
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 29 November 2023
Sec. Coronary Artery Disease
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1275710
This article is part of the Research Topic Insights in Coronary Artery Disease: 2023 View all 9 articles
 Feng Dai1,†
Feng Dai1,† Xianzhi Xu2,†
Xianzhi Xu2,† Chunxue Zhou1
Chunxue Zhou1 Cheng Li1
Cheng Li1 Zhaoxuan Tian1
Zhaoxuan Tian1 Zhaokai Wang1
Zhaokai Wang1 Shuping Yang3
Shuping Yang3 Gege Liao1
Gege Liao1 Xiangxiang Shi3
Xiangxiang Shi3 Lili Wang1
Lili Wang1 Dongye Li1
Dongye Li1 Xiancun Hou4
Xiancun Hou4 Junhong Chen1*
Junhong Chen1* Tongda Xu1*
Tongda Xu1*
Background: Acute ST-segment elevation myocardial infarction (STEMI) patients after primary PCI were readmitted for revascularization due to non-culprit lesion (NCL) progression.
Objective: To develop and validate a nomogram that can accurately predict the likelihood of NCL progression revascularization in STEMI patients following primary PCI.
Methods: The study enrolled 1,612 STEMI patients after primary PCI in our hospital from June 2009 to June 2018. Patients were randomly divided into training and validation sets in a 7:3 ratio. The independent risk factors were determined by LASSO regression and multivariable logistic regression analysis. Multivariate logistic regression analysis was utilized to develop a nomogram, which was then evaluated for its performance using the concordance statistics, calibration plots, and decision curve analysis (DCA).
Results: The nomogram was composed of five predictors, including age (OR: 1.007 95% CI: 1.005–1.009, P < 0.001), body mass index (OR: 1.476, 95% CI: 1.363–1.600, P < 0.001), triglyceride and glucose index (OR: 1.050, 95% CI: 1.022–1.079, P < 0.001), Killip classification (OR: 1.594, 95% CI: 1.140–2.229, P = 0.006), and serum creatinine (OR: 1.007, 95% CI: 1.005–1.009, P < 0.001). Both the training and validation groups accurately predicted the occurrence of NCL progression revascularization (The area under the receiver operating characteristic curve values, 0.901 and 0.857). The calibration plots indicated an excellent agreement between prediction and observation in both sets. Furthermore, the DCA demonstrated that the model exhibited clinical efficacy.
Conclusion: A convenient and accurate nomogram was developed and validated for predicting the occurrence of NCL progression revascularization in STEMI patients after primary PCI.
Coronary artery disease causes 8.9 million patient deaths and 264 million workforce losses annually (1). Among the various manifestations of coronary artery disease, ST-elevation myocardial infarction (STEMI) is the most severe type characterized by rapid onset, progression, and high morbidity and mortality (2). Fortunately, the availability of thrombolytic therapy and primary percutaneous coronary intervention (PCI) has significantly improved STEMI patients' prognosis. Although the utilization of drug-eluting stents has contributed to reducing the occurrences of in-stent restenosis (3). Revascularization after PCI remains a common clinical problem. The rate of revascularization after PCI was 12% at 1 year, 15% at 2 years, 20% at 4 years, and 32.3% at 5 years (4, 5). The in-stent restenosis is not the sole cause of repeat revascularization. One study highlighted that non-culprit lesion (NCL) progression accounts for over half of all revascularizations (4). NCL progression refers to developing or worsening atherosclerotic lesions outside the culprit lesions that cause ischemia, angina, and myocardial infarction. Such progression can lead to recurrent ischemia, angina, myocardial infarction, or death. Nonetheless, not all non-culprit lesions necessitate revascularization. Hence, predicting the likelihood of revascularization for NCL progression in STEMI patients is crucial.
Several studies have examined risk factors associated with repeat revascularization, such as fasting glucose, peak cTnI levels, and complex lesions (6, 7). Compared to conventional statistical models, the nomogram provides several advantages such as validation of effectiveness, visual representation, and personalized risk assessment (8). The nomogram effectively quantifies risk factors and synthesizes them into a predictive score to inform clinical decisions.
However, there are no studies for developing and validating a nomogram specifically for NCL progression revascularization. The study aims to build and validate a nomogram to predict the likelihood of NCL progression revascularization in STEMI patients after complete revascularization.
In this retrospective study, a nomogram was established to screen high-risk groups based on our clinical results, which may provide guidance for clinicians and assesses STEMI patients' prognosis.
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (XYFY2022-KL375-01). Since the study was retrospective, the committee waived the requirement for written informed consent. Personal private information was removed prior to the data is analyzed.
7,277 patients who underwent primary PCI for STEMI between 2009 and 2018 were initially screened in the affiliated hospital of Xuzhou medical university. Patients showing multivessel disease at the first CAG were included in the study only if NCL with diameter stenosis greater than 70% were treated. Secondly, 2,469 patients combined of at least one NCL (diameter stenosis between 50% and 70%) were readmitted to the hospital within 5 years after primary PCI for clinical symptoms such as chest pain and chest tightness. These patients underwent review coronary angiography (CAG). Finally, 1,612 patients were included in the study according to the exclusion and inclusion criteria.
Inclusion criteria: (1) diagnosis of acute ST-segment elevation myocardial infarction (9), (2) successful drug-eluting stent implantation procedure, (3) immediate complete revascularization or elective completion of complete revascularization within 2 months (10), (4) no in-hospital cardiovascular events such as cardiogenic shock, recurrent myocardial infarction, or death, (5) follow-up CAG within 5 years after the first PCI, (6) no anticoagulant or antiplatelet contraindications, (7) over 18 years old, (8). Combination of at least one NCL (diameter stenosis between 50% and 70%).
Exclusion criteria: (1) incomplete clinical data (n=576), (2) follow-up CAG showing in-stent restenosis or recommendation for coronary artery bypass graft (n=258), (3) severe hepatic insufficiency or renal insufficiency (n=23).
