
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 18 December 2023
Sec. Cardiovascular Imaging
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1282597
Abdominal vascular compression syndrome (AVCS) is caused by the compression of abdominal blood vessels by adjacent structures or the compression of abdominal organs by neighboring blood vessels. Such compressions can result in a variety of clinical symptoms. They are not commonly seen in ultrasound practices, and their presence may have been underrecognized and underdiagnosed. This article reviews the clinical features, ultrasound characteristics, and diagnostic criteria of four types of AVCS, namely, celiac artery compression syndrome, renal vein compression syndrome, iliac vein compression syndrome, and superior mesenteric artery syndrome to increase awareness of these conditions among ultrasound practitioners. The ultrasound criteria for AVCS are primarily based on studies with small sample sizes, and therefore, it is important to exercise caution if these criteria are used.
Blood vessels in the abdomen may be compressed by adjacent anatomical structures, or they may cause compression of organs in the abdomen. Abdominal vascular compression phenomena are referred to the identification of such compressions with or without clinical symptoms. When the compression causes relevant clinical symptoms, it is regarded as an abdominal vascular compression syndrome (AVCS) (1–5).
AVCS involving the compression of blood vessels includes celiac artery compression syndrome (CACS), renal vein compression syndrome (RVCS), and iliac vein compression syndrome (IVCS). AVCS involving the compression of organs consists of superior mesenteric artery syndrome (SMAS), ureteropelvic junction obstruction, and retrocaval ureter (Table 1) (3, 6, 7).
AVCS are uncommon, and perhaps underrecognized and underdiagnosed. We review the clinical features, ultrasound characteristics, and diagnostic criteria of the first four types of AVCS to help sonographers and sonologists become familiar with these conditions and encourage them to take them into consideration when making diagnostic assessments in their ultrasound practice. Of note, all of these syndromes are primarily observed in young and middle-aged women (3, 4, 8–10).
The last two AVCS are not included in this review due to the limited information on their ultrasound findings in the literature.
The celiac artery compression syndrome (CACS), also known as median arcuate ligament syndrome (MALS) and Dunbar syndrome, is caused by the external compression of the median arcuate ligament (MAL) on the celiac artery (CA). The MAL is the fibrous arc of the aortic hiatus in the diaphragm which connects the left and right diaphragmatic crura and usually lies superior to the origin of celiac artery (Figure 1A). When the MAL has a low insertion, it can compress the celiac artery. The compression usually worsens during expiration as the diaphragm moves caudally (Figures 1B,C) (8, 11). Patents with MAL compression may have no symptoms. Postprandial abdominal pain and weight loss are the most common symptoms in symptomatic patients with CACS. Other symptoms include nausea and vomiting (3). Most CACS are diagnosed when other chronic abdominal pain-related diseases are excluded.
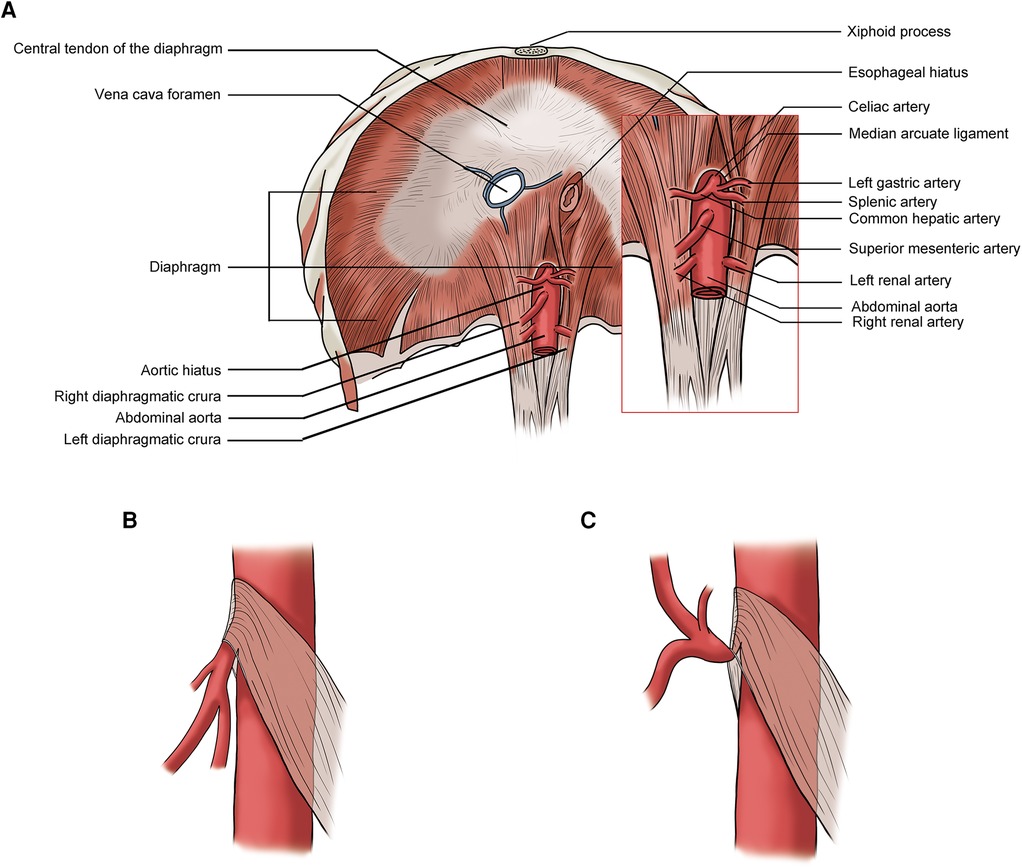
Figure 1. (A) Normal median arcuate ligament (MAL) position; (B) lower MAL position: no significant celiac artery compression during inspiration; (C) lower MAL position: celiac artery compressed by the MAL during expiration.
