- Division of Cardiology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Background: Late gadolinium enhancement (LGE) cardiac magnetic resonance (CMR) imaging has emerged as an important tool for assessment of patients with dilated cardiomyopathy (DCM). Electrocardiography (ECG) is an accessible, reproducible, low-cost diagnostic and prognostic tool. This study aimed to investigate the ECG characteristics associated with LGE, as well as to assess the prognostic significance of ECG in patients with DCM.
Methods: Consecutive patients diagnosed with DCM by CMR [left ventricular ejection fraction (LVEF) < 50%] between 2011 and 2020 were included. Multivariable analysis was conducted to evaluate ECG predictors associated with LGE. Receiver operating characteristic (ROC) analysis was performed to assess the diagnostic performance of ECG in combination of clinical data and LVEF for LGE. Two composite outcomes were also assessed among patients with and without ECG predictors: (1) sudden cardiac death (SCD), sustained ventricular arrhythmia, or appropriate implantable cardioverter-defibrillator (ICD) therapy, and (2) all-cause death or hospitalization for heart failure.
Results: A total of 422 patients, with a mean age of 59.5 ± 16.3 years (58.3% male), were included. LGE was present in 169 (40%) of the patients. Multivariable analysis identified lateral inverted T-waves, intraventricular conduction delay, low voltage, and fragmented QRS as independent predictors of LGE. ROC analysis showed a significant increase in the area under the curve (AUC) when ECG predictors of the four aforementioned characteristics were added to the clinical-LVEF model (AUC 0.66, 95% CI 0.59–0.71 vs. 0.72, 95% CI 0.67–0.78, p = 0.003). During a median follow-up of 2.7 years (IQR 0.8, 5.2), 16 events of SCD, sustained ventricular arrhythmia, or appropriate ICD therapy, and 70 events of all-cause death or hospitalization for heart failure occurred. ECG predictors were independently associated with SCD, sustained ventricular arrhythmia, or appropriate ICD therapy (HR 4.84, 95% CI 1.34–17.40, p = 0.01). However, ECG predictors were not associated with all-cause death or hospitalization for heart failure (HR 1.22, 95% CI 0.76–1.96, p = 0.39).
Conclusion: In patients with DCM, lateral inverted T-waves, intraventricular conduction delay, low voltage, and fragmented QRS were independently associated with LGE. Additionally, these ECG predictors had prognostic value for predicting SCD, sustained ventricular arrhythmia, or appropriate ICD therapy, assisting clinicians in stratifying SCD risk and identifying primary prevention ICD implantation candidates.
Introduction
Non-ischemic dilated cardiomyopathy (DCM) is a heterogeneous group of conditions, mostly caused by a genetic predisposition, consisting in left ventricular (LV) dilatation and dysfunction. DCM is an important cause of sudden cardiac death (SCD) and heart failure. Several previous studies have demonstrated the prognostic value of late gadolinium enhancement (LGE) with cardiac magnetic resonance (CMR) in patients with DCM (1–4). A meta-analysis by Becker et al., including 4,554 patients with DCM, demonstrated that LGE substantially worsens the prognosis for adverse cardiovascular (CV) events in DCM patients, and its absence indicates LV reverse remodeling (4).
The most recent European Society of Cardiology (ESC) guidelines have recommended that CMR with LGE should be considered in patients with DCM for assessing the etiology and the risk of SCD (5). However, in developing countries, the ability to send all DCM patients for CMR is limited. Electrocardiography (ECG) is an accessible, reproducible, low-cost diagnostic and prognostic tool. Previous studies have reported that ECG findings such as low QRS amplitude, anterolateral inverted T-waves, and fragmented QRS complex carry a heightened risk for major ventricular arrhythmias and adverse cardiac events (6–9).
Despite the potential of several ECG features to indicate a susceptibility to life-threatening cardiac arrhythmias, there is a lack of data regarding specific ECG characteristics associated with LGE. Additionally, none of these ECG characteristics are incorporated into the recommendations for primary prevention implantation of implantable cardioverter-defibrillators (ICDs), which currently rely solely on left ventricular ejection fraction (LVEF) and the New York Heart Association (NYHA) class (5).
In this study, our objective was to evaluate specific ECG characteristics associated with LGE in patients with DCM undergoing CMR, along with ECG predictors for adverse events. The results could enhance the risk stratification for DCM patients, assisting in the selection of candidates for CMR and future ICD implantation.
Methods
Study population
Consecutive patients aged 18 years or above with known or suspected DCM referred for CMR in a tertiary center in Thailand during 2010 and 2020 were studied. DCM was defined in accordance with the criteria of the World Health Organization (10). Patients were excluded if they had severe primary valvular disease, a diagnosis of other nonischemic disorders such as hypertrophic or infiltrative cardiomyopathy, or congenital heart disease. Additionally, those with CMR-confirmed subendocardial or transmural LV scarring indicative of previous myocardial infarction, a positive pharmacologic stress test indicating myocardial ischemia, or an LVEF ≥ 50%, were also excluded (Figure 1). The study was done in accordance with the Declaration of Helsinki. The institutional ethics committee [Siriraj Institutional Review Board (SIRB), Faculty of Medicine Siriraj Hospital, Mahidol University] approved this retrospective study and waived the need for additional written informed consent.
