- 1Department of Ultrasound, The Fourth Clinical Medical College of Guangzhou University of Chinese Medicine (Shenzhen Traditional Chinese Medicine Hospital), Shenzhen, China
- 2Department of Ultrasound, The First Affiliated Hospital of Shenzhen University (Shenzhen Second People’s Hospital), Shenzhen, China
Objective: Patients with heart failure with pulmonary edema may have declining left atrial (LA) function. Left atrial strain (LAS) imaging enables quantitative assessment of LA function. The aim of this prospective study was to assess the LA function and pulmonary edema in patients with heart failure evaluated by cardiopulmonary ultrasonography.
Methods: Two-dimensional speckle-tracking echocardiography for LAS was performed in 115 consecutive patients with congestive heart failure. A semiquantitative B-lines score of pleural effusions was derived by pulmonary ultrasound almost at the same time by combined cardiopulmonary ultrasound.
Results: Compared with those who did not have pulmonary edema, patients with pulmonary edema had lower LAS (LASreservoir, 21.5 ± 4.9% vs. 9.2 ± 3.7% [P < 0.001]; LASconduit, 10.7 ± 3.5% vs. 5.1 ± 2.1% [P < 0.001]; LASpump, 11.3 ± 5.4% vs. 4.0 ± 2.7% [P < 0.001]), lower LVEF, TAPSE; and higher SPAP, E/e′, larger LA, LV, RV; more severe MR. However, there were no significant between-group differences with respect to sex and body surface area. In patients with pulmonary edema, B-lines score was independently associated with LASreservoir (R = −0.71, P < 0.001); LASpump (R = −0.66, P < 0.001) and LASconduit (R = −0.56, P < 0.001). On multiple linear regression, decreased LASreservoir (beta = −0.61, B = −0.71, P < 0.001) and elevated SPAP (beta = 0.31, B = 0.13, P = 0.01) were significantly associated with B-lines score in heart failure.
Conclusion: Declining LA function, especially the reservoir function, assessed by speckle-tracking echocardiography is related to the degree and occurrence of pulmonary edema in patients with left heart failure.
Introduction
Pulmonary edema is one of the most serious consequences of left heart failure. It is the main determinant of adverse outcomes, higher risk of hospitalization, and emergency department visits, and is evaluated as a treatment target in patients with heart failure (1). In patients with left heart failure, when fluid filtration increases due to left ventricular (LV) filling pressure, increased extravascular lung water (EVLW) results in cardiogenic pulmonary edema. B-lines detected by lung ultrasound have been demonstrated to change dynamically with EVLW content (2). Studies have shown that pulmonary ultrasound can allow for semi-quantitative evaluation of the degree of pulmonary edema and shows a good correlation with x-ray extravascular pulmonary water score (3, 4).
The main role of the left atrium (LA) is to regulate left ventricular filling and cardiovascular function. As a complex process, the LA acts as a reservoir during systole, a conduit for direct blood flow from the pulmonary veins into the left ventricle during the early diastolic period, and as an active pump in the late diastolic period of the left ventricle. LA function has been shown to predict left ventricular filling pressure and adverse cardiac outcome (5). Decline in LA function in patients with heart failure may induce the typical symptoms of heart failure (6). Speckle tracking echocardiography (STE) is a novel imaging tool that can objectively quantify the contributions of reservoir, conduit, and active pump functions of the LA. The role of LA function as a biomarker is increasingly being evaluated by strain analysis.
However, there is a paucity of studies evaluating the prevalence and correlation of left atrial dysfunction in patients with heart failure who have pulmonary edema. In this study, we used cardiopulmonary ultrasonography for comprehensive and simultaneous assessment of the degree of pulmonary edema, left atrium strain, and other echocardiographic parameters in patients with congestive heart failure to explore the correlation of these indices with cardiogenic pulmonary edema.
Materials and methods
Baseline characteristics
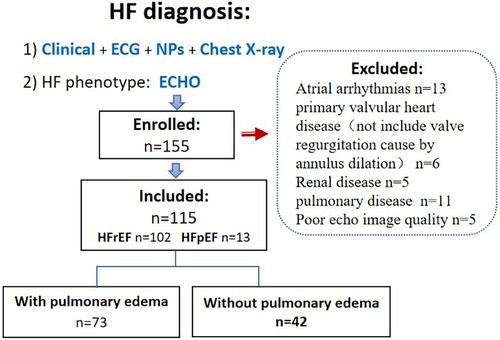
This was a prospective study conducted at an affiliated hospital of a medical college. Patients with congestive heart failure [diagnosis based on the 2016 ESC Guidelines (7)] who were admitted to our Internal Medicine-Cardiovascular Department between October 2020 and August 2022 were eligible for inclusion in this study. The Ethics Committee of the Shenzhen Traditional Chinese Medicine Hospital approved this study, and all participants provided written informed consent. The exclusion criteria were: age < 18 years, primary valvular heart disease, atrial arrhythmias, systemic disease associated with pleural edema (such as renal disease), and pulmonary disease (Figure 1).
