- 1Department of Cardiothoracic Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 2Department of Ultrasound, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
Background: Ultrasound-guided percutaneous device closure of perimembranous ventricular septal defects (PmVSD) is a minimally invasive recent treatment approach. Perventricular PmVSD device closure is an emerging radiation-free intervention, yet it comes with certain limitations. No studies compared both of these treatment approaches.
Methods: We performed a retrospective institutional data comparison of percutaneous (PCP Group, n = 138) and perventricular (PVP Group, n = 67) ultrasound-guided device closure procedures in 205 patients with PmVSD between March 2017 and December 2022.
Results: Patients of the PCP and PVP groups had a median age of 4.9 years (IQR, 3.1–14.0) and 5.3 years (IQR, 3.4–13.1) respectively. The median PmVSD diameter in the PCP Group was 4.0 mm (IQR, 3.3–5.3) and 5.2 mm (IQR, 4.0–7.0) in the PVP Group (p = 0.001). There was no significant difference in success rates between the PCP and PVP Groups (intention-to-treat population, 88.4% vs. 92.5%, p = 0.36; as-treated population, 88.4% vs. 89.3%, p = 0.84). 5/8 failed percutaneous cases that were shifted to the perventricular approach were successful. Compared to the PVP Group, patients of the PCP group experienced a significant decrease in ventilation time, drainage volume, and postoperative hospital stay (p < 0.001). The median follow-up period was 24 months (IQR, 6–42) for the PCP group and 61 months (IQR, 53–65) for the PVP group. The overall severe adverse event rate was 0% in the PCP group and 3.0% in the PVP group.
Conclusions: Perventricular and percutaneous ultrasound-guided device closure of PmVSD are both effective and safe treatment options. The percutaneous approach offers less trauma and faster recovery and may be the preferred approach in selected patients.
Introduction
Perimembranous ventricular septal defects (PmVSD) represent 80% of ventricular septal defects (VSDs) (1) and are closed using percutaneous transcatheter devices with good medium-term outcomes (2, 3). Over the past decade, Chinese surgeons have proposed a perventricular ultrasound-guided device closure to avoid latent radiation-associated hazards (4–8). However, this procedure is associated with surgical morbidity and scarring. To avoid that, Wang et al. performed ultrasound-guided percutaneous PmVSD closure and reported the safety and feasibility of this approach with satisfactory results (9).
Despite these encouraging findings, studies on ultrasound-guided percutaneous PmVSD closures are still rare. Moreover, no previous studies have compared the outcomes between perventricular and percutaneous approaches for ultrasound-guided PmVSD closure. Therefore, we aimed to compare our institutional experience with perventricular and percutaneous ultrasound-guided device closure of PmVSD and describe the outcomes of these interventions.
Patients and methods
Study design
We retrospectively reviewed the clinical data of patients who underwent transcatheter percutaneous and perventricular ultrasound-guided PmVSD closure at our institution between March 2017 and December 2022. We divided the patients into percutaneous (PCP) and perventricular (PVP) procedure groups according to the applied closure approach. Standard safety and midterm outcomes were compared. All procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation, and with the Helsinki Declaration of 1975, as revised in 2008. Approval from the institutional review board was obtained. Written informed consent was signed by the patients or their legal guardians to perform the procedure and to use their clinical records for eventual publication. We extracted in-hospital data from the institutional information system. Echocardiography and electrocardiography reports completed by other health organizations were collected.
Patient selection and pre-procedure ultrasound evaluation
Patients with congenital PmVSD who met the following criteria were sent for ultrasound-guided device closure: (1) age ≥ 6 months, (2) PmVSD diameter > 2 mm on ultrasound, and (3) absence of cardiovascular malformations requiring surgical repair. Patients with (1) severe pulmonary hypertension, (2) significant aortic valve prolapse without a deep aneurysm or aortic regurgitation ≥ Grade 1, and (3) active infective endocarditis were excluded.
Echocardiographic assessment
A comprehensive transthoracic echocardiography (TTE) assessment was performed preoperatively by an experienced echocardiologist (J. W.) using a Philips iE33 ultrasound machine (S5-1 probe, 1–5 MHz; S8-3 probe, 3–8 MHz; Philips Medical Systems, Cleveland, Ohio, USA). The GE Vivid E95 (M5SC-D probe, 1.5–4.6 MHz; 6VT probe, 3.0–8.0 MHz; 9 T probe, 4.0–10.0 MHz; GE Healthcare, Chicago, Illinois, USA) was used for intraoperative guidance. We measured the left ventricular entry and right ventricular exit diameters of the pmVSD and focused on the exit diameter as a reference for selecting the occluder size. Residual shunts were classified according to the width of the colored jet. Tricuspid and aortic regurgitations were evaluated using a color-flow Doppler signal and classified into four grades: none or trivial (0/4), mild (1/4), moderate (2/4), and severe (3/4) (10, 11).