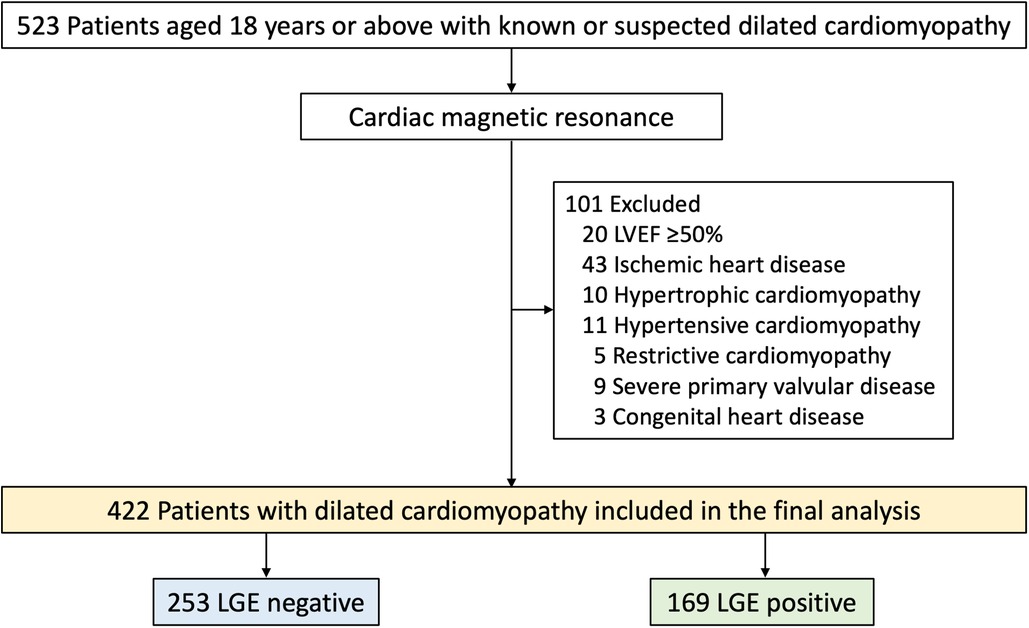
Figure 1. Flow chart of study population. LVEF, left ventricular ejection fraction; LGE, late gadolinium enhancement.
Electrocardiographic analysis
A twelve-lead ECG (GE MAC 1200; GE Marquette Medical Systems, Milwaukee, WI, USA) was obtained on the CMR date as part of the routine CMR protocol. Each ECG was reviewed by two trained cardiologists who were blinded to the patients’ data, and discordant results were resolved by a senior cardiologist. The ECGs were coded according to the Minnesota Code. ECG measurements included PR, RR, QT, and QRS intervals. The presence of a left bundle branch block (LBBB), complete right bundle branch block (RBBB), or interventricular conduction delay (IVCD) was defined according to the Minnesota Code. A premature ventricular contraction (PVC) recorded on a 12-lead ECG is characterized by a wide and atypical QRS complex, occurring earlier than expected in the regular heart rhythm and not preceded by a premature P wave. A fragmented QRS complex was defined based on a published article (11).
CMR protocol and image analysis
CMR was performed on 1.5-T or 3.0-T systems. (Philips Medical Systems, Best, the Netherlands) A standardized protocol as described by the Society for Cardiovascular Magnetic Resonance for the assessment of LV structure and function and myocardial scarring with cine and LGE imaging, respectively, was used (12). In brief, cine imaging was performed using a segmented steady-state free precession (SSFP) sequence with short-axis images acquired throughout the entire left ventricle. Long-axis images were obtained in standard 2-, 3-, and 4-chamber orientations. LGE imaging was performed 10 min after 0.15 mmol/kg gadolinium contrast administration using a standard 3D segmented inversion-recovery sequence and images were obtained in short- and long-axis locations matching those of cine imaging. Typical CMR parameters for 1.5-T for cine images included 8 mm slice thickness, 70-degree flip angle, repetition time (TR)/echo time (TE)/number of excitations = 3.7/1.8/2, 390 × 312 mm field of view, 256 × 240 matrix, and 1.52 × 1.3 reconstruction pixel. The parameters for LGE images were 3D segmented-gradient-echo inversion- recovery sequence with 8 mm slice thickness, 15-degree flip angle, 1.5 SENSitivity Encoding (SENSE) factor, TR/TE = 4.1/1.25 ms, 303 × 384 mm field of view, 240 × 256 matrix, and 1.26 × 1.5 mm reconstruction pixel.
Standard LV volumes, mass, and EF were quantitatively measured from the stack of short-axis SSFP cine images. LVEF was calculated from left ventricular end-systolic (LVESV) and end-diastolic volume (LVEDV) data. LV mass was calculated from the summation of subtraction of left ventricular end-diastolic epicardial and endocardial area and slice thickness of each slide. LGE images were analyzed using visual assessment. LGE was considered present only if confirmed on both the short-axis and at least one other orthogonal plane (13). Midwall LGE was only considered present if the area of LGE was confined to the intermural (3). Subepicardial LGE was only considered present if the area of LGE was confined to subepicardial layers (3), and isolated LGE was defined as focal LGE (14).
Clinical follow-up
Follow-up data were collected from clinical visits and medical records. Clinical event adjudication was conducted while maintaining complete blindness to clinical, ECG, and CMR data. Patients were followed for two composite outcomes, which included: (1) SCD, sustained ventricular arrhythmia, or appropriate ICD therapy; and (2) all-cause death or hospitalization for heart failure (15).
Statistical analysis
All statistical analyses were performed using SPSS Statistics for Windows version 20.0 (SPSS, Inc., Chicago, IL, USA). Continuous variables with normal distribution were presented as mean ± standard deviation, and continuous variables with non-normal distribution were presented as median and interquartile ranges (IQR). The normality of the distribution of variables was examined by the Kolmogorov-Smirnov test. Categorical variables were present as absolute numbers and percentages. Differences between patients with and without LGE in terms of baseline, ECG and image characteristics were compared using the Student’s unpaired t-test or the Mann-Whitney U test for continuous variables, while the chi-square test or Fisher’s exact test was used for categorical variables, as appropriate.
Binary logistic regression analysis was performed to identify significant predictors of LGE from baseline and ECG characteristics. Variables with a p-value < 0.05 from univariable analysis were entered into multivariable analysis. The results of the univariable and multivariable analyses are given as odds ratios (OR) along with their respective 95% confidence intervals (CI). A p-value less than 0.05 was considered statistically significant for all tests. We performed receiver operating characteristic (ROC) curve analysis to assess the diagnostic performance of clinical data alone and when combined with ECG to predict LGE. A comparison of the area under the curve (AUC) was made for these two approaches.
