
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 27 November 2023
Sec. Lipids in Cardiovascular Disease
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1286286
 Chengxi Liu1,2,†
Chengxi Liu1,2,† Mi Dai3,†
Mi Dai3,† Kunming Tian2,4
Kunming Tian2,4 Shiyu Zhou4
Shiyu Zhou4 Lei Luo2
Lei Luo2 Zhiying Zeng1
Zhiying Zeng1 Xuelian Yan3
Xuelian Yan3 Ying Xiao3
Ying Xiao3 Yiying Wang5
Yiying Wang5 Renli Deng4
Renli Deng4 Xiuhong Lei1*
Xiuhong Lei1* Tao Liu5*
Tao Liu5*
Background: Emerging evidence has indicated that remnant cholesterol (RC) could predict cardiovascular disease (CVD) incidence. Nevertheless, the relationship between RC and CVD risk, especially within the general Chinese population, remains scarce.
Objective: The present research aimed to assess whether RC concentrations and CVD outcomes in general Chinese adults are related.
Methods: The Cox proportional hazard model was established to explore the relationship between RC and the outcomes of CVD and CVD subgroups. A restricted cubic spline (RCS) was utilized to investigate the dose–response connection between RC and the risk of CVD outcomes, and the ROC curve was used to calculate the corresponding cutoff values. Moreover, stratified analysis was conducted to investigate the potential effect modification in the association between RC and CVD outcomes.
Results: Significant positive associations were found between elevated categorical RC and increased risk of CVD (HR Q4, 1.80; 95% CI 1.15–2.79; P-value = 0.008), atherosclerotic cardiovascular disease (HR Q4, 2.00; 95% CI 1.22–3.27; P-value = 0.007), stroke (HR Q4, 1.66; 95% CI 1.02–2.69; P-value = 0.040), and ischemic stroke (HR Q4, 1.87, 95% CI 1.08–3.25; P-value = 0.034), respectively. Our study suggested that the incidence of CVD outcomes increased when RC levels were above 0.75 mmol/L. Importantly, the CVD risks related to RC were more likely to be those found in subjects aged above 60 years, women, subjects with BMI <24 kg/m2, and subjects with hypertension and unhealthy diet patterns.
Conclusions: Aberrant high level of RC is associated with elevated CVD risk, independent of low-density lipoprotein cholesterol (LDL-C). Our data reveal urgent primary prevention for subjects with high RC levels to a low incidence of CVD, especially for the elderly, women, and those with hypertension and unhealthy diet patterns.
Cardiovascular disease (CVD) is the dominant cause of global mortality (1), accounting for 40% of deaths in China (2). More importantly, developing countries, such as China and India, suffer the enormous burden of CVD (3). Therefore, early identification and management of patients with high CVD risk are critical for planning effective interventions for the susceptible population. It is well known that dyslipidemia greatly influences the pathogenesis of atherosclerotic cardiovascular disease (ASCVD). The association between low-density lipoprotein cholesterol (LDL-C) and ASCVD has been extensively studied (4, 5). Lowering plasma LDL-C levels has been recommended as a priority measure to prevent ASCVD for decades (6, 7). Unfortunately, although statins or other lipid-lowering drugs have controlled the LDL-C levels to the target range, a significant residual risk of CVD remains (8, 9). Therefore, the residual plasma lipid may partially contribute to the residual CVD risk. Emerging evidence has indicated that the deposition of RC on arterial walls may induce atherosclerosis.
Remnant cholesterol (RC), a critical member of triglyceride-rich lipoproteins (TRLs), which are partially lipidated by lipoprotein lipase, generally consists of very low-density lipoproteins (VLDLs), intermediate-density lipoproteins (IDLs), and chylomicron residues (10). Mounting evidence indicates that RC, besides LDL cholesterol, is a primary causative agent of ASCVD (11, 12). Recent evidence suggests that RC was strongly related to cardiovascular events and total mortality (11–17). However, most studies investigating the connection between RC and CVD were carried out in Western populations, and the evidence for the Asian population is scarce (13, 18). Several studies have addressed the relationship between RC and CVD events in peritoneal dialysis (PD) (19), percutaneous coronary intervention (PCI) (20), type 2 diabetes, and incident diabetic nephropathy patients in China. Currently, quite limited studies have been established regarding the risk of RC with CVD among the general Chinese population. Only one longitudinal cohort in rural northeast China was explored to study the association between RC and CVD (14). Considering the high variation of demographic differences, more evidence is needed to identify the risk of RC for CVD using the representative population. In addition, an acceptable range or cutoff value of RC is also crucial for early prevention and treatment for the high-risk population. Also, these limitations have yet to be explored.
This investigation was carried out to elaborate on the relationship between RC and CVD in a large-sample prospective cohort study that contains a representative southwest China population. We systematically explored the relationship between RC and risks of CVD and its subtypes, and the cutoff value for the above associations was also identified. Stratified analysis was further performed to explore the modification factors impacting the RC and CVD relationships. Our study is of great scientific proof to identify the economic biomarker for diagnosing the population with high risk for CVD.
This is a multistage and randomly stratified cluster sample design including 9,280 adults (age ≥18 years) enlisted between October 2010 and August 2012 in Guizhou Province's 48 townships across 12 districts. A total of 8,165 study participants completed at least one follow-up visit in this 10-year follow-up study. The following individuals were excluded from this study: (1) those who were diagnosed with baseline CVD [n = 88, incidence of combined ischemic heart disease (IHD) and stroke]; (2) those with missing data of baseline lipid (n = 1,203); and (3) those with a lack of information on population baseline covariates (n = 110). Finally, 6,764 subjects were included in this study. Figure 1 depicts the study flow. To carry out this investigation, we received permission from the Guizhou Center for Disease Control and Prevention's institutional review committee (No. S2017-02). Each participant signed an informed consent form. The principles of the Declaration of Helsinki were followed in this investigation.
