- 1Department of Cardiology, Westmead Hospital, Sydney, NSW, Australia
- 2Centre for Heart Research, Westmead Institute for Medical Research, University of Sydney, Westmead, Sydney, NSW, Australia
- 3University of Adelaide, The Queen Elizabeth Hospital, Adelaide, SA, Australia
- 4Research and Education Network, Western Sydney Local Health District, Westmead Hospital, Sydney, NSW, Australia
- 5Kolling Institute, Royal North Shore Hospital, Sydney, NSW, Australia
- 6Charles Perkins Centre, University of Sydney, Sydney, NSW, Australia
Background: Inducible ventricular tachycardia (VT) at electrophysiology study (EPS) predicts sudden cardiac death because of ventricular tachyarrhythmia, the single greatest cause of death within 2 years after myocardial infarction (MI).
Objectives: We aimed to assess the association between standard modifiable risk factors (SMuRFs) and inducible VT at EPS early after MI.
Methods: Consecutive patients with left ventricle ejection fraction ≤40% on days 3–5 after ST elevation MI (STEMI) who underwent EPS were prospectively recruited. Positive EPS was defined as induced sustained monomorphic VT cycle length ≥200 ms for ≥10 s or shorter if hemodynamically compromised. The primary outcome was inducibility of VT at EPS, and the secondary outcome was all-cause mortality on follow-up.
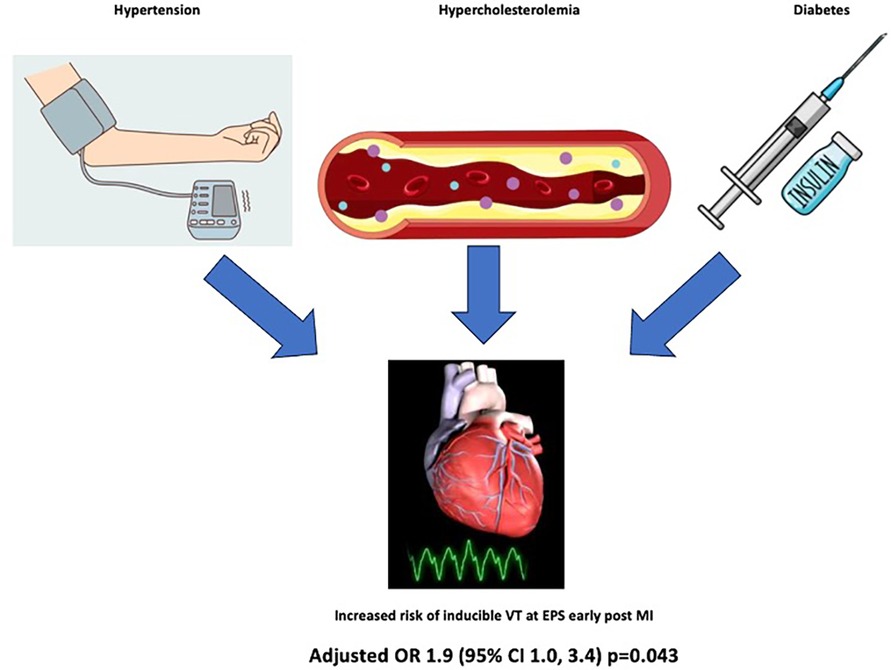
Results: In 410 eligible patients undergoing EPS soon (median of 9 days) after STEMI, 126 had inducible VT. Ex-smokers experienced an increased risk of inducible VT [multivariable logistic regression adjusted odds ratio (OR) 2.0, p = 0.033] compared with current or never-smokers, with comparable risk. The presence of any SMuRFs apart from being a current smoker conferred an increased risk of inducible VT (adjusted OR 1.9, p = 0.043). Neither the number of SMuRFs nor the presence of any SMuRFs was associated with mortality at a median follow-up of 5.4 years.
Conclusions: In patients with recent STEMI and impaired left ventricular function, the presence of any SMuRFs, apart from being a current smoker, conferred an increased risk of inducible VT at EPS. These results highlight the need to modify SMuRFs in this high-risk subset of patients.
Introduction
Patients without standard modifiable risk factors (SMuRFs) have reported higher all-cause mortality at 30 days after ST elevation myocardial infarction (STEMI) compared with those with at least one standard modifiable coronary artery risk factor, including hypertension, diabetes, hypercholesterolemia, and smoking (1–3). This was not driven by recurrent ischemic events, stroke, or bleeding, suggesting potential heightened susceptibility to fatal arrhythmia as the cause of the unexpected differences. Sudden cardiac death because of ventricular tachyarrhythmia is the single greatest cause of death within 2 years after myocardial infarction (MI), especially early in the first 30 days (4, 5).