We assessed two composite outcomes: (1) SCD, sustained ventricular arrhythmia, or appropriate ICD therapy; and (2) all-cause death or hospitalization for heart failure. We employed the Kaplan-Meier method to compare the rates of these composite outcomes between patients with and without ECG predictors, using the log-rank test. To analyze the predictors of composite outcomes, we performed a Cox regression analysis to assess univariable predictors. Variables with a p-value < 0.05 from the univariable analysis were included in the multivariable analysis.
Results
Study population
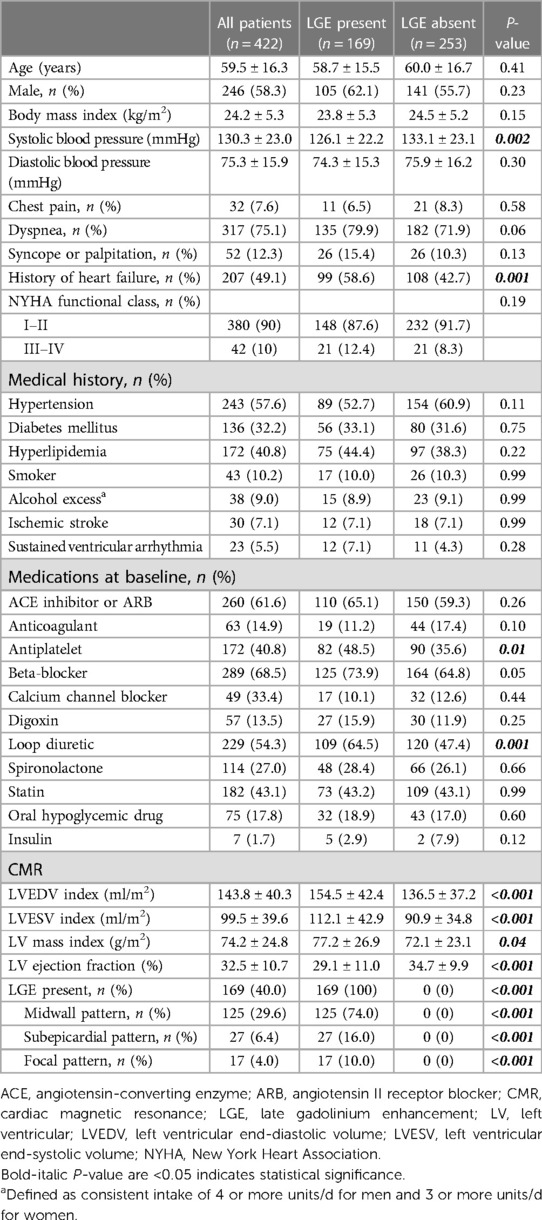
A total of 523 patients with known or suspected DCM were clinically referred for CMR (Figure 1). Of these, 101 were excluded and 422 were included in the final analysis. Baseline characteristics are summarized in Table 1. Mean age was 59.5 ± 16.3 years (range 18–89 years) and 58% were male. The most common symptoms of DCM included dyspnea (75.1%), syncope or palpitation (12.3%), and chest pain (7.6%). Forty-nine percent had a history of heart failure and 10% had NYHA functional class III or IV. The mean LVEF was 32.5 ± 10.7% and 169 patients (40.0%) had LGE present. Patients with LGE exhibited lower systolic blood pressure (BP), a higher prevalence of heart failure, and a greater usage of loop diuretics compared to those without LGE. Additionally, patients with LGE demonstrated significantly more severe LV dilatation and systolic dysfunction than those without LGE.
Electrocardiographic characteristics
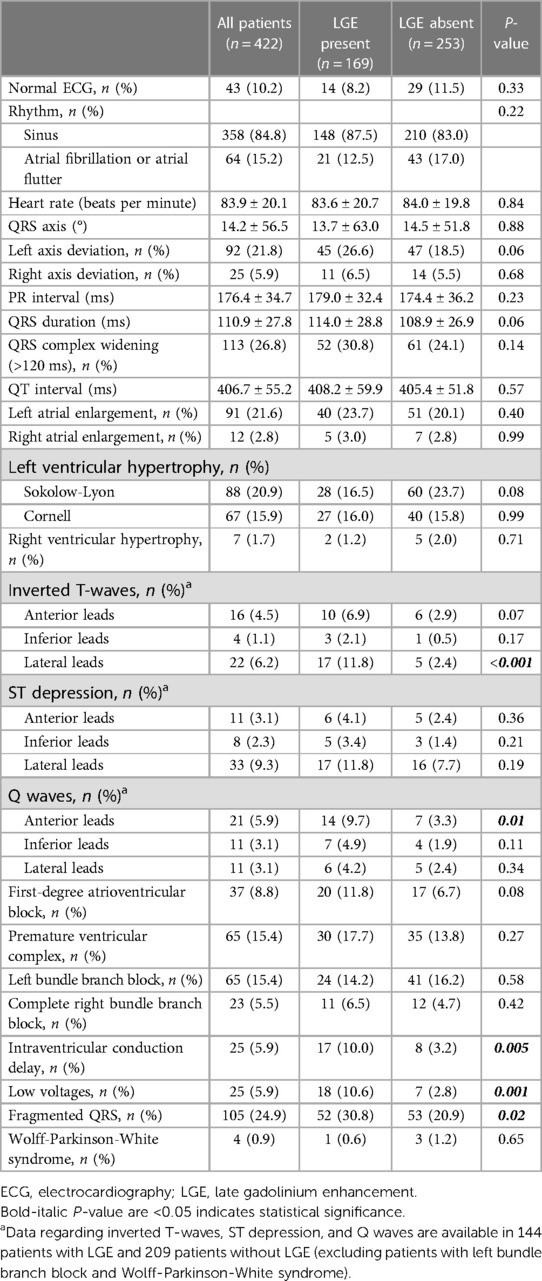
Table 2 shows ECG characteristics in DCM patients, with only 10% having a completely normal ECG and 15% demonstrating atrial fibrillation or flutter. Approximately 20% exhibited left atrial enlargement or LV hypertrophy. Patients with LGE showed a significantly higher prevalence of lateral inverted T-waves (11.8% vs. 2.4%, p < 0.001) and anterior Q waves (9.7% vs. 3.3%, p = 0.001) compared to those without LGE. Additionally, patients with LGE had a higher prevalence of IVCD (10.0% vs. 3.2%, p = 0.005), low voltages (10.6% vs. 2.8%, p = 0.001), and fragmented QRS (30.8% vs. 20.9%, HR p = 0.02) than those without LGE. However, patients with LGE seemed to exhibit lower rates of LV hypertrophy compared to those without LGE (Sokolow-Lyon criteria: 16.5% vs. 23.7%, p = 0.08).