Participants providing venous blood samples were instructed to fast overnight for at least 8 h to measure triglyceride (TG), total cholesterol (TC), LDL-C, and HDL cholesterol (HDL-C) levels. A fully automated hematology analyzer (Sysmex, XN-9000, Japan) was used to detect blood parameters, and all tests were conducted by professional laboratory personnel. All testing equipment met quality control requirements. RC was obtained through the following formula: RC = TC-LDL-C‒HDL-C (21).
The main terminus was the composite of CVD events—including IHD and stroke. IHD was recognized as sudden cardiac death (death within 1 h of commencement, cardiac arrest, or sudden collapse without 1 h of symptoms), definite or probable myocardial infarction, and angina pectoris (22). A stroke was described as a focal nerve disease that developed suddenly and persisted for at least 24 h or till death. The specific incidence of IS and IHD was also documented. Participants were defined as ASCVD if they had a definite or probable myocardial infarction, coronary death, and stroke. The information to identify endpoints was derived from self-reported previous diagnoses and medical records or extracted from the Death Registration Information System. ICD-10, or the International Classification of Diseases, Tenth Revision, was used to categorize and encode the diagnosis. IHD is I20.x–125.x, stroke is I60.x–I69.x, and IS is I61.x–I68.x. ASCVD is ICD-9 410–414, 430–438, and 440.
Covariate information, including age, sex, BMI, LDL-C, excessive drinking (yes or no), current smoking status (yes or no), physical activity (never, 1–2 days/week, ≥3 days/week), insufficient cereal intake (yes or no), insufficient vegetable and fruit intake (yes or no), excessive meat intake (yes or no), bean product intake (yes or no), animal entrail intake (yes or no), lipid-lowering drug use (yes or no), diabetes mellitus (yes or no), and hypertension (yes or no), was referenced from previous literature studies and derived from questionnaires used by the interviewer face to face at baseline. According to the 2016 Dietary Guidelines for Chinese Residents (23), insufficient cereal intake was defined as daily intake of cereals <250 g, insufficient vegetable and fruit intake was defined as daily vegetable intake <300 g and daily fruit intake <200 g, excessive red meat intake was defined as daily meat intake >75 g, and excessive alcohol consumption was defined as >25 g/day for men and >15 g/day for women. Anthropometric measurements were used by skilled professionals to obtain data on height, weight, and blood pressure (BP). BMI was calculated by dividing weight (in kilograms) by the square of height (in meters). Based on the recommendation of the Working Group on Obesity in China (24), low weight, normal weight, overweight, and obesity were defined as BMI <18.5 kg/m2, BMI 18.5–23.9 kg/m2, BMI 24.0–27.9 kg/m2, and BMI ≥28 kg/m2, respectively. BP was calculated as the average of three readings using the same brand of an electronic sphygmomanometer. Participants were defined as having hypertension if they met the following criteria of JNC 7 (25): (1) self-reported diagnosis of hypertension or intake of hypotensive drugs; and/or (2) systolic blood pressure of 140 mmHg or higher and diastolic blood pressure of 90 mmHg or higher. Diabetes was defined by the American Diabetes Association diagnostic criterion (26): (1) self-reported diagnosis of diabetes, (2) FPG of 7.0 mmol/L (126 mg/dL) or higher, (3) 2-h glucose of 11.1 mmol/L (200 mg/dL) or higher, or (4) HbA1c concentration of higher than 6.5%.
Normally distributed variables and skewed data were presented as means ± standard deviations (SDs) and medians (25%, 75%), respectively. RC levels divided all subjects into four subgroups (Quartile 1, Quartile 2, Quartile 3, and Quartile 4). The difference among baseline characteristics of groups was compared through the Kruskal–Wallis test. Categorical data were presented as n (%) and compared utilizing the χ2 test.
The follow-up time was calculated and expressed as the interval between enrollment in the cohort and the incident CVD outcomes or the last visit time, whichever came first. Cox proportional hazard models were fitted to evaluate hazards ratios (HRs) and 95% confidence intervals (CIs) for the associations of RC (considered as both categorical and continuous variables) with CVD, ASCVD, CHD, stroke, and IS events. Schoenfeld residuals were utilized to investigate the proportionality of hazards per variable. Potential confounding factors were selected according to previous studies regarding the relation of RC with CVD events and biological plausibility. Three adjustment models were fitted for Cox analysis: (1) Model 1—adjusted for age and sex; (2) Model 2—Model 1 plus present smoking status, excessive drinking status, physical activity, insufficient cereal intake, insufficient vegetable and fruit intake, excessive meat intake, bean product intake, and animal entrail intake; (3) Model 3—Model 2 plus BMI, history of lipid-lowering drug use, LDL-C, diabetes, and hypertension. The p-value was obtained by assigning the median value as a continuous variable to each category. The dose–response relationship of RC with the risk of CVD outcomes was investigated using RCS, and the ROC curve was constructed to calculate the cutoff value to provide evidence for early screening of the susceptible population. Stratified analyses were further carried out to explore the potential effect modification by baseline age (≤60 years vs. >60 years), sex, current smoking status (yes or no), BMI subgroups (<24 kg/m2 vs. ≥24 kg/m2), insufficient vegetable and fruit intake (yes or no), excessive meat intake (yes or no), bean product intake (yes or no), animal entrail intake (yes or no), and hypertension (yes or no) on the association between different RC levels (Quartile 1 as the reference) and risks of CVD outcomes.
All statistical analyses were performed using SPSS version 25.0 and R 4.1.1, and statistical significance was defined by setting the two-sided P-value at 0.05.
We finally included 6,764 subjects for the present study, and Table 1 presents the baseline characteristics. Based on the RC concentration, we divided all subjects into four groups: Quartile 1 (RC ≤0.25 mmol/L), Quartile 2 (RC 0.25–0.67 mmol/L), Quartile 3 (RC 0.67–1.00 mmol/L), and Quartile 4 (RC >1.00 mmol/L). Among all participants, 47.7% were men; the mean age and mean BMI were 44.81 ± 15.09 years and 22.88 ± 3.25 kg/m2, respectively. The prevalence rates of hypertension and diabetes were 27.0% and 8.9%, respectively. Subjects with higher levels of RC tended to be older than those with low levels and more likely to be present smokers, excessive drinkers, diabetes, and hypertension. Moreover, individuals who consumed animal entrails, excessive meat, fewer vegetables and fruits, and insufficient cereals have higher RC levels. Meanwhile, serum levels of TC and TG increased along with the level of RC, while sex did not differ significantly across different groups.