Extensive work from our group has shown that in patients with left ventricular ejection fraction (LVEF) ≤40% early after reperfusion for STEMI, the presence of inducible ventricular tachycardia (VT) during electrophysiology (EP) studies (EPS) is a marker for the future occurrence of spontaneous ventricular tachyarrhythmias (6–8). No previous study has assessed the impact of the presence or absence of SMuRFs on the risk of inducible VT early after MI during EPS. We aimed to evaluate the incidence of inducible VT at EPS in individuals with LVEF ≤40% shortly after MI, comparing those with standard modifiable coronary artery risk factors to those without.
Methods
Study protocol
The protocol for the collection of data utilized for this study, comprising patient recruitment, follow-up and implantable cardioverter–defibrillator (ICD) implantation and programming, has previously been described in detail (6, 9–13). The Western Sydney Local Health District Human Research Ethics Committee has approved the study, and all patients gave their informed consent. Consecutive patients with LVEF ≤40% on days 3–5 after MI who underwent EPS were prospectively recruited.
A positive EPS was defined as sustained monomorphic VT with cycle length (CL) ≥200 ms (7, 12) for ≥10 s or shorter if hemodynamically compromised (11). Inducible ventricular fibrillation (VF) or ventricular flutter with CL <200 ms was considered non-inducible VT (9, 14). The full protocol for the programmed ventricular stimulation is provided in the Supplementary Material. Predischarge ICD implantation was recommended for patients with inducible VT at EPS (7, 8, 15).
Statistical analysis
IBM SPSS Statistics version 28 was used to analyze the data. SMuRFs were defined as being a current smoker, hypercholesterolemia, diabetes, or hypertension as per the criteria set out in Figtree et al. (1) and also provided in the Supplementary Material.
The chi-squared test or exact permutation tests, when appropriate, were used to assess the univariable association between each categorical variable and VT at EPS status (present vs. absent). The Mann–Whitney test was used to evaluate the univariable association between VT at EPS and the continuous variables age and LVEF (%). LVEF (%), gender, previous coronary artery bypass grafting (CABG) status, and previous percutaneous transluminal coronary angioplasty (PTCA) status were the non-SMuRF variables demonstrating univariable association with VT at EPS at the p ≤ 0.2 level. These variables were selected as candidates for inclusion along with either the number of SMuRFs (0–4) or the presence of any SMuRFs in multiple logistic regression models of VT at EPS. Adjusted odds ratios (ORs) and 95% confidence intervals (95% CIs) from the best-fitting logistic regression models were used to quantify the association of each variable with VT at EPS.
Multiple logistic regression models were fitted, incorporating LVEF (%), gender, previous CABG status, and previous PTCA status along with smoking history and either the number of other SMuRFs (0–3, excluding current smoking status) or the presence of any other SMuRFs as candidates for inclusion. Adjusted ORs with 95% CIs were reported.
Log-rank tests were used to assess the univariable association between each of the categorical variables and the right-censored outcome “years from EPS to death” [overall survival (OS)]. Cox proportional hazards (PH) models were used to assess the univariable association between OS and each continuous variable. The non-SMuRF variables demonstrating univariable association with OS at the p ≤ 0.2 level were age, LVEF (%), family history of cardiovascular disease (CVD), previous ischemic heart disease (IHD), previous CABG, previous PTCA, previous cerebrovascular accident (CVA), and VT at EPS. These variables were selected as candidates for inclusion along with either the number of SMuRFs (0–4) or the presence of any SMuRFs in multiple Cox PH models. For the removal, backward stepwise variable selection with p > 0.1 was used to identify the best-fitting model containing the independent predictors of OS. Hazard ratios (HRs) with 95% CI were used to quantify the strength of the association. Two-tailed tests with a significance level of 5% were used throughout.