Clinical and electrocardiographic predictors of LGE
Supplementary Table S1 presents the results of the univariable Cox regression analysis of clinical and ECG predictors associated with LGE. Systolic BP, history of heart failure, lateral inverted T-waves, IVCD, low voltages, and fragmented QRS were found to be associated with LGE in the univariable analysis. Table 3 demonstrates the multivariable Cox regression analyses of ECG predictors associated with LGE. Four ECG characteristics, including lateral inverted T-waves (OR 5.80, 95% CI 2.02–16.65, p = 0.001), IVCD (OR 4.99, 95% CI 1.86–13.39, p = 0.001), low voltages (OR 3.60, 95% CI 1.69–7.67, p = 0.001), and fragmented QRS (OR 2.77, 95% CI 1.41–5.46, p = 0.003), were independently associated with LGE.
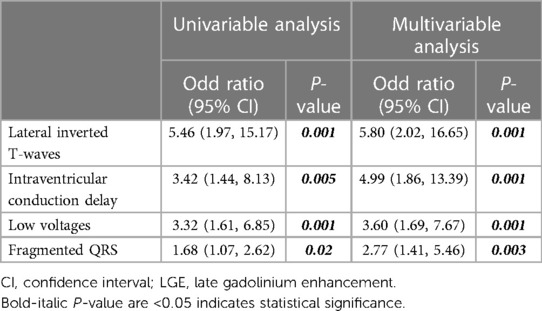
Table 3. Univariable and multivariable Cox regression analyses of electrocardiographic predictors associated with LGE.
Figure 2 displays the ROC analysis for LGE, comparing clinical factors (systolic BP and history of heart failure, which were associated with LGE in the univariable analysis) + LVEF, and clinical factors + LVEF + ECG (lateral inverted T-waves, IVCD, low voltages, or fragmented QRS). The AUC for clinical factors + LVEF + ECG was significantly higher than that for clinical factors + LVEF (AUC: 0.66, 95% CI 0.59–0.71 vs. 0.72, 95% CI 0.67–0.78, p = 0.003).
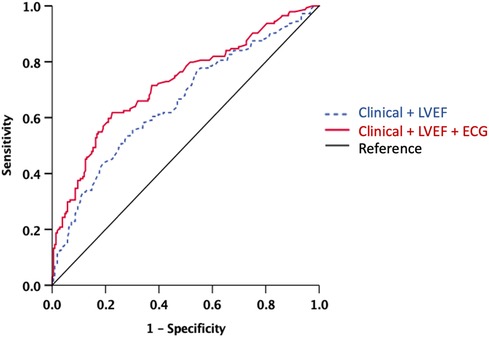
Figure 2. ROC analysis for the presence of LGE. Note the significant increase of the AUC when ECG model was added to the clinical-LVEF model. The AUC for clinical factors + LVEF + ECG was significantly higher than that for clinical factors + LVEF (AUC: 0.66, 95% CI 0.59−0.71 vs. 0.72, 95% CI 0.67−0.78, p = 0.003). AUC, area under the curve; ECG, electrocardiography; LVEF, left ventricular ejection fraction; ROC, receiver-operating curve. Clinical model includes systolic blood pressure and history of heart failure. ECG includes lateral inverted T-waves, intraventricular conduction delay, low voltage, and fragmented QRS.
Clinical outcomes
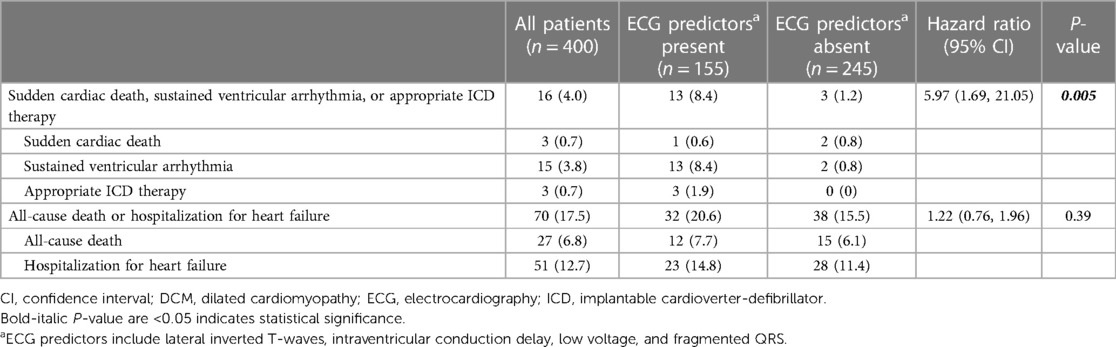
Twenty-two patients did not have follow-up data; therefore, 400 patients were included in the outcome analysis. During a median follow-up period of 2.7 years (IQR 0.8, 5.2), 16 events of SCD, sustained ventricular arrhythmia, or appropriate ICD therapy and 70 events of all-cause death or hospitalization for heart failure occurred. Thirty patients underwent ICD implantation after CMR. Among them, three received appropriate therapy, while three received inappropriate therapy. Table 4 shows the rates of outcomes of patients with and without ECG predictors (lateral inverted T-waves, IVCD, low voltages, or fragmented QRS), respectively. Patients with ECG predictors demonstrated markedly higher rates of SCD, sustained ventricular arrhythmia, or appropriate ICD therapy [8.4% vs. 1.2%, hazard ratio (HR) 5.97, 95% CI 1.69–21.05, p = 0.005], while experiencing similar rates of all-cause death or hospitalization for heart failure (20.6% vs. 15.5%, HR 1.22, 95% CI 0.76–1.96, p = 0.39). Figure 3 shows Kaplan-Meier curves for composite outcomes in DCM patients, stratified by the presence or absence of ECG predictors.