Radiological examinations including computed tomography angiography (CTA), magnetic resonance angiography (MRA), and selective angiography can be used to confirm the presence of MAL compression (7, 12, 13). It is important to assess the CA with inspiration along with expiration as CA compression may only occur at the end of the expiration. CT and MRI may also be used to rule out other causes of CA compression (14) such as lymph node metastases.
Many patients with CACS are treated using conservative management approaches. The surgical options for CACS include open or laparoscopic surgery to relieve MAL compression, open or endovascular revascularization of the CA, and celiac ganglion resection (3, 10, 11). More recently, robotic-assisted MAL release has been used in patients with MALS (15).
Gruber and colleagues (16) compared the findings of duplex ultrasound in six patients with CACS with 20 age-matched asymptomatic volunteers and found that a peak systolic velocity (PSV) of the CA during expiration greater than 350 cm/s had an 83% positive predictive value (PPV) and a 95% negative predictive value (NPV). The CA deflection angle (end-expiratory upturn angle) of 50° or more has a PPV of 43% and an NPV of 100%, and the combination of CA PSV (>350 cm/s) and deflection angle (>50°) has a PPV of 100% and NPV of 95%. Saleem and coauthors (17) described the ultrasound criteria that are supportive in diagnosing CACS, which include expiratory CA PSV of > 200 cm/s and a CA deflection angle of > 50°. The changes of CA deflection angle during inspiration and expiration in a patient with CACS are shown in Figure 2. Another ultrasound criterion for diagnosing CACS includes measuring the PSV of the CA during the expiratory phase. A PSV of over 200 cm/s or a ratio of PSV of CA to aorta of greater than 3:1 in the expiratory phase can also indicate CACS (18–23). In patients with an optimal sonographic window, the “hook” or “J” shape of the proximal CA caused by extrinsic compression by the MAL can be demonstrated in the sagittal plane with a B-mode or color Doppler imaging (24).
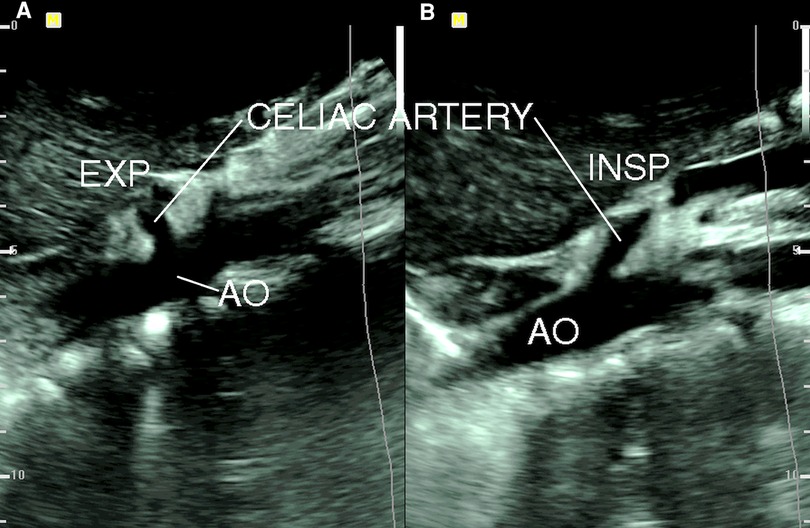
Figure 2. B-mode ultrasound image of celiac artery compression. (A) Celiac artery deflection angle > 50° during expiration (EXP); (B) celiac artery deflection angle < 50° during inspiration (INSP). AO, aorta [from: Youssef (18). Reprint with permission from Dr Youssef].
The use of intravascular ultrasound (IVSU) to assess patients with MALS has been reported to show substantial luminal stenosis at the origin of the celiac artery with wall thickening (25). This finding is suggestive of CA intimal hyperplasia from chronic irritation by the MAL.
The renal vein compression syndrome (RVCS), also known as left renal vein entrapment syndrome and nutcracker syndrome (NCS), refers to the compression of the left renal vein (LRV) by the superior mesenteric artery (SMA) and abdominal aorta (AA), like the compression of a nut between the jaws of a nutcracker (Figure 3) (26). This type of RVCS is also known as the anterior nutcracker syndrome (ANCS). In addition, when the retroaortic LRV is compressed by the AA and the vertebral body, it is referred to as the posterior nutcracker syndrome (PNCS) (27). There is also a known anatomical variant involving two LRVs that pass anteriorly and posteriorly to the AA. In these patients, the simultaneous compression of both veins is referred to as the combined nutcracker syndrome (14).