Patients undergoing the procedure were routinely administered stress dose medications (300 mg of aspirin, 600 mg of clopidogrel, or 180 mg of ticagrelor). To ensure proper anticoagulation, 3,000 IU of unfractionated heparin and 200 ug of nitroglycerin were administered prior to CAG. The administration of heparin was initiated at 100 IU/kg before the operation, with the option of adding 1,000 IU/h during the procedure. Additionally, intraoperative medications such as nitroprusside, nitroglycerin, tirofiban, and other drugs aimed at improving coronary ischemia were selectively administered based on the patient's condition.
Following the operation, medications were prescribed according to established guidelines. These included: (1) a dual antiplatelet regimen involving aspirin (100 mg once daily) combined with either clopidogrel (75 mg once daily) or ticagrelor (90 mg twice daily); (2) statin; (3) angiotensin converting enzyme inhibitor or angiotensin receptor blocker; and (4) beta-blocker.
Senior cardiologists performed both CAG and PCI procedures. Two skilled clinicians thoroughly analyzed all CAG images.
The clinical endpoint was that STEMI patient readmitted with ischemic symptoms found to have NCL progression (diameter stenosis progresses from between 50%–70% to >70%) requiring revascularization within 5 years after PCI. NCL was defined as a vessel with stenosis between 50% and 70% at the first CAG (7). All patients underwent primary PCI of the culprit lesions successfully. Patients showing multivessel disease at the first CAG were included in the study only if they had completed complete revascularization or selective complete revascularization. Complete revascularization was defined as successful revascularization of all coronary artery lesions or segments ≥1.5 mm in diameter with ≥70% diameter stenosis regardless of their functional significance (11). Related definitions included the following: Calcified lesions were defined as the presence of moderate or severe calcification in the vessel wall (12); bifurcation lesions were defined as stenosis adjacent to and/or involving the opening of a major branch (13); ostial lesions were defined as lesions within 3 mm of the origin of the major coronary arteries (14); angular distortion lesions were defined as angles of at least one major branch of the coronary artery ≥45° along the direction of the main coronary artery (15).
All patient clinical features (including demographics, previous history, laboratory indices, two-times coronary angiography images, and medication use at discharge) were collected. Each patient's blood was collected within 24 h of admission, and the central laboratory tested all laboratory parameters before operation. Data collection during PCI consisted of door-to-balloon time, calcified lesions, bifurcation lesions, open and angular distortion lesions, and culprit lesions. Echocardiography measures the left ventricular ejection fraction.
Categorical variables were displayed as counts and percentages and then compared using either the χ2 or Fisher exact test. Meanwhile, continuous variables were presented as mean ± standard deviation. If the variables adhered to a normal distribution pattern, they were compared using the t-test. However, if the variables exhibited non-normal distribution, they were compared using the Mann–Whitney U-test. Univariate logistic regression was analyzed to filter significant variables in the training cohort. This part was done using SPSS version 25.0 (SPSS Inc., Chicago, IL, USA). LASSO regression was used to screen out non-zero coefficient characteristics, and multivariate logistic regression backward stepwise regression was used to analyze the independent predictors. The restricted cubic spline was performed to investigate the linear relationships between continuous variables and NCL progression revascularization. A nomogram was developed using variables with P < 0.05 based on the result of the multivariate logistic regression. The discrimination capability of the model was gauged by the concordance index, which is equal to the area under the receiver operating characteristics curve (AUC-ROC). This metric was used to assess the effectiveness of the nomogram's discrimination capacity. In order to determine the level of accuracy of the calibration, Hosmer-Lemeshow tests and calibration plots were carried out. Clinical efficacy was evaluated using decision curve analysis. The statistical threshold for determining significance was established at a level of P < 0.05 for the two-sided test. This part was analyzed using R Studio version 4.1.3 (https://cran.r-project.org).
The study flow chart is shown in Figure 1. There are 1,612 STEMI patients were enrolled based on inclusion and exclusion criteria. Patients were randomly divided into training and validation sets in a 7:3 ratio. Among them, 14.8% (163/1,097) of patients in the training set underwent repeat PCI due to NCL progression, similar to 14.3% (74/515) of patients in the validation set. Table 1 presents a comprehensive sight of the baseline characteristics of the two sets. Table 2 displays the results of the univariate logistic regression. Univariate logistic regression analysis showed that the following factors were consistently associated with NCL revascularization in STEMI patients following primary PCI: age (OR: 1.09, 95% CI: 1.07–1.11, P < 0.001), body mass index (BMI) (OR: 1.55, 95% CI: 1.44–1.67, P < 0.001), triglyceride and glucose index (OR: 1.03, 95% CI: 1.01–1.05, P = 0.005), Killip classification (OR: 1.76, 95% CI: 1.31–2.35, P < 0.001), Hypertension (OR: 0.62, 95% CI: 0.44–0.86, P = 0.005), serum creatinine (SCr) (OR: 1.01, 95% CI: 1.00–1.01, P < 0.001), Lipoprotein-a (OR: 1.00, 95% CI: 1.00–1.00, P < 0.001), creatine kinase MB (OR: 1.00, 95% CI: 1.00–1.01, P = 0.001), total cholesterol (OR: 2.59, 95% CI: 2.18–3.08, P < 0.001). LASSO regression analysis showed that age, BMI, triglyceride and glucose index, Killip classification, and SCr were the more important predictors with non-zero coefficient, as shown in Figure 2. Multivariate logistic regression analysis identified in Table 3 that age (OR: 1.007, 95% CI: 1.005–1.009, P < 0.001), BMI (OR: 1.476, 95% CI: 1.363–1.600, P < 0.001), triglyceride and glucose index (OR: 1.050, 95% CI: 1.022–1.079, P < 0.001), Killip classification (OR: 1.594, 95% CI: 1.140–2.229, P = 0.006), and SCr (OR: 1.007, 95% CI: 1.005–1.009, P < 0.001). A nomogram was built and displayed in Figure 3. A point value is projected at the top of the nomogram for each independent predictor. This process helps obtain a score ranging between 0 and 100. The scores of all variables are added to get a total score, which is projected down to a vertical line on the “Risk” line, indicating the risk of NCL revascularization. A higher overall score indicates a higher likelihood of NCL revascularization. Consequently, the model could visually predict the occurrence of NCL progression revascularization.
