- 1Division of Cardiology, Department of Internal Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
- 2Medical Department, Healthcare Division, Merck Pty Ltd, Modderfontein, South Africa
- 3Medical Affairs EMEA, Merck Serono Middle East FZ-LLC, Dubai, United Arab Emirates
Introduction: Compared with first-line antihypertensives, beta-blockers (BB) have been reported to lower the central aortic blood pressure suboptimally and are associated with increased stroke risk. This observation has not been investigated in hypertensives of African ancestry. We hypothesised that an individual patient data meta-analysis (IPD-MA) on the efficacy of second- or third-generation beta-blockers (STGBBs) in hypertensives of African descent may provide new insights.
Methods: A single-stage IPD-MA analysed the efficacy of STGBB in lowering the mean arterial blood pressure and reducing the composite outcomes: cardiovascular death, stroke, and myocardial infarction.
Results: A total of 11,860 participants from four randomised control trials were included in the analysis. Second- or third-generation beta-blockers reduced the mean arterial pressure by 1.75 mmHg [95% confidence interval (CI):1.16–2.33; P < 0.001] in all participants included in the analysis, and by 1.93 mmHg (95% CI: 0.86–3.00; P < 0.001) in hypertensive Africans. In patients with established cardiovascular disease, where the benefits of BB therapy are well established, STGBBs were associated with an adjusted odds ratio of 1.33 (95% CI: 1.06–1.65; P = 0.015) of the composite outcome, most likely due to confounding. Similarly, the risk of total myocardial infarction was 1.76 times higher (95% CI: 1.15–2.68; P = 0.008) in hypertensives of African ancestry on STGBBs.
Conclusion: The STGBBs reduced the mean arterial pressure comparably to other antihypertensives, and they were not associated with an increased risk of stroke.
1 Introduction
Beta-blockers are classified by generation as follows: first-generation beta-blockers refer to non-selective beta-blockers without any additional vasodilatory effects; second-generation beta-blockers generally refer to β1-selective beta-blockers; and third-generation beta-blockers refer to beta-blockers that possess additional vasodilatory action, often mediated by the release of nitric oxide.
Beta-blockers (BBs) are a pharmacologically heterogeneous class of drugs widely used over the past five decades (1). The ability of BBs to modulate the sympathetic nervous system (SNS) through the adrenergic blockade of β1 and β2 receptors, and variable vasodilatory properties, position these agents as efficacious first-line antihypertensives. However, several clinical guidelines for the management of hypertension have recently withdrawn BBs as a first-line therapy (2–5), citing that, compared with other classes of antihypertensives, BBs reduce central aortic pressure suboptimally and offer less protection against fatal and non-fatal strokes (6).
This contentious decision to withdraw BB as a first-line therapy has been found to be controversial and met with some opposition. No prospective randomised control trials (RCTs) have investigated the efficacy of, specifically, second or third-generation beta-blockers (STGBBs) in reducing morbidity and mortality in hypertensives. Furthermore, individuals of African ancestry are usually underrepresented in RCTs (7). Yet, they have a higher prevalence of hypertension and are more likely to experience target organ damage caused by poorly controlled hypertension (8–11). This increase in hypertension-related morbidity and mortality may be because of the interplay between biological factors and myriad social drivers (12–15). We conducted an individual patient data meta-analysis (IPD-MA) of RCTs evaluating the efficacy of STGBBs in hypertensives of African ancestry in lowering blood pressure and the risk of cardiovascular death, fatal and non-fatal myocardial infarction (MI), and strokes.
2 Materials and methods
The study protocol was registered on the International Prospective Register for systematic reviews (PROSPERO ID = CRD42022344733). This IPD-MA adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for individual patient data systematic reviews (PRISMA-IPD) statement (16).
2.1 Selection of randomised control trials and participants included in the individual patient data meta-analysis
Following a prespecified search strategy, we performed a comprehensive systematic literature search in multiple clinical trial registries, search engines, and dataset repositories. (Supplementary material 1). All published and unpublished RCTs that assessed the efficacy of BBs, or antihypertensives, including STGBBs, compared with placebo, standard-of-care (SoC), or other antihypertensives (including first-generation BBs) in hypertensive participants were eligible for inclusion. Eligible RCTs were required to report the efficacy of BBs in lowering blood pressure and outcomes such as cardiovascular death, MI, and strokes in hypertensive individuals. Furthermore, RCTs required a proportion of the study participants to be of African descent. We excluded RCTs with a follow-up duration shorter than 1 year, participants younger than 18 years of age, studies conducted on healthy volunteers, and those without participants of African descent.
The risk of confounding was minimised by excluding participants with a history of previous major cardiovascular events. Participants were excluded from the analysis if they had a prior history of MI, congestive heart failure (CHF), arrhythmia, history of coronary revascularisation, and transient ischaemic attacks (TIAs) or stroke. Two reviewers independently examined the eligibility of all potential RCTs and assessed the risk of bias (RoB). Differences were resolved through discussion and consensus, and, where required, a third clinical expert arbitrator was consulted.
2.2 Individual patient data collection and assessment
The individuals listed as corresponding authors were contacted via email for each RCT study that met the inclusion criteria (Supplementary material 2). The integrity of the obtained datasets was checked for consistency and completeness (17). Also, summary statistics of received datasets were matched with published results, and internal summary statistics were generated as part of the IPD-MA to identify discrepancies. The variables of interest were extracted, standardised, and merged into a single dataset, ensuring that standard scales and definitions were used (Supplementary material 3). The study participants self-identified their ethnicity. The RoB 2 tool adapted for IPD-MA was used to assess the RoB in each study included in the meta-analysis (18, 19) (Supplementary material 4). All datasets included in this study were obtained from repositories within the National Institute of Health umbrella or Vivli.org. No IPD integrity or RoB 2 concerns were identified (Supplementary material 4). The internal and external summary statistics of the individual datasets matched, verifying the accuracy of the datasets employed in the final analysis.