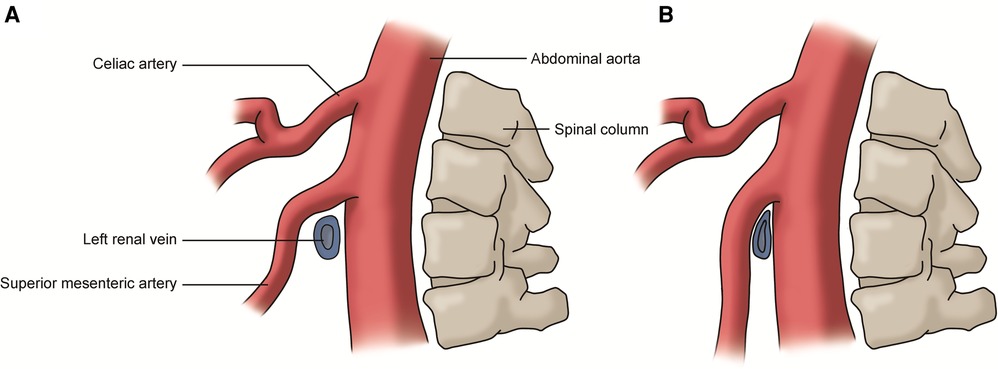
Figure 3. (A) Normal left renal vein: the vein passes between the superior mesenteric artery and abdominal aorta without compression; (B) renal vein compression syndrome: the left renal vein is compressed by the superior mesenteric artery and the abdominal aorta.
The clinical manifestations of the RVCS include left flank pain, hematuria, proteinuria, and symptoms related to left gonadal vein reflux such as pelvic congestion syndrome in the female and varicocele in the male. CT venography and contrast venography are useful radiological modalities for diagnosis (3, 26, 28). The conservative management involves gaining weight and exercising the back muscles. Open surgical options such as renocaval bypass and transposition of the LRV to the inferior vena cava and endovascular interventions including stent implantation and ovarian vein embolization are used when conservative management has failed (3, 29). Laparoscopic extravascular stent placement has been reported as an alternative to open surgery or endovascular intervention (30).
Kim and coworkers (31, 32) analyzed duplex ultrasound results of the left renal vein in 16 patients with RVCS and 18 healthy control subjects. They measured the diameters and PSVs in two regions of the left renal vein: firstly, between the SMA and the aorta (MA) and secondly, near the hilar (NH) (Figure 4). They recommended ultrasound criteria for RVCS of diameter ratio (NH/MA) > 5 (sensitivity: 69% and specificity: 89%) and PSV ratio (MA/NH) > 5 (sensitivity: 80% and specificity: 94%). Yang and colleagues (33) reported eight cases of PNCS and described their ultrasound diagnostic criteria for PNCS as follows: firstly, the finding of a retroaortic left renal vein; secondly, the diameter of the dilated part of the left renal vein (D-VD): diameter of stenotic part of the vein (S-VD) >3:1 in the supine position, or >4:1 in the spinal extension position; and thirdly, the PSV in the stenotic part of the left renal vein: PSV in the dilated part of the vein >3:1, or >4:1 in the spinal extension position.
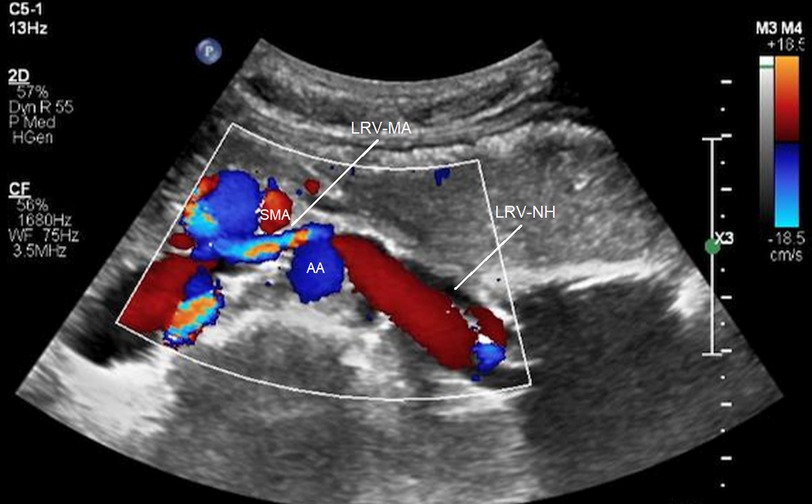
Figure 4. Compression of the left renal vein on color Doppler imaging. SMA, superior mesenteric artery; AA, abdominal aorta; LRV-MA, left renal vein between the SMA and AA; LRV-NH, left renal vein near the hilar [from: Kim (32). Reprinted with permission from Dr Kim].
The left gonadal vein is a tributary of the LRV. LRV hypertension may lead to incompetence of the left gonadal vein (34). Left ovarian vein reflux in a patient with RVCS is shown in Figure 5.

Figure 5. Ovarian vein reflux on color Doppler imaging. L RV, left renal vein; L OV, left ovarian vein.
The iliac vein compression syndrome (IVCS), also known as May–Thurner syndrome (MTS) and Cockett syndrome, is a clinical condition caused by the extrinsic compression of the left common iliac vein (CIV) by the right common iliac artery (CIA) and vertebral body (Figure 6) (9). The clinical symptoms include swelling of the left lower limb, edema, and varicose veins. In the event of the development of deep vein thrombosis (DVT), symptoms related to acute DVT, post-thrombotic syndrome, and pulmonary embolism may result (3, 9, 35). CT/MR venography and contrast venography are diagnostic tools used to assess IVCS. However, contrast venography is mainly used in conjunction with intervention (3, 9, 36, 37). The conservative management includes the use of compression stockings and anticoagulation therapy. Iliac vein stenting and DVT thrombolysis may be considered when conservative treatment has failed (3).