Device and delivery system
The HeartR™ VSD occluder (Lifetech Scientific Corporation, Shenzhen, China), had been described in detail previously (12). It is a self-expandable double-disk device with a 3 mm long connecting waist. The waist diameter correspond to the size of the VSD occluder. Three types of occluders were used: symmetrically concentric, asymmetrically concentric, and eccentric (Supplementary Figure S1). In the symmetrically concentric type, the flanges of both disks are 2 mm wide. In the eccentric type, the aortic flange of the left disk is 0.5 mm wide, whereas its opposite flange is 5 mm wider than the waist. In the asymmetrically concentric type, which is used for PmVSDs with multiple exits, the flange of the left disk is 4 mm and that of the right disk is 2 mm wide. The device selection was based on the protocol that had been previously described (12). An occluder 1–2 mm larger than the targeted shunt of the PmVSD was chosen.
A 20 cm long delivery system with a trocar, a 0.035 inch guidewire, dilator, and loading sheath were chosen for perventricular PmVSD closure. A 90 cm long delivery system was selected for percutaneous PmVSD closure.
Procedures
All procedures were performed by a hybrid team of cardiac surgeons and echocardiographers under general anesthesia, heparinization, and antibiotic prophylaxis in a routine operating room. Since 2017, we've used perventricular closure for eligible patients. From 2018, with increased percutaneous experience, we've employed the transfemoral retrograde approach for PmVSD closure if the femoral artery diameter is sufficient and the aortic valve interference risk is low (PmVSD with a deep aneurysm or sufficient subaortic rim). For cases where the symmetrical occluder might interfere with the aortic valve, we attempted closure using an eccentric occluder via the femoral vein. If unsuccessful, we resort to perventricular closure.
Perventricular device closure
Transesophageal echocardiography (TEE) was used to guide the procedure. A 3 cm incision was made in the inferior median sternum. After the sternum was split and retracted, the pericardium was incised and suspended. The optimal puncture site was identified using TEE. After systemic heparinization (1 mg/kg), the rest of the procedure was performed using a previously described protocol (5, 6).
Percutaneous device closure
Figure 1 shows the steps of closure under TTE guidance. Before the occluder was unscrewed, the correct position of the device, presence of a residual shunting, and valve regurgitation were identified using multiple echocardiographic views. TEE was performed when TTE images were unclear during the procedure.