2.3 Outcomes and treatment group allocation
The efficacy of BBs was assessed by evaluating the rate of cardiovascular mortality, myocardial infarction, and non-fatal and fatal strokes in hypertensives included in the IPD-MA prescribed STGBB compared with those on a placebo or first-generation BB therapy. To estimate the risk of composite events, odds ratios (OR) were extracted from each study, and the reduction in blood pressure (BP) was calculated by measuring the difference between the baseline and exit mean arterial blood pressure (MAP).
The treatment arm consisted of the whole BB class, which was partitioned into a newer second- and third-generation BB (STGBB group), defined by the β1 adrenergic receptor affinity (second generation) and/or vasodilatory properties mediated by the release of Nitric oxide (third generation) (20), and their respective individual components of non-selective, selective β-receptor properties and vasodilatory properties. For studies that did not have a BB treatment arm, we reviewed the documented concomitant medication to allocate participants to their respective treatment groups. Participants were included in the STGBB group if they were on a BB for at least 18 months.
2.4 Statistical analysis
The respective RCT data were combined, creating a standardised IPD dataset. Exploratory and descriptive analysis preceded the one-stage IPD-MA. Categorical variables were summarised with frequencies and percentages. The mean and standard deviation and the median with minimum and maximum values were used to summarise numerical variables. The intraclass coefficient (ICC) was calculated by quantifying the degree to which participants within RCTs were alike, based on proportional variance. The generalised linear mixed effects model (GLMM) building process, evaluating both the fit of random intercept and/or random slopes in the modelling process was used. Known confounders (age, gender, ethnicity, diabetes, and smoking) for cardiovascular death were included in the analysis if they were statistically significant, clinically relevant, or the inclusion improved the model's goodness of fit (GoF) statistics. The iterative process assessed each covariate in this manner. Assumptions of GLMMs were robustly evaluated to curb any violations that may negatively influence the validity of the results (Supplementary material 5). Univariate logistic regression analysis was conducted and the odds ratios were adjusted for confounding by including covariates traditionally regarded as predictors of cardiovascular outcomes. Confidence intervals (CIs) were set at 95% and a P-value <0.05 was set as a threshold for statistical significance. Sensitivity analysis quantifying study heterogeneity and trends were performed as part of the statistical analysis. All data manipulation and analyses were done in R (version 4.2.1) (21) using the Tidyverse (22) and lme4 (23) packages.
2.5 Role of the funding
The funder of this research (Merck) had no role in data collection, statistical analysis, data wrangling, data interpretation, and manuscript writing. The funders gave their consent towards the publication of the manuscript.
2.6 Ethics
Permission to conduct the study was obtained from the University of the Witwatersrand Human Research Ethics Committee (Medical), ethics clearance certificate number: W-CBP-211102-01.
3 Results
3.1 Study cohort selection and baseline characteristics
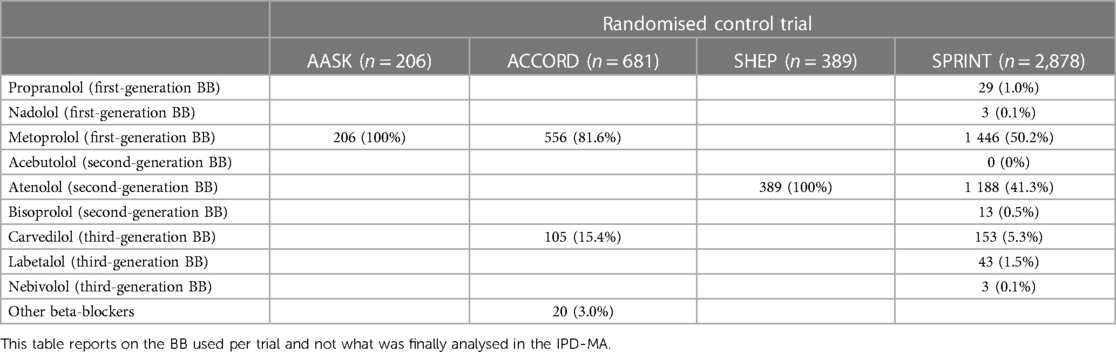
The search strategy resulted in 30 eligible RCTs (Figure 1). All authors listed in the respective trials were contacted, of which 23 authors responded. Of the 30 RCT datasets requested, access was granted to seven datasets. We excluded 19,494 participants with established severe CVD. The final study cohort in the IPD-MA comprised 11,860 hypertensives from four RCTs (Table 1). The STGBB arm had 3,864 (32.6%) hypertensives with a mean age of 66.4 ± 9.2 years, of which 1,339 (34.7%) hypertensives were of African ancestry. Similarly, the non-STGBB arm had 2,541 (31.8%) hypertensives of African ancestry. The STGBB arm had more individuals who had been prescribed concomitant antihypertensive medication; with diuretics prescribed to 3,131 (81.1%) vs. 3,781 (47.3%) in the non-STGBB arm. Of note, 2,075 (53.7%) participants in the STGBB arm were prescribed calcium channel blockers (CCB), compared with the 2,827 (35.4%) in the non-STGBB arm. The baseline mean systolic and diastolic blood pressures were comparable in both arms of the study. Table 2 depicts the type of BBs prescribed in each RCT.