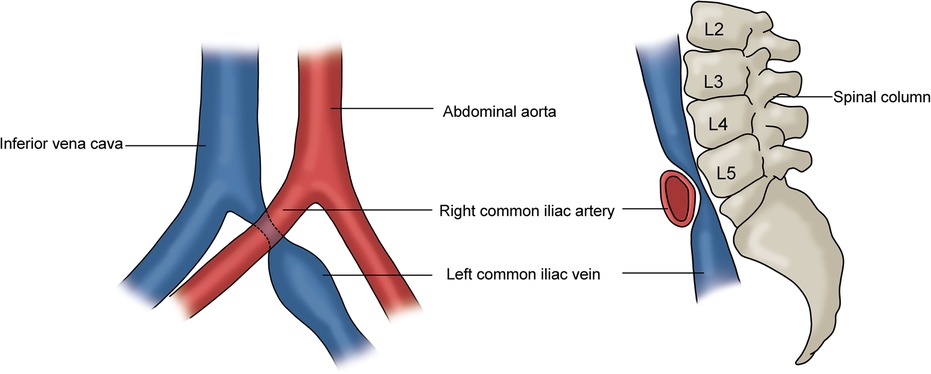
Figure 6. Iliac vein compression syndrome. Left: the left common iliac vein is compressed by the right common iliac artery; Right: the compressed left CIV is located between the right common iliac artery and the vertebral body.
The compression of the left CIV by the right CIA can be demonstrated with duplex ultrasound when a good acoustic window is obtained (38, 39). Figure 7 shows the anatomical relationship of the right and left CIAs and CIVs. In this image, the left CIV is compressed by the right CIA. However, direct visualization of a severe stenosis of the left CIV at the point that the right CIA crosses the vein is not always possible on B-mode ultrasound imaging. Duplex ultrasound may demonstrate venous flow without respiratory variation in the iliac vein below the compression point, and flow velocity increases at the location of the right CIA compressing the left CIV (40). If the PSV gradient is more than 2.5, the findings are significant. Flow reversal in the ipsilateral internal iliac vein can be associated with CIV compression (3, 41). The first clinical presentation of some patients with IVCS can be left iliac vein thrombosis. Confirming the presence of deep vein thrombosis may not be difficult, but demonstrating the left iliac vein compression is not generally possible when the vessel is thrombosed.
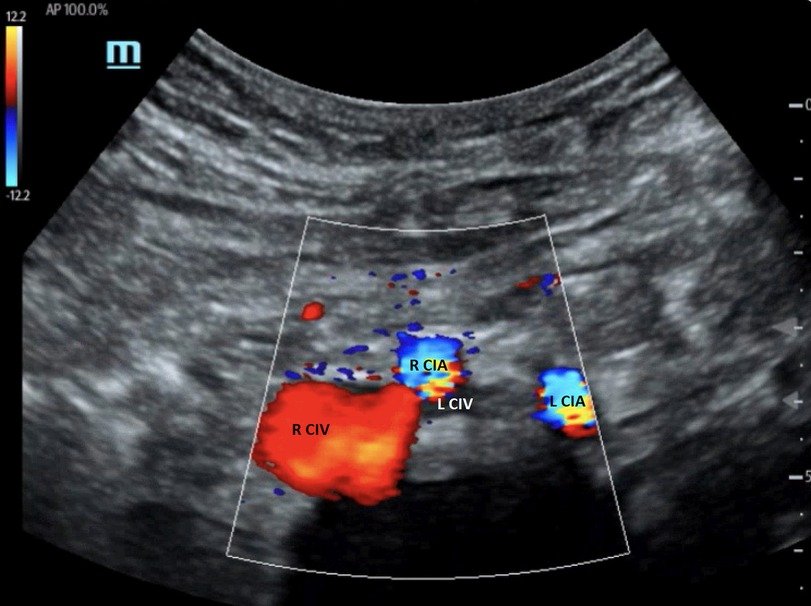
Figure 7. Color Doppler image of iliac vein compression syndrome. The left common iliac vein (L CIV) is compressed by the right common iliac artery (R CIA). R CIV, right common iliac vein; L CIA, left common iliac artery [From: Brown et al. (39). Reprint under a Creative Commons Attribution 4.0 International License].
IVUS has been used successfully to detect iliac vein compression. It provides information about the spatial relationships of anatomic structures and compression of the left CIV by the right CIA in the case of IVCS. As the modality assesses the vessel wall directly, venous spurs thought to be caused by a combination of mechanical compression and arterial pulsations on the trapped vein can also be visualized (41–44).
The superior mesenteric artery syndrome (SMAS), also known as Cast syndrome and Wilkie syndrome, is a vascular compression syndrome that presents clinically with upper small bowel obstruction due to entrapment of the third part of the duodenum between the SMA and AA (Figures 8A,B) (42). The common symptoms include postprandial epigastric pain, weight loss, nausea, and vomiting (3, 45). SMAS may be accompanied by RVCS as they share the same pathogenesis (Figures 8C,D) (46).