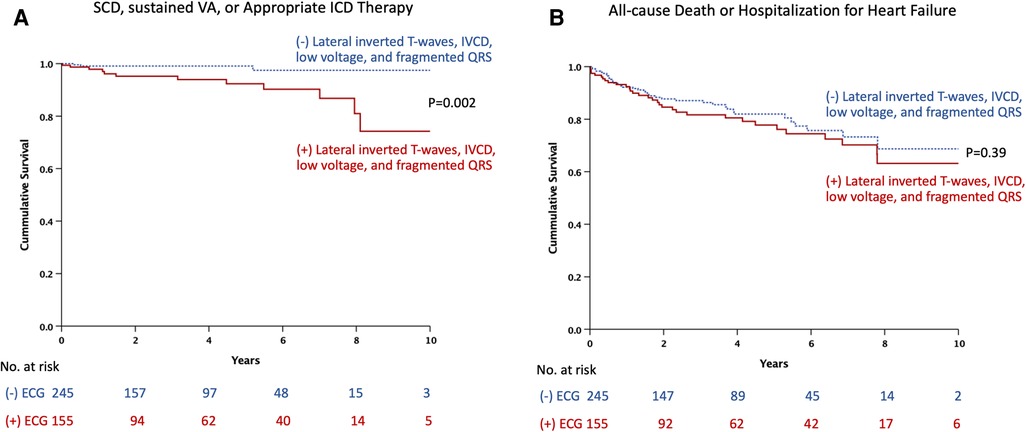
Figure 3. Kaplan-Meier estimates of the time to events by presence or absence of ECG predictors: (A) sudden cardiac death, sustained ventricular arrhythmia, or appropriate ICD therapy. (B) All-cause death or hospitalization for heart failure. ECG, electrocardiography; ICD, implantable cardioverter-defibrillator; IVCD, intraventricular conduction delay; SCD, sudden cardiac death; VA, ventricular arrhythmia. ECG predictors include lateral inverted T-waves, intraventricular conduction delay, low voltage, and fragmented QRS.
Multivariable analyses of composite outcomes
Table 5 demonstrates univariable and multivariable analyses of variables associated with SCD, sustained ventricular arrhythmia, or appropriate ICD therapy. The multivariable analysis revealed that history of sustained ventricular arrhythmia (HR 12.15, 95% CI 4.19–35.21, p < 0.001), history of heart failure (HR 5.40, 95% CI 1.20–24.31, p = 0.03), LVEDV index (HR 1.01, 95% CI 1.003–1.02, p = 0.02), and ECG predictors (HR 4.84, 95% CI 1.34–17.40, p = 0.01) were independently associated with SCD, sustained ventricular arrhythmia, or appropriate ICD therapy.
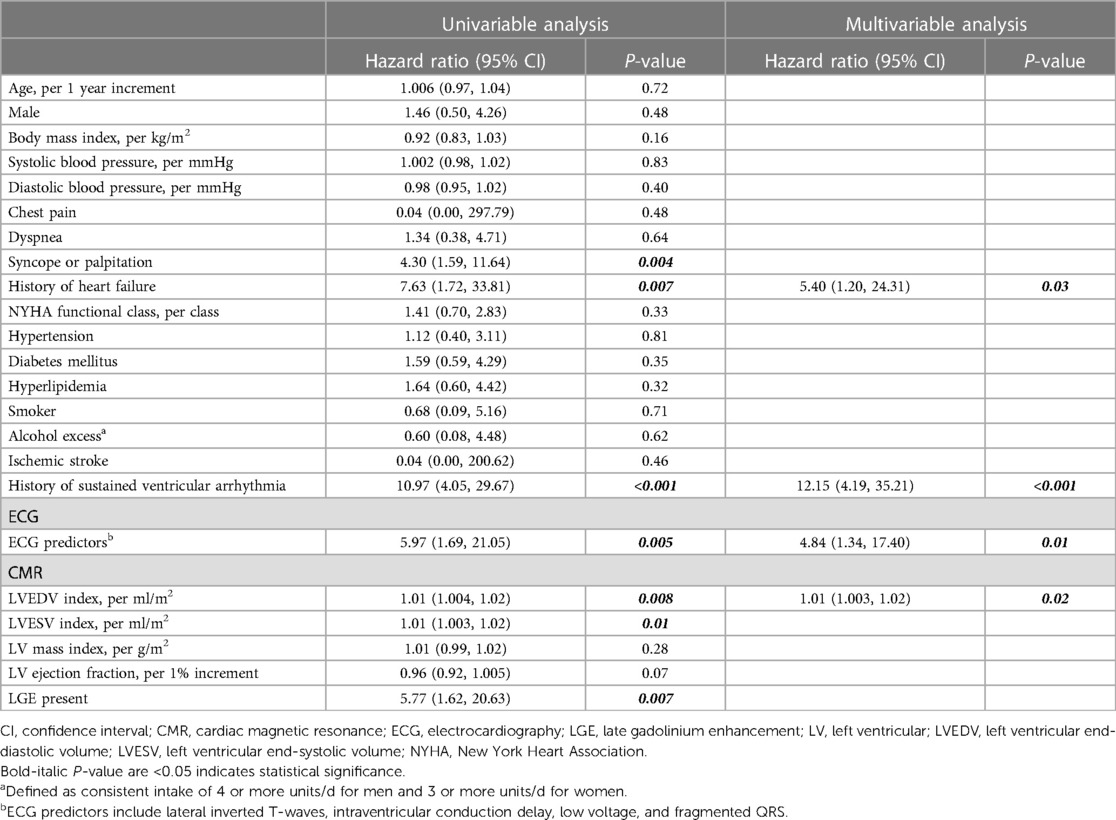
Table 5. Univariable and multivariable Cox regression analyses of clinical, electrocardiographic, and CMR predictors associated with sudden cardiac death, sustained ventricular arrhythmia, or appropriate ICD therapy.
