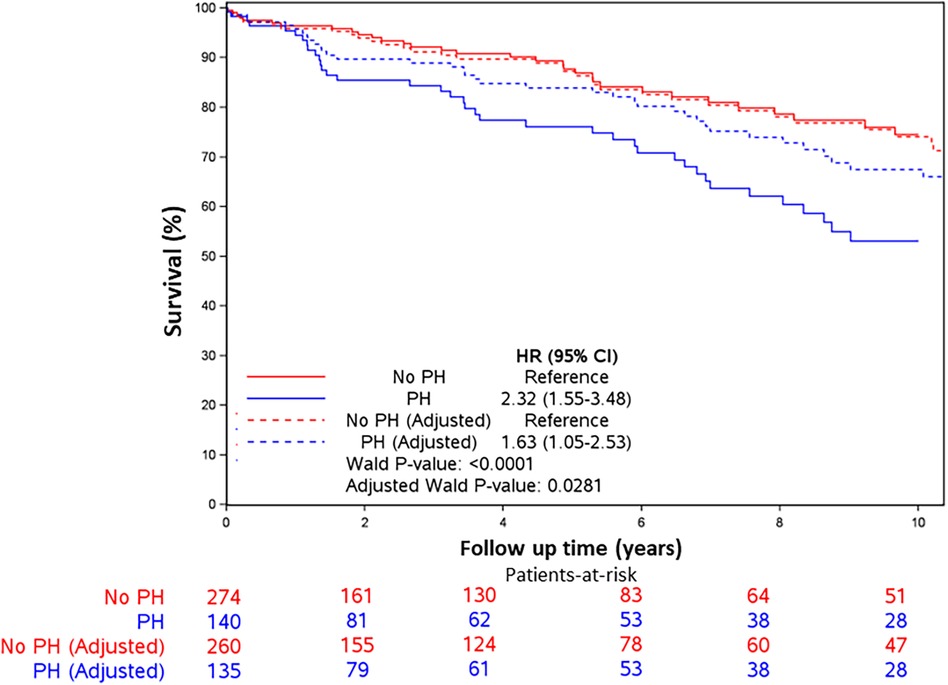
Figure 3. Kaplan–Meier survival curves. Kaplan–Meier survival curves for all-cause mortality by PH (PASP > 36 vs. <36 mmHg) in unadjusted and adjusted models. Patients with PASP > 36 mmHg had higher all-cause mortality in unadjusted models and after adjusting for age, sex, comorbidity index, and left ventricular filling pressures. Hazard ratios were 2.32 (95% CI 1.55, 3.48, p < 0.001) and 1.63 (95% CI 1.05, 2.53, p = 0.028), respectively.
PH, when present, was also found to be independently associated with symptoms (NYHA functional Class II–IV) [OR 2.28, 95% CI (1.40, 3.71), p < 0.001] in addition to the diagnosis of congestive heart failure [OR 5.01, 95% CI (2.30, 10.89), p < 0.001] (Table 4). PH was also independently associated with the presence of significant symptoms (NYHA Class III and IV) [OR 2.73 (1.60, 4.64), p < 0.001].
This study, which involved a large cohort of patients with ApHCM, has identified several significant findings: (1) PH as defined by a PASP value of > 36 mmHg on TTE was present in one-third of the patients, (2) female sex, atrial fibrillation, diagnosis of congestive heart failure, and elevated filling pressures were independently associated with PH in ApHCM patients, and (3) PH, when present, was associated with worse symptoms and higher all-cause mortality even after adjusting for known risk factors.
ApHCM is an understudied variant of HCM characterized by hypertrophy localized to the LV apex. It was previously considered “benign” due to the absence of obstruction, but studies have shown an increased risk of heart failure, arrhythmias, thromboembolic events, and death (4, 6, 7, 13). Although dynamic LV outflow tract obstruction is infrequent in ApHCM, elevated filling pressures due to diastolic dysfunction are frequently observed (7, 13–15) and serve as a mechanism for PH. In HCM, PH is thought to be post-capillary initially, and then over time, develop pre-capillary remodeling, leading to combined pre- and post-capillary PH (8, 9, 11). When present, PH is associated with worse outcomes in both patients with obstructive and non-obstructive HCM (8–11); however, there has been no specific research of the prevalence, risk associations, and implications of PH in ApHCM.