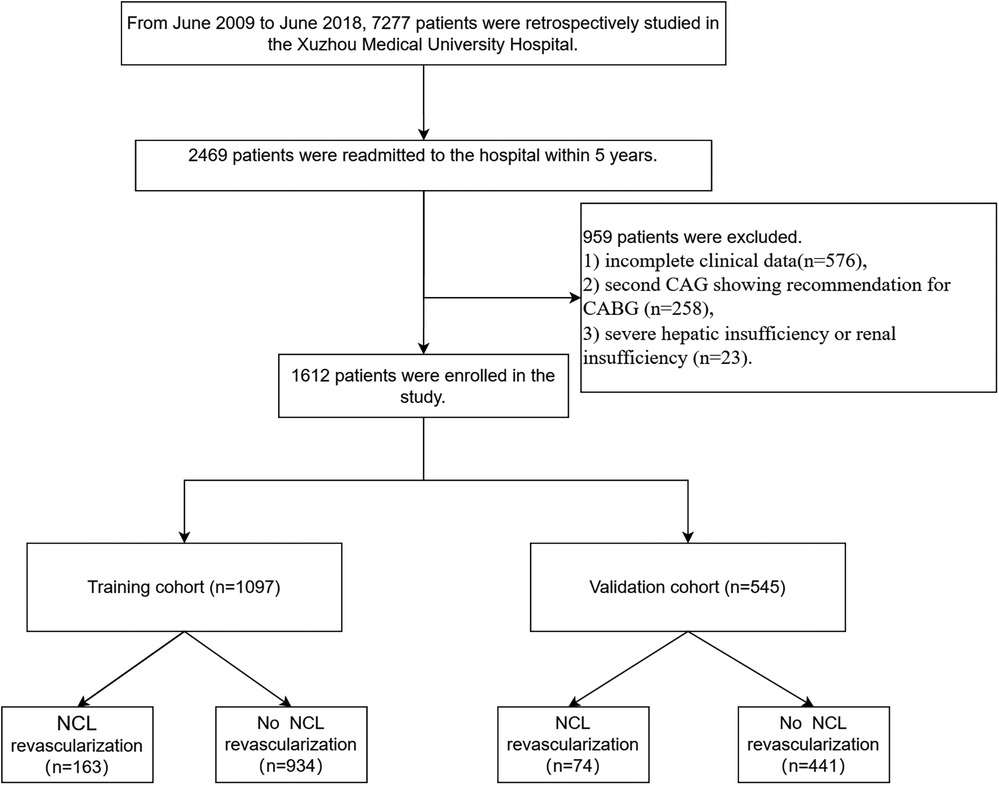
Figure 1. The study flowchart for developing and validating nomograms. CAG, coronary angiography; CABG, coronary artery bypass graft.
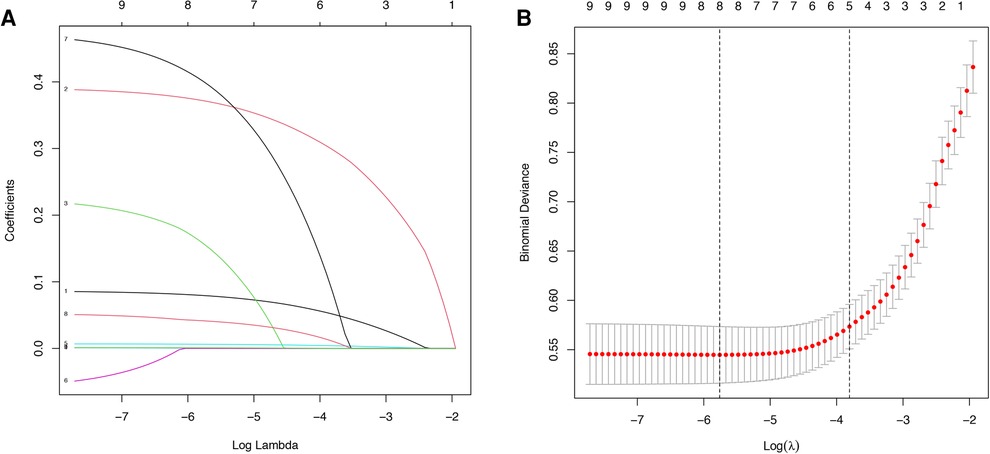
Figure 2. Variable screening based on lasso regression. (A) Characterization of the variation of variable coefficients; (B) The process of selecting the optimal value of the parameter λ in the Lasso regression model by the cross-validation method.