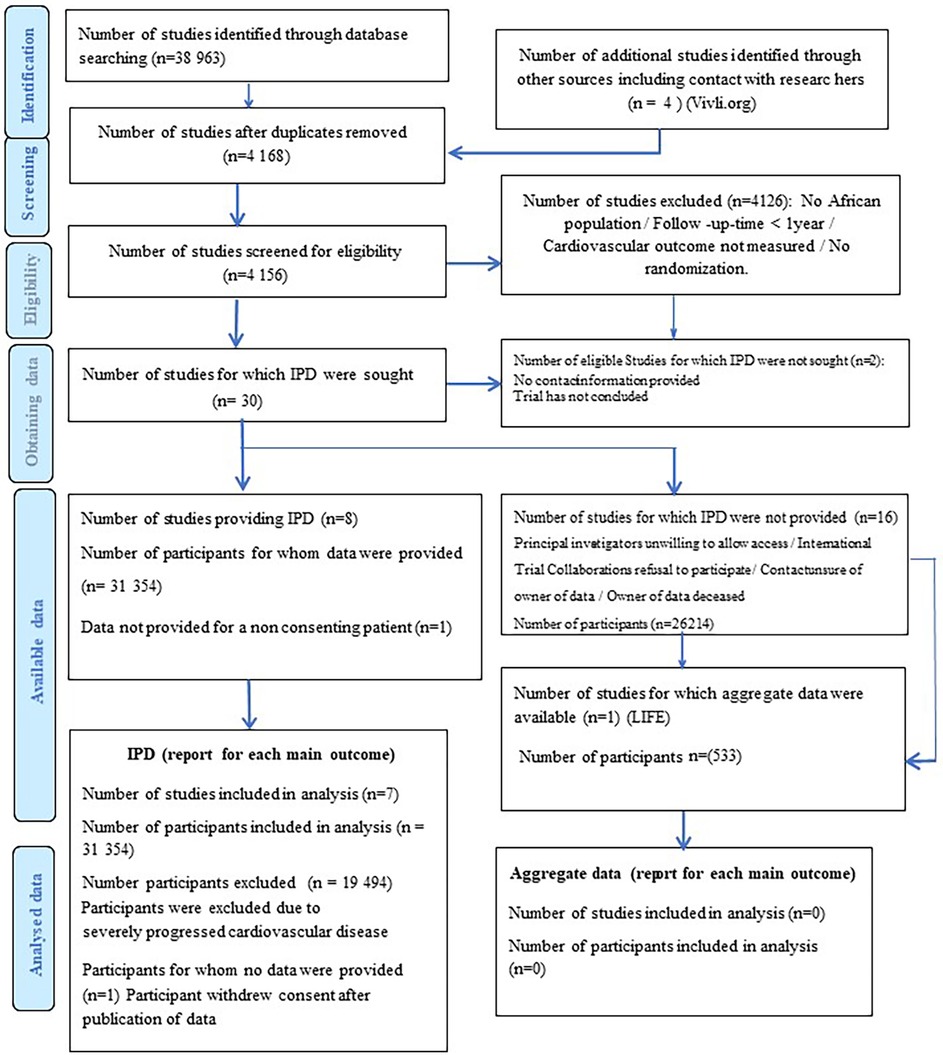
Figure 1. PRISMA flow chart showing the selection of studies and participants included in the meta-analysis.
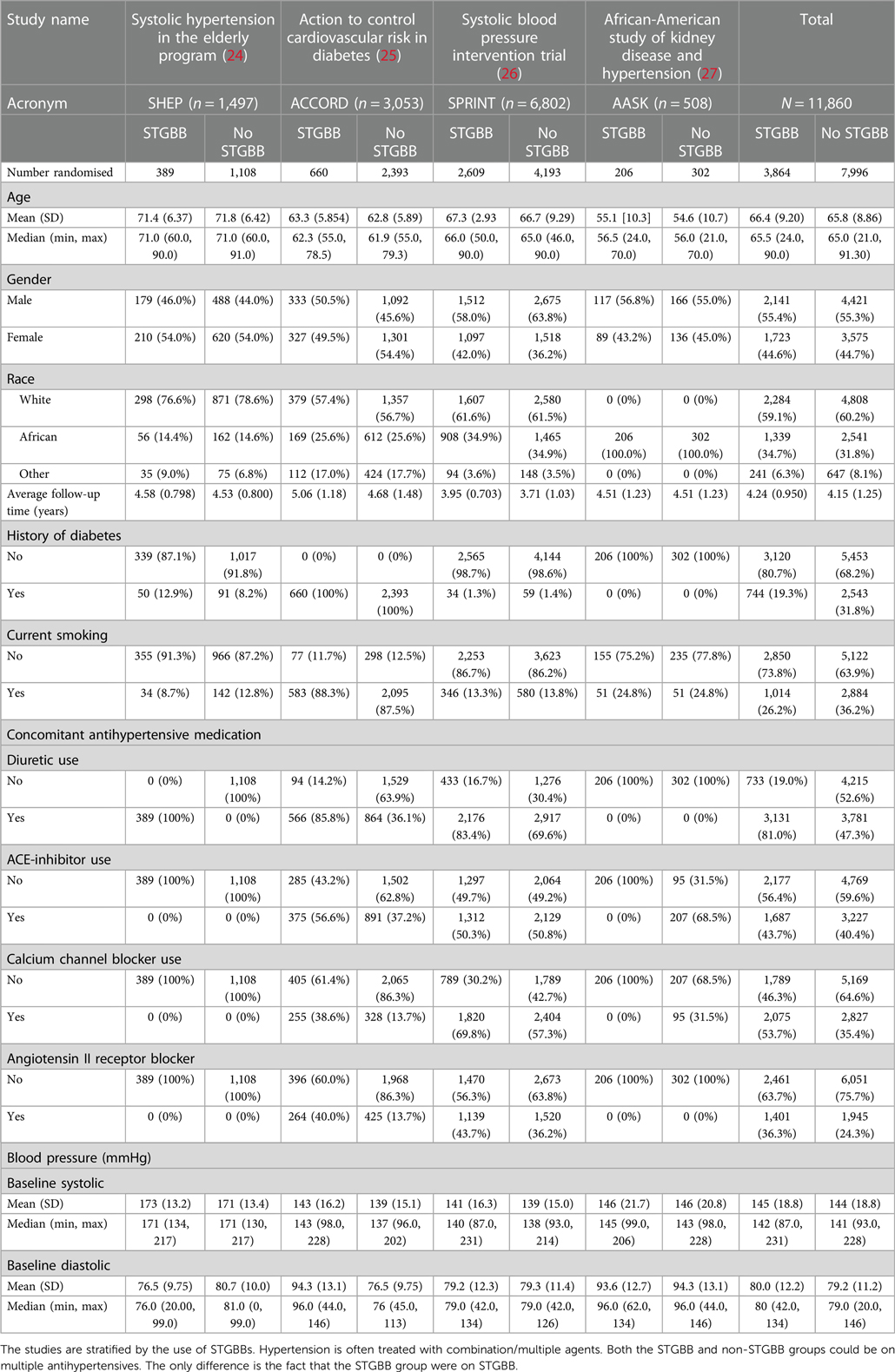
Table 1. Standardised demographic and clinical characteristics of patients included in the meta-analysis stratified by the trial providing the patient data.
3.2 Study outcomes
Hypertensives included in the IPD-MA had a mean follow-up duration of 4.13 years. The STGBB arm recorded 217 (5.6%) primary events compared with 374 (4.7%) events in the non-STGBB arm (Table 3). Myocardial infarction occurred in 134 (3.5%) and 178 (2.2%) hypertensives in the STGBB and non-STGBB arms, respectively. In the included RCTs, strokes were more likely to be non-fatal, occurring in 81 (2.1%) hypertensives in the STGBB arm, compared with 140 (1.7%) in the non-STGBB arm.