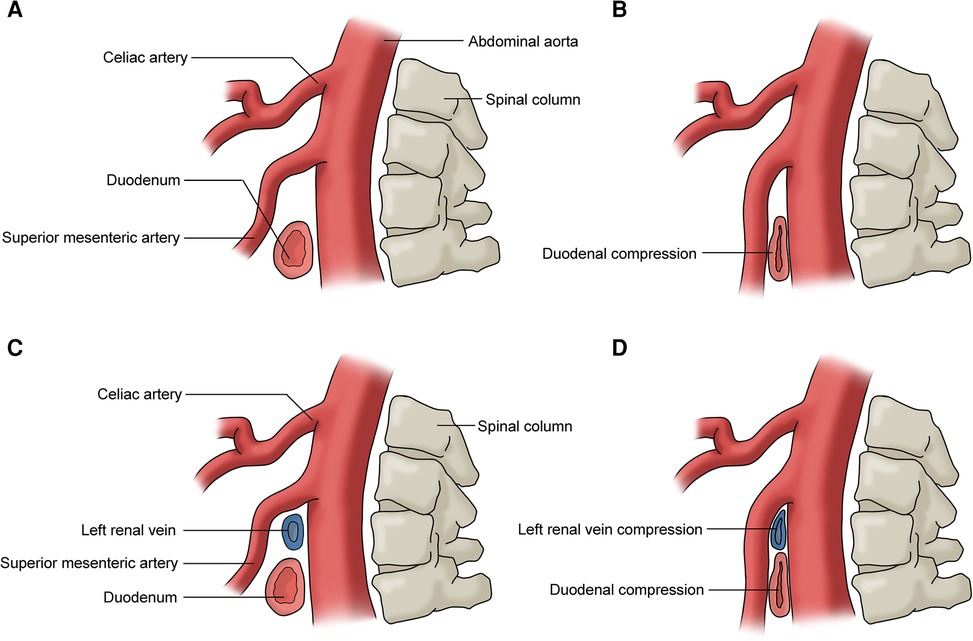
Figure 8. (A) No duodenum compression; (B) duodenum compression (superior mesmeric artery compression syndrome); (C) no duodenum or left renal vein compression; (D) duodenum and left renal vein compression (superior mesmeric artery compression syndrome and renal vein compression syndrome).
Barium testing, abdominal x-ray, CT/CTA, MRI/MRA, and endoscopy play important roles in the diagnosis and differential diagnosis of SMAS (47–49). The main therapeutic goal is eliminating obstructions and obtaining optimal weight gains. The initial management is usually conservative, including decompression of the duodenum with a nasogastric tube and nutritional support. Surgical interventions consist of Strong's procedure to release the Treitz ligament, gastrojejunostomy, or duodenojejunostomy to bypass the duodenum obstruction (3, 10).
The ultrasound characteristics of SMAS are reduced SMA-AA distance and reduced SMA-AA angle. Unal and coworkers (50) reported cutoff values of 8 mm for SMA-AA distance (100% sensitivity and specificity), and 22° for SMA-AA angle (42.8% sensitivity, 100% specificity). Similar thresholds of aorto-mesenteric distance (<8 mm) and aorto-mesenteric angle (<25°) were reported by Neri and colleagues (51). Figure 9 shows a case of SMAS with an aorto-mesenteric angle of 8°. Mauceri et al. (52) demonstrated a statistically significant correlation between the use of power Doppler ultrasound and CT for detecting reduced aorto-mesenteric angle and suggested that duplex ultrasound can be used for screening of reduced aorto-mesenteric angle to diagnose suspected cases of SMAS in patients with inexplicable abdominal pain. The application of duplex ultrasound to assess for SMA syndrome has been carried out not only in the outpatient setting but also in the emergency department (53).
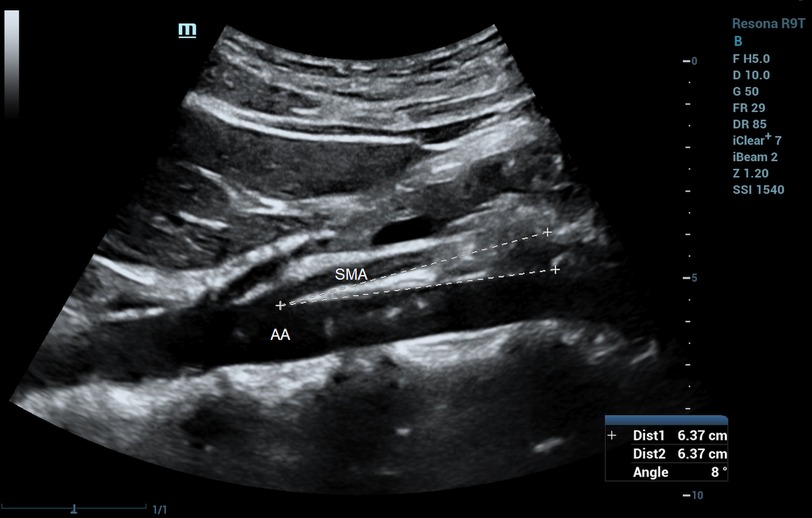
Figure 9. Superior mesenteric artery syndrome on B-mode imaging. Aorto-mesenteric angle = 8°. SMA, superior mesenteric artery; AA, abdominal aorta.
The duplex ultrasound scan is a non-invasive, dynamic, and low-cost diagnostic examination. Ultrasound scanning is easily reproducible with the patient positioned in various postures or assessed during different respiratory phases. It provides morphological information regarding the anatomical structures and hemodynamic changes in the examined arteries or veins (3).