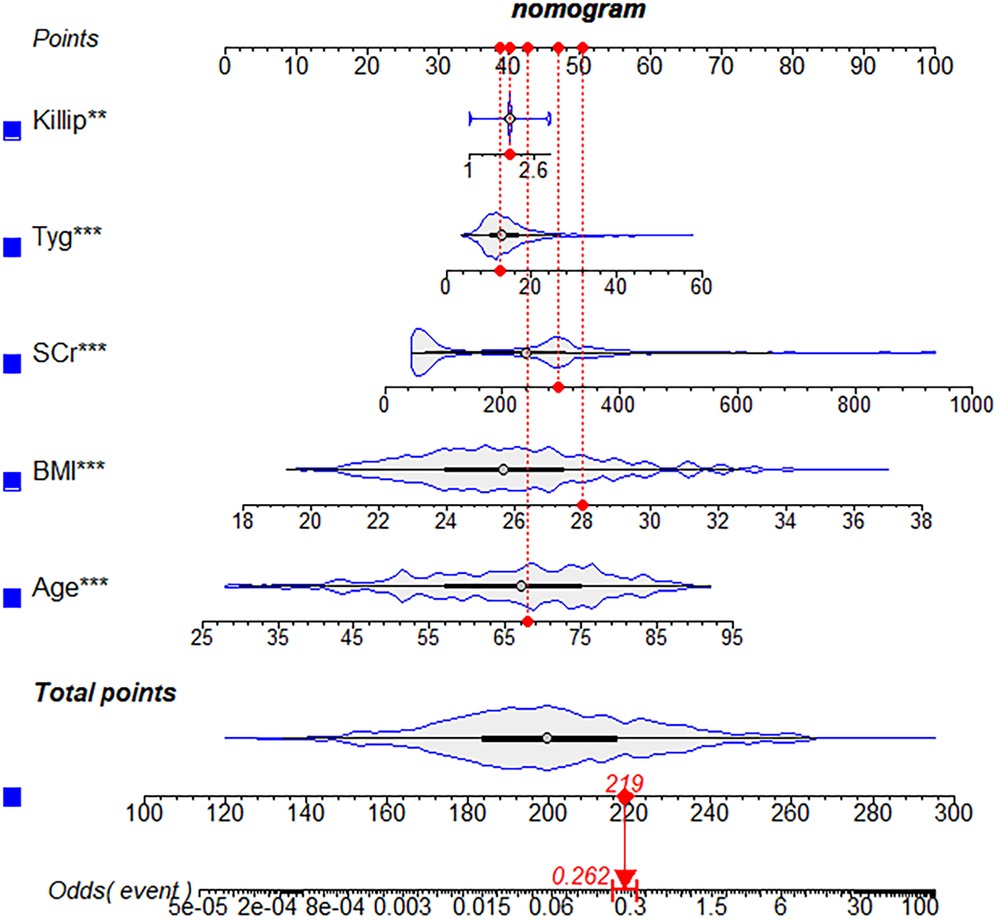
Figure 3. A nomogram for predicting the possibility of revascularization for non-culprit lesions progression. BMI, body mass index; SCr, serum creatinine.
The AUC-ROC for the nomogram and validation sets (Figures 4A,B) was calculated to be 0.9014 (95% CI: 0.8795–0.9233) and 0.8574 (95% CI: 0.8103–0.9046), showing a good discrimination ability of the nomogram. The Hosmer-Lemeshow test produced χ2 values of the nomogram (7.3528, P = 0.4991) and the validation group (2.961, P = 0.9368), respectively. These results attest to the model's excellent calibration capability. Additionally, the calibration curve demonstrates high consistency between the predicted and actual probabilities (Figures 5A,B).
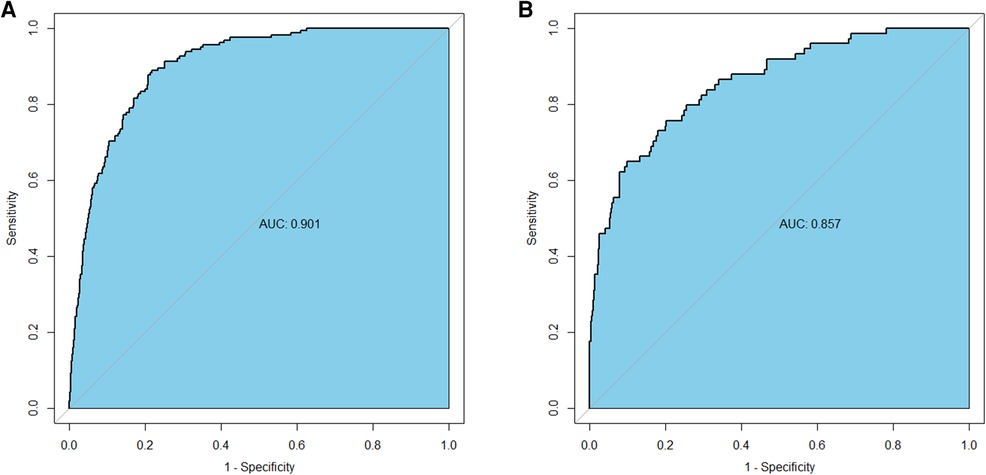
Figure 4. Receiver operating characteristics curve of the nomogram in the training group (A) and the validation group (B).
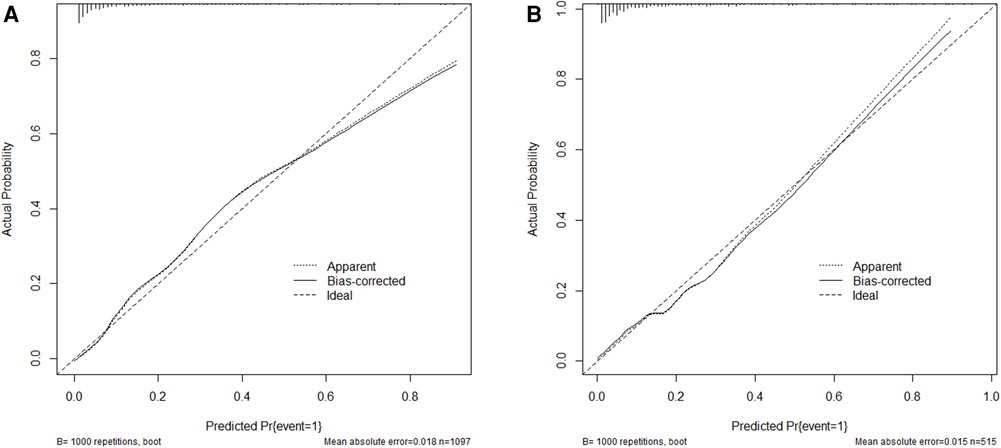
Figure 5. Calibration curve for the training group (A) and the validation group (B) the horizontal axis denotes the overall predicted probability of revascularization in STEMI patients after percutaneous coronary intervention due to non-culprit lesions, and the vertical axis displays the actual probability.