Table 6 demonstrates univariable and multivariable analyses of variables associated with all-cause death or hospitalization for heart failure. The multivariable analysis revealed that age (HR 1.02, 95% CI 1.005–1.04, p = 0.01), NYHA functional class (HR 1.85, 95% CI 1.39–2.48, p < 0.001), hyperlipidemia (HR 1.89, 95% CI 1.15–3.08, p = 0.01), and LGE (HR 2.10, 95% CI 1.28–3.44, p = 0.003) were independently associated with all-cause death or hospitalization for heart failure.
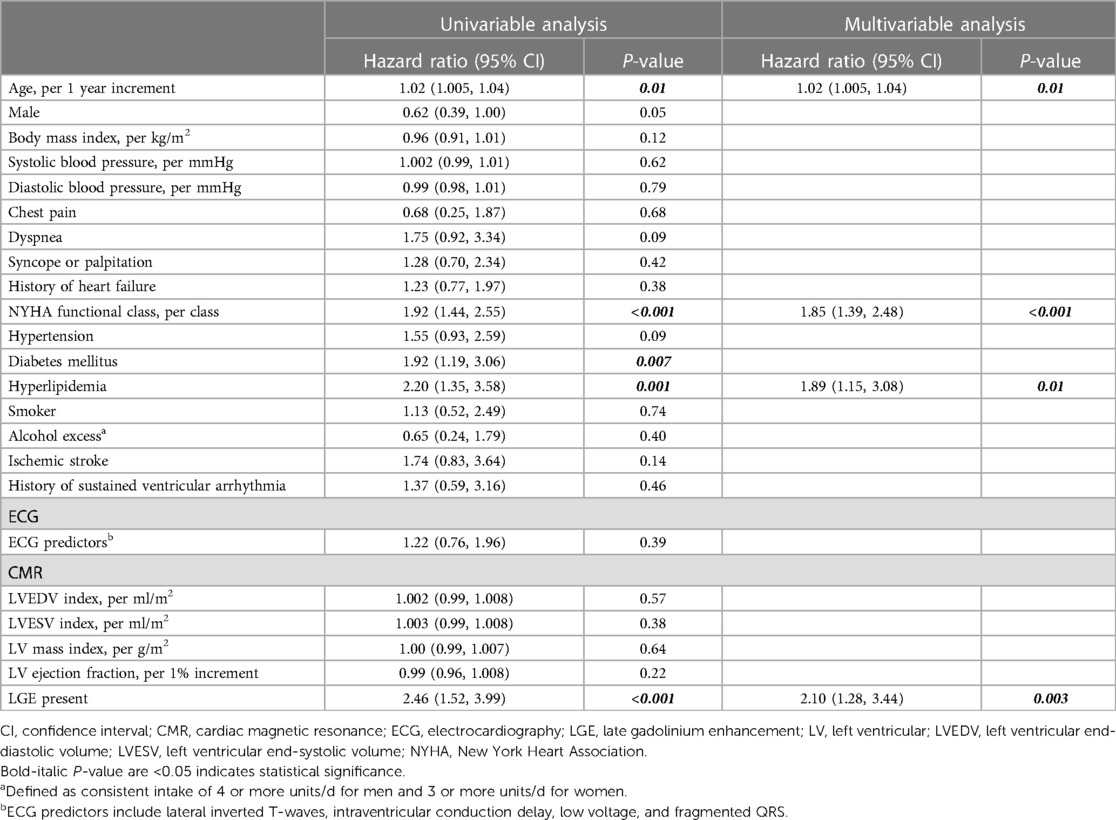
Table 6. Univariable and multivariable Cox regression analyses of clinical, electrocardiographic, and CMR predictors associated with all-cause death or hospitalization for heart failure.
Discussion
The main findings of this study can be summarized as follows: In patients with DCM who underwent CMR, ECG predictors, including lateral inverted T-waves, IVCD, low voltage, and fragmented QRS, were independently associated with LGE. Additionally, ECG predictors added diagnostic value for the identification of LGE, beyond clinical factors and LVEF. Furthermore, ECG predictors were independently associated with an increased risk of SCD, sustained ventricular arrhythmia, or the need for appropriate ICD therapy.
LGE and cardiovascular events in DCM
LGE CMR is a noninvasive method used to determine the underlying cause of DCM, and previous studies have reported its prognostic value in identifying patients at risk of cardiac events (1–3, 9, 16). Gulati et al. suggested that midwall LGE is independently associated with all-cause mortality, heart failure, as well as SCD (3). Di Marco et al. also reported that LGE is independently associated with SCD or ventricular arrhythmia in a meta-analysis encompassing 2,948 patients with non-ischemic DCM (16). Therefore, LGE can potentially improve risk stratification for cardiac events in patients with DCM. In our study, 40% of DCM patients demonstrated LGE, with the majority exhibiting midwall LGE. The prevalence of LGE in DCM patients varied among studies. Gulati et al. demonstrated that 30% of DCM patients had LGE, focusing solely on midwall LGE (3). Tateishi et al. found that 50.7% of DCM patients had LGE (14). In a meta-analysis conducted by Becker et al., which included 4,554 patients with DCM, LGE was present in 44.8% of cases (4). The prevalence of LGE in our study was comparable to that reported in previous studies. Patients with LGE had a higher prevalence of a history of heart failure and exhibited more severe degrees of LV remodeling, including higher LV volumes and lower LVEF.
Patients with LGE demonstrated significantly higher rates of all-cause death and heart failure hospitalization. These findings are consistent with prior studies (2–4). Patients with LGE were also associated with SCD and sustained ventricular arrhythmia in the univariable analysis, but this was not shown in multivariable analysis. However, given the relatively low number of events, caution should be exercised when interpreting these findings.