Cardiopulmonary ultrasonography
Examinations were performed by qualified sonographers using the same equipment (Philips APEC 7C, Philips Healthcare, Bothell, Washington, USA) and transducers (S5-1 phased-array probe [1–5 MHz] and L12-3 linear probe [3–12 MHz]). Cardiopulmonary ultrasonography was performed prior to initiation of intravenous diuretic therapy. Echocardiography was performed in the left decubitus position, supine, or near-to-supine position. Pulmonary ultrasound was performed with patients in supine or near-to-supine position.
Echocardiography
Two-dimensional, M-mode, and Doppler measures and indices were obtained using standard techniques outlined by the American Society of Echocardiography (8). Assessment of LV diastolic function grade included the following variables: mitral flow velocities, mitral annular e′ velocity, E/e′ ratio (Peak E wave was measured from the apical 4-chamber in Doppler velocities of early diastolic flow, and a 1.5 mm sample volume was placed at the septal and lateral corner of the mitral annulus; early diastolic velocity e′ was measured; average E/e′ ratio was then calculated), peak velocity of TR jet, and LA maximum volume index (9).
Three-heart-cycles were recorded to obtain images suitable for strain analysis. LAS analysis was performed on the Apical four- and two-chamber views with a frame rate ≥40 frames/s. Surface tracing was automatically generated by the system. Tracking was considered satisfactory if it covered the entire LA wall and with visible motion of the speckles; segments that failed to be tracked by the software were adjusted manually as required. Strain analysis was performed in all studies with adequate image quality. The automated strain detection measures LA conduit strain (LASconduit, a “positive” wave describing LA myocardial lengthening during passive conduit function); LA pump strain (LASpump, a contraction shown as a “negative” strain); and LA reservoir strain (LASreservoir, “total” strain when the LA has the maximum volume) at both end-diastole (10) (all the parameters of strain refer only to the absolute value). Offline strain analysis was performed using the Auto Strain LA algorithm (QLAB version 13.0; Philips Healthcare, Andover, MA) (Figure 2). All strain measurements were performed by two investigators. Reproducibility was assessed using a random sample of 20 patients.
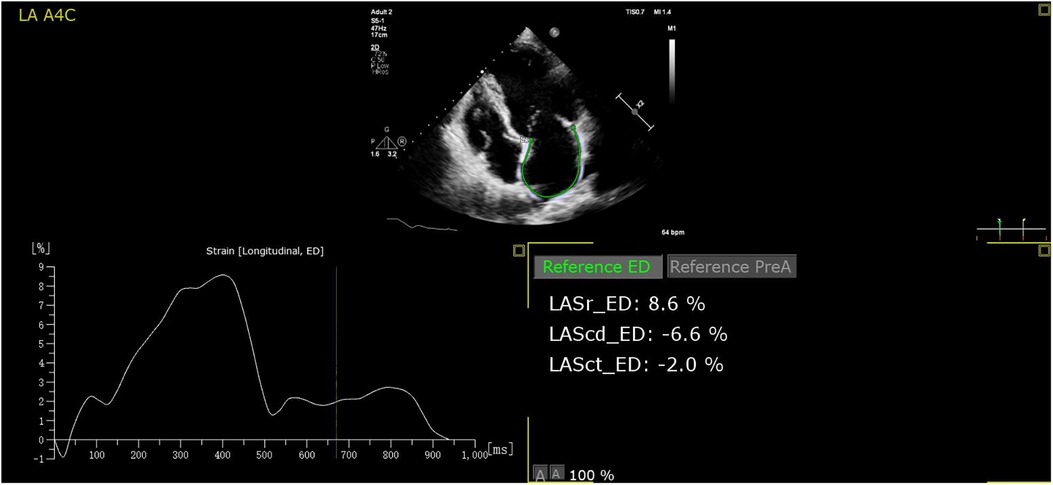
Figure 2. The auto strain LA algorithm acquires the LA strain parameters at both end-diastole automatically. LASr, LASreservoir; LAScd, LASconduit; LASct, LASpump.