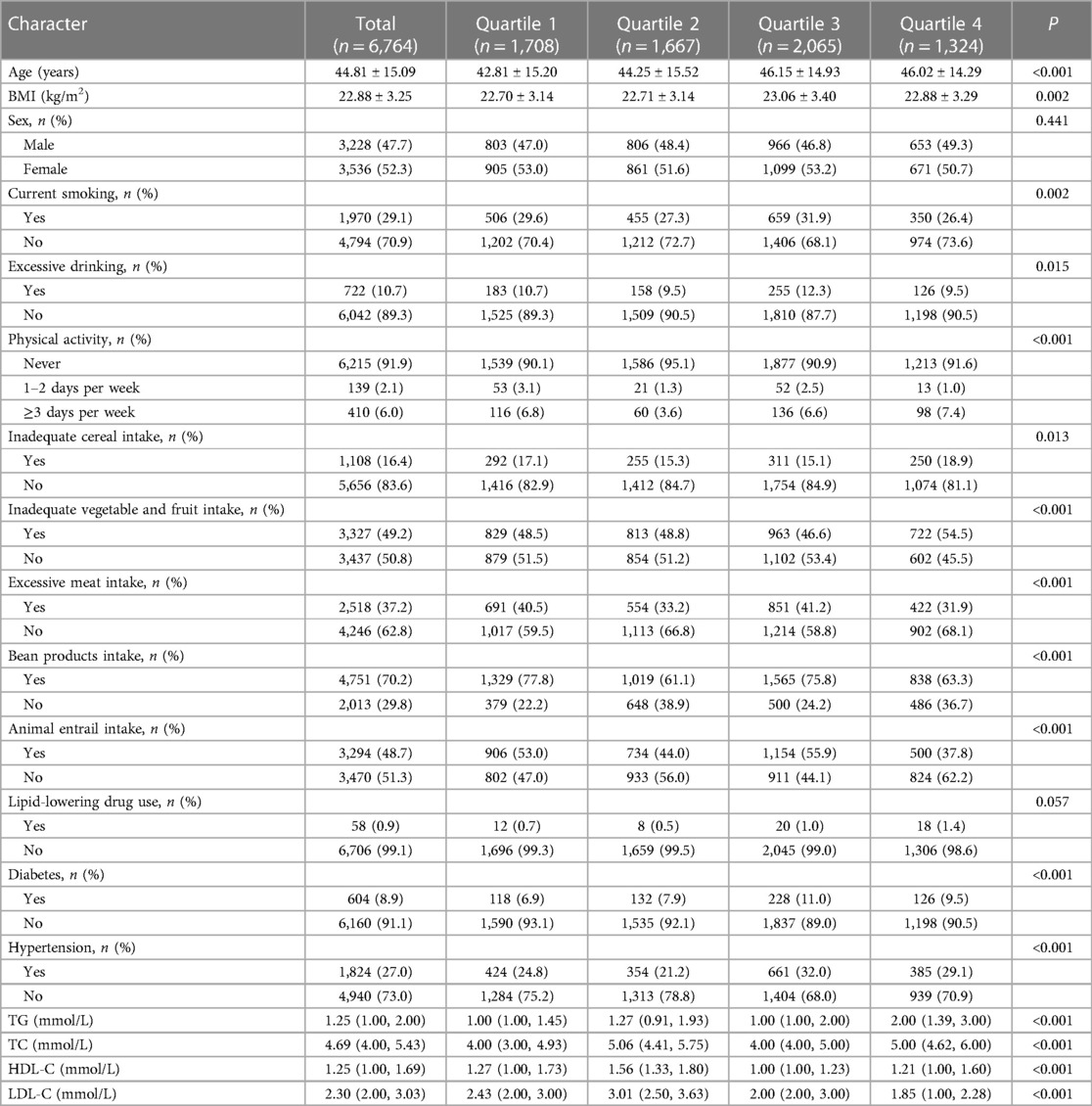
Table 1. Basic characteristics of the participants according to different groups of remnant cholesterol.
The difference in baseline characteristics of the included and excluded populations in this study are shown in Supplementary Table S1. In most baseline features, we found no statistically significant difference in the included and excluded groups. However, participants had a slightly higher rate of excessive drinking and a lower rate of inadequate cereal intake.
Among 6,764 individuals enrolled in the present study with a follow-up to 10 years (median 9.4 years), 199 participants had CVD, 160 had ASCVD, 171 had stroke, 37 had CHD, and 133 had IS. We explored the HRs for the incidence of various CVD outcomes based on the RC concentration. When RC was modeled as a categorical variable, individuals with a higher concentration of RC showed an increased risk of CVD, ASCVD, stroke, and IS. In the present study, for participants with RC >1.00 mmol/L vs. RC ≤0.25 mmol/L, the multivariable-adjusted results for CVD (HR, 1.79; 95% CI 1.15–2.79; P-value = 0.008), ASCVD (HR, 2.00; 95% CI 1.22–3.27; P-value = 0.007), stroke (HR, 1.66; 95% CI 1.02–2.69; P-value = 0.040), and IS (HR, 1.87, 95% CI 1.08–3.25; P-value = 0.034) were observed. No significant association between RC and CHD was found (HR, 2.11; 95% CI 0.83–5.37; P-value = 0.118) (Table 2).