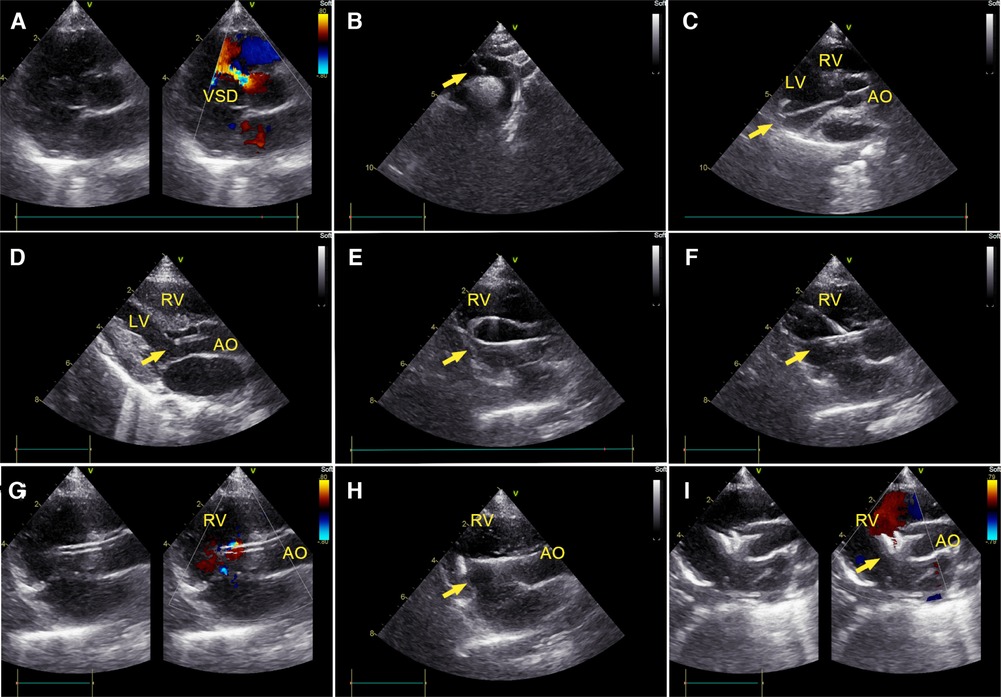
Figure 1. Percutaneous ultrasound-guided perimembranous ventricular septal defect (PmVSD) closure. (A) Image of PmVSD in the short- axis view. (B) A 5-French trimmed pigtail catheter was introduced along a 0.035-inch angled hydrophilic guidewire (Radifocus Guidewire M, Terumo Medical Corporation, Tokyo, Japan) into the descending aorta, then advanced across the aortic arch in the suprasternal aortic arch long-axis view of TTE. (C) Using the long-axis view of the LV, the guidewire crossed the aortic valve, then the pigtail catheter would be advanced into the left ventricle (LV). (D) The cut-pigtail catheter was pulled to the level of the PmVSD, and its trimmed tip was rotated toward the ventricular septum in the LV long axis or the apical five-chamber view. (E) After the pigtail catheter was advanced to the right ventricle (RV) along the hydrophilic guidewire, a 260 cm exchange J-tip guidewire (Cordis Corporation, Miami Lakes, Florida, USA) was advanced along the catheter into the RV. Its soft tip (arrow) was confirmed in the parasternal long axis view of the RV inflow tract. (F) The long delivery sheath (arrow) was inserted over the exchange J-tip guidewire into the RV. (G) The guidewire was pulled back and the “double track sign” was viewed. The tip of sheath was confirmed in the RV. (H) The occluder was placed inside the delivery sheath, and its right disc (arrow) was deployed. (I) The PmVSD was closed after both discs of device were deployed. LV, left ventricle; RV, right ventricle; AO, ascending aorta.
When an asymmetrically concentric or eccentric occluder was used, an antegrade approach via the femoral vein was performed according to the method described by Bu et al. (13). We defined device time as the duration from guidewire entry of the guiding sheath to delivery sheath retrieval.
Postoperative management and follow-up
A daily dose of 3–5 mg/kg oral aspirin was administered after the procedure and continued for six consecutive months. All patients underwent electrocardiogram and TTE before discharge and at 1, 3, 6, and 12 months postoperatively and yearly thereafter.
Adverse event assessments
We defined major adverse events as all-cause death, cardiovascular perforation, device embolization, large residual shunting at last follow up, new-onset of ≥Grade 2 valvular regurgitation, new-onset of grade II or III atrioventricular block, new-onset complete left bundle branch block, new-onset junctional rhythm, and device-related endocarditis. We defined minor adverse events as small or moderate residual shunting at last follow up, device-related grade 1 valvular regurgitation, pericardial effusion, new-onset left anterior fascicular block, new-onset right bundle branch block, thromboembolism, hemolysis, delayed wound healing, and groin hematoma.