Results
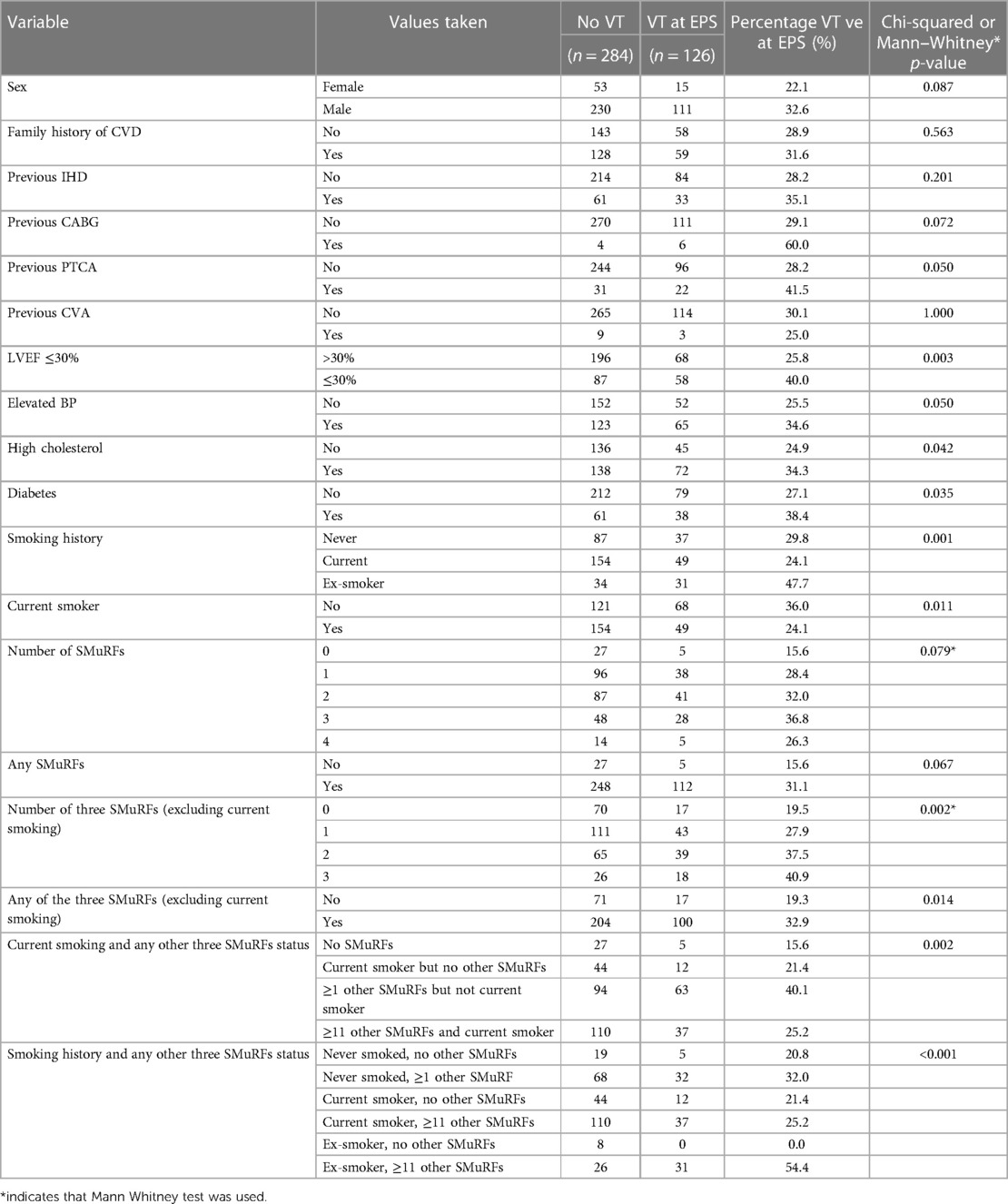
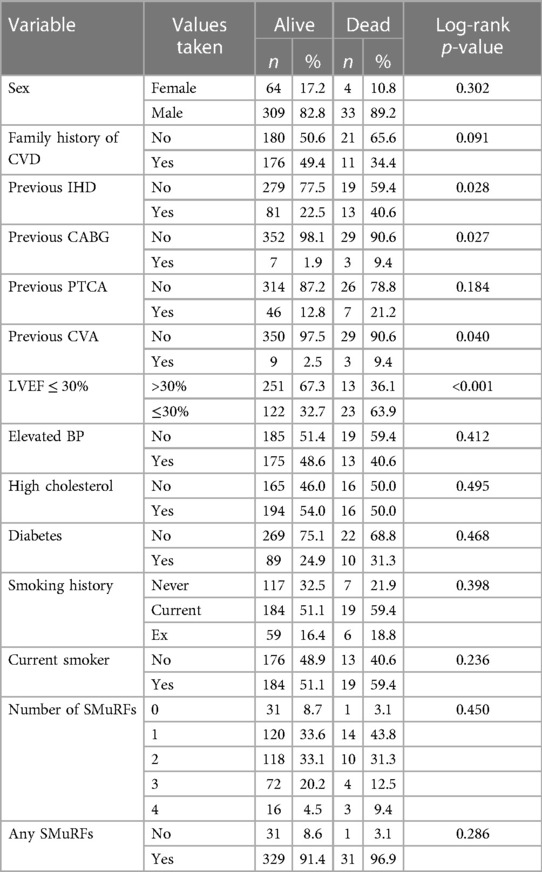
Tables 1A,B show the association between potential risk factors and VT at EPS in 410 participants.