This study found a high prevalence of PH in up to one-third of patients with ApHCM. The results are similar to the reported prevalence rate of 38% in patients with other subtypes of HCM (predominantly septal, reverse-curve, neutral) using similar echocardiographic criteria (8). The factors associated with PH in ApHCM patients were found to be female sex, atrial fibrillation, diagnosis of congestive heart failure, and elevated filling pressures on echocardiogram, similar to what has been previously reported in non-selected HCM cohorts (8–10). The association between elevated filling pressures on echocardiogram (medial E/e') and a larger left atrial volume index, which reflects long-standing elevated left-sided filling pressures, suggests a group 2 mechanism of PH. Although a systematic approach to defining PH etiology was not performed in this retrospective assessment, a thorough chart review did not reveal alternative etiologies of PH. There are data showing worse outcomes in females with HCM (16, 17), and data herein clarify that this relationship persists in patients with ApHCM (7).
PH, when present, was independently associated with all-cause mortality, with other significant factors being female sex and Charlson comorbidity index. In the spline curve analysis, there was a continuous increase in the risk of mortality above a cutoff of PASP > 35–36 mmHg. This cutoff corresponds to a mean PA pressure of 25 mmHg, is recommended by the society guidelines for detecting PH with high sensitivity, and is previously shown to be associated with an increased risk of death in non-obstructive and obstructive HCMs without prior septal reduction treatment (8, 18, 19). PH has been shown to be associated with worse outcomes in left-sided heart disease including heart failure with a reduced and preserved ejection fraction and left-sided valve diseases (20). There are a few studies including one large study with over 1,500 patients showing worse outcomes associated with PH in patients with HCM (8–11), and to our knowledge, the current study is the first to systematically evaluate the prognostic significance of PH in patients with an apical subtype of HCM.
The novel myosin inhibitor, Mavacamten, was recently evaluated in a phase 2 trial (MAVERICK-HCM), which included a small group of patients with non-obstructive HCM (21). The study found that the treatment was well tolerated by the majority of patients and was associated with a reduction in cardiac biomarkers, indicating a decrease in wall stress. Mavacamten is currently under further investigation to assess improvements in clinical parameters (22). In addition, there is a need to explore its effects in patients with ApHCM.
Our study has several limitations—retrospective study design, single-center experience, and unavailability of TR jet to estimate PASP in approximately 25% of the patients from the original cohort which may change the true prevalence of PH in this population. The retrospective design limits the assessment of predictors, and only risk associations can be determined. The interobserver agreement of echocardiographic measurements is unavailable, but it is unlikely to affect the study results. Hemodynamic right heart catheterization, labs (autoimmune antibodies, NT-pro brain natriuretic peptide), pulmonary function tests, and ventilation perfusion scan were unavailable in most patients, which limits the exact characterization of the type (pre-capillary, post-capillary, or combined pre- and post-capillary) and etiology of PH (13). However, a systematic chart review of this large cohort did not reveal an obvious alternate etiology of PH in these patients. Some comorbidities, such as coronary artery disease and lung disease, could also potentially be associated with PH. However, the proportion of patients with these comorbidities was small, and their association with PH was not significant in the univariate analyses. Genetic testing for sarcomere mutations was not routinely available due to the retrospective nature of the study; therefore, genetic associations could not be tested.
The present study shows a high prevalence rate of PH of 34% as determined by echocardiography in patients with ApHCM, with significantly higher prevalence in females vs. males (44% vs. 26%). The factors associated with PH were found to be female sex, atrial fibrillation, diagnosis of congestive heart failure, and elevated filling pressures on echocardiogram. PH when present was associated with both worse symptoms and higher all-cause mortality. There is a need for larger prospective studies to further evaluate the sex differences in PH, as well as the role of PH in categorizing risk and deciding the most suitable timing for intervention in these patients.
The datasets presented in this article are not readily available until they receive approval from the institution and the IRB. Requests to access the datasets should be directed to the corresponding author.