The duplex ultrasound plays an important role in the diagnosis of various vascular disorders and can be successfully used to assess AVCS. Many authors recommended ultrasound as a first line imaging modality to assist the diagnosis of AVCS (14, 16, 54, 55). The article provides a concise summary of the ultrasound characteristics and diagnostic criteria for AVCS, which may be useful to sonographers and sonologists who may not be familiar with these conditions. However, it is noted that most of the ultrasound criteria for AVCS are based on studies with relatively small sample sizes. Therefore, caution should be taken when these criteria are used.
YL: Writing – original draft, Writing – review & editing, Data curation. HZ: Project administration, Supervision, Writing – review & editing. XW: Writing – review & editing. ZW: Writing – review & editing. QZ: Writing – review & editing, Formal Analysis, Project administration. CW: Project administration, Writing – review & editing, Conceptualization. YT: Data curation, Formal Analysis, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant No. 81771833).
The authors would like to thank Jayne Chambers for her proofreading of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fong JK, Poh AC, Tan AG, Taneja R. Imaging findings and clinical features of abdominal vascular compression syndromes. AJR Am J Roentgenol. (2014) 203(1):29–36. doi: 10.2214/AJR.13.11598
2. Zahid M, Nepal P, Nagar A, Ojili V. Abdominal vascular compression syndromes encountered in the emergency department: cross-sectional imaging spectrum and clinical implications. Emerg Radiol. (2020) 27(5):513–26. doi: 10.1007/s10140-020-01778-1
3. D'Oria M, Zlatanovic P, Anthony A, Dua A, Flores AM, Tanious A, et al. International Union of Angiology consensus document on vascular compression syndromes. Int Angiol. (2023) 42(4):282–309. doi: 10.23736/S0392-9590.23.05100-3
4. Kurklinsky AK, Rooke TW. Nutcracker phenomenon and nutcracker syndrome. Mayo Clin Proc. (2010) 85(6):552–9. doi: 10.4065/mcp.2009.0586
5. Granata A, Distefano G, Sturiale A, Figuera M, Foti PV, Palmucci S, et al. From nutcracker phenomenon to nutcracker syndrome: a pictorial review. Diagnostics (Basel). (2021) 11(1):101. doi: 10.3390/diagnostics11010101
6. Lamba R, Tanner DT, Sekhon S, McGahan JP, Corwin MT, Lall CG. Multidetector CT of vascular compression syndromes in the abdomen and pelvis. Radiographics. (2014) 34(1):93–115. doi: 10.1148/rg.341125010 Erratum in: Radiographics. 2015 May-Jun;35(3):973.24428284
7. Gozzo C, Giambelluca D, Cannella R, Caruana G, Jukna A, Picone D, et al. CT Imaging findings of abdominopelvic vascular compression syndromes: what the radiologist needs to know. Insights Imaging. (2020) 11(1):48. doi: 10.1186/s13244-020-00852-z
8. Kim EN, Lamb K, Relles D, Moudgill N, DiMuzio PJ, Eisenberg JA. Median arcuate ligament syndrome-review of this rare disease. JAMA Surg. (2016) 151(5):471–7. doi: 10.1001/jamasurg.2016.0002
9. Brinegar KN, Sheth RA, Khademhosseini A, Bautista J, Oklu R. Iliac vein compression syndrome: clinical, imaging and pathologic findings. World J Radiol. (2015) 7(11):375–81. doi: 10.4329/wjr.v7.i11.375
10. Merrett ND, Wilson RB, Cosman P, Biankin AV. Superior mesenteric artery syndrome: diagnosis and treatment strategies. J Gastrointest Surg. (2009) 13(2):287–92. doi: 10.1007/s11605-008-0695-4
11. Goodall R, Langridge B, Onida S, Ellis M, Lane T, Davies AH. Median arcuate ligament syndrome. J Vasc Surg. (2020) 71(6):2170–6. doi: 10.1016/j.jvs.2019.11.012
12. Nasr LA, Faraj WG, Al-Kutoubi A, Hamady M, Khalifeh M, Hallal A, et al. Median arcuate ligament syndrome: a single-center experience with 23 patients. Cardiovasc Intervent Radiol. (2017) 40(5):664–70. doi: 10.1007/s00270-016-1560-6
13. Chou SQH, Kwok KY, Wong LS, Fung DHS, Wong WK. Imaging features of median arcuate ligament syndrome. J Hong Kong Col Radiol. (2010) 13:101–3.
14. Farina R, Foti PV, Pennisi I, Vasile T, Clemenza M, Rosa G, et al. Vascular compression syndromes: a pictorial review. Ultrasonography. (2022) 41(3):444–61. doi: 10.14366/usg.21233
15. Roberts B, Pevsner R, Alkhoury F. Robotic approach for median arcuate ligament release in pediatrics. J Laparoendosc Adv Surg Tech A. (2020) 30(1):92–6. doi: 10.1089/lap.2019.0337
16. Gruber H, Loizides A, Peer S, Gruber I. Ultrasound of the median arcuate ligament syndrome: a new approach to diagnosis. Med Ultrason. (2012) 14(1):5–9.22396932
17. Saleem T, Katta S, Baril DT. Celiac artery compression syndrome. In: Statpearls. Treasure Island (FL): StatPearls Publishing (2023). Available at: https://www.ncbi.nlm.nih.gov/books/NBK470601/ (Updated Apr 26, 2023).