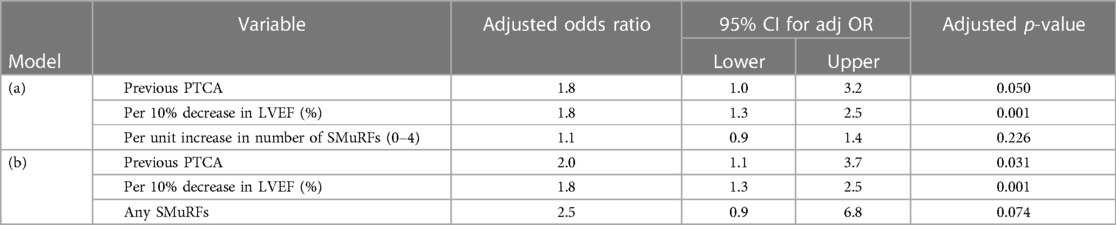
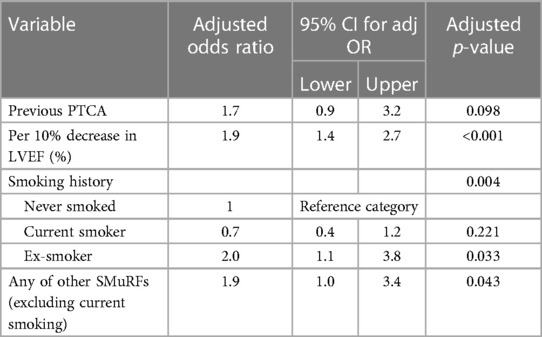
The non-SMuRF variables demonstrating a univariable association with VT at EPS at the p ≤ 0.2 level [namely, LVEF (%), gender, previous CABG status, and previous PTCA status] were selected as candidates for inclusion along with either the number of SMuRFs (0–4) or the presence of any SMuRFs in multiple logistic regression models. Table 2 shows the ORs for VT at EPS with 95% CIs for (a) each unit increase in the number of SMuRFs and (b) the presence of any SMuRFs adjusted for the independent predictors LVEF (%) and previous PTCA. The only independent predictors of VT at EPS were previous PTCA and LVEF (%).
When multiple logistic regression models were fitted incorporating LVEF (%), gender, previous CABG status, and previous PTCA status along with smoking history and either the number of other SMuRFs (0–3, excluding current smoking status) or the presence of any other SMuRFs as candidates for inclusion, the independent predictors of VT at EPS in the best-fitting model were LVEF (%), previous PTCA, smoking history, and any other SMuRFs (0–3 excluding current smoking status). The fit of this model was significantly better than that based on only LVEF (%) and previous PTCA [change in −2 × log (likelihood was 17.3, chi-squared with 3 degrees of freedom, p < 0.001)]. Table 3 shows the adjusted ORs with 95% CIs for VT at EPS in this best-fitting model.
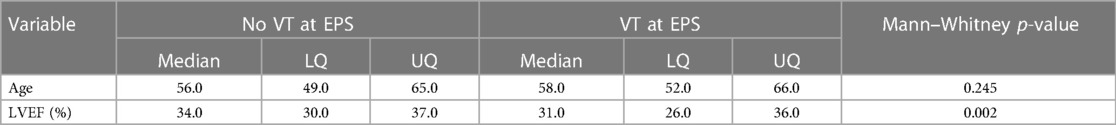
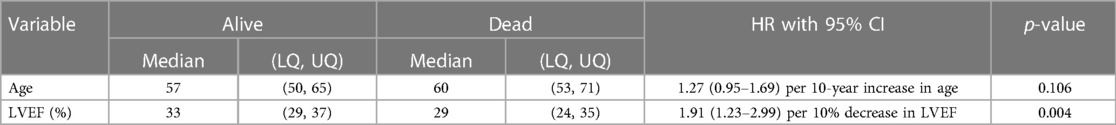
A total of 410 patients who underwent EPS were followed up for a median of 5.4 years; 37 patients died during the follow-up period. In total, 196 patients were followed up for at least 5 years and 66 patients for at least 10 years. Tables 4A,B show the patient characteristics during the EPS by mortality status.
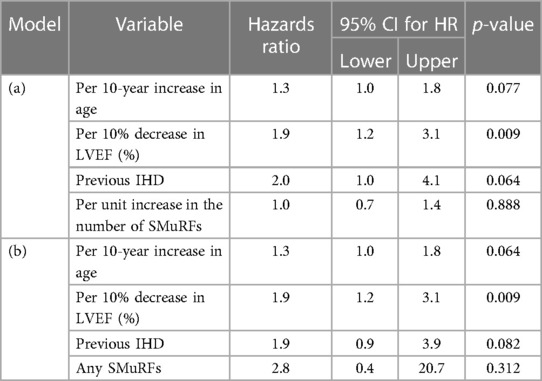
The non-SMuRF variables demonstrating a univariable association with mortality at the p ≤ 0.2 level [namely, age, LVEF (%), family history of CVD, previous IHD, previous CABG, previous PTCA, and previous CVA status] were selected as candidates for inclusion along with either the number of SMuRFs (0–4) or the presence of any SMuRFs in multiple Cox PH models of the right-censored outcome years from EPS to death. The only independent predictors were previous IHD, age, and LVEF (%). Table 5 shows the HRs with 95% CIs for (a) each unit increase in the number of SMuRFs and (b) the presence of any SMuRFs adjusted for the independent predictors age, LVEF (%), and previous IHD.