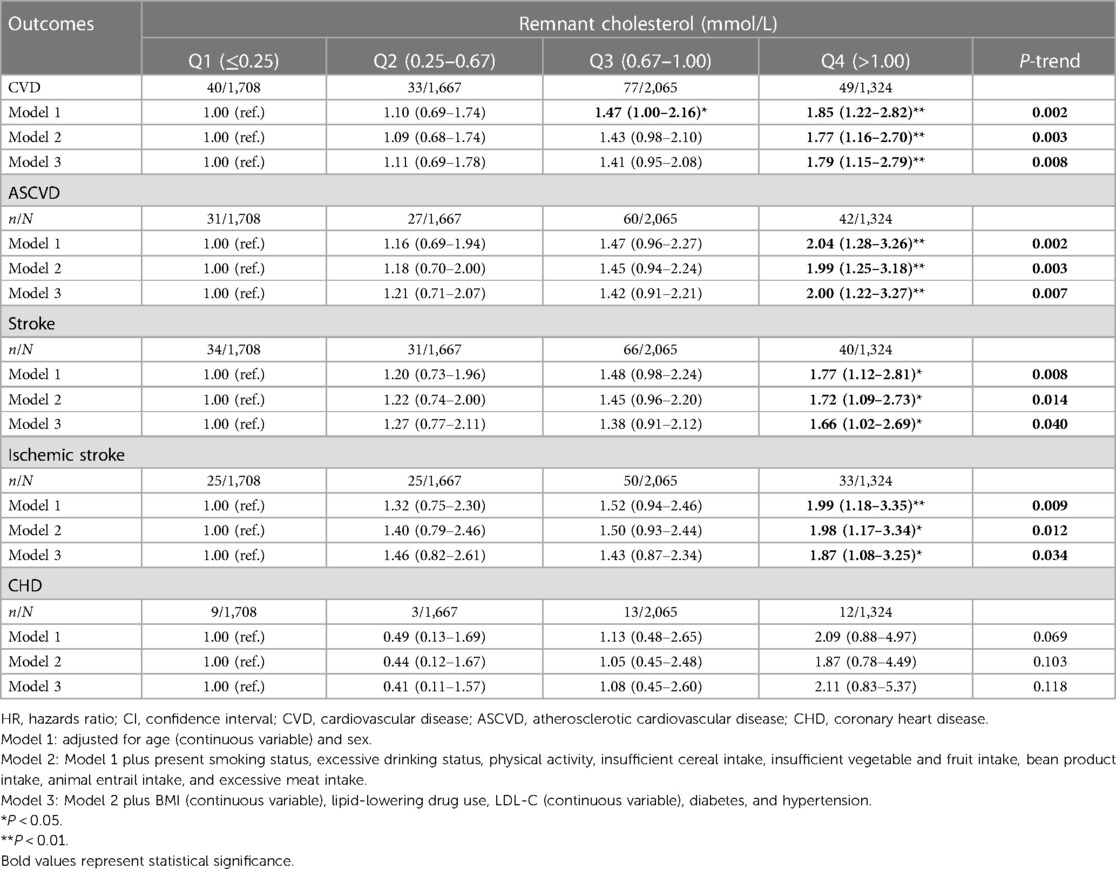
Table 2. Incident risk of CVD outcomes associated with the baseline concentration of remnant cholesterol (categorical variable).
When RC was modeled as a continuous variable, participants had a significantly increased risk of CVD by 24.6% (HR, 1.25, 95% CI 1.02–1.52) and ASCVD by 28.0% (HR, 1.28, 95% CI 1.03–1.56) after fully adjusting for the models. Significantly higher risks for stroke (HR, 1.23; 95% CI 1.00–1.49), IS (HR, 1.26; 95% CI 1.01–1.56), and CHD (HR, 1.49; 95% CI 1.00–2.22) were found for participants after adjusting for Model 2 covariates, but the significance vanished in Model 3 (Table 3).
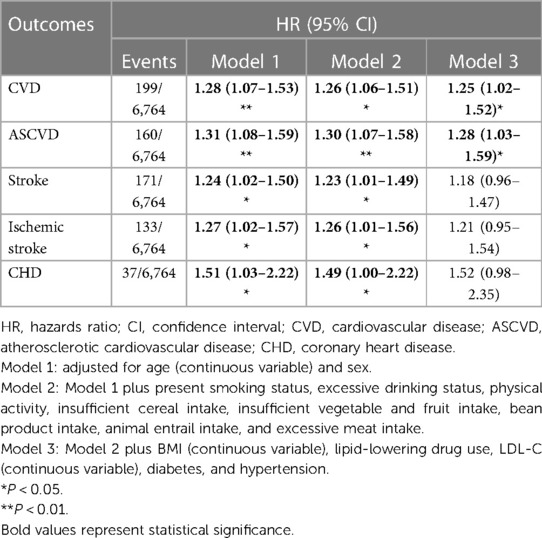
Table 3. Incident risk of CVD outcomes associated with the baseline concentration of remnant cholesterol (continuous variable).
After fully adjusting for the models, RCS regression presented significant overall associations of RC with CVD (P-overall <0.001), ASCVD (P-overall <0.001), stroke (P-overall <0.001), IS (P-overall <0.001), and CHD (P-overall <0.001) (Figure 2). More importantly, when RC concentration was >0.75 mmol/L, the risk of CVD dramatically increased.