18. Youssef AT. Evaluation of asymptomatic patients with median arcuate ligament syndrome (mals) using color duplex ultrasound and computed tomographic (CT) angiography. Am J Cardiovasc Dis Res. (2013) 1(1):7–11. doi: 10.12691/ajcdr-1-1-2
19. Ozel A, Toksoy G, Ozdogan O, Mahmutoglu AS, Karpat Z. Ultrasonographic diagnosis of median arcuate ligament syndrome: a report of two cases. Med Ultrason. (2012) 14(2):154–7.22675717
20. Aswani Y, Thakkar H, Anandpara KM. Imaging in median arcuate ligament syndrome. BMJ Case Rep. (2015) 2015:bcr2014207856. doi: 10.1136/bcr-2014-207856
21. Wu E. Median arcuate ligament syndrome. J Diagn Med Sonogr. (2019) 35(2):141–5. doi: 10.1177/8756479318818061
22. Farina R, Foti PV, Conti A, Iannace FA, Pennisi I, Santonocito S, et al. The role of ultrasound in dunbar syndrome: lessons based on a case report. Am J Case Rep. (2020) 21:e926778. doi: 10.12659/AJCR.926778
23. Acampora C, Di Serafino M, Iacobellis F, Trovato P, Barbuto L, Sangiuliano N, et al. Insight into Dunbar syndrome: color-Doppler ultrasound findings and literature review. J Ultrasound. (2021) 24(3):317–21. doi: 10.1007/s40477-019-00422-0
24. Cernigliaro J, Chen F, Bhatt S. The “hook” sign of median arcuate ligament syndrome on ultrasound. Abdom Radiol (NY). (2021) 46(5):2251–2. doi: 10.1007/s00261-020-02842-7
25. de Lara FV, Higgins C, Hernandez-Vila EA. Median arcuate ligament syndrome confirmed with the use of intravascular ultrasound. Tex Heart Inst J. (2014) 41(1):57–60. doi: 10.14503/THIJ-12-2495
26. Ananthan K, Onida S, Davies AH. Nutcracker syndrome: an update on current diagnostic criteria and management guidelines. Eur J Vasc Endovasc Surg. (2017) 53(6):886–94. doi: 10.1016/j.ejvs.2017.02.015
27. Skeik N, Gloviczki P, Macedo TA. Posterior nutcracker syndrome. Vasc Endovascular Surg. (2011) 45(8):749–55. doi: 10.1177/1538574411419376
28. Velasquez CA, Saeyeldin A, Zafar MA, Brownstein AJ, Erben Y. A systematic review on management of nutcracker syndrome. J Vasc Surg Venous Lymphat Disord. (2018) 6(2):271–8. doi: 10.1016/j.jvsv.2017.11.005
29. Neupane S, Ambulgekar N, Edla S, Torey J, Gottam N, Yamasaki H. Intravascular ultrasound-guided endovascular stenting of renal vein in nutcracker syndrome. Vasc Endovascular Surg. (2018) 52(5):355–6. doi: 10.1177/1538574418765387
30. Sorokin I, Nelson J, Rectenwald JE, Cadeddu JA. Robot-assisted laparoscopic extravascular stent for nutcracker syndrome. J Robot Surg. (2018) 12(3):561–5. doi: 10.1007/s11701-017-0744-7
31. Kim SH, Cho SW, Kim HD, Chung JW, Park JH, Han MC. Nutcracker syndrome: diagnosis with Doppler US. Radiology. (1996) 198(1):93–7. doi: 10.1148/radiology.198.1.8539413
32. Kim SH. Doppler US and CT diagnosis of nutcracker Syndrome. Korean J Radiol. (2019) 20(12):1627–37. doi: 10.3348/kjr.2019.0084
33. Yang YR, Ling WW, Shi SH, Li YZ, Zhou JJ. Ultrasound features of posterior nutcracker syndrome: case series and literature analysis. Quant Imaging Med Surg. (2022) 12(10):4984–9. doi: 10.21037/qims-22-166
34. Inal M, Karadeniz Bilgili MY, Sahin S. Nutcracker syndrome accompanying pelvic congestion syndrome; color Doppler sonography and multislice CT findings: a case report. Iran J Radiol. (2014) 11(2):e11075. doi: 10.5812/iranjradiol.11075
35. Farina R, Foti PV, Iannace FA, Fanzone L, Pennisi I, Conti A, et al. May–Thurner syndrome: description of a case with unusual clinical onset. J Ultrasound. (2022) 25(2):309–13. doi: 10.1007/s40477-020-00497-0
36. Wu WL, Tzeng WS, Wu RH, Tsai WL, Chen MC, Lin PC, et al. Comprehensive MDCT evaluation of patients with suspected May–Thurner syndrome. AJR Am J Roentgenol. (2012) 199(5):W638–45. doi: 10.2214/AJR.11.8040
37. Hsu YC, Huang YK, Hsu LS, Chen PY, Chen CW. Using non-contrast-enhanced magnetic resonance venography for the evaluation of May–Thurner syndrome in patients with renal insufficiency: a case report. Medicine (Baltimore). (2019) 98(52):e18427. doi: 10.1097/MD.0000000000018427
38. Barry A. Sonography’s role in the diagnosis of May–Thurner syndrome. J Diagn Med Sonogr. (2018) 34(1):65–70. doi: 10.1177/8756479317714796
39. Brown M, Hsu E, McCoy C, Whited M. A case report of May–Thurner syndrome identified on abdominal ultrasound. J Educ Teach Emerg Med. (2022) 7(3):V14–9. doi: 10.21980/J8C64K
40. Oğuzkurt L, Ozkan U, Tercan F, Koç Z. Ultrasonographic diagnosis of iliac vein compression (May–Thurner) syndrome. Diagn Interv Radiol. (2007) 13(3):152–5.