For every 10-year increase in age, the risk of death increased by a multiplicative factor of 1.3. For every 10% decrease in LVEF (%), the risk of death increased by a multiplicative factor of 1.9, and in patients with previous IHD, the risk of death was doubled. Neither the number of SMuRFs nor the presence of any SMuRFs was associated with mortality.
This study highlights the following significant results:
• Patients who are ex-smokers or have any other SMuRFs apart from smoking have double the odds of having inducible VT at EPS early after STEMI.
• Neither the presence of any SMuRFs nor the number of SMuRFs was associated with an increased risk of mortality.
Discussion
In this study, we were able to examine potential differences in susceptibility to ventricular arrhythmias in individuals who presented with atherosclerotic MI in the absence of traditional risk factors. This subgroup of MI patients had been observed to have cardiac arrest at presentation and an excess mortality rate in the first 30 days (1–3), with most of this difference driven in the first 48 h and not explained by recurrent MI, heart failure, or stroke (1). However, our findings contradicted the hypothesis that STEMI patients without SMuRFs may be more sensitive to inducible VT, clearly demonstrating lower rates than those observed in patients with at least one standard risk factor. The rigorous approach we have validated over the last decade strengthened our study (6, 8, 15–17).
The apparent contradiction of our findings, despite the observed higher rates of cardiac arrest and mortality in SMuRF-less STEMI patients compared with those with at least one risk factor, may be attributed to the timing of our EPS. The underlying biological mechanism of VT in the first 48 h is known to be different from that driving VT at later timepoints. Indeed, VT in the first 48 h is not strongly predictive of VT/VF beyond this timepoint (18). Our team is dedicated to conducting early EP stimulation studies to detect the long-term risk of sudden cardiac death to guide preventative strategies, including automated implantable cardioverter defibrillator (AICD) insertion (11, 13, 16, 19). In this study, the earliest time post-STEMI was 4 days, with a median of 9 days. In contrast, as reported by Figtree et al. (1), most deaths in the SWEDEHEART cohort occurred in the first 48 h, with a difference in cardiac arrest identified at the time of presentation. If individuals survived to 30 days, mortality was less in SMuRF-less STEMI patients vs. those with at least one risk factor.
In addition to this potential explanation, our study population significantly differs from that of the Figtree et al. (1) paper, which excluded patients with pre-existing coronary artery disease (CAD), while 23% of our population had previous CAD. We also had a group of higher-risk patients because we only included those with LVEF ≤40% early after MI. Furthermore, only 8% of our patient population had no SMuRFs, compared with 15% of the SWEDEHEART cohort.
In our study cohort of patients undergoing EPS early following STEMI, the current smoking status SMuRF was associated with a decreased risk of VT at EPS. Since we have defined the current smoking SMuRF as current smokers vs. never and ex-smokers, these findings are unsurprising, given our patient population. Nearly 50% of all ex-smokers had inducible VT, while a similar proportion of never-smokers (30%) and current smokers (25%) had induced VT. Furthermore, 54% of ex-smokers with inducible VT had at least one other SMuRF, while only 32% of never-smokers and 25% of current smokers did. When smoking history was considered, ex-smokers were identified at an increased risk of VT at EPS, while those who never smoked or were current smokers had a comparable risk, as seen in our multiple regressions model. However, we also found that the presence of any SMuRFs apart from the current smoker status also conferred a similarly increased risk of inducible VT.
SMuRFs have been shown to worsen cardiovascular morbidity and mortality. It has been shown that patients with hypertension have an increased frequency of ventricular tachyarrhythmias (20, 21). Hypertension in itself is not arrhythmogenic but likely exerts its potential through its effect of pressure overload on the ventricle (22). Hypertension is a known risk factor for sudden cardiac death (SCD), especially with increasing LV mass (23). Diabetes can lead to myocardial dysfunction through mechanical abnormalities and electrical remodeling (24). Arrhythmogenesis is also exacerbated in patients with diabetes because of autonomic dysregulation (25) and inflammation (26). The AVID study (27) has previously shown that lipid-lowering therapy had a positive impact on decreasing ventricular arrhythmia in patients with atherosclerotic heart disease. Smoking has been shown to have deleterious effects on the myocardium with the predisposition to an arrhythmogenic substrate in an animal model (28). Smoking cessation improves cardiovascular outcomes, but it can take up to 5 (29) and even 15 (30) years for outcomes to stabilize to levels of never-smokers. The effect of previous smoking may confound the data as we did not have data on the time of smoking cessation and the number of cigarettes consumed.