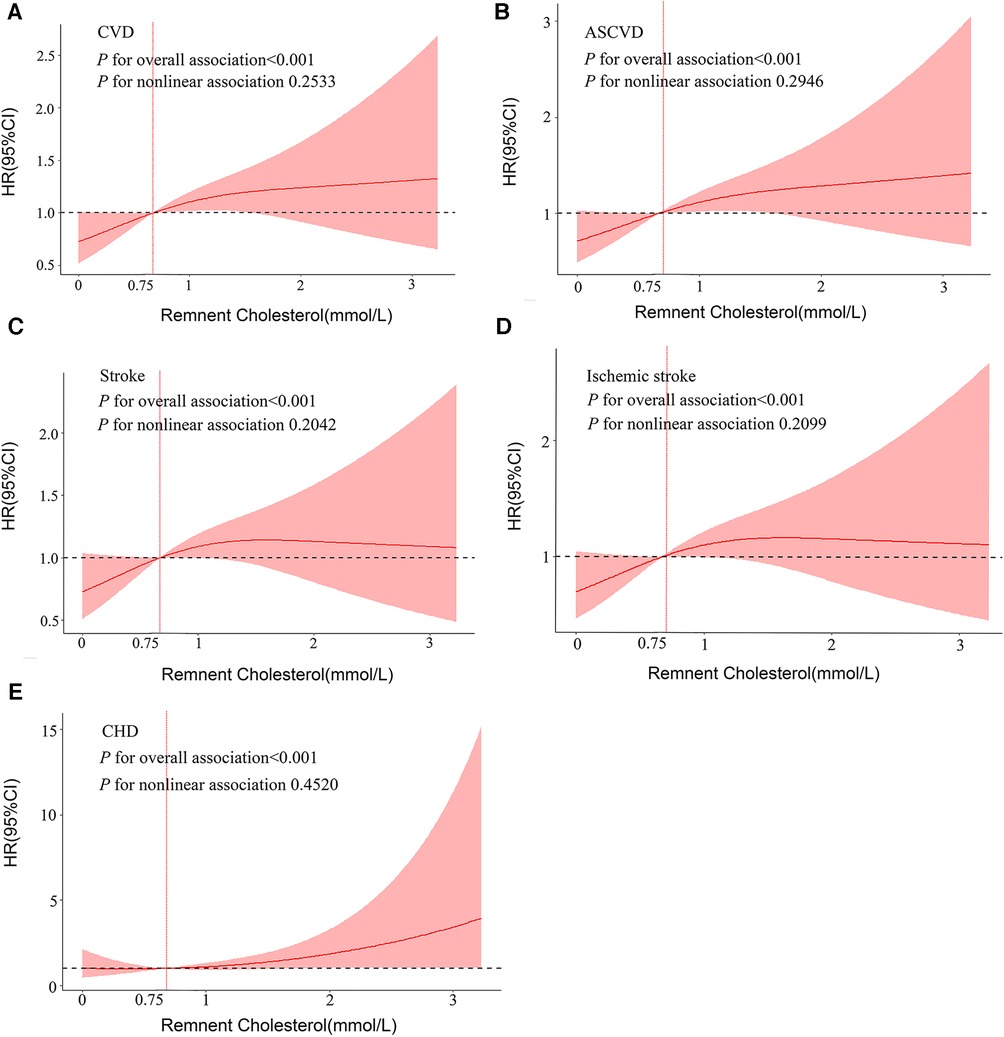
Figure 2. Dose–response associations between RC and cardiovascular outcomes. Restricted cubic splines display the HR of cardiovascular outcomes with 95% confidence intervals according to the concentration of RC. All analyses were adjusted for Model 3 covariates. HR, hazards ratio; CI, confidence interval; CVD, cardiovascular disease; ASCVD, atherosclerotic cardiovascular disease; CHD, coronary heart disease.
After fully adjusting for potential confounders, individuals in the highest RC quartile had a significantly higher incidence of CVD than those in the lowest RC quartile in the following subgroups (Figure 3): women (HR: 2.84, 95% CI: 1.34–6.00; Pvalue = 0.007), aged >60 years (HR: 2.92, 95% CI:1.45–5.88; P-value = 0.003), noncurrent smokers (HR: 2.23, 95% CI 1.27–3.92; P-value = 0.004), BMI <24 kg/m2 (HR: 2.01, 95% CI: 1.15–3.52; P-value = 0.018), inadequate vegetable and fruit intake (HR: 2.40, 95% CI: 1.26–4.58; P-value = 0.004), excessive meat intake (HR: 3.62, 95% CI: 1.46–8.97; P-value = 0.002), animal entrail intake (HR: 2.43, 95% CI: 1.28–4.61; P-value = 0.003), and bean product intake (HR: 2.19, 95% CI: 1.33–3.17; P-value = 0.002). The same trend was found for ASCVD, stroke, and IS prevalence among the following subgroups: aged >60 years, women, noncurrent smokers, insufficient vegetable and fruit intake, excessive meat intake, and animal entrail intake (Supplementary Figures S1–S3).
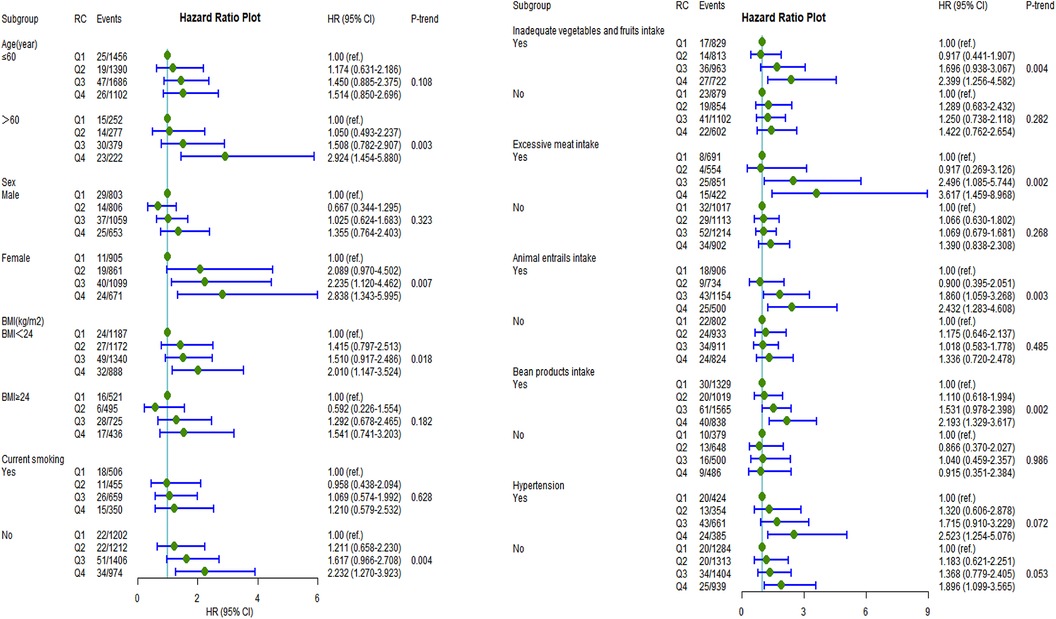
Figure 3. Incident risk of CVD associated with remnant cholesterol by age, sex, BMI, current smoking, inadequate vegetable and fruit intake, excessive meat intake, bean product intake, animal entrail intake, and hypertension (yes or no). All analyses were adjusted for Model 3 covariates. HR, hazards ratio; CI, confidence interval; CVD, cardiovascular disease; BMI, body mass index.