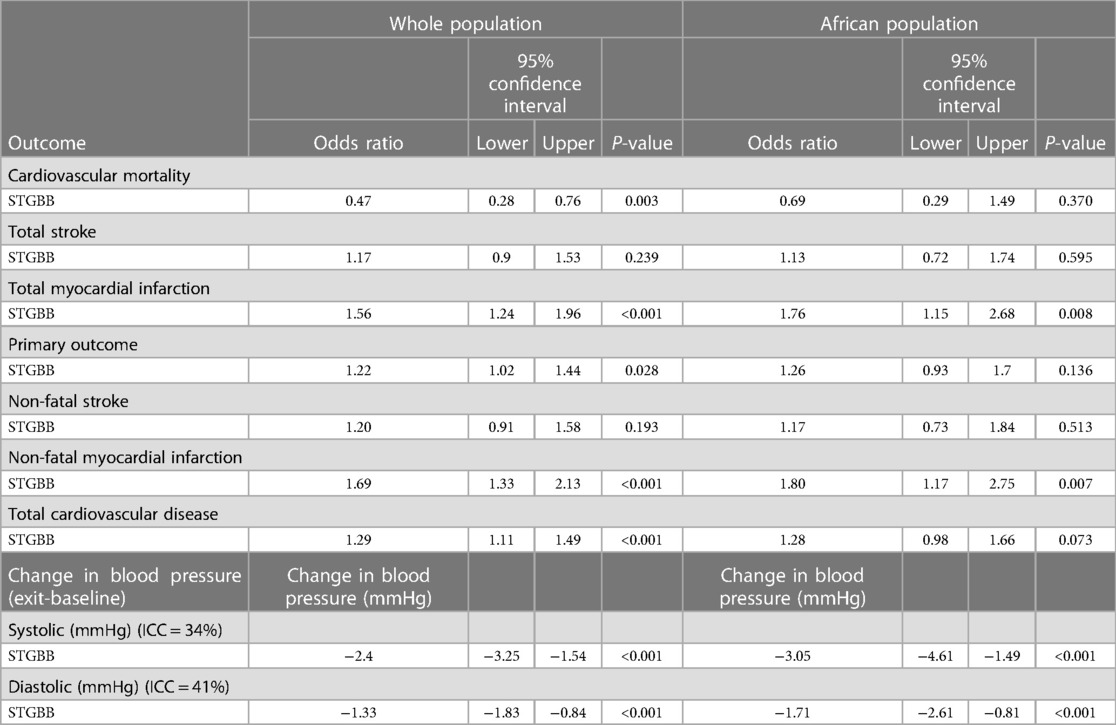
Hypertensives on STGBB were 1.2 times more likely to experience composite primary outcomes (95% CI: 1.02–1.44; P-value = 0.028) compared with those in the non-STGBB arm (Table 4). Myocardial infarctions were 1.8 times (95% CI: 1.15–2.68; P-value = 0.008) more likely to occur in hypertensives of African ancestry compared to the entire population (95% CI: 1.24–1.96; P-value < 0.001) on STGBB. After adjusting the odds ratios for confounders such as age, gender, and smoking (Table 5), there were no statistically significant differences in the risk of composite primary outcomes in the STGBB arm when comparing hypertensives of African ancestry and other racial groups.