Pulmonary ultrasonography
After echocardiography, all patients underwent pulmonary ultrasonography with the S5-1 transducer for detection of B-lines. When multiple B-lines were observed, an L12-3 linear probe (3–12 MHz) was used to observe the pleural lines (smooth and regular hyperechoic linear structure in cardiogenic pulmonary edema) with a linear-probe. Abnormal pleural lines can also generate B-lines such as due to interstitial lung disease (11) or pneumonia (12, 13); thus, patients in whom abnormal pleural lines were observed by linear probe were excluded.
The following B-line scoring method was used: The anterior and lateral chest wall was divided into seven-zones. B-line score was then calculated according to the degree of pulmonary edema (Figure 3). A score of 0 (zero) was defined as absence of B-lines. A score of 1 corresponded to septal syndrome (B-lines at regular distances, corresponding to pleural projection of the subpleural septa, at approximately 7 mm distance). Interstitial-alveolar syndrome was assigned a score of 2 (B-lines more confluent, separated by <7 mm). A score of 3 referred to white lung (B-lines coalesced, confluent B-lines >80%, resulting in an almost completely white image). Images with the highest score in each zone were recorded. A final ultrasound B-lines score was obtained by summing the B-lines scores of the seven zones of anterolateral chest scan (3).
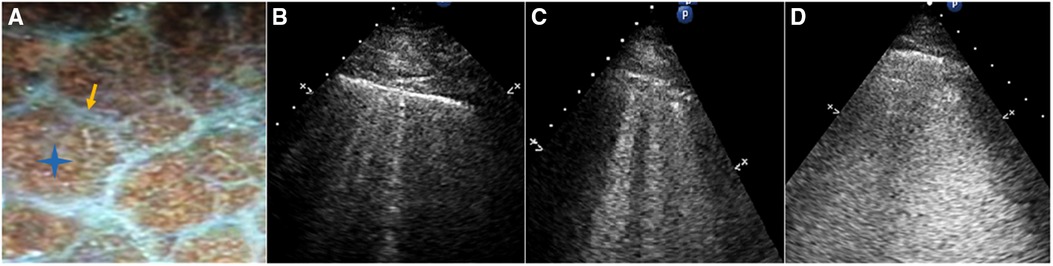
Figure 3. (A) Gross anatomy of the lung surface: the white line pointed by the yellow arrow is the subpleural interlobular septum which is approximately 7 mm apart. The blue star indicates the location of the alveoli. (B) Septal syndrome: B-lines are approximately 7 mm apart, corresponding to subpleural interlobular septa. (C) Interstitial-alveolar syndrome: B-lines are confluent. (D) White lung. B-lines have coalesced, resulting in an almost completely white echographic image.
Statistical analysis
Normally distributed continuous variables are expressed as mean ± standard deviation, while those with skewed distribution are expressed as median (range). Categorical variables are expressed as frequency (percentage). Between-group differences were assessed using unpaired t test, Wilcoxon rank-sum test, Chi-squared or Fisher's exact test as appropriate. Simple linear regression was used to assess relationship of B-lines score with LA reservoir, conduit, and pump strain and other echocardiographic and clinical parameters. Stepwise multivariate logistic regression analysis was applied to identify the independent predictors of pulmonary edema. All variables associated with P values <0.05 were included in multivariable analysis, and collinearity between dependent factors was checked. Model performance of LAS for detecting pulmonary edema was assessed using area under the curve (AUC) along with its 95% confidence interval (CI). Intra- and inter-observer variability for LA strain were assessed using analysis of limits of agreement and the intraclass correlation coefficient in 20 patients randomly selected from the study population by the same observer (H.L.) and a second observer (J.C.). Both observers were blinded to the results of pulmonary ultrasound assessments at the time of strain analysis. Analysis was performed using SPSS 20.0 software (IBM-SPSS Inc., Chicago, IL, USA). P values <0.05 were considered indicative of statistical significance.