Moreover, participants who took lipid-lowering drugs at baseline were excluded for sensitive analysis. The relationship between RC and the risks of CVD outcomes did not markedly change. Individuals with higher RC concentrations still showed a higher risk for CVD, ASCVD, stroke, and IS (Supplementary Table S2, Model 3). When RC was regarded as a continuous variable, the CVD (HR, 1.24; 95% CI 1.02–1.51) and ASCVD (HR, 1.26; 95% CI 1.02–1.57) risks remained increased (Supplementary Tables S2–S3, Model 3).
After adequate adjustment for confounders (including HDL-C), the association between RC and CVD remained robust (Supplementary Tables S4–S5, Model 3), and the results remained robust after including diabetic nephropathy in the adjusted model (Supplementary Tables S6–S7, Model 3).
This is the first large-scale prospective cohort study to investigate the association between RC and the incidence of CVD outcomes based on the representative population in southwest China. The Cox fully adjusted model indicated a roughly twofold increased risk for CVD, ASCVD, and IS with elevated RC concentration. This relationship remained significant even after adjusting for LDL-C, indicating that the correlation between RC and incidence of CVD outcomes was independent of LDL-C. Importantly, our study found that the increasing trend of CVD risk began when RC ≥0.75 mmol/L. Furthermore, a stronger association was observed among participants aged >60 years, women, hypertension patients, and participants with unhealthy diet patterns.
It has been demonstrated through several observational studies and Mendelian randomization experiments that RC is crucial to the pathophysiology of CVD. A randomized controlled trial at the Spanish PREDIMED Research Center suggested that the decreased triglycerides (which can be used as a marker of RC) caused by icosapent ethyl, a highly purified omega-3 fatty acid, can contribute to a 25% reduction in the risk of ASCVD (27), which is consistent with our findings. Similar results were found in the US and Copenhagen cohort populations by Joshi and Duran et al. (13, 15, 28). Notably, the relationship of RC with CVD risk was independent of LDL-C in the above studies. These results imply that proper management of RC could provide cardiovascular benefits. However, in current clinical practice, most clinicians use statins to reduce LDL-C concentrations without considering residual CVD risk (11). Although there is no consensus on whether treating high levels of RC could prevent cardiovascular disease, some consensus statements and guidelines recommend such treatment as a priority strategy (29). There is a large amount of new research studying new treatments focusing on statins, PcsK9 inhibitors, fibrates, and omega-3 fatty acids (OM3FAs) for elevated RC. Although beta-blockers and fish oils have lowered RC, they have not been consistently successful in reducing CVD. For targeted drug therapy, genetic studies are likewise being vigorously pursued. For instance, angiopoietin–like protein 3 (ANGPTL3), apolipoprotein C3 (APOC3), angiopoietin-like protein 4 (ANGPTL4). APOC3 and ANGPTL3 have been targeted for inhibition by antibody, antisense RNA, or RNAi approaches. Inhibition of any of these molecules decreases RC but increases or lowers HDL levels (30). Although no precisely targeted therapies are available, many novel agents in late-stage research have been developed. Large clinical trials of such drugs in patients with high cardiovascular risk and elevated RC levels are urgently needed to demonstrate the effectiveness of these approaches. Based on the large-scale prospective study conducted in a representative Southwest Chinese general population, our results further support that RC might as a potential preventive/therapeutic target for the CVD susceptibility population, especially those with RC concentrations greater than 0.75 mmol/L. Consistently, in the PREDIMED elderly subjects and Northeast Rural Cardiovascular Health Study, CVD risk increased when RC concentrations were greater than 0.78 mmol/L and 0.84 mmol/L, respectively (14, 18). Importantly, our study covered both urban and rural representative populations in southwest China; thus, our results were more generalizable than previous studies.
TRL-transformed lipoprotein remnants are vital for the development and progression of atherosclerosis (31). Compared with LDL, RC may be preferentially retained in the intima due to its larger size and higher cholesterol content (29, 32). Once RC enters the arterial wall, it is subsequently directly phagocyted by the macrophage, making it more likely to be retained in the endarterium and eventually lead to the formation of atherosclerotic plaques (33, 34). In addition, RC derived from the hydrolysis of TRLs induces the production of IL-8, IL-1, cytokines (TNF-a), and proatherogenic adhesion molecules (35). The activated coagulation cascade via prothrombinase and excessive inflammation also contributes to increased CVD risk (36, 37). Taken together, all the above aberrant processes elicited by RC well-orchestrated lead to the occurrence of CVD.
In the subgroup analysis, the adverse correlation between RC and CVD outcomes in women, participants aged >60 years, and participants with BMI <24 kg/m2 was stronger, which is partially controversial with previous studies (13, 14). Compared to men, this may be related to the lack of estrogen in older women and unfavorable lipid changes after menopause (38). It is noteworthy that compared to Western populations, the Chinese have a smaller body size and lower BMI but higher visceral fat and a lower fat-free mass (39). This race difference contributes to more abnormal metabolic at the same BMI in China compared with people from other countries (40). Thus, our results suggest that Chinese people with BMI <24 kg/m2 and abnormal metabolism should be paid more attention. More importantly, our study observed a stronger association among those with low vegetable and fruit intake, excessive meat intake, and consumption of animal entrails. This is consistent with prior research showing that a healthy diet (such as the Mediterranean diet) could reduce the risk of CVD.