The clinical effectiveness of the model was evaluated, which is depicted in Figures 6A,B. The horizontal line indicates a net benefit of 0, which occurs when no intervention is performed and all samples are negative (Pi < Pt). Conversely, the green line corresponds to a scenario where all interventions are applied, and all samples are positive. The DCA demonstrates that the nomogram can achieve a net benefit over a wide range of threshold probabilities, indicating our approach's clinical utility.
With the aging of the population, there has been an annual increase in the incidence of acute ST-segment elevation myocardial infarction (STEMI). Fortunately, the prognosis for STEMI patients has improved with the widespread availability of percutaneous coronary intervention (PCI). Nevertheless, STEMI patients who have undergone PCI are still at risk of readmission due to in-stent restenosis or the non-culprit lesion (NCL) progression. The use of drug-eluting stents reduces incidences of in-stent restenosis as compared to bare metal stents. However, the application of drug-eluting stents has not demonstrated a commensurate improvement in the rate of revascularization. In two randomized controlled trials (16, 17), there was no difference between bare metal stent and drug-eluting stent applications for revascularization due to NCL progression in acute myocardial infarction (AMI) patients. Notably, numerous clinical trials (18–20) revealed that the rate of revascularization associated with NCL ranges from approximately 11.6%–19%, accounting for 42%–57% of all repeat procedures during the five years after PCI. It has been observed that while there have been several studies focusing on the progression of NCL, the repeated PCI for NCL progression has not received much attention. The study focuses on predicting repeat PCI due to NCL. One study of 480 patients identified fasting glucose and creatinine as independent risk factors for NCL progression (6), while another study of 492 patients suggested that several chronic stress and inflammation-related factors such as serum catecholamines, and C-reactive protein, as well as complex lesion rates, were related to the progression of NCL (7). We had a larger sample size (n = 1,612 vs. 480) and more complete clinical data (including angiographic, laboratory, and follow-up data) than previous studies, which increased the statistical power and generalizability of our results. Compared with traditional independent risk factors, nomograms could visualize the effect of predictors on NCL revascularization, which is a user-friendly tool to help clinicians and patients estimate the individualized risks and benefits of NCL PCI. Unlike previous studies that did not validate independent risk factors, we evaluated the validity of our model using ROC curve, calibration curve, and decision curve analyses both in the training and validation sets. The results showed that our model had good discrimination, calibration, and clinical utility. Therefore, the nomogram was useful and meaningful to predict NCL progression revascularization in STEMI patients who have undergone primary PCI.
To determine independent risk factors, a multivariate logistic regression analysis was performed. The variables analyzed included age, body mass index (BMI), triglycerides and glucose index (TyG), Killip classification, and serum creatinine (SCr). Furthermore, these five independent risk factors were integrated to build a nomogram to predict the revascularization for the progression of NCL in STEMI patients after PCI.
In the baseline characteristics, the population's age in the training and validation sets was 67 (57, 75) and 66 (56, 75) years. Notably, multivariate logistic regression results displayed that the likelihood of revascularization for NCL progression increased with advancing age. Based on statistical data, it is evident that the aging of the population has contributed to a steady rise in the occurrence of STEMI among older adults over the years. Currently, more than one-third of STEMI patients are over the age of 75 (21). In non-culprit lesions, plaque burden increases with age (22). Patients are mostly readmitted from acute coronary syndromes. In most acute coronary syndromes, thrombotic occlusion due to plaque rupture is considered a major pathological process (23). These plaques are generally characterized by a large plaque burden rich in lipid content. Unplanned revascularization is significantly more likely to occur in patients with large lipid plaques (24). The visceral and intramuscular fat ratio increases with age, reaching a peak between 60 and 75 years (25).
BMI is a valid indicator of obesity. According to the World Health Organization reported, worldwide obesity rates have almost tripled since 1975 (26). The risk ratios for repeat revascularization augment progressively with BMI increase, in other words, underweight patients have lower risk and severely obese patients possess higher risk (27). The mechanisms underlying the high risk of revascularization for NCL associated with high BMI are unclear. However, one potential explanation may be high BMI are associated with the impaired endothelium-dependent function of microvascular coronary arteries (28).
The TyG index is calculated from fasting triglycerides and blood glucose. A meta-analysis that included 5,731,29 patients showed that a higher TyG index may be independently associated with a higher incidence of atherosclerotic cardiovascular disease (29). TyG index is also a reliable alternative biomarker for assessing insulin resistance (30). Insulin resistance is a condition in which body tissues become resistant to insulin, leading to disturbances in lipid and glucose metabolism (31). These metabolic disturbances promote endothelial dysfunction, cardiovascular remodeling, oxidative stress, inflammatory factor release, and exacerbated blood pressure elevation, all of which may contribute to NCL revascularization after PCI (32, 33).
Killip classification is a simple clinical tool proposed by Killip et al. (34) in 1979 to quickly and effectively evaluate cardiac function. One study (35) found that Killip classification was associated with adverse cardiovascular events, including the progression of NCL. Another study (36) found that patients classified as a higher Killip classification tend to have more severe coronary artery injuries, which could eventually increase the likelihood of NCL progression. The coronary vascular endothelium in patients with a higher Killip classification is damaged by activation of the renin-angiotensin-aldosterone system and inflammatory storms (37, 38). These damages may be the cause of repeated PCI for NCL. Meanwhile, DeGeare et al. (39) reported that patients with high Killip classification were more susceptible to renal impairment after PCI. SCr can be a good indicator of kidney function. The prevalence of renal impairment increases with age. Severe renal impairment was reported that in 7% of patients aged 70%–80% and 11% of patients aged 80 years or older (40). Miyagi et al. (41) found a significant correlation between an increased percentage of lipid volume and decreased percentage of fibrous volume in NCL of patients with renal insufficiency. Furthermore, Hayano et al. (42) found that moderate chronic kidney disease (CKD) patients had more lipid and less fibrous volume in NCL. It has also been found that after standard lipid-lowering therapy after PCI in patients with CKD), lipid plaque regression occurred in CKD stage 1–2, while lipid plaque increase occurred in CKD stage 3–5. Fibrous plaques were also increased in CKD stage 4–5. People with CKD frequently present with obesity, hyperlipidemia, hyperglycemia, and hypertension, all of which are risk factors that make them susceptible to atherosclerosis development. At the same time, chronic inflammation and stress can lead to maladaptive repair responses that destabilize plaque. Unstable coronary atheromatous plaque rupture often leads to downstream coronary artery obstruction (43). Besides, SCr concentrations are thought to be associated with oxidative stress, endothelial dysfunction, and more progressive atherosclerosis (44, 45). These biological abnormalities may also contribute to the risk of NCL revascularization.