Results
Baseline characteristics
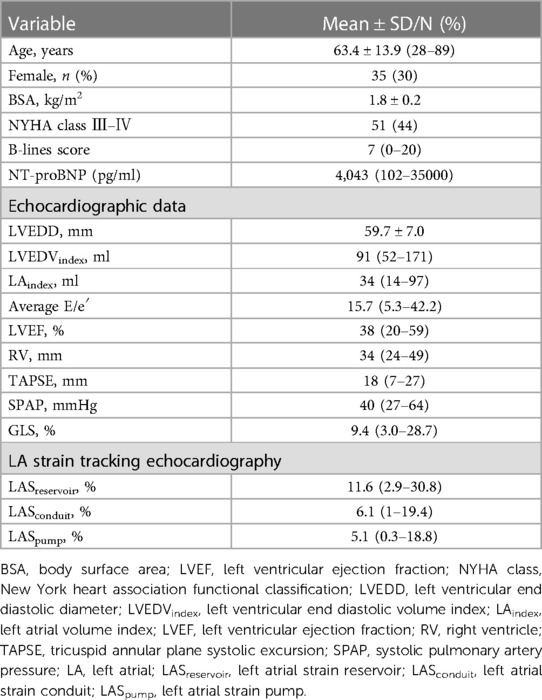
Of the 155 consecutive patients screened, 13 patients were excluded because of atrial arrhythmias, 11 because of concomitant lung disease, 6 because of primary severe valvular heart disease, 5 because of renal failure, and 5 because of poor echocardiographic windows. Therefore, 115 patients (mean age, 67 ± 11 years; 30.4% female) qualified the eligibility criteria. Eleven patients had HFpEF (LVEF > 50%) and 104 patients had HFrEF; among patients with pulmonary edema (n = 73), four patients had HFpEF. The majority of patients had coronary heart disease (52%) and hypercholesterolemia (52%), while 20% patients had type Ⅱ diabetes mellitus, and 32% patients had hypertension. Median (range) values of LA reservoir, conduit, and pump strain were 11.6 (2.9–30.8), 6.1 (1–19.4), and 5.1 (0.3–18.8), respectively. The baseline characteristics of the study population are summarized in Table 1.
Parameters in groups with and without pulmonary edema
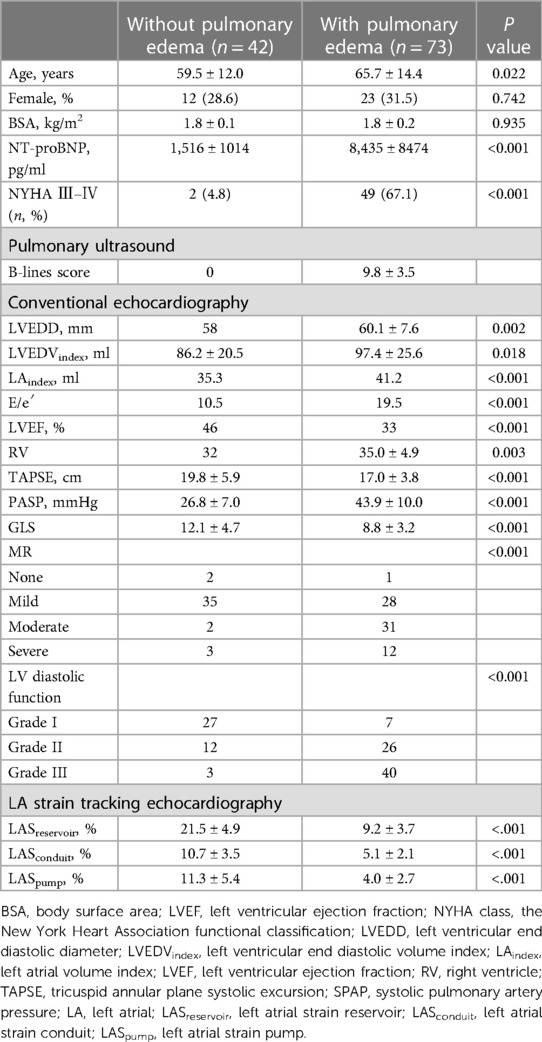
Patients were divided into two groups based on the presence or absence of pulmonary edema according to the pulmonary ultrasonography findings. Patients in the pulmonary edema group had bilateral septal syndrome, interstitial-alveolar syndrome, or white lung (14). Patients with pulmonary edema were significantly older and had higher NT-proBNP levels and NYHA class (Table 2).
Patients with pulmonary edema had significantly reduced LASreservoir, LASconduit, LASpump (LASreservoir, 21.5 ± 4.9% vs. 9.2 ± 3.7% [P < 0.001]; LASconduit, 10.7 ± 3.5% vs. 5.1 ± 2.1% [P < 0.001]; LASpump, 11.3 ± 5.4% vs. 4.0 ± 2.7% [P < 0.001]). Moreover, patients with pulmonary edema had lower LVEF, TAPSE; higher SPAP, E/e′, larger LA, LV, RV; and more severe MR. However, there was no significant between-group difference with respect to sex and BSA (Table 2). On stepwise multiple logistic regression analysis, LASreservoir (OR (95% CI) = 0.53[0.35–0.80], P < 0.01), PASP (OR (95% CI) = 1.33[1.05–1.68], P < 0.05) were the independent predictors of pulmonary edema.