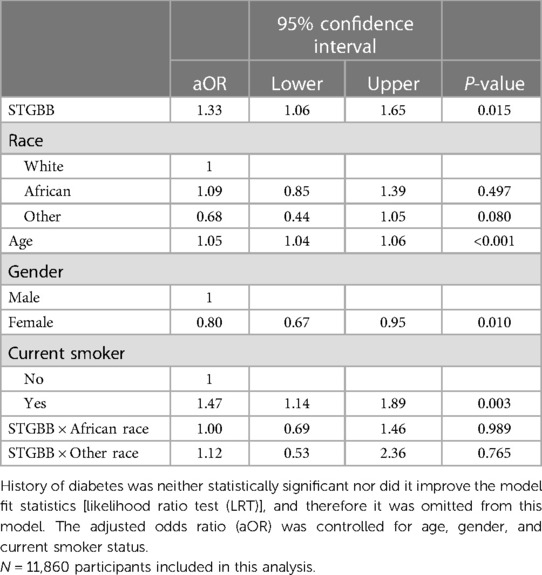
Table 5. Multivariable logistic regression model predicting the primary outcome: total cardiovascular mortality, total stroke, and total myocardial infarction in 11,860 patients included in the meta-analysis.
3.3 Efficacy of second- and third-generation beta-blockers in lowering the mean arterial pressure
Second- and third-generation BBs reduced the MAP in the whole population by 1.75 mmHg (95% CI: 1.16–2.33; P < 0.001) compared with 1.93 mmHg (95% CI: 0.86–3.00; P < 0.001) in hypertensives of African ancestry (Table 6). The multivariable generalised mixed effects model also demonstrated a statistically significant reduction in the MAP in hypertensives prescribed angiotensin-converting enzyme inhibitors (ACE-I), angiotensin II receptor blockers (ARB), calcium channel blockers, and diuretics. The highest reduction in the MAP was 3.88 mmHg (95% CI: 3.23–4.54; P < 0.001) and was associated with diuretic use. In hypertensives of African ancestry, diuretics decreased the MAP by 4.17 mmHg (95% CI: 2.78–5.50; P < 0.001). Despite a relatively smaller sample size in the African population, diuretics, ARBs, and STGBBs reduced the MAP more effectively in the African population than in the whole study population.
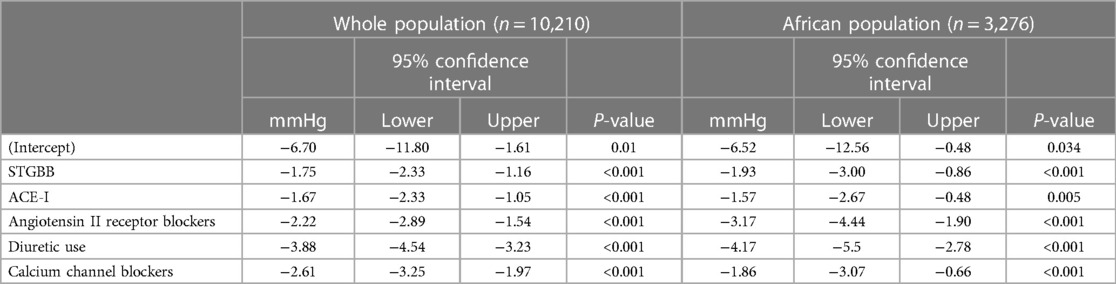
Table 6. Multivariable generalised mixed effects model with the respective RCT as a random effect (random intercept).
4 Discussion
In this IPD-MA, we evaluated the efficacy of STGBBs in reducing the risk of cardiovascular death, strokes, and MI in hypertensives of African ancestry compared with other racial groups on non-STGBBs. The composite primary outcome was higher in the STGBB arm (5.6%), compared with the 4.7% in the non-STGBB arm. In the entire hypertensive study population, including hypertensives of African ancestry, STGBBs were efficacious in reducing the risk of cardiovascular death. Although the trend was evident in African hypertensive, the estimate was not statistically significant. Furthermore, STGBBs had a greater MAP reduction in the African population.
Data supporting the recommendation to withdraw BBs as first-line therapy suggested that BBs failed to reduce the central aortic pressure and were associated with a greater risk of cardiovascular death or stroke (28–31). In this IPD-MA, we found that STGBBs reduced the MAP as efficiently as other antihypertensives in the African population and other racial groups included in the analysis. The findings from our meta-analysis indicate a need for prospective outcomes-driven RCTs to definitively examine the role of STGBBs in treating uncomplicated hypertension.
Beta-blockers prevent complications in patients with hypertension by lowering blood pressure and reducing cardiovascular events with an efficacy similar to other antihypertensives (30, 32–40). In this IPD-MA, we found that STGBBs significantly reduced the MAP in participants of African ancestry. However, the risk of MI was higher in hypertensives of African ancestry who were prescribed STGBB. A meta-analysis by Lindholm et al. published in 2005 evaluating whether BBs should remain first-line agents in the treatment of hypertension found that the relative risk of stroke was 26% higher in participants prescribed atenolol vs. other antihypertensive treatment (41). However, in this study, atenolol was not associated with an increased risk of stroke.