This study, to our knowledge, is the first sizeable longitudinal cohort sample based on the Southwest Chinese general population to assess the relationship between RC and CVD and its subtypes. Moreover, we first adjusted for dietary habits in the multivariable model. Notably, we calculated the cutoff values for RC's increased susceptibility to CVD, which may facilitate clinical practice or early screening of people at risk for CVD. However, several limitations should also be acknowledged. First, we calculated the RC using Friedewald's formula instead of directly measuring the RC. Second, even if most confounding factors were adjusted, unmeasured or unidentified factors such as GFR, urine albumin-to-creatinine ratio (UACR), and fetal-liver infusion (FIL) may have potential confounding effects. Third, because our participants were enrolled from Southwest China, caution must be made when extrapolating our result to other nationwide populations. Fourth, due to the observational nature, the causality of RC on CVD risk should be supported in additional randomized controlled trial studies. Fifth, we failed to consider how lipid profiles vary over time in this investigation.
In this large-scale prospective cohort study based on the Southwest China general population, we found that a high level of RC is a risk factor for developing CVD, independent of LDL-C. RC may serve as an important and new intervention target biomarker to reduce CVD, especially for a sensitive subject whose RC level is >0.75 mmol/L. More importantly, the importance of RC, which could be one low-cost and wide-availability lipid biochemical biomarker, must be considered. We believe that combining traditional influencing factors with new lipid markers constitutes a predictive model or screening strategy that may improve detection and early intervention in high-risk populations and reduce cardiovascular detection rates and the incidence of poor prognosis.
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
The studies involving humans were approved by the Guizhou Center for Disease Control and Prevention's institutional review committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
CL: data curation, writing – original draft. MD: data curation, writing – original draft. KT: data curation, supervision, writing – review and editing. SZ: writing – review and editing, validation. LL: validation, writing – review and editing. ZZ: writing – review and editing, data curation. XY: writing – review and editing, supervision. YX: supervision, writing – review and editing. YW: supervision, writing – review and editing. RD: supervision, writing – review and editing. XL: supervision, writing – review and editing, validation. TL: supervision, writing – review and editing, conceptualization, project administration.
The authors declare that financial support was received for the research, authorship, and/or publication of this article.
The Guizhou Province Science and Technology Support Program [Qiankehe (2018) 2819 and (2021) General 446].
Provincial Key Construction Discipline Project by Guizhou Health Commission.
The Science and Technology Department of Guizhou Province (Qiankehe JichuZK[2022]597.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1286286/full#supplementary-material
1. Sacco RL, Roth GA, Reddy KS, Arnett DK, Bonita R, Gaziano TA, et al. The heart of 25 by 25: achieving the goal of reducing global and regional premature deaths from cardiovascular diseases and stroke: a modeling study from the American Heart Association and World Heart Federation. Circulation. (2016) 133(23):e674–90. doi: 10.1161/CIR.0000000000000395
2. Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, et al. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. (2016) 387(10015):251–72. doi: 10.1016/S0140-6736(15)00551-6
3. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010
4. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. (2016) 37(39):2999–3058. doi: 10.1093/eurheartj/ehw272
5. Ferhatbegovic L, Mrsic D, Kusljugic S, Pojskic B. LDL-C: the only causal risk factor for ASCVD. Why is it still overlooked and underestimated? Curr Atheroscler Rep. (2022) 24(8):635–42. doi: 10.1007/s11883-022-01037-3
6. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. (2016) 316(12):1289–97. doi: 10.1001/jama.2016.13985
7. Byrne P, Demasi M, Jones M, Smith SM, O'Brien KK, DuBroff R. Evaluating the association between low-density lipoprotein cholesterol reduction and relative and absolute effects of statin treatment: a systematic review and meta-analysis. JAMA Intern Med. (2022) 182(5):474–81. doi: 10.1001/jamainternmed.2022.0134
8. Fonseca L, Paredes S, Ramos H, Oliveira JC, Palma I. Apolipoprotein B, and non-high-density lipoprotein cholesterol reveal a high atherogenicity in individuals with type 2 diabetes and controlled low-density lipoprotein-cholesterol. Lipids Health Dis. (2020) 19(1):127. doi: 10.1186/s12944-020-01292-w
9. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. (2018) 379(22):2097–107. doi: 10.1056/NEJMoa1801174
10. Varbo A, Benn M, Tybjaerg-Hansen A, Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. (2013) 61(4):427–36. doi: 10.1016/j.jacc.2012.08.1026
11. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. (2014) 384(9943):626–35. doi: 10.1016/S0140-6736(14)61177-6
12. Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. (2016) 118(4):547–63. doi: 10.1161/CIRCRESAHA.115.306249
13. Wadstrom BN, Wulff AB, Pedersen KM, Jensen GB, Nordestgaard BG. Elevated remnant cholesterol increases the risk of peripheral artery disease, myocardial infarction, and ischaemic stroke: a cohort-based study. Eur Heart J. (2021) 43(34):3258–69. doi: 10.1093/eurheartj/ehab705
14. Chen Y, Li G, Guo X, Ouyang N, Li Z, Ye N, et al. The effects of calculated remnant-like particle cholesterol on incident cardiovascular disease: insights from a general Chinese population. J Clin Med. (2021) 10(15). doi: 10.3390/jcm10153388
15. Joshi PH, Khokhar AA, Massaro JM, Lirette ST, Griswold ME, Martin SS, et al. Remnant lipoprotein cholesterol and incident coronary heart disease: the Jackson Heart and Framingham Offspring cohort studies. J Am Heart Assoc. (2016) 5(5):e002765. doi: 10.1161/JAHA.115.002765
16. Varbo A, Freiberg JJ, Nordestgaard BG. Extreme nonfasting remnant cholesterol vs extreme LDL cholesterol as contributors to cardiovascular disease and all-cause mortality in 90,000 individuals from the general population. Clin Chem. (2015) 61(3):533–43. doi: 10.1373/clinchem.2014.234146
17. Jepsen AM, Langsted A, Varbo A, Bang LE, Kamstrup PR, Nordestgaard BG. Increased remnant cholesterol explains part of residual risk of all-cause mortality in 5,414 patients with ischemic heart disease. Clin Chem. (2016) 62(4):593–604. doi: 10.1373/clinchem.2015.253757
18. Castaner O, Pinto X, Subirana I, Amor AJ, Ros E, Hernaez A, et al. Remnant cholesterol, not LDL cholesterol, is associated with incident cardiovascular disease. J Am Coll Cardiol. (2020) 76(23):2712–24. doi: 10.1016/j.jacc.2020.10.008
19. Deng J, Tang R, Chen J, Zhou Q, Zhan X, Long H, et al. Remnant cholesterol as a risk factor for all-cause and cardiovascular mortality in incident peritoneal dialysis patients. Nutr Metab Cardiovasc. (2023). 33(5):1049–56. doi: 10.1016/j.numecd.2023.02.009
20. Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, et al. Prognostic impact of estimated remnant-like particle cholesterol in patients with differing glycometabolic status: an observational cohort study from China. Lipids Health Dis. (2020) 19(1):179. doi: 10.1186/s12944-020-01355-y
21. Sandesara PB, Virani SS, Fazio S, Shapiro MD. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr Rev. (2019) 40(2):537–57. doi: 10.1210/er.2018-00184
22. WHO Expert Committee. Arterial hypertension and ischemic heart disease, preventive aspect. Geneva: World Health Organization (1962). (WHO technical report series no. 231).