Statistical analysis
Statistical analyses were performed using R software version 4.2.2. Categorical variables were reported as frequency and percentage and continuous variables were represented as median with interquartile range (IQR). Statistical analyses for continuous variables were conducted using Mann–Whitney U and by chi-square test for categorical variables. Missing data were addressed using the chained equations method for multiple imputations (14, 15). Regression analyses, both linear and logistic, were employed to determine the relationship between closure approaches and various outcomes, after adjusting for confounding factors in two separate models. Sensitivity analyses were conducted on the complete dataset without imputations. A p-value < 0.05 was considered statistically significant. All reported p values are two-sided.
Results
Patient characteristics
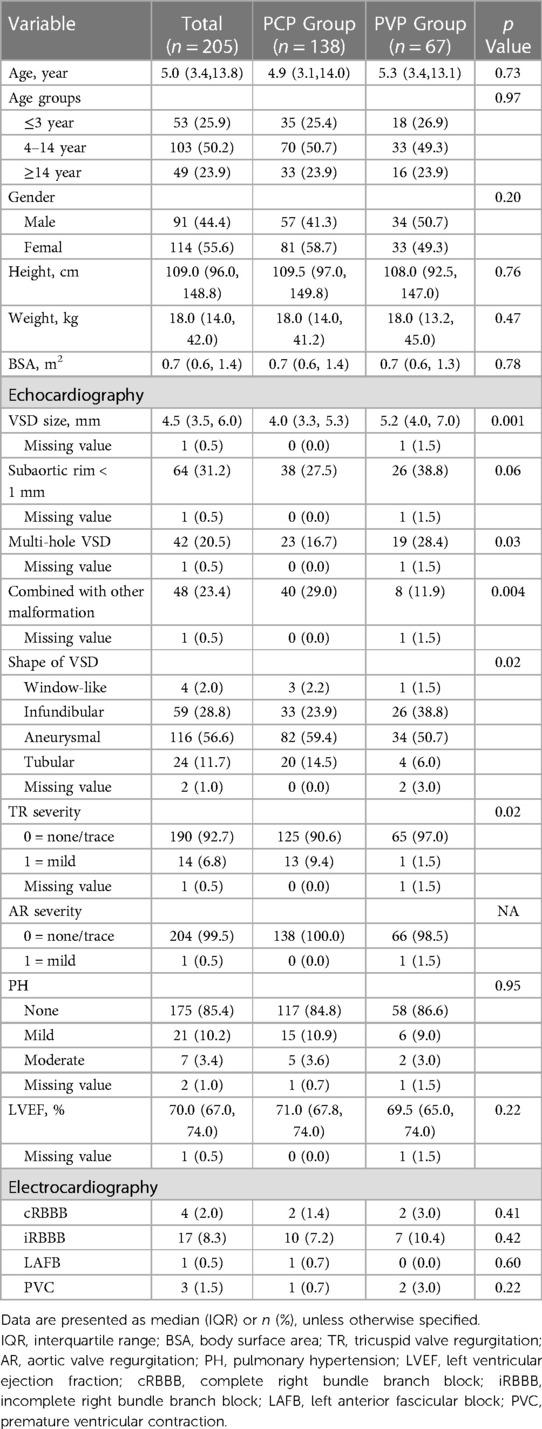
We studied 205 consecutive patients (55.6% women/girls) with a median age of 5.0 years (IQR: 3.4–13.8). Among them, 138 (67.3%) had percutaneous procedures, and 67 (32.7%) had perventricular procedures. The median PmVSD diameter in the PCP group was 4.0 mm (IQR, 3.3–5.3) and 5.2 mm (IQR, 4.0–7.0) in the PVP group (p = 0.001). Multihole PmVSDs were more common in the perventricular group (28.4% vs. 16.7%, p = 0.03) (Table 1).
Intraoperative and postoperative results
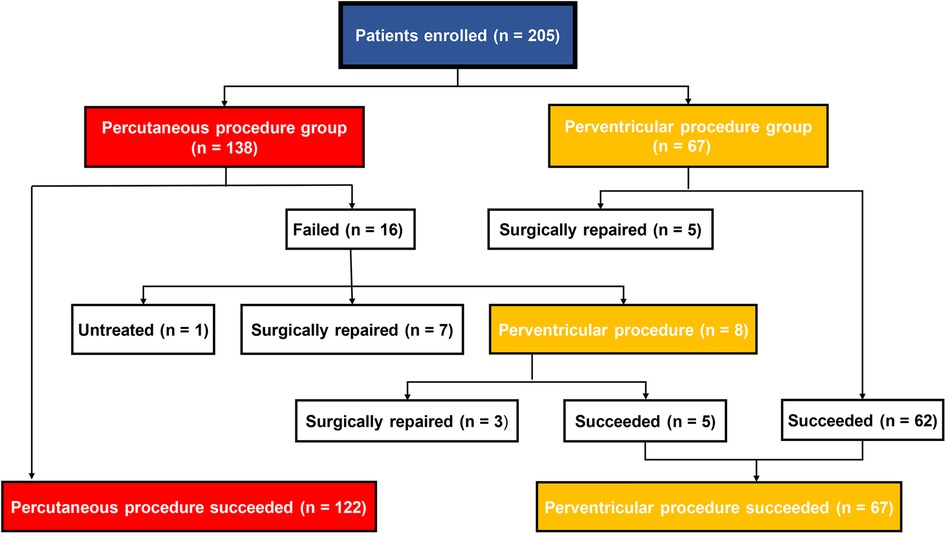
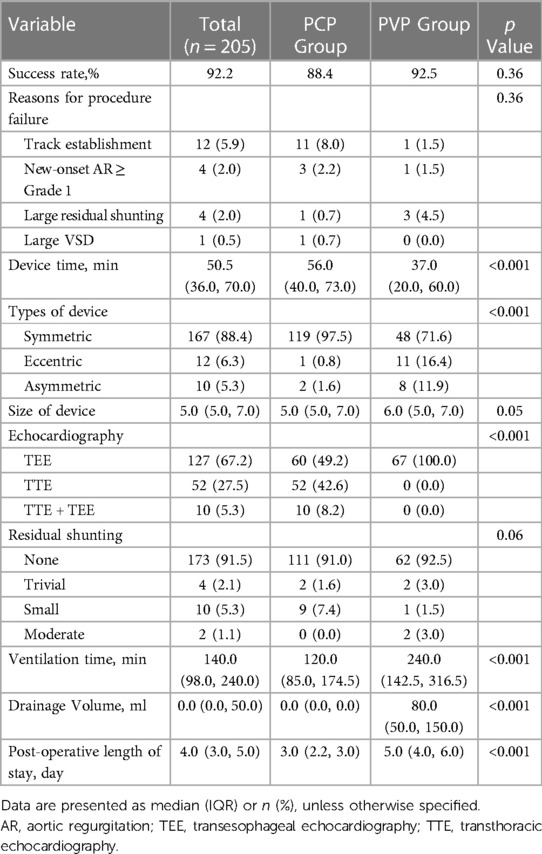
The patient’s flow pattern throughout the study period is illustrated in Figure 2. In the intention to treat the population, the success rates were not significantly different between the two groups, with a success rate of 88.4% (122/138) in the PCP group and 92.5% (62/67) in the PVP group (p = 0.36). In the as-treated population, the success rates were 88.4% (122/138) in the PCP group and 89.3% (67/75) in the PVP group (p = 0.84). In the PCP group, the procedure was completed via antegrade femoral access in 14.8% (18/122) of the patients. Among the 16 patients in the PCP group who experienced failure during the percutaneous procedure, 8 were subsequently converted to a perventricular approach due to difficulties in track establishment. However, out of these 8 patients, we were unable to successfully establish a track in three. In contrast, no patients in the PVP group were converted to a percutaneous procedure.