Electrocardiography as a predictor of LGE
The majority of patients with DCM exhibit abnormal ECG findings, including cardiac chamber enlargement, bundle branch block, and inverted T-waves. In our study only 10% had normal ECG, which was consistent with a study of Merlo (7). ECG findings in DCM can result from either abnormal cardiac structure or genetic factors associated with DCM (9). Our study was the first to demonstrate ECG characteristics associated with LGE including lateral inverted T-waves, IVCD, low voltage, and fragmented QRS. The reduction in QRS amplitude, particularly in the precordial leads, can be attributed to the loss of vital myocardium and diffuse LV fibrosis (7, 17, 18). In cases of DCM caused by muscular dystrophies, posterior or inferior Q waves often manifest, reflecting transmural myocardial fibrosis (19). Inverted T-waves, especially in the lateral leads, is a recognized feature of certain genetic forms (such as filamin C or desmosomal disease) (20). DCM resulting from prior myocarditis presents a wide spectrum of non-specific ECG findings, including low voltages, conduction abnormalities, lateral inverted T-waves, increased QRS duration, and PVC/nonsustained ventricular tachycardia (4). Although we did not have genetic data to prove an association between ECG findings and specific genetic factors, our findings contribute ECG predictors of LGE, potentially aiding clinicians in selecting patients with DCM for CMR when its availability is limited, particularly in developing countries. We also believe that certain ECG findings could provide additional prognostic value, complemented by benefits from clinical and CMR assessments.
Prognostic value of electrocardiography in DCM
Several studies have demonstrated the prognostic value of ECG in patients with DCM. Merlo et al. demonstrated that anterolateral inverted T-waves predicted study outcomes, including death or heart transplant, as well as sudden death or malignant ventricular arrhythmias (7). A CMR study by Marume et al. showed that the combination of LGE and a wide QRS complex provided additional prognostic stratification compared to LGE status alone, potentially enhancing the appropriate use of ICD therapy in patients with DCM (21). Low QRS amplitude and fragmented QRS complex also carry a heightened risk for major ventricular arrhythmias and adverse cardiac events (6, 8, 9). Our study demonstrated that ECG predictors were independently associated with SCD, sustained ventricular arrhythmia, or appropriate ICD therapy. This is largely consistent with the aforementioned studies. Lateral inverted T-waves were one of the ECG characteristics that were consistently associated with SCD and malignant ventricular arrhythmia in both our study and the study by Merlo (7). The mechanism behind this could be related to DCM caused by post-inflammatory processes. Another mechanism could arise from specific genotypes, such as intercellular junction protein mutations, which underlie possible overlapping phenotypes between DCM and arrhythmogenic cardiomyopathy. Consequently, the overall prognostic role of lateral inverted T-waves was mainly driven by their association with major arrhythmic events (4, 7, 22). In our study, ECG predictors were not associated with all-cause death or hospitalization for heart failure. This could be explained by several reasons, such as ECG findings like atrial fibrillation or left atrial enlargement may be consequences of severe heart failure rather than predictive factors.
Study limitations
This study had some limitations. Firstly, single-time ECG data may change dynamically during a follow-up period. However, using a single ECG is practical for clinicians to assess. Secondly, the study population was limited to a CMR center within a tertiary hospital, introducing selection bias, and the characteristics of the patients analyzed may differ from those of the general population with DCM. Thirdly, we have not included the impact of different LGE patterns in our analysis, as the majority of patients (>70%) exhibited midwall LGE. Other types of LGE could represent specific cardiomyopathies such as burnout hypertrophic cardiomyopathy or varying degrees of hypertensive heart disease. However, the prevalence of these populations was low, reflecting real-world data that are consistent with prior published research. Lastly, we did not have T1 mapping and extracellular volume fraction data that had prognostic value in patients with DCM (23, 24). However, there is little evidence of incremental value when LGE is already a routine part of the scanning protocol (24, 25).
Conclusions
In patients with DCM, lateral inverted T-waves, IVCD, low voltage, and fragmented QRS were independently associated with LGE. Additionally, these ECG predictors had prognostic value for predicting SCD, sustained ventricular arrhythmia, or appropriate ICD therapy, assisting clinicians in stratifying SCD risk and identifying primary prevention ICD implantation candidates.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Siriraj Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this is a retrospective study.
Author contributions
PC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. TB: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review and editing. NP: Data curation, Investigation, Methodology, Writing – review and editing. SP: Data curation, Investigation, Methodology, Writing – review and editing. YK: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors gratefully acknowledge Khemajira, Karaketklang and Dittapol Muntham for their assistance with statistical analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1281563/full#supplementary-material
Abbreviations
AUC, area under the curve; CMR, cardiac magnetic resonance; DCM, dilated cardiomyopathy; ECG, electrocardiography; ICD, implantable cardioverter-defibrillator; IVCD, intraventricular conduction delay; IQR, interquartile range; LBBB, left bundle branch block; LGE, late gadolinium enhancement; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; NYHA, New York Heart Association; RBBB, right bundle branch block; ROC, receiver operating characteristic; SCD, sudden cardiac death.