Association of the degree of pulmonary edema and echocardiographic measurements
This analysis only included the group with pulmonary edema. Considering that only four patients had HFpEF in this group, we analyzed all cases including HFpEF (n = 4) and HFrEF (n = 69).
B-lines score was independently associated with LASreservoir (R = −0.71, B = −0.60, P < 0.001); LASpump (R = −0.66, B = −0.57, P < 0.001); LASconduit (R = −0.56, B = −0.95, P < 0.001); MR (R = 0.26, B = 1.20, P = 0.03); PASP (R = 0.54, B = 0.23, P < 0.001); E/e′ (R = 0.51, B = 0.22, P < 0.001); LV diastolic function (R = 0.41, B = 2.05, P = 0.001); LAindex (R = 0.32, B = 0.73, P = 0.011); LVGLS (R = −0.29, B = −0.36, P = 0.014); RV (R = 0.31, B = 0.22, P = 0.01); TAPSE (R = −0.27, B = −0.25, P = 0.04); and LVEF (R = −0.24, B = −0.10, P = 0.04). However, B-lines score was not associated with age (P = 0.38), LVEDD (P = 0.86), or LVEDVindex (P = 0.74).
Multiple linear regression showed that decreased LASreservoir (beta = −0.61, B = −0.71, P < 0.001) and elevated SPAP (beta = 0.31, B = 0.13, P = 0.01) were significantly associated with B-lines score in heart failure (Table 3).
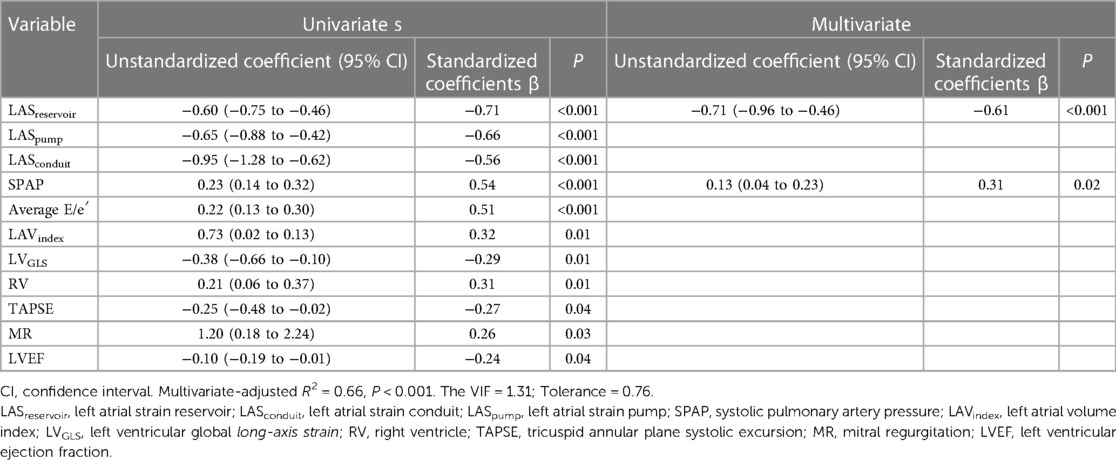
Table 3. Univariate and multiple linear regression analysis of LAS and other echocardiographic variables for pulmonary edema.
Performance of LAS parameters for identification of pulmonary edema
We further investigated the value of these echocardiographic indices for identifying pulmonary edema by performing receiver operating characteristics (ROC) curve analyses. Area under the curve (AUC), optimal cutoff values, and corresponding sensitivity and specificity for pulmonary edema. Among all clinical and echocardiographic parameters analyzed, LASreservoir showed the highest diagnostic accuracy. Use of LASreservoir cutoff value of less than 16.2% for pulmonary edema was associated with 97.3% sensitivity and 85.7% specificity (AUC = 0.96, P < 0.001). LASconduit cut-off value of less than 7.5% for pulmonary edema had 87.7% sensitivity and 85.7% specificity (AUC = 0.92, P < 0.001). LASpump cut-off value of less than 6.5% for pulmonary edema had 87.7% sensitivity and 81.0% specificity (AUC = 0.86, P < 0.001).