The studies involving humans were approved by Mayo Clinic IRB number 20-008555. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
VA: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. MC: Data curation, Writing – review & editing. US: Data curation, Writing – review & editing. CS: Formal Analysis, Methodology, Validation, Writing – review & editing. AL: Writing – review & editing. RF: Writing – review & editing. NA: Writing – review & editing. JG: Writing – review & editing. AA-O: Data curation, Writing – review & editing. KK: Methodology, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This research was funded by a grant from Mayo Clinic CV Prospective Award.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1288747/full#supplementary-material
Supplementary Figure 1
Spline curves for PASP cutoff for males and females. Spline curves demonstrate mortality risk across the range of measured PASP in A. Males B. Females. The risk increased for PASP > 35–36 mmHg in both males and females.
Supplementary Figure 2
Kaplan–Meier survival curves for males and females. Kaplan–Meier survival curves for all-cause mortality by PH (PASP > 36 vs. <36 mmHg) in unadjusted and adjusted models. Patients with PASP > 36 mmHg had higher all-cause mortality in unadjusted models and after adjusting for age, comorbidity index, and left ventricular filling pressures. Unadjusted and adjusted hazard ratios were 2.26 (95% CI 1.18,4.31, p = 0.013) and 2.03 (95% CI 1.03, 4.01, p = 0.040), respectively, for males and 1.91 (95% CI 1.10–3.32, p = 0.021) and 1.71 (95% CI 0.94, 3.11), p = 0.078), respectively, for females.
1. Kitaoka H, Doi Y, Casey SA, Hitomi N, Furuno T, Maron BJ. Comparison of prevalence of apical hypertrophic cardiomyopathy in Japan and the United States. Am J Cardiol. (2003) 92:1183–6. doi: 10.1016/j.amjcard.2003.07.027
2. Hughes RK, Knott KD, Malcolmson J, Augusto JB, Mohiddin SA, Kellman P, et al. Apical hypertrophic cardiomyopathy: the variant less known. J Am Heart Assoc. (2020) 9:e015294. doi: 10.1161/JAHA.119.015294
3. Jan MF, Todaro MC, Oreto L, Tajik AJ. Apical hypertrophic cardiomyopathy: present status. Int J Cardiol. (2016) 222:745–59. doi: 10.1016/j.ijcard.2016.07.154
4. Sakamoto T. Apical hypertrophic cardiomyopathy (apical hypertrophy): an overview. J Cardiol. (2001) 37(Suppl 1):161–78.11433822
5. Hebl VB, Miranda WR, Ong KC, Hodge DO, Bos JM, Gentile F, et al. The natural history of nonobstructive hypertrophic cardiomyopathy. Mayo Clin Proc. (2016) 91:279–87. doi: 10.1016/j.mayocp.2016.01.002
6. Webb JG, Sasson Z, Rakowski H, Liu P, Wigle ED. Apical hypertrophic cardiomyopathy: clinical follow-up and diagnostic correlates. J Am Coll Cardiol. (1990) 15:83–90. doi: 10.1016/0735-1097(90)90180-W
7. Klarich KW, Attenhofer Jost CH, Binder J, Connolly HM, Scott CG, Freeman WK, et al. Risk of death in long-term follow-up of patients with apical hypertrophic cardiomyopathy. Am J Cardiol. (2013) 111:1784–91. doi: 10.1016/j.amjcard.2013.02.040
8. Ong KC, Geske JB, Hebl VB, Nishimura RA, Schaff HV, Ackerman MJ, et al. Pulmonary hypertension is associated with worse survival in hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. (2016) 17:604–10. doi: 10.1093/ehjci/jew024
9. Musumeci MB, Mastromarino V, Casenghi M, Tini G, Francia P, Maruotti A, et al. Pulmonary hypertension and clinical correlates in hypertrophic cardiomyopathy. Int J Cardiol. (2017) 248:326–32. doi: 10.1016/j.ijcard.2017.07.010