References
1. Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. (2006) 48(10):1977–85. doi: 10.1016/j.jacc.2006.07.049
2. Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. (2008) 51(25):2414–21. doi: 10.1016/j.jacc.2008.03.018
3. Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. (2013) 309(9):896–908. doi: 10.1001/jama.2013.1363
4. Becker MAJ, Cornel JH, van de Ven PM, van Rossum AC, Allaart CP, Germans T. The prognostic value of late gadolinium-enhanced cardiac magnetic resonance imaging in nonischemic dilated cardiomyopathy: a review and meta-analysis. JACC Cardiovasc Imaging. (2018) 11(9):1274–84. doi: 10.1016/j.jcmg.2018.03.006
5. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. (2022) 43(40):3997–4126. doi: 10.1093/eurheartj/ehac262
6. Goldberger JJ, Subačius H, Patel T, Cunnane R, Kadish AH. Sudden cardiac death risk stratification in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol. (2014) 63(18):1879–89. doi: 10.1016/j.jacc.2013.12.021
7. Merlo M, Zaffalon D, Stolfo D, Altinier A, Barbati G, Zecchin M, et al. ECG in dilated cardiomyopathy: specific findings and long-term prognostic significance. J Cardiovasc Med (Hagerstown). (2019) 20(7):450–8. doi: 10.2459/JCM.0000000000000804
8. Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC, et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. (2019) 16(11):e301–e72. doi: 10.1016/j.hrthm.2019.05.007
9. Finocchiaro G, Merlo M, Sheikh N, De Angelis G, Papadakis M, Olivotto I, et al. The electrocardiogram in the diagnosis and management of patients with dilated cardiomyopathy. Eur J Heart Fail. (2020) 22(7):1097–107. doi: 10.1002/ejhf.1815
10. Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O'Connell J, et al. Report of the 1995 world health organization/international society and federation of cardiology task force on the definition and classification of cardiomyopathies. Circulation. (1996) 93(5):841–2. doi: 10.1161/01.CIR.93.5.841
11. Konno T, Hayashi K, Fujino N, Oka R, Nomura A, Nagata Y, et al. Electrocardiographic QRS fragmentation as a marker for myocardial fibrosis in hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol. (2015) 26(10):1081–7. doi: 10.1111/jce.12742
12. Kramer CM, Barkhausen J, Bucciarelli-Ducci C, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson. (2020) 22(1):17. doi: 10.1186/s12968-020-00607-1
13. Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance—2020 update. J Cardiovasc Magn Reson. (2020) 22(1):19. doi: 10.1186/s12968-020-00610-6
14. Tateishi E, Noguchi T, Goto Y, Morita Y, Ishibashi-Ueda H, Yamada N, et al. Prognostic impact of blood pressure response plus gadolinium enhancement in dilated cardiomyopathy. Heart. (2015) 101(10):774–80. doi: 10.1136/heartjnl-2014-307007
15. Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, et al. 2017 cardiovascular and stroke endpoint definitions for clinical trials. Circulation. (2018) 137(9):961–72. doi: 10.1161/CIRCULATIONAHA.117.033502
16. Di Marco A, Anguera I, Schmitt M, Klem I, Neilan TG, White JA, et al. Late gadolinium enhancement and the risk for ventricular arrhythmias or sudden death in dilated cardiomyopathy: systematic review and meta-analysis. JACC Heart Fail. (2017) 5(1):28–38. doi: 10.1016/j.jchf.2016.09.017
17. Hamby RI, Raia F. Vectorcardiographic aspects of primary myocardial disease in 50 patients. Am Heart J. (1968) 76(3):304–15. doi: 10.1016/0002-8703(68)90225-1
18. Roberts WC, Siegel RJ, McManus BM. Idiopathic dilated cardiomyopathy: analysis of 152 necropsy patients. Am J Cardiol. (1987) 60(16):1340–55. doi: 10.1016/0002-9149(87)90618-7
19. Rapezzi C, Arbustini E, Caforio AL, Charron P, Gimeno-Blanes J, Heliö T, et al. Diagnostic work-up in cardiomyopathies: bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC working group on myocardial and pericardial diseases. Eur Heart J. (2013) 34(19):1448–58. doi: 10.1093/eurheartj/ehs397
20. Gigli M, Merlo M, Graw SL, Barbati G, Rowland TJ, Slavov DB, et al. Genetic risk of arrhythmic phenotypes in patients with dilated cardiomyopathy. J Am Coll Cardiol. (2019) 74(11):1480–90. doi: 10.1016/j.jacc.2019.06.072
21. Marume K, Noguchi T, Tateishi E, Morita Y, Kamakura T, Ishibashi K, et al. Mortality and sudden cardiac death risk stratification using the noninvasive combination of wide QRS duration and late gadolinium enhancement in idiopathic dilated cardiomyopathy. Circ Arrhythm Electrophysiol. (2018) 11(4):e006233. doi: 10.1161/CIRCEP.117.006233
22. Sen-Chowdhry S, Syrris P, Prasad SK, Hughes SE, Merrifield R, Ward D, et al. Left-dominant arrhythmogenic cardiomyopathy: an under-recognized clinical entity. J Am Coll Cardiol. (2008) 52(25):2175–87. doi: 10.1016/j.jacc.2008.09.019
23. Li S, Zhou D, Sirajuddin A, He J, Xu J, Zhuang B, et al. T1 mapping and extracellular volume fraction in dilated cardiomyopathy: a prognosis study. JACC Cardiovasc Imaging. (2022) 15(4):578–90. doi: 10.1016/j.jcmg.2021.07.023
24. Puntmann VO, Carr-White G, Jabbour A, Yu CY, Gebker R, Kelle S, et al. T1-mapping and outcome in nonischemic cardiomyopathy: all-cause mortality and heart failure. JACC Cardiovasc Imaging. (2016) 9(1):40–50. doi: 10.1016/j.jcmg.2015.12.001
25. Chen Z, Sohal M, Voigt T, Sammut E, Tobon-Gomez C, Child N, et al. Myocardial tissue characterization by cardiac magnetic resonance imaging using T1 mapping predicts ventricular arrhythmia in ischemic and non-ischemic cardiomyopathy patients with implantable cardioverter-defibrillators. Heart Rhythm. (2015) 12(4):792–801. doi: 10.1016/j.hrthm.2014.12.020
Keywords: cardiac magnetic resonance, dilated cardiomyopathy, electrocardiography, late gadolinium enhancement, prognostic value, sudden cardiac death
Citation: Chayanopparat P, Boonyasirinant T, Prapan N, Phoopattana S and Kaolawanich Y (2023) Electrocardiographic characteristics associated with late gadolinium enhancement and prognostic value in patients with dilated cardiomyopathy. Front. Cardiovasc. Med. 10:1281563. doi: 10.3389/fcvm.2023.1281563
Received: 22 August 2023; Accepted: 4 October 2023;
Published: 18 October 2023.
Edited by:
Masaki Izumo, St. Marianna University School of Medicine, JapanReviewed by:
Alexander H. Maass, University Medical Center Groningen, NetherlandsYukio Sato, St. Marianna University School of Medicine, Japan
© 2023 Chayanopparat, Boonyasirinant, Prapan, Phoopattana and Kaolawanich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yodying Kaolawanich eW9keWluZy5rYW9AZ21haWwuY29t
 Punyanuch Chayanopparat
Punyanuch Chayanopparat Thananya Boonyasirinant
Thananya Boonyasirinant Yodying Kaolawanich
Yodying Kaolawanich