Progression of NCL is responsible for more than half of the causes of revascularization after PCI in STEMI patients, but not all NCLs require revascularization. In this regard, identifying high-risk groups is critical. The nomogram in the study was constructed using 5 variables that were easy to obtain at the time of patient admission. The model can accurately and reliably assess the risk of NCL revascularization in STEMI patients after PCI. In clinical practice, clinicians can use the nomogram can be used to quantify the weighting of risk factors to obtain a total score based on the patient's condition. The total score could be used to locate the corresponding 5-year probability of NCL revascularization on the nomogram scale. Further, clinicians should use the probability of NCL revascularization to guide the management of NCL in patients with STEMI. For example, if the probability is high (>50%), clinicians may choose to intensify medical treatment and follow-up of NCL to avoid possible intervention. If the probability is very low (<10%), it can reduce the patient's concern and psychological burden. If the probability is intermediate (10%–50%), clinicians can discuss the importance of medical therapy follow-up with patients, and make a joint decision based on the patient's preferences and clinical situation. Therefore, the tool is useful for clinicians to identify and target high-risk groups as early as possible.
Limitations of the study should be acknowledged. Firstly, this is a retrospective study. Not all STEMI patients who received PCI at our hospital were included in the study, as some patients may choose to seek consultation elsewhere when NCL progression occurred again. This situation may have resulted in some bias in the study's findings. Secondly, although the study incorporated common clinical indicators, there were certain factors that were not taken into consideration. For instance, the research did not account for serum catecholamines, a risk factor for NCL progression. This omission may have implications for the interpretation of the study's findings. Thirdly, our study involved a retrospective analysis of readmitted patients who underwent NCL PCI. Therefore, our focus was on NCL revascularization as the primary endpoint, which is a common and clinically relevant outcome in patients with STEMI. Other endpoints, such as death, acute myocardial infarction, and culprit revascularization, were also important and could potentially impact the outcome of NCL PCI. However, we did not include them in this study. In terms of adjudicating endpoint events, we followed the definitions and methods documented in the hospital records. We acknowledge that this reliance on retrospective data may introduce inherent bias, a limitation inherent in this type of study. Fourthly, even when NCL progression occurs, patients do not tend to seek immediate medical care. Therefore, the time of readmission is not necessarily the time of NCL progression. We performed logistic regression rather than Cox regression for the detection of predictors of 5-year NCL-related revascularization because we were interested in the binary outcome of whether or not the revascularization occurred within 5 years, not in the time to revascularization. However, we also recognize that time to revascularization and revascularization should be considered together, but as a retrospective study, the absence of a rigorous follow-up mechanism is a major limitation. Finally, although the study had external validation, the data analyzed were collected from different periods within a single hospital. The nomogram's clinical value should be evaluated further using multicenter and larger sample sizes in future studies.
A convenient and accurate nomogram was developed and validated using five factors for predicting the revascularization due to NCL progression in STEMI patients after primary PCI. The nomogram enables clinicians to make appropriate disease management decisions by assessing the risk of NCL progression revascularization for STEMI patients after primary PCI.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The Ethics Committee of the Affiliated Hospital of Xuzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the study was retrospective, thus the committee waived the requirement for written informed consent. Personal private information was removed prior to the data is analyzed.
FD: Conceptualization, Writing – original draft, Writing – review & editing, Methodology. XX: Writing – original draft, Writing – review & editing. CZ: Data curation, Writing – review & editing. CL: Data curation, Writing – review & editing. ZT: Data curation, Writing – review & editing. ZW: Visualization, Writing – review & editing. SY: Data curation, Writing – review & editing, Supervision. GL: Writing – review & editing. XS: Writing – review & editing. LW: Writing – review & editing. DL: Writing – review & editing. XH: Writing – review & editing. JC: Funding acquisition, Project administration, Writing – review & editing. TX: Funding acquisition, Project administration, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
The study was funded by the Jiangsu Traditional Chinese Medicine Science and Technology Development Plan Project (Grant number: YB201988), Jiangsu Provincial Science and Technology Department Social Development Fund (Grant number: BE2019639), Jiangsu Provincial Health Commission Project Fund (Grant number: M2020015), Research and Practice Innovation Plan for Postgraduates in General Colleges and Universities in Jiangsu Province (Grant number: SJCX22_1267), and Xuzhou innovative plan to promote science technology (Grant number: KC21192).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ralapanawa U, Sivakanesan R. Epidemiology and the magnitude of coronary artery disease and acute coronary syndrome: a narrative review. J Epidemiol Glob Health. (2021) 11(2):169–77. doi: 10.2991/jegh.k.201217.001