Hypertension is common in individuals of African ancestry and tends to follow a severe course associated with a higher rate of morbidity and mortality (14). The higher prevalence of hypertension in the African population compared with their counterparts has been attributed to biological factors, increased psychological stress, poor socioeconomic status, disparities in salt retention, and a higher rate of obesity among individuals of African ancestry (42, 43). Recent evidence suggests that there may be no racial disparities in the response to antihypertensive treatment (44).
Some of the trials that demonstrated higher adverse events in hypertensives treated with BBs include the Cardiovascular Morbidity and Mortality in the Losartan Intervention For Endpoint (LIFE) Reduction in Hypertension Trial and the Anglo-Scandinavian Cardiac Outcomes Trial—Blood Pressure Lowering Arm (ASCOT-BPLA). The LIFE trial randomised 9,222 hypertensives to atenolol or losartan. After a follow-up duration of 4.8 years, the rate of cardiovascular mortality was 10.6 per 1,000 patient-years of follow-up in the atenolol arm vs. 9.2 per 1,000 patient-years in the losartan arm (28). The stroke rate was also higher in the atenolol arm (14.5 vs. 10.8 per 1,000 patient-years of follow-up). In the ASCOT-BPLA, participants were randomly assigned to either atenolol plus a thiazide diuretic or amlodipine and perindopril. Strokes, cardiovascular events, and all-cause mortality rates were higher in participants randomised to atenolol and a thiazide diuretic (29). Although these studies highlight a higher risk of stroke in patients prescribed BBs, both RCTs had suboptimal dosing of atenolol.
In this IPD-MA, confounding was partially controlled for by excluding participants with previous cardiovascular events and adjusting the odds ratio in the multivariable regression model. For example, we relied on documented baseline clinical history and examination findings to identify high-risk hypertensives with previous MI, strokes, or CHF, requiring exclusion from the study. As such, the significant increase in the risk of MI in hypertensives could be accounted for by including undocumented high-risk hypertensives in the IPD-MA. Although reasonable attempts were made to minimise confounding, the STGBB group was still a higher risk group than the non-STGBB group. The patients in the STGBB group were on more antihypertensive medication than those in the non-STGBB group. This may explain the increased risk of cardiovascular outcomes associated with the STGBB group found in this study.
Individual patient data meta-analysis is considered the most reliable and robust method for obtaining evidence (45). Despite this, most authors prefer conducting systematic reviews and meta-analyses based on aggregated data from various research studies instead of requesting individual participant data from the data custodians. The major drawback of conducting IPD-MA is the unpredictable access to data. Despite receiving a response from 77% of the authors contacted, we could only access data from seven RCTs and eventually only analysed four. Some reasons cited in the literature restricting access to data include a lack of response from the authors or data custodians contacted, operational constraints, staff relocation, and lack of communication (46). Data-sharing policies vary with each country, and authors requesting access to data may be expected to apply for ethical clearance in multiple institutions prior to accessing data. The median time from the first request to accessing data to fully receiving the data can also be excessively long and may take up to 242 days (46). Realising that obtaining data for IPD-MA comes with many challenges, Ventresca et al. recommend requesting data through personal contact, offering incentives such as coauthorship and setting up a data-sharing agreement (47). Furthermore, consenting authors could also deposit de-identified data in a common data repository site. Data confidentiality breach and leakage are some of the key areas that need to be addressed before implementing such data repositories.
5 Limitations
This IPD-MA's chief limitation is the incomplete acquisition of IPD from previously conducted RCTs, leading to a smaller sample size. Although generalisability is improved, severe confounding was introduced through the surrogacy BB treatment, since BBs are traditionally prescribed in individuals with established or advanced cardiovascular risk factors. A higher proportion of ACE-I, CCB, ARB, and diuretics use in participants in the STGBB arm demonstrated this BB surrogacy phenomenon. Including the daily cumulative dose of each BB used in the analysis may have provided more insights into the added benefit of STGBBs compared to other BBs in reducing the MAP.
6 Conclusions
Second- and third-generation BBs effectively reduced the MAP in hypertensives of African ancestry as well as in other racial groups. Compared with other racial groups, the risk of stroke was not increased in hypertensives of African descent who were prescribed STGBBs. However, the risk of myocardial infarction was higher in hypertensives of African descent on STGBBs. This IPD-MA suggests that the cardiovascular outcomes associated with STGBB use in managing essential hypertension may differ according to ethnicity and generation of BB therapy.
Data availability statement
Data was obtained from the Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC), and requests to access the data should be directed towards the data repository. Further enquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The University of the Witwatersrand Human Research Ethics Committee (Medical), ethics clearance certificate number: W-CBP-211102-01. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the study analysed pre-existing datasets from various trials.
Author contributions
NT: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. RDN: Conceptualization, Supervision, Writing – review & editing, Methodology. SM: Conceptualization, Supervision, Writing – review & editing, Methodology.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article.
The authors declare that this study received funding from Merck KGaA. The funder was not involved in the study design, data collection, data analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgements
We would like to acknowledge the Biologic Specimen and Data Repository Information Coordinating Center, Vivli.org, and the National Institute of Diabetes and Digestive and Kidney Disease. This analysis would not be possible without their research input and data-sharing initiatives. We also thank Japie van Tonder, Xan Swart, and Cornelia van Graan for their role in compiling and conducting the analysis and manuscript writing; and Chiara Centonze, Ulrike Gottwald-Hostalek, and Anna Perminova for their invaluable inputs during the manuscript review process.