The unadjusted assessment of the patients in the PCP group vs. PVP group showed a longer device time (56 min vs. 37 min, p < 0.001), shorter ventilation time, less drainage volume, and shorter postoperative length of stay (all p < 0.001). While 100% of the cases involving the perventricular procedure were performed under TEE guidance, TTE guidance alone was employed in 42.6% of the percutaneous procedures (p < 0.001) (Table 2).
After multivariable adjustment (models 1 and 2), the outcomes were consistent with those of the unadjusted population (Supplementary Table S1). We also performed a sensitivity analysis of 180 patients without missing values, and the results were consistent with the primary findings (Supplementary Table S2).
Adverse events and follow-up data
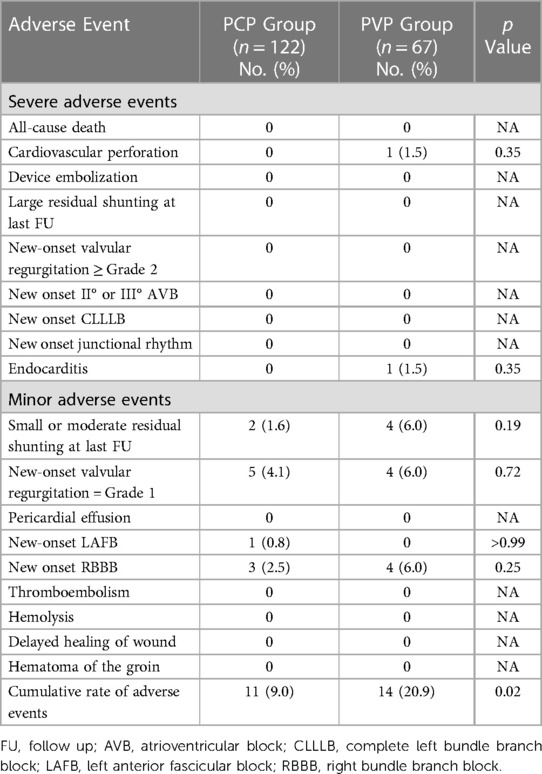
For the PCP Group, the median follow-up was 24 months (IQR: 6–42 months), while for the PVP Group, it was 61 months (IQR: 53–65 months). Adverse events during procedure and follow-up are listed in Table 3. The severe adverse event rate was 0% in the PCP Group and 3.0% in the PVP Group. Two major adverse events were observed in the PVP group. In one patient, an ascending aortic perforation caused by the short sheath was found immediately after successful pmVSD closure. The incision was extended, and a full sternotomy was performed. The injured ascending aorta was repaired without removal of the occluder. One patient developed acute endocarditis during the fourth month of follow-up. The patient had aortic valve perforation and new-onset moderate aortic valve regurgitation. He underwent replacement of the aortic valve and removal of the occluder. No occluder impingement was observed on the aortic valve during surgery.
No severe arrythmia events (new-onset II° or III° atrioventricular block, complete left bundle branch block, or junctional rhythm) occurred in either group. One of the five patients with new-onset Grade 1 valvular regurgitation had mild aortic valve regurgitation. When analyzed separately, the rates of adverse events did not differ significantly between the two groups. However, when taken together, the overall rate of adverse events differed significantly between the PCP and PVP groups (p = 0.02).
Discussion
In this study, we compared two approaches of ultrasound-guided PmVSD closure. Our results showed that percutaneous ultrasound-guided closure has comparable feasibility, safety, and event-free survival outcomes to the perventricular approach. Patients who underwent percutaneous ultrasound-guided procedures experienced reduced surgical trauma, faster procedures, and quicker recovery.