10. Wu X, Cui H, Xiao MH, Lu J, Zhu CS, Wang SY, et al. Prevalence of pulmonary hypertension in patients with hypertrophic obstructive cardiomyopathy: a case-control study. Zhonghua Xin Xue Guan Bing Za Zhi. (2016) 44:1010–4. doi: 10.3760/cma.j.issn.0253-3758.2016.12.004
11. Mitra A, Ghosh RK, Bandyopadhyay D, Ghosh GC, Kalra A, Lavie CJ. Significance of pulmonary hypertension in hypertrophic cardiomyopathy. Curr Probl Cardiol. (2020) 45:100398. doi: 10.1016/j.cpcardiol.2018.10.002
12. Geske JB, Ong KC, Siontis KC, Hebl VB, Ackerman MJ, Hodge DO, et al. Women with hypertrophic cardiomyopathy have worse survival. Eur Heart J. (2017) 38:3434–40. doi: 10.1093/eurheartj/ehx527
13. Moon J, Shim CY, Ha JW, Cho IJ, Kang MK, Yang WI, et al. Clinical and echocardiographic predictors of outcomes in patients with apical hypertrophic cardiomyopathy. Am J Cardiol. (2011) 108:1614–9. doi: 10.1016/j.amjcard.2011.07.024
14. Aslannif R, Suraya K, Koh HB, Tey YS, Tan KL, Tham CH, et al. Diastolic dysfunction grading, echocardiographic and electrocardiogram findings in 50 patients with apical hypertrophic cardiomyopathy. Med J Malaysia. (2019) 74:521–6.31929479
15. Finocchiaro G, Haddad F, Pavlovic A, Magavern E, Sinagra G, Knowles JW, et al. How does morphology impact on diastolic function in hypertrophic cardiomyopathy? A single centre experience. BMJ Open. (2014) 4:e004814. doi: 10.1136/bmjopen-2014-004814
16. Siontis KC, Ommen SR, Geske JB. Sex, survival, and cardiomyopathy: differences between men and women with hypertrophic cardiomyopathy. J Am Heart Assoc. (2019) 8:e014448. doi: 10.1161/JAHA.119.014448
17. Trongtorsak A, Polpichai N, Thangjui S, Kewcharoen J, Yodsuwan R, Devkota A, et al. Gender-Related differences in hypertrophic cardiomyopathy: a systematic review and meta-analysis. Pulse (Basel). (2021) 9:38–46. doi: 10.1159/000517618
18. Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. (2009) 30:2493–537. doi: 10.1093/eurheartj/ehp297
19. Bossone E, D'Andrea A, D'Alto M, Citro R, Argiento P, Ferrara F, et al. Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. J Am Soc Echocardiogr. (2013) 26:1–14. doi: 10.1016/j.echo.2012.10.009
20. Vachiéry JL, Tedford RJ, Rosenkranz S, Palazzini M, Lang I, Guazzi M, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. (2019) 53:1801897. doi: 10.1183/13993003.01897-2018
21. Ho CY, Mealiffe ME, Bach RG, Bhattacharya M, Choudhury L, Edelberg JM, et al. Evaluation of mavacamten in symptomatic patients with nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. (2020) 75:2649–60. doi: 10.1016/j.jacc.2020.03.064
Keywords: apical hypertrophic cardiomyopathy, pulmonary hypertension, pulmonary artery systolic pressure, sex differences, all-cause mortality
Citation: Anand V, Covington MK, Saraswati U, Scott CG, Lee AT, Frantz RP, Anavekar NS, Geske JB, Arruda-Olson AM and Klarich KW (2024) Prevalence, sex differences, and implications of pulmonary hypertension in patients with apical hypertrophic cardiomyopathy. Front. Cardiovasc. Med. 10:1288747. doi: 10.3389/fcvm.2023.1288747
Received: 4 September 2023; Accepted: 11 December 2023;
Published: 11 January 2024.
Edited by:
Sanjeev Bhattacharyya, Barts Heart Centre, United KingdomReviewed by:
Yosef Manla, Cleveland Clinic Abu Dhabi, United Arab Emirates© 2024 Anand, Covington, Saraswati, Scott, Lee, Frantz, Anavekar, Geske, Arruda-Olson and Klarich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vidhu Anand YW5hbmQudmlkaHVAbWF5by5lZHU=
Abbreviations ApHCM, apical hypertrophic cardiomyopathy; PH, pulmonary hypertension; PASP, pulmonary artery systolic pressure; TTE, transthoracic echocardiogram.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.