2. Thrane PG, Olesen KKW, Thim T, Gyldenkerne C, Mortensen MB, Kristensen SD, et al. Mortality trends after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. J Am Coll Cardiol. (2023) 82(10):999–1010. doi: 10.1016/j.jacc.2023.06.025
3. Ferenc M, Buettner HJ, Gick M, Comberg T, Rothe J, Khoury F, et al. Clinical outcome after percutaneous treatment of de novo coronary bifurcation lesions using first or second generation of drug-eluting stents. Clin Res Cardiol. (2016) 105(3):230–8. doi: 10.1007/s00392-015-0911-7
4. Taniwaki M, Stefanini GG, Silber S, Richardt G, Vranckx P, Serruys PW, et al. 4-year clinical outcomes and predictors of repeat revascularization in patients treated with new-generation drug-eluting stents: a report from the RESOLUTE all-comers trial (A randomized comparison of a zotarolimus-eluting stent with an everolimus-eluting stent for percutaneous coronary intervention). J Am Coll Cardiol. (2014) 63(16):1617–25. doi: 10.1016/j.jacc.2013.12.036
5. Glaser R, Selzer F, Faxon DP, Laskey WK, Cohen HA, Slater J, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation. (2005) 111(2):143–9. doi: 10.1161/01.CIR.0000150335.01285.12
6. Wang J, Liu JH, Zhu XL, Zhang M, Wang SP, Zheng Z. Nonculprit lesion progression in patients with ST elevation myocardial infarction after primary percutaneous coronary intervention. Int Heart J. (2014) 55(1):48–52. doi: 10.1536/ihj.13-081
7. Wang J, Yan CY, Wang W, Wang TZ. The clinical prediction factors for non-culprit lesion progression in patients with acute ST elevation myocardial infarction after primary percutaneous coronary intervention. BMC Cardiovasc Disord. (2022) 22(1):529. doi: 10.1186/s12872-022-02974-2
8. Park SY. Nomogram: an analogue tool to deliver digital knowledge. J Thorac Cardiovasc Surg. (2018) 155(4):1793. doi: 10.1016/j.jtcvs.2017.12.107
9. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42(14):1289–367. doi: 10.1093/eurheartj/ehaa575
10. Mehta SR, Wood DA, Storey RF, Mehran R, Bainey KR, Nguyen H, et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. (2019) 381(15):1411–21. doi: 10.1056/NEJMoa1907775
11. Gaba P, Gersh BJ, Ali ZA, Moses JW, Stone GW. Complete versus incomplete coronary revascularization: definitions, assessment and outcomes. Nat Rev Cardiol. (2021) 18(3):155–68. doi: 10.1038/s41569-020-00457-5
12. Shlofmitz RA, Galougahi KK, Jeremias A, Shlofmitz E, Thomas SV, Ali ZA. Calcium modification in percutaneous coronary interventions. Interv Cardiol Clin. (2022) 11(4):373–81. doi: 10.1016/j.iccl.2022.06.001
13. Medina A, de Lezo J S, Pan M. A new classification of coronary bifurcation lesions. Rev Esp Cardiol. (2006) 59(2):183. doi: 10.1157/13084649
14. Musallam A, Chezar-Azerrad C, Torguson R, Case BC, Yerasi C, Forrestal BJ, et al. Procedural outcomes of patients undergoing percutaneous coronary intervention for de novo lesions in the ostial and proximal left circumflex coronary artery. Am J Cardiol. (2020) 135:62–7. doi: 10.1016/j.amjcard.2020.08.014
15. Turgut O, Yilmaz A, Yalta K, Yilmaz BM, Ozyol A, Kendirlioglu O, et al. Tortuosity of coronary arteries: an indicator for impaired left ventricular relaxation? Int J Cardiovasc Imaging. (2007) 23(6):671–7. doi: 10.1007/s10554-006-9186-4
16. Leon MB, Allocco DJ, Dawkins KD, Baim DS. Late clinical events after drug-eluting stents: the interplay between stent-related and natural history-driven events. JACC Cardiovasc Interv. (2009) 2(6):504–12. doi: 10.1016/j.jcin.2009.04.004
17. Chacko R, Mulhearn M, Novack V, Novack L, Mauri L, Cohen SA, et al. Impact of target lesion and nontarget lesion cardiac events on 5-year clinical outcomes after sirolimus-eluting or bare-metal stenting. JACC Cardiovasc Interv. (2009) 2(6):498–503. doi: 10.1016/j.jcin.2009.03.013
18. Gada H, Kirtane AJ, Newman W, Sanz M, Hermiller JB, Mahaffey KW, et al. 5-year Results of a randomized comparison of XIENCE V everolimus-eluting and TAXUS paclitaxel-eluting stents: final results from the SPIRIT III trial (clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc Interv. (2013) 6(12):1263–6. doi: 10.1016/j.jcin.2013.07.009
19. Parodi G, Memisha G, Valenti R, Trapani M, Migliorini A, Santoro GM, et al. Five year outcome after primary coronary intervention for acute ST elevation myocardial infarction: results from a single centre experience. Heart. (2005) 91(12):1541–4. doi: 10.1136/hrt.2004.054692
20. Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. (2011) 364(3):226–35. doi: 10.1056/NEJMoa1002358
21. Gabriel R, Alonso M, Reviriego B, Muñiz J, Vega S, López I, et al. Ten-year fatal and non-fatal myocardial infarction incidence in elderly populations in Spain: the EPICARDIAN cohort study. BMC Public Health. (2009) 9:360. doi: 10.1186/1471-2458-9-360
22. Schoenenberger AW, Urbanek N, Toggweiler S, Stuck AE, Resink TJ, Erne P. Ultrasound-assessed non-culprit and culprit coronary vessels differ by age and gender. World J Cardiol. (2013) 5(3):42–8. doi: 10.4330/wjc.v5.i3.42
23. Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. (2006) 47(8 Suppl):C13–8. doi: 10.1016/j.jacc.2005.10.065
24. Tashiro H, Tanaka A, Ishii H, Sakakibara K, Tobe A, Kataoka T, et al. Lipid-rich large plaques in a non-culprit left main coronary artery and long-term clinical outcomes. Int J Cardiol. (2020) 305:5–10. doi: 10.1016/j.ijcard.2020.01.072