We have previously confirmed the utility of the inducibility of VT in predicting future arrhythmic events (6, 7, 10). Conversely, patients with a negative EPS, meaning no induced arrhythmia or ventricular flutter/VF, have demonstrated a good long-term prognosis without an ICD insertion (8, 13). Confirming an EPS-guided strategy for the primary prevention of sudden cardiac death requires a large, multicenter, randomized, controlled trial such as the current PROTECT-ICD trial, which has a VT induction protocol similar to our study (31). Gatzoulis et al. have also shown the utility of EPS after STEMI in a population with LVEF >40% (32).
The limitations of this study include the lack of randomization and the small sample size from a single center. Although we have used the same definitions from the seminal Lancet paper (1) to define SMuRFs, we acknowledge that individual-level characterization of each risk factor would enhance the results. Characterizing the infarct substrate using late gadolinium enhancement on cardiac magnetic resonance imaging may provide additional and novel insights into this high-risk population. Although a relatively small study sample was analyzed, statistically significant results regarding VT inducibility were obtained. The routine use of EPS shortly after MI to guide ICD implantation is part of a study protocol and is limited by its invasiveness and costs. However, it might offer a rational and cost-effective approach to the expensive long-term therapy with ICD following MI. This approach of using EPS early after MI in individuals with impaired left ventricular function is under investigation in the randomized PROTECT ICD trial (31).
Conclusions
In our cohort of patients with LVEF ≤40% early after STEMI who had an EPS to assess for inducible VT at a median of 9 days, either the presence of any of the three SMuRFs (hypertension, hypercholesterolemia, or diabetes mellitus) or previous smoking doubled the chance of having inducible VT at EPS. Neither the presence of any SMuRFs nor the number of SMuRFs was associated with an increased mortality risk on long-term follow-up. These results further highlight the need to modify SMuRFs in a high-risk subset of patients with IHD to reduce the significant risk of ventricular tachyarrhythmias following MI.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Western Sydney Local Health District Human Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TD: Data curation, Formal analysis, Methodology, Validation, Writing – Original draft, Writing – Review & editing. JK: Data curation, Formal analysis, Methodology, Writing – Original draft, Writing – Review & editing. KB: Data curation, Formal analysis, Methodology, Writing – Review & editing. CC: Methodology, Validation, Visualization, Writing – Review & editing. SZ: Formal analysis, Methodology, Supervision, Validation, Writing – Review & editing. JC: Investigation, Methodology, Validation, Writing – Review & editing. GF: Conceptualization, Data curation, Investigation, Validation, Writing – Review & editing. AT: Conceptualization, Formal analysis, Investigation, Supervision, Validation, Writing – Original draft, Writing – Review & editing. PK: Conceptualization, Data curation, Investigation, Project administration, Supervision, Validation, Visualization, Writing – Original draft, Writing – Review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article.
TD is currently supported by a National Health and Research Medical Council Postgraduate Scholarship (APP2002783) and Heart Foundation Health Professionals Scholarship (104615). JK currently holds The Hospital Research Foundation/Basil Hetzel Institute Scholarship from the University of Adelaide, Adelaide, South Australia, Australia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1283382/full#supplementary-material
References
1. Figtree GA, Vernon ST, Hadziosmanovic N, Sundström J, Alfredsson J, Arnott C, et al. Mortality in STEMI patients without standard modifiable risk factors: a sex-disaggregated analysis of SWEDEHEART registry data. Lancet. (2021) 397(10279):1085–94. doi: 10.1016/S0140-6736(21)00272-5
2. Chong B, Goh R, Kong G, Ng Cheng H, Foo Roger S-Y, Low A, et al. Prevalence and outcomes of patients without standard modifiable risk factors following acute coronary syndrome: a systematic review and meta-analysis. J Am Coll Cardiol. (2022) 79(9_Supplement):1091. doi: 10.1016/S0735-1097(22)02082-4