Hijazi et al. first reported transcatheter PmVSD closure using an Amplatzer PmVSD occluder in 2002; this procedure has since become an alternative to traditional surgery (16). Transcatheter PmVSD closure has shown proven medium-term follow-up outcomes in a large number of patients who underwent the procedure (2, 3, 17–19). Recently, perventricular PmVSD closure through a minimal incision under TEE guidance has been performed in China (4, 5). This hybrid procedure offers advantages such as avoidance of cardiopulmonary bypass and potential radiation damage, less surgical trauma, and no limitation in patient weight and peripheral vessels. However, existing surgical injuries can counteract the benefits of fluoroscopy-free guidance. Although Wang and colleagues showed promising clinical results for percutaneous PmVSD closure using TTE alone, the adoption of percutaneous approach was rare (9).
Comparable success rates were found between the two approaches in our study. Since percutaneous and perventricular PmVSD cloure have been standard procedures for over 10 years, the VSD anatomy suitable for closure has been well established (20). We followed these standards in our study. It should be noted that the success rate of percutaneous PmVSD closure in our study were lower than the pooled estimate rate of 97.8% reported in a meta-analysis report (21). This could be attributed to our less experience in operative ultrasound guidance and instrument manipulation in the early phase due to our learning curve with exclusive ultrasound guidance.
Technical considerations
The greatest challenge in percutaneous PmVSD closure without fluoroscopic guidance is tracking the guidewire and sheath. Unlike fluoroscopy, which can display all interventional instruments on the screen, echocardiographic assessments yield 2-D images; therefore, ultrasonologist experience is an important factor for accurate monitoring of the instruments. We emphasized three aspects of procedural safety: tracking the tips of interventional instruments with multiple echocardiographic sections, guidewire protection when the catheter or sheath was advancing, and safe distance measurements for the sheath. However, blind or uncertain manipulation should be avoided. Finally, TEE was used as a supplementary modality when TTE could not yield clear images. None of the patients had to undergo surgery because of dim ultrasonic images. One child experienced an aortic injury during the perventricular procedure in the early phase of the implementation of this technique. No patient experienced bleeding complications due to accidental instrument injury during the percutaneous procedure.
Perventricular device closure via a minimal lower-sternal incision offers a short path and a maneuverable approach that is easy to accomplish. However, a full sternotomy incision is inevitable if the procedure fails. The use of a right thoracic minimal incision can avoid this limitation but is more suitable for children (7, 8). In addition, a lower health-related quality of life in the early postoperative stage of a minimally invasive procedure indicates greater physiological and psychological traumas (22). The present study demonstrated similar results, with the percutaneous group showing a comparable success rate, shorter ventilation time, and shorter postoperative length of stay. The procedure had no limitations in the peripheral vessels when the patients were older than 2 years of age. Therefore, both children and adults can undergo percutaneous PmVSD device closure without fluoroscopy. In most cases, once the procedure fails, a PmVSD repair is performed using a minimally thoracic incision.
Complications
The anatomical morphology of a PmVSD determines its success rate and long-term outcomes. A previous study showed that the most common reason for crossing over to surgery was new-onset or worsening aortic regurgitation (23). Although the eccentric occluder was designed for VSDs with an insufficient subaortic rim (<1 mm), the success rate of the procedure was associated with prolapse of the aortic valve and sinus (6, 24). In our study, 27.5% of PCP group patients and 38.8% of PVP group patients had a insufficient subaortic rim. Not all cases used eccentric occluders, as symmetrically concentric VSD occluder could close the infundibular or aneurysmal structure without affecting the aortic valve. Despite previous encouraging results by other operators using mixed guidance, VSD closure was avoided in cases without an aneurysmal structure and when the subaortic rim was less than 1 mm (25).
Serious arrythmia rarely occurs during follow-up. However, a higher incidence of serious postoperative arrhythmia has been reported in patients treated using an eccentric occluder (26). The proposed explanation is that the occurrence of a heart block after closure is due to conduction impairments caused by occluder compression. Kaur and colleagues utilized a mapping system to demonstrate several positional relationships between conduction bundles and the PmVSD (27). Therefore, eccentric and asymmetrically concentric occluders may have a higher rate of arrhythmia postoperatively because of their wider disks. We have always been cautious about utilizing these two types of occluders in PmVSD cloure. This may explain the low rate of postoperative arrhythmias observed in our cohort.
Limitations
First, this was a single-center retrospective study; therefore, selection bias was inevitable. We could not clarify whether these findings could be extended to low-weight children, since our study did not include children weighing less than 10 kg in the percutaneous group. Second, prospective randomized controlled studies are needed to compare these two procedures in patients with similar PmVSD morphologies. Third, a longer follow-up period was required because of the uncertainty of severe complications, such as a complete atrioventricular block or left bundle branch block.