Conflict of interest
RDN and SM are Merck employees. NT has received consulting & speaker fees from Merck, Acino Health Care Group, Boehringer-Ingelheim, Boston Scientific, Eli Lilly, Novartis Pharmaceuticals, NovoNordisk, Pfizer, Phillips, Servier, and Takeda. NT has also received educational grants from Biotronik, Boston Scientific, Medtronic, and Vertice Health Care Group.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1280953/full#supplementary-material
Abbreviations
STGBB, second- or third-generation beta-blocker; SNS, sympathetic nervous system; LIFE, Losartan Intervention For Endpoint reduction in hypertension; ASCOT, Anglo-Scandinavian Cardiac Outcomes Trial; IPD, individual patient-level data; BioLINCC, Biologic Specimen and Data Repository Information Coordinating Center; IPD-MA, individual patient data meta-analysis; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RoB, risk of bias; GoF, goodness of fit; GLMM, generalised linear mixed effects models [also known as mixed models (MM), hierarchical or multi-level models, or random effects models].
References
1. Waal-Manning HJ. Hypertension: which beta-blocker? Drugs. (1976) 12:412–41. doi: 10.2165/00003495-197612060-00002
2. The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). 2018 practice guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC task force for the management of arterial hypertension: erratum. J Hypertens. (2019) 37(2):456. doi: 10.1097/HJH.0000000000002026
3. World Health Organization. Guideline for the pharmacological treatment of hypertension in adults [Internet]. Geneva: World Health Organization (2021). Available from: https://www.ncbi.nlm.nih.gov/books/NBK573631/ (Accessed January 24, 2023).
4. Jones NR, McCormack T, Constanti M, McManus RJ. Diagnosis and management of hypertension in adults: NICE guideline update 2019. Br J Gen Pract. (2020) 70(691):90–1. doi: 10.3399/bjgp20X708053
5. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens. (2020) 38(6):982–1004. doi: 10.1097/HJH.0000000000002453
6. Thomopoulos C, Bazoukis G, Tsioufis C, Mancia G. Beta-blockers in hypertension: overview and meta-analysis of randomised outcome trials. J Hypertens. (2020) 38(9):1669–81. doi: 10.1097/HJH.0000000000002523
7. Park IU, Taylor AL. Race and ethnicity in trials of antihypertensive therapy to prevent cardiovascular outcomes: a systematic review. Ann Fam Med. (2007) 5(5):444–52. doi: 10.1370/afm.708
8. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. (2003) 42(6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2
9. Stewart S, Wilkinson D, Hansen C, Vaghela V, Mvungi R, McMurray J, et al. Predominance of heart failure in the Heart of Soweto Study cohort: emerging challenges for urban African communities. Circulation. (2008) 118(23):2360–7. doi: 10.1161/CIRCULATIONAHA.108.786244
10. Ojji D, Stewart S, Ajayi S, Manmak M, Sliwa K. A predominance of hypertensive heart failure in the Abuja Heart Study cohort of urban Nigerians: a prospective clinical registry of 1515 de novo cases. Eur J Heart Fail. (2013) 15(8):835–42. doi: 10.1093/eurjhf/hft061
11. Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. (2017) 136(21):e393–423. doi: 10.1161/CIR.0000000000000534
12. Din-Dzietham R, Couper D, Evans G, Arnett DK, Jones DW. Arterial stiffness is greater in African Americans than in whites: evidence from the Forsyth County, North Carolina, ARIC cohort. Am J Hypertens. (2004) 17(4):304–13. doi: 10.1016/j.amjhyper.2003.12.004
13. Houghton JL, Smith VE, Strogatz DS, Henches NL, Breisblatt WM, Carr AA. Effect of African-American race and hypertensive left ventricular hypertrophy on coronary vascular reactivity and endothelial function. Hypertension. (1997) 29(3):706–14. doi: 10.1161/01.HYP.29.3.706
14. Lindhorst J, Alexander N, Blignaut J, Rayner B. Differences in hypertension between blacks and whites: an overview. Cardiovasc J Afr. (2007) 18(4):241–7.17940670
15. Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The department of veterans affairs cooperative study group on antihypertensive agents. N Engl J Med. (1993) 328(13):914–21. doi: 10.1056/NEJM199304013281303
16. Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA. (2015) 313(16):1657–65. doi: 10.1001/jama.2015.3656
17. Tierney JF, Vale C, Riley R, Smith CT, Stewart L, Clarke M, et al. Individual participant data (IPD) meta-analyses of randomised controlled trials: guidance on their use. PLoS Med. (2015) 12(7):e1001855. doi: 10.1371/journal.pmed.1001855
18. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
19. Tierney JF, Riley RD, Rydzewska LHM, Stewart LA. Running an IPD meta-analysis project: from developing the protocol to preparing data for meta-analysis. In: Riley RD, Tierney JF, Stewart LA, editors. Individual Participant Data Meta-Analysis: a Handbook for Healthcare Research. Wiley-Blackwell Publishing Ltd. (2021). p. 45–80. doi: 10.1002/9781119333784.ch4
20. Oliver E, Mayor F Jr., D'Ocon P. Beta-blockers: historical perspective and mechanisms of action. Rev Esp Cardiol (Engl Ed). (2019) 72(10):853–62. doi: 10.1016/j.recesp.2019.02.023
21. Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing (2022).