41. Radaideh Q, Patel NM, Shammas NW. Iliac vein compression: epidemiology, diagnosis and treatment. Vasc Health Risk Manag. (2019) 15:115–22. doi: 10.2147/VHRM.S203349
42. Ahmed HK, Hagspiel KD. Intravascular ultrasonographic findings in May–Thurner syndrome (iliac vein compression syndrome). J Ultrasound Med. (2001) 20(3):251–6. doi: 10.7863/jum.2001.20.3.251
43. Forauer AR, Gemmete JJ, Dasika NL, Cho KJ, Williams DM. Intravascular ultrasound in the diagnosis and treatment of iliac vein compression (May–Thurner) syndrome. J Vasc Interv Radiol. (2002) 13(5):523–7. doi: 10.1016/s1051-0443(07)61535-8
44. Knuttinen MG, Naidu S, Oklu R, Kriegshauser S, Eversman W, Rotellini L, et al. May–Thurner: diagnosis and endovascular management. Cardiovasc Diagn Ther. (2017) 7(Suppl 3):S159–64. doi: 10.21037/cdt.2017.10.14
45. Jain R. Superior mesenteric artery syndrome. Curr Treat Options Gastroenterol. (2007) 10(1):24–7. doi: 10.1007/s11938-007-0053-8
46. Farina R, Gozzo C, Foti PV, Conti A, Vasile T, Pennisi I, et al. A man with the rare simultaneous combination of three abdominal vascular compression syndromes: median arcuate ligament syndrome, superior mesenteric artery syndrome, and nutcracker syndrome. Radiol Case Rep. (2021) 16(6):1264–70. doi: 10.1016/j.radcr.2021.02.065
47. Ganss A, Rampado S, Savarino E, Bardini R. Superior mesenteric artery syndrome: a prospective study in a single institution. J Gastrointest Surg. (2019) 23(5):997–1005. doi: 10.1007/s11605-018-3984-6
48. Warncke ES, Gursahaney DL, Mascolo M, Dee E. Superior mesenteric artery syndrome: a radiographic review. Abdom Radiol (NY). (2019) 44(9):3188–94. doi: 10.1007/s00261-019-02066-4
49. Al Faqeeh AA, Syed MK, Ammar M, Almas T, Syed S. Wilkie’s syndrome as a rare cause of duodenal obstruction: perspicacity is in the radiological details. Cureus. (2020) 12(9):e10467. doi: 10.7759/cureus.10467
50. Unal B, Aktaş A, Kemal G, Bilgili Y, Güliter S, Daphan C, et al. Superior mesenteric artery syndrome: CT and ultrasonography findings. Diagn Interv Radiol. (2005) 11(2):90–5.15957095
51. Neri S, Signorelli SS, Mondati E, Pulvirenti D, Campanile E, Di Pino L, et al. Ultrasound imaging in diagnosis of superior mesenteric artery syndrome. J Intern Med. (2005) 257(4):346–51. doi: 10.1111/j.1365-2796.2005.01456.x
52. Mauceri B, Misseri M, Tsami A, Vecchio C, Di Pino A, Galati D, et al. [Ultrasound in diagnosis of superior mesenteric artery syndrome]. Clin Ter. (2010) 161(1):35–7.20393676
53. Le D, Stirparo JJ, Magdaleno TF, Paulson CL, Roth KR. Point-of-care ultrasound findings in the diagnosis and management of superior mesenteric artery (SMA) syndrome. Am J Emerg Med. (2022) 55(233):e1–233.e4. doi: 10.1016/j.ajem.2022.02.018
54. Farina R, Foti PV, Conti A, Iannace FA, Pennisi I, Fanzone L, et al. The role of ultrasound imaging in vascular compression syndromes. Ultrasound J. (2021) 13(1):4. doi: 10.1186/s13089-020-00202-6
Keywords: abdominal vascular compression syndrome, celiac artery compression syndrome, iliac vein compression syndrome, renal vein compression syndrome, superior mesenteric artery syndrome, ultrasound
Citation: Liu Y, Zheng H, Wang X, Wang Z, Zhu Q, Wen C and Tong Y (2023) Ultrasound characteristics of abdominal vascular compression syndromes. Front. Cardiovasc. Med. 10:1282597. doi: 10.3389/fcvm.2023.1282597
Received: 13 September 2023; Accepted: 30 November 2023;
Published: 18 December 2023.
Edited by:
Tharmarajan Ramprasath, Georgia State University, United StatesReviewed by:
Petar Zlatanovic, University of Belgrade, Serbia© 2023 Liu, Zheng, Wang, Wang, Zhu, Wen and Tong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaoyang Wen d2VuY3lwa3VpaEAxNjMuY29t Yisha Tong eWlzaGEudG9uZ0BhdXN0aW4ub3JnLmF1
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.