Conclusions
Perventricular and percutaneous ultrasound-guided device closure of PmVSD are both effective and safe treatment options. The percutaneous approach offers less trauma, faster recovery and may be the preferred approach in selected patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of First Affiliated Hospital of Guangxi Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This research is a retrospective objective investigation.
Author contributions
LH: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft. MC: Project administration, Writing – review & editing. DZ: Project administration, Writing – review & editing, Data curation. CS: Data curation, Project administration, Writing – review & editing, Investigation. CJ: Data curation, Project administration, Writing – review & editing. BZ: Writing – review & editing, Conceptualization, Supervision. JW: Supervision, Writing – review & editing, Validation. SL: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This research was sponsored by Middle/young aged teachers' research ability improvement project of Guangxi Higher Education (No. 2022KY0102) and National Key Clinical Specialty Discipline Construction Program of China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1281860/full#supplementary-material
References
1. Roos-Hesselink JW, Meijboom FJ, Spitaels SE, Van Domburg R, Van Rijen EH, Utens EM, et al. Outcome of patients after surgical closure of ventricular septal defect at young age: longitudinal follow-up of 22–34 years. Eur Heart J. (2004) 25(12):1057–62. doi: 10.1016/j.ehj.2004.04.012
2. Butera G, Carminati M, Chessa M, Piazza L, Micheletti A, Negura DG, et al. Transcatheter closure of perimembranous ventricular septal defects: early and long-term results. J Am Coll Cardiol. (2007) 50(12):1189–95. doi: 10.1016/j.jacc.2007.03.068
3. Houeijeh A, Godart F, Jalal Z, Ovaert C, Heitz F, Mauran P, et al. Transcatheter closure of a perimembranous ventricular septal defect with nit-occlud lê vsd coil: a French multicentre study. Arch Cardiovasc Dis. (2020) 113(2):104–12. doi: 10.1016/j.acvd.2019.11.004
4. Xing Q, Pan S, An Q, Zhang Z, Li J, Li F, et al. Minimally invasive perventricular device closure of perimembranous ventricular septal defect without cardiopulmonary bypass: multicenter experience and mid-term follow-up. J Thorac Cardiovasc Surg. (2010) 139(6):1409–15. doi: 10.1016/j.jtcvs.2010.01.018
5. Ou-Yang WB, Li SJ, Wang SZ, Zhang DW, Liu Y, Zhang Z, et al. Echocardiographic guided closure of perimembranous ventricular septal defects. Ann Thorac Surg. (2015) 100(4):1398–402. doi: 10.1016/j.athoracsur.2015.05.036
6. Ou-Yang WB, Wang SZ, Hu SS, Zhang FW, Zhang DW, Liu Y, et al. Perventricular device closure of perimembranous ventricular septal defect: effectiveness of symmetric and asymmetric occluders. Eur J Cardiothorac Surg. (2017) 51(3):478–82. doi: 10.1093/ejcts/ezw352.28082474
7. Song S, Fan T, Li B, Liang W, Dong H, Wu K, et al. Minimally invasive peratrial device closure of perimembranous ventricular septal defect through a right infraaxillary route: clinical experience and preliminary results. Ann Thorac Surg. (2017) 103(1):199–204. doi: 10.1016/j.athoracsur.2016.05.069
8. Chen Q, Qiu HF, Zhang GC, Chen LW. Intraoperative device closure of a perimembranous ventricular septal defect using the right thoracic ventricle approach. Ann Thorac Surg. (2019) 107(3):817–22. doi: 10.1016/j.athoracsur.2018.08.038
9. Wang S, Ouyang W, Liu Y, Zhang F, Guo G, Zhao G, et al. Transcatheter perimembranous ventricular septal defect closure under transthoracic echocardiographic guidance without fluoroscopy. J Thorac Dis. (2018) 10(9):5222–31. doi: 10.21037/jtd.2018.08.03
10. Boutin C, Musewe NN, Smallhorn JF, Dyck JD, Kobayashi T, Benson LN. Echocardiographic follow-up of atrial septal defect after catheter closure by double-umbrella device. Circulation. (1993) 88(2):621–7. doi: 10.1161/01.CIR.88.2.621
11. Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, et al. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2013) 14(7):611–44. doi: 10.1093/ehjci/jet105
12. Jiang D, Han B, Zhao L, Yi Y, Zhang J, Fan Y, et al. Transcatheter device closure of perimembranous and intracristal ventricular septal defects in children: medium- and long-term results. J Am Heart Assoc. (2021) 10(11):e020417. doi: 10.1161/JAHA.120.020417