22. Wickham HAM, Bryan J, Chang W, McGowan LD, François R, Grolemund G, et al. Welcome to the Tidyverse. J Open Source Softw. (2019) 4(43):1686. doi: 10.21105/joss.01686
23. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. (2015) 67(1):1–48. doi: 10.18637/jss.v067.i01
24. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. (1991) 265(24):3255–64. doi: 10.1001/jama.1991.03460240051027
25. Group AS, Cushman WC, Evans GW, Byington RP, Goff DC Jr., Grimm RH Jr., et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. (2010) 362(17):1575–85. doi: 10.1056/NEJMoa1001286
26. SPRINT Research Group, Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A randomised trial of intensive versus standard blood-pressure control. N Engl J Med. (2015) 373(22):2103–16. doi: 10.1056/NEJMoa1511939
27. Wright JT Jr., Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. (2002) 288(19):2421–31. doi: 10.1001/jama.288.19.2421
28. Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. (2002) 359(9311):995–1003. doi: 10.1016/S0140-6736(02)08089-3
29. Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. (2005) 366(9489):895–906. doi: 10.1016/S0140-6736(05)67185-1
30. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. (2016) 387(10022):957–67. doi: 10.1016/S0140-6736(15)01225-8
31. Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. (2006) 113(9):1213–25. doi: 10.1161/CIRCULATIONAHA.105.595496
32. Wilhelmsen L, Berglund G, Elmfeldt D, Fitzsimons T, Holzgreve H, Hosie J, et al. Beta-blockers versus diuretics in hypertensive men: main results from the HAPPHY trial. J Hypertens. (1987) 5(5):561–72. doi: 10.1097/00004872-198710000-00009
33. Wikstrand J, Warnold I, Tuomilehto J, Olsson G, Barber HJ, Eliasson K, et al. Metoprolol versus thiazide diuretics in hypertension. Morbidity results from the MAPHY study. Hypertension. (1991) 17(4):579–88. doi: 10.1161/01.HYP.17.4.579
34. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 4. Effects of various classes of antihypertensive drugs–overview and meta-analyses. J Hypertens. (2015) 33(2):195–211. doi: 10.1097/HJH.0000000000000447
35. Pepine CJ, Handberg EM, Cooper-DeHoff RM, Marks RG, Kowey P, Messerli FH, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomised controlled trial. JAMA. (2003) 290(21):2805–16. doi: 10.1001/jama.290.21.2805
36. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. (2008) 359(15):1577–89. doi: 10.1056/NEJMoa0806470
37. Hansson L, Lindholm LH, Ekbom T, Dahlof B, Lanke J, Schersten B, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish trial in old patients with hypertension-2 study. Lancet. (1999) 354(9192):1751–6. doi: 10.1016/S0140-6736(99)10327-1
38. Blood Pressure Lowering Treatment Trialists’ Collaboration, Turnbull F, Neal B, Ninomiya T, Algert C, Arima H, et al. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. Br Med J. (2008) 336(7653):1121–3. doi: 10.1136/bmj.39548.738368.BE
39. Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. (2014) 384(9943):591–8. doi: 10.1016/S0140-6736(14)61212-5
40. Medical Research Council Working Party. MRC trial of treatment of mild hypertension: principal results. Medical Research Council Working Party. Br Med J (Clin Res Ed). (1985) 291(6488):97–104. doi: 10.1136/bmj.291.6488.97
41. Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet. (2005) 366(9496):1545–53. doi: 10.1016/S0140-6736(05)67573-3
42. Spence JD, Rayner BL. Hypertension in blacks: individualised therapy based on renin/aldosterone phenotyping. Hypertension. (2018) 72(2):263–9. doi: 10.1161/HYPERTENSIONAHA.118.11064
43. Lackland DT. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci. (2014) 348(2):135–8. doi: 10.1097/MAJ.0000000000000308
44. Brewster LM, Seedat YK. Why do hypertensive patients of African ancestry respond better to calcium blockers and diuretics than to ACE inhibitors and beta-adrenergic blockers? A systematic review. BMC Med. (2013) 11:141. doi: 10.1186/1741-7015-11-141
45. Stewart LA, Clarke MJ. Practical methodology of meta-analyses (overviews) using updated individual patient data. Cochrane Working Group. Stat Med. (1995) 14(19):2057–79. doi: 10.1002/sim.4780141902
46. Scutt P, Woodhouse LJ, Montgomery AA, Bath PM. Data sharing: experience of accessing individual patient data from completed randomised controlled trials in vascular and cognitive medicine. BMJ Open. (2020) 10(9):e038765. doi: 10.1136/bmjopen-2020-038765
Keywords: hypertension, beta-blocker, antihypertensive, individual patient data meta-analysis, cardiovascular outcomes, Africa, blood pressure (BP)
Citation: Tsabedze N, Naicker RD and Mrabeti S (2024) Efficacy of beta-blockers on blood pressure control and morbidity and mortality endpoints in hypertensives of African ancestry: an individual patient data meta-analysis. Front. Cardiovasc. Med. 10:1280953. doi: 10.3389/fcvm.2023.1280953
Received: 21 August 2023; Accepted: 11 December 2023;
Published: 23 January 2024.
Edited by:
Mahdi Garelnabi, University of Massachusetts Lowell, United StatesReviewed by:
Maciej Siński, Medical University of Warsaw, PolandMarek Klocek, Jagiellonian University Medical College, Poland
© 2024 Tsabedze, Naicker and Mrabeti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nqoba Tsabedze bnFvYmEudHNhYmVkemVAd2l0cy5hYy56YQ==
 Nqoba Tsabedze
Nqoba Tsabedze R. Darshni Naicker
R. Darshni Naicker Sanaa Mrabeti3
Sanaa Mrabeti3