3. Sia CH, Ko J, Zheng H, Ho AF, Foo D, Foo LL, et al. Comparison of mortality outcomes in acute myocardial infarction patients with or without standard modifiable cardiovascular risk factors. Front Cardiovasc Med. (2022) 9:876465. doi: 10.3389/fcvm.2022.876465
4. Zaman S, Kovoor P. Sudden cardiac death early after myocardial infarction. Circulation. (2014) 129(23):2426–35. doi: 10.1161/CIRCULATIONAHA.113.007497
5. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: developed by the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) endorsed by the Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. (2022) 43(40):3997–4126. doi: 10.1093/eurheartj/ehac262
6. Zaman S, Kumar S, Narayan A, Sivagangabalan G, Thiagalingam A, Ross DL, et al. Induction of ventricular tachycardia with the fourth extrastimulus and its relationship to risk of arrhythmic events in patients with post-myocardial infarct left ventricular dysfunction. Europace. (2012) 14(12):1771–7. doi: 10.1093/europace/eus199
7. Zaman S, Kumar S, Sullivan J, Narayan A, Thiagalingam A, Ross David L, et al. Significance of inducible very fast ventricular tachycardia (cycle length 200–230 ms) after early reperfusion for ST-segment–elevation myocardial infarction. Circ Arrhythm Electrophysiol. (2013) 6(5):884–90. doi: 10.1161/CIRCEP.113.000213
8. Zaman SNA, Thiagalingam A, Sivagangabalan G, Thomas S, Ross DL, Kovoor P. Long-term arrhythmia-free survival in patients with severe left ventricular dysfunction and no inducible ventricular tachycardia after myocardial infarction. Circulation. (2014) 129:848–54. doi: 10.1161/CIRCULATIONAHA.113.005146
9. Zaman S, Kumar S, Sullivan J, Narayan A, Thiagalingam A, Ross DL, et al. Significance of inducible very fast ventricular tachycardia (cycle length 200–230 ms) after early reperfusion for ST-segment-elevation myocardial infarction. Circ Arrhythm Electrophysiol. (2013) 6(5):884–90. doi: 10.1161/CIRCEP.113.000213
10. Zaman S, Narayan A, Thiagalingam A, Sivagangabalan G, Thomas S, Ross DL, et al. Significance of repeat programmed ventricular stimulation at electrophysiology study for arrhythmia prediction after acute myocardial infarction. Pacing Clin Electrophysiol. (2014) 37(7):795–802. doi: 10.1111/pace.12391
11. Deshmukh T, Zaman S, Narayan A, Kovoor P. Duration of inducible ventricular tachycardia early after ST-segment-elevation myocardial infarction and its impact on mortality and ventricular tachycardia recurrence. J Am Heart Assoc. (2020) 9(13):e015204. doi: 10.1161/JAHA.119.015204
12. Kumar S, Sivagangabalan G, Thiagalingam A, West EB, Narayan A, Sadick N, et al. Effect of reperfusion time on inducible ventricular tachycardia early and spontaneous ventricular arrhythmias late after ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart Rhythm. (2011) 8(4):493–9. doi: 10.1016/j.hrthm.2010.11.046
13. Kumar S, Sivagangabalan G, Zaman S, West EB, Narayan A, Thiagalingam A, et al. Electrophysiology-guided defibrillator implantation early after ST-elevation myocardial infarction. Heart Rhythm. (2010) 7(11):1589–97. doi: 10.1016/j.hrthm.2010.07.019
14. Kumar S, Sivagangabalan G, Choi MC, Eipper V, Thiagalingam A, Kovoor P. Long-term outcomes of inducible very fast ventricular tachycardia (cycle length 200–250 ms) in patients with ischemic cardiomyopathy. J Cardiovasc Electrophysiol. (2010) 21(3):262–9. doi: 10.1111/j.1540-8167.2009.01624.x
15. Zaman SSG, Narayan N, Thiagalingam A, Ross DL, Kovoor P. Outcomes of early risk stratification and targeted implantable cardioverter–defibrillator implantation after ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Circulation. (2009) 120:194–200. doi: 10.1161/CIRCULATIONAHA.108.836791
16. Nalliah CJ, Narayan A, Thiagalingam A, Ross DL, Sivagangabalan G, Zaman S, et al. What is the optimal left ventricular ejection fraction cut-off for risk stratification for primary prevention of sudden cardiac death early after myocardial infarction? EP Europace. (2014) 16(9):1315–21. doi: 10.1093/europace/euu026
17. Narayan A, Nalliah CJ, Sullivan J, Zaman S, Kovoor P. Coronary artery reperfusion for ST elevation myocardial infarction is associated with shorter cycle length ventricular tachycardia and fewer spontaneous arrhythmias. EP Europace. (2013) 16(7):1053–60. doi: 10.1093/europace/eut307
18. Mehta RH, Yu J, Piccini JP, Tcheng JE, Farkouh ME, Reiffel J, et al. Prognostic significance of postprocedural sustained ventricular tachycardia or fibrillation in patients undergoing primary percutaneous coronary intervention (from the HORIZONS-AMI trial). Am J Cardiol. (2012) 109(6):805–12. doi: 10.1016/j.amjcard.2011.10.043