25. Li CW, Yu K, Shyh-Chang N, Jiang Z, Liu T, Ma S, et al. Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review. J Cachexia Sarcopenia Muscle. (2022) 13(2):781–94. doi: 10.1002/jcsm.12901
26. Boutari C, Mantzoros CS. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism. (2022) 92(98):107–96. doi: 10.1016/j.metabol.2022.155217
27. Wang ZJ, Gao F, Cheng WJ, Yang Q, Zhou YJ. Body mass index and repeat revascularization after percutaneous coronary intervention: a meta-analysis. Can J Cardiol. (2015) 31(6):800–8. doi: 10.1016/j.cjca.2015.01.031
28. Koliaki C, Liatis S, Kokkinos A. Obesity and cardiovascular disease: revisiting an old relationship. Metab Clin Exp. (2019) 92:98–107. doi: 10.1016/j.metabol.2018.10.011
29. Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol. (2021) 20(1):76. doi: 10.1186/s12933-021-01268-9
30. Sánchez-García A, Rodríguez-Gutiérrez R, Mancillas-Adame L, González-Nava V, Díaz González-Colmenero A, Solis RC, et al. Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: a systematic review. Int J Endocrinol. (2020) 2020:4678526. doi: 10.1155/2020/4678526
31. Brown AE, Walker M. Genetics of insulin resistance and the metabolic syndrome. Curr Cardiol Rep. (2016) 18(8):75. doi: 10.1007/s11886-016-0755-4
32. Markus MRP, Rospleszcz S, Ittermann T, Baumeister SE, Schipf S, Siewert-Markus U, et al. Glucose and insulin levels are associated with arterial stiffness and concentric remodeling of the heart. Cardiovasc Diabetol. (2019) 18(1):145. doi: 10.1186/s12933-019-0948-4
33. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. (2018) 17(1):122. doi: 10.1186/s12933-018-0762-4
34. Killip T 3rd, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. (1967) 20(4):457–64. doi: 10.1016/0002-9149(67)90023-9
35. Li M, Wang X, Mi SH, Chi Z, Chen Q, Zhao X, et al. Short-term prognosis of fragmented QRS complex in patients with non-ST elevated acute myocardial infarction. Chin Med J. (2016) 129(5):518–22. doi: 10.4103/0366-6999.176989
36. Shiraishi J, Kohno Y, Sawada T, Takeda M, Arihara M, Hyogo M, et al. Predictors of in-hospital outcome after primary percutaneous coronary intervention for recurrent myocardial infarction. Circ J. (2008) 72(8):1225–9. doi: 10.1253/circj.72.1225
37. Bednár F, Widimský P, Groch L, Aschermann M, Zelízko M, Krupicka J. Acute myocardial infarction complicated by early onset of heart failure: safety and feasibility of interhospital transfer for coronary angioplasty. Subanalysis of Killip II-IV patients from the PRAGUE-1 study. J Interv Cardiol. (2003) 16(3):201–8. doi: 10.1034/j.1600-0854.2003.8047.x
38. Khot UN, Jia G, Moliterno DJ, Lincoff AM, Khot MB, Harrington RA, et al. Prognostic importance of physical examination for heart failure in non-ST-elevation acute coronary syndromes: the enduring value of Killip classification. Jama. (2003) 290(16):2174–81. doi: 10.1001/jama.290.16.2174
39. DeGeare VS, Boura JA, Grines LL, O'Neill WW, Grines CL. Predictive value of the Killip classification in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol. (2001) 87(9):1035–8. doi: 10.1016/S0002-9149(01)01457-6
40. Devlin G, Gore JM, Elliott J, Wijesinghe N, Eagle KA, Avezum A, et al. Management and 6-month outcomes in elderly and very elderly patients with high-risk non-ST-elevation acute coronary syndromes: the global registry of acute coronary events. Eur Heart J. (2008) 29(10):1275–82. doi: 10.1093/eurheartj/ehn124
41. Miyagi M, Ishii H, Murakami R, Isobe S, Hayashi M, Amano T, et al. Impact of renal function on coronary plaque composition. Nephrol Dial Transplant. (2010) 25(1):175–81. doi: 10.1093/ndt/gfp423
42. Hayano S, Ichimiya S, Ishii H, Kanashiro M, Watanabe J, Kurebayashi N, et al. Relation between estimated glomerular filtration rate and composition of coronary arterial atherosclerotic plaques. Am J Cardiol. (2012) 109(8):1131–6. doi: 10.1016/j.amjcard.2011.11.052
43. Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers. (2019) 5(1):56. doi: 10.1038/s41572-019-0106-z
44. Becker BN, Himmelfarb J, Henrich WL, Hakim RM. Reassessing the cardiac risk profile in chronic hemodialysis patients: a hypothesis on the role of oxidant stress and other non-traditional cardiac risk factors. J Am Soc Nephrol. (1997) 8(3):475–86. doi: 10.1681/ASN.V83475
Keywords: non-culprit lesion, percutaneous coronary intervention, nomogram, ST-segment elevation myocardial infarction, primary PCI
Citation: Dai F, Xu X, Zhou C, Li C, Tian Z, Wang Z, Yang S, Liao G, Shi X, Wang L, Li D, Hou X, Chen J and Xu T (2023) Development and validation of a nomogram to predict the five-year risk of revascularization for non-culprit lesion progression in STEMI patients after primary PCI. Front. Cardiovasc. Med. 10:1275710. doi: 10.3389/fcvm.2023.1275710
Received: 10 August 2023; Accepted: 13 November 2023;
Published: 29 November 2023.
Edited by:
Tommaso Gori, Johannes Gutenberg University Mainz, GermanyReviewed by:
Long Jiang, Second Affiliated Hospital of Nanchang University, China© 2023 Dai, Xu, Zhou, Li, Tian, Wang, Yang, Liao, Shi, Wang, Li, Hou, Chen and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junhong Chen Y2poMTAyOUBzaW5hLmNvbQ== Tongda Xu eHlmeXh5c0AxNjMuY29t
†These authors have contributed equally to this work
Abbreviations STEMI, ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; NCL, non-culprit lesion; DCA, decision curve analysis; CAG, coronary angiography; BMI, body mass index; TyG, triglycerides and glucose index; SCr, serum creatinine; CKD, chronic kidney disease.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.