23. Wang SS, Lay S, Yu HN, Shen SR. Dietary guidelines for Chinese residents (2016): comments and comparisons. J Zhejiang Univ Sci B. (2016) 17(9):649–56. doi: 10.1631/jzus.B1600341
24. Chen C, Lu FC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. (2004) 17(Suppl):1–36.15807475
25. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JJ, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. (2003) 289(19):2560–72. doi: 10.1001/jama.289.19.2560
26. American, Diabetes, Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. (2019). 42(Suppl 1):S13–28. doi: 10.2337/dc19-S002
27. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. (2019) 380(1):11–22. doi: 10.1056/NEJMoa1812792
28. Duran EK, Aday AW, Cook NR, Buring JE, Ridker PM, Pradhan AD. Triglyceride-rich lipoprotein cholesterol, small dense LDL cholesterol, and incident cardiovascular disease. J Am Coll Cardiol. (2020) 75(17):2122–35. doi: 10.1016/j.jacc.2020.02.059
29. Hegele RA, Ginsberg HN, Chapman MJ, Nordestgaard BG, Kuivenhoven JA, Averna M, et al. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol. (2014) 2(8):655–66. doi: 10.1016/S2213-8587(13)70191-8
30. Tall AR, Thomas DG, Gonzalez-Cabodevilla AG, Goldberg IJ. Addressing dyslipidemic risk beyond LDL-cholesterol. J Clin Invest. (2022) 132(1). doi: 10.1172/JCI148559
31. Zilversmit DB. A proposal linking atherogenesis to the interaction of endothelial lipoprotein lipase with triglyceride-rich lipoproteins. Circ Res. (1973) 33(6):633–8. doi: 10.1161/01.RES.33.6.633
32. Nordestgaard BG, Wootton R, Lewis B. Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler Thromb Vasc Biol. (1995) 15(4):534–42. doi: 10.1161/01.ATV.15.4.534
33. Varbo A, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. (2013) 128(12):1298–309. doi: 10.1161/CIRCULATIONAHA.113.003008
34. Varbo A, Benn M, Nordestgaard BG. Remnant cholesterol as a cause of ischemic heart disease: evidence, definition, measurement, atherogenicity, high risk patients, and present and future treatment. Pharmacol Ther. (2014) 141(3):358–67. doi: 10.1016/j.pharmthera.2013.11.008
35. Doi H, Kugiyama K, Oka H, Sugiyama S, Ogata N, Koide SI, et al. Remnant lipoproteins induce proatherothrombogenic molecules in endothelial cells through a redox-sensitive mechanism. Circulation. (2000) 102(6):670–6. doi: 10.1161/01.CIR.102.6.670
36. Reiner Z. Hypertriglyceridaemia and risk of coronary artery disease. Nat Rev Cardiol. (2017) 14(7):401–11. doi: 10.1038/nrcardio.2017.31
37. Olufadi R, Byrne CD. Effects of VLDL and remnant particles on platelets. Pathophysiol Haemost Thromb. (2006) 35(3–4):281–91. doi: 10.1159/000093221
38. Schenck-Gustafsson K. Risk factors for cardiovascular disease in women: assessment and management. Eur Heart J. (1996) 17(Suppl D):2–8. doi: 10.1093/eurheartj/17.suppl_D.2
39. Janghorbani M, Salamat MR, Amini M, Aminorroaya A. Risk of diabetes according to the metabolic health status and degree of obesity. Diabetes Metab Syndr. (2017) 11(Suppl 1):S439–44. doi: 10.1016/j.dsx.2017.03.032
Keywords: remnant cholesterol, cardiovascular disease, general population, cohort study, China
Citation: Liu C, Dai M, Tian K, Zhou S, Luo L, Zeng Z, Yan X, Xiao Y, Wang Y, Deng R, Lei X and Liu T (2023) Association of remnant cholesterol with CVD incidence: a general population cohort study in Southwest China. Front. Cardiovasc. Med. 10:1286286. doi: 10.3389/fcvm.2023.1286286
Received: 12 September 2023; Accepted: 6 November 2023;
Published: 27 November 2023.
Edited by:
Mohamad Navab, UCLA Health System, United StatesReviewed by:
Long Jiang, Second Affiliated Hospital of Nanchang University, China© 2023 Liu, Dai, Tian, Zhou, Luo, Zeng, Yan, Xiao, Wang, Deng, Lei and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Liu bGl1dGFvOTA5OU5vXzJAMTYzLmNvbQ== Xiuhong Lei bGVpeGl1aG9uZ0AxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.