19. Zaman S, Deshmukh T, Aslam A, Martin C, Kovoor P. Sex differences in electrophysiology, ventricular tachyarrhythmia, cardiac arrest and sudden cardiac death following acute myocardial infarction. Heart Lung Circ. (2019) 29(7):1025–31. doi: 10.1016/j.hlc.2019.07.017
20. Zehender M, Meinertz T, Hohnloser S, Geibel A, Gerisch U, Olschewski M, et al. Prevalence of circadian variations and spontaneous variability of cardiac disorders and ECG changes suggestive of myocardial ischemia in systemic arterial hypertension. Circulation. (1992) 85(5):1808–15. doi: 10.1161/01.CIR.85.5.1808
21. Simpson RJ Jr., Cascio WE, Crow RS, Schreiner PJ, Rautaharju PM, Heiss G. Association of ventricular premature complexes with electrocardiographic-estimated left ventricular mass in a population of African-American and white men and women (the atherosclerosis risk in communities). Am J Cardiol. (2001) 87(1):49–53. doi: 10.1016/S0002-9149(00)01271-6
22. Lip GYH, Coca A, Kahan T, Boriani G, Manolis AS, Olsen MH, et al. Hypertension and cardiac arrhythmias: a consensus document from the European Heart Rhythm Association (EHRA) and ESC Council on Hypertension, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). EP Europace. (2017) 19(6):891–911. doi: 10.1093/europace/eux091
23. Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. (1998) 32(5):1454–9. doi: 10.1016/S0735-1097(98)00407-0
24. Gallego M, Zayas-Arrabal J, Alquiza A, Apellaniz B, Casis O. Electrical features of the diabetic myocardium. Arrhythmic and cardiovascular safety considerations in diabetes. Front Pharmacol. (2021) 12. doi: 10.3389/fphar.2021.687256
25. Chen C, Wang W, Zhou W, Jin J, Chen W, Zhu D, et al. Nocturnal ventricular arrhythmias are associated with the severity of cardiovascular autonomic neuropathy in type 2 diabetes. J Diabetes. (2019) 11(10):794–801. doi: 10.1111/1753-0407.12908
26. Karam BS, Chavez-Moreno A, Koh W, Akar JG, Akar FG. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc Diabetol. (2017) 16(1):120. doi: 10.1186/s12933-017-0604-9
27. Mitchell LB, Powell JL, Gillis AM, Kehl V, Hallstrom AP. Are lipid-lowering drugs also antiarrhythmic drugs?: an analysis of the antiarrhythmics versus implantable defibrillators (AVID) trial. J Am Coll Cardiol. (2003) 42(1):81–7. doi: 10.1016/S0735-1097(03)00498-4
28. Qiu H, Zhang H, Han DD, Derakhshandeh R, Wang X, Goyal N, et al. Increased vulnerability to atrial and ventricular arrhythmias caused by different types of inhaled tobacco or marijuana products. Heart Rhythm. (2023) 20(1):76–86. doi: 10.1016/j.hrthm.2022.09.021
29. Duncan MS, Freiberg MS, Greevy RA Jr., Kundu S, Vasan RS, Tindle HA. Association of smoking cessation with subsequent risk of cardiovascular disease. JAMA. (2019) 322(7):642–50. doi: 10.1001/jama.2019.10298
30. Ahmed AA, Patel K, Nyaku MA, Kheirbek RE, Bittner V, Fonarow GC, et al. Risk of heart failure and death after prolonged smoking cessation. Circ Heart Failure. (2015) 8(4):694–701. doi: 10.1161/CIRCHEARTFAILURE.114.001885
31. Zaman S, Taylor AJ, Stiles M, Chow C, Kovoor P. Programmed ventricular stimulation to risk stratify for early cardioverter–defibrillator implantation to prevent tachyarrhythmias following acute myocardial infarction (PROTECT-ICD): trial protocol, background and significance. Heart Lung Circ. (2016) 25(11):1055–62. doi: 10.1016/j.hlc.2016.04.007
Keywords: myocardial infarction, ventricular tachycardia, electrophysiology study, standard modifiable risk factors, LVEF (left ventricular ejection fraction)
Citation: Deshmukh T, Kovoor JG, Byth K, Chow CK, Zaman S, Chong JJH, Figtree GA, Thiagalingam A and Kovoor P (2023) Influence of standard modifiable risk factors on ventricular tachycardia after myocardial infarction. Front. Cardiovasc. Med. 10:1283382. doi: 10.3389/fcvm.2023.1283382
Received: 25 August 2023; Accepted: 27 September 2023;
Published: 24 October 2023.
Edited by:
Konstantinos Athanasios Gatzoulis, National and Kapodistrian University of Athens, GreeceReviewed by:
Giorgos Oikonomou, Hippokration General Hospital, GreecePetros Arsenos, National and Kapodistrian University of Athens, Greece
© 2023 Deshmukh, Kovoor, Byth, Chow, Zaman, Chong, Figtree, Thiagalingam and Kovoor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pramesh Kovoor cHJhbWVzaC5rb3Zvb3JAc3lkbmV5LmVkdS5hdQ==
 Tejas Deshmukh
Tejas Deshmukh Joshua G. Kovoor3
Joshua G. Kovoor3 Gemma A. Figtree
Gemma A. Figtree Pramesh Kovoor
Pramesh Kovoor