13. Bu H, Yang Y, Wu Q, Jin W, Zhao T. Echocardiography-guided percutaneous closure of perimembranous ventricular septal defects without arterial access and fluoroscopy. BMC Pediatr. (2019) 19(1):302. doi: 10.1186/s12887-019-1687-0
14. van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. (1999) 18(6):681–94. doi: 10.1002/(SICI)1097-0258(19990330)18:6%3C681::AID-SIM71%3E3.0.CO;2-R
15. van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. (2011) 45(3):1–67. doi: 10.18637/jss.v045.i03
16. Hijazi ZM, Hakim F, Haweleh AA, Madani A, Tarawna W, Hiari A, et al. Catheter closure of perimembranous ventricular septal defects using the new amplatzer membranous vsd occluder: initial clinical experience. Catheter Cardiovasc Interv. (2002) 56(4):508–15. doi: 10.1002/ccd.10292
17. Bai Y, Xu XD, Li CY, Zhu JQ, Wu H, Chen SP, et al. Complete atrioventricular block after percutaneous device closure of perimembranous ventricular septal defect: a single-center experience on 1046 cases. Heart Rhythm. (2015) 12(10):2132–40. doi: 10.1016/j.hrthm.2015.05.014
18. Chen Q, Hong ZN, Zhang GC, Chen LW, Zhang QL, Lin ZW, et al. Intraoperative device closure of isolated ventricular septal defects: experience on 1,090 cases. Ann Thorac Surg. (2018) 105(6):1797–802. doi: 10.1016/j.athoracsur.2018.02.059
19. Bergmann M, Germann CP, Nordmeyer J, Peters B, Berger F, Schubert S. Short- and long-term outcome after interventional vsd closure: a single-center experience in pediatric and adult patients. Pediatr Cardiol. (2021) 42(1):78–88. doi: 10.1007/s00246-020-02456-2
20. Turner ME, Bouhout I, Petit CJ, Kalfa D. Transcatheter closure of atrial and ventricular septal defects: JACC focus seminar. J Am Coll Cardiol. (2022) 79(22):2247–58. doi: 10.1016/j.jacc.2021.08.082
21. Santhanam H, Yang L, Chen Z, Tai BC, Rajgor DD, Quek SC. A meta-analysis of transcatheter device closure of perimembranous ventricular septal defect. Int J Cardiol. (2018) 254:75–83. doi: 10.1016/j.ijcard.2017.12.011
22. Yuan Y, Pan B, Liang X, Lv T, Tian J. Health-related quality of life in children with congenital heart disease following interventional closure versus minimally invasive closure. Front Cardiovasc Med. (2022) 9:974720. doi: 10.3389/fcvm.2022.974720
23. Yang P, Wu Z, Liu Z, Zhang J, Zhou H, Ji X, et al. Unplanned surgery after transcatheter closure of ventricular septal defect in children: causes and risk factors. Front Pediatr. (2021) 9:772138. doi: 10.3389/fped.2021.772138
24. Yang CZ, Hua C, Yuan Ji M, Qiang C, Wen Zhi P, Wan Hua C, et al. Transfemoral and perventricular device closures and surgical repair for doubly committed subarterial ventricular septal defects. Ann Thorac Surg. (2015) 99(5):1664–70. doi: 10.1016/j.athoracsur.2015.01.051
25. Haddad RN, Saliba ZS. Comparative outcomes of two competitive devices for retrograde closure of perimembranous ventricular septal defects. Front Cardiovasc Med. (2023) 10:1215397. doi: 10.3389/fcvm.2023.1215397
26. Zheng H, Lin A, Wang L, Xu Y, Zhang Z. The long-term change of arrhythmias after transcatheter closure of perimembranous ventricular septal defects. Cardiol Res Pract. (2021) 2021:1625915. doi: 10.1155/2021/1625915
Keywords: congenital heart defect, ventricular septal defect (perimembranous type), percutaneous (catheter-based) treatment, perventricular device closure, echocardiography guidance
Citation: Huang LL, Chen M, Zeng DC, Su CX, Jiang CL, Zheng BS, Wu J and Li SK (2023) Comparison of perventricular and percutaneous ultrasound-guided device closure of perimembranous ventricular septal defects. Front. Cardiovasc. Med. 10:1281860. doi: 10.3389/fcvm.2023.1281860
Received: 23 August 2023; Accepted: 26 October 2023;
Published: 6 November 2023.
Edited by:
Gianfranco Butera, Bambino Gesù Children’s Hospital (IRCCS), ItalyReviewed by:
Raymond N. Haddad, M3C-Necker, Hôpital Universitaire Necker-Enfants Malades, FranceMara Pilati, Bambino Gesù Children’s Hospital (IRCCS), Italy
© 2023 Huang, Chen, Zeng, Su, Jiang, Zheng, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shi Kang Li c2hpa2FuZ2xpQGhvdG1haWwuY29t
 Liu Liu Huang1
Liu Liu Huang1 De Cai Zeng
De Cai Zeng Ji Wu
Ji Wu Shi Kang Li
Shi Kang Li