Reproducibility
For assessment of inter-observer variability, two investigators (H.L. and J.C) independently performed strain measurements in a random sample of 20 patients in a blinded manner. The intraclass correlation coefficients of LASreservoir, LASconduit, LASpump were 0.95 (95% CI, 0.87–98), 0.91 (95% CI, 0.73–0.97), and 0.90 (95% CI, 0.74–0.96), respectively, for intraobserver variability assessment and 0.93 (95% CI, 0.84–0.97), 0.84 (95% CI, 0.64–0.93), and 0.88 (95% CI, 0.73–0.95) for interobserver variability assessment.
Discussion
In this study, we evaluated LA strain using a strain software that was developed for the left atrium, which is different from that used only for the evaluation of left ventricle. We found that patients with pulmonary edema had reduced LASreservoir, LASpump, LASconduit values compared to patients with heart failure who did not have pulmonary edema. Compared to those without pulmonary edema, patients with pulmonary edema were older, had lower LVEF, TAPSE; and higher SPAP, E/e′, larger LA, LV, RV; more severe MR. On stepwise logistic regression analysis, LASreservoir and SPAP were identified as independent predictors of pulmonary edema. We further analyzed the correlation of left atrial function and other echocardiographic and clinical parameters with the degree of pulmonary edema in the group with pulmonary edema. We found a consistent reduction in LA strain levels in patients with more severe pulmonary edema; in addition, there was no association between the degree of pulmonary edema and LV dimension or volume. On multiple linear regression, decreased LASreservoir and elevated SPAP were significantly associated with the degree of pulmonary edema.
The 2016 guidelines for assessment of left ventricular diastolic function mention E/e′ as an important and easily-obtained evaluation parameter; however, it is a less accurate parameter for estimating the LV filling pressure compared to invasive catheters (15–17). Clinical conditions, such as LV hypertrophy and dilatation, severe reduction of LV systolic function, and mitral annular calcification are liable to affect the accuracy of the filling pressure estimated from E/e′ (15, 18). Left atrial volume index (LAVI) is another important indicator of diastolic dysfunction which can be acquired with high feasibility and reproducibility. It reflects the chronic elevation of LV filling pressure; however, LAVI alone cannot be used as a marker of improved diastolic dysfunction due to the slow and incomplete remodeling of LA (19). Unlike E/e′ and LAVI, analysis of LA strain parameters allows discrimination between active and passive myocardial tissue movement, independent of tethering effects and is less load dependent compared to conventional parameters for evaluation of LA function, enabling the evaluation of LA reservoir, conduit, and pump function (20). Cameli et al. measured LA reservoir and E/e′ and invasive pulmonary artery wedge pressure (PAWP) simultaneously, and found that LASreservoir rather than E/e′ was closely related to PAWP (21). LA strain is a sensitive marker of early diastolic dysfunction and can reflect LA pressure and LV filling pressure changes instantaneously (22, 23). It was recommended to incorporate it into the 2016 EACVI/ASE criteria to improve the diagnostic efficiency of diastolic dysfunction (23).
It was reported that pulmonary edema develops acutely with LA hypertension (24). When left heart fails, increased LV filling pressure causes a backward failure process. Elevated LV pressure transmits back to LA and further to the pulmonary vessels, which leads to an increased hydrostatic force, resulting in an imbalance in the Starling equilibrium. Consequently, excessive EVLW accumulates in the lung interstitium and even in the alveoli, leading to pulmonary edema and pulmonary hypertension. Changes in EVLW can be detected by lung ultrasound and semi-quantified (25). Elevated PASP leads to RV afterload, promoting progression of RV failure. This may explain the higher PASP, larger RV, decreased TAPSE, and more severe dyspnea in patients with pulmonary edema.
LA reservoir and conduit function changes with LV compliance and impaired relaxation. In the univariate analysis, the association of LA reservoir, conduit strain, and B-line score reflects the mechanism of association of pulmonary edema with increased LA pressure and tension and impaired LA compliance. Cardiogenic pulmonary edema is the decompensated stage of left heart failure. Occurrence of pulmonary edema indicates severe increase in LA pressure, which impairs the atrial reservoir and conduit functions. In addition, our study showed a better correlation between LA reservoir strain decrease and increase in B-lines score in patients with pulmonary edema. This may be explained by the greater sensitivity of LASreservoir compared to other LA function and standard diastolic markers of elevated LA pressure and left ventricular filling pressure (26). Patients with worse left atrial function, especially LASreservoir, may remain at high risk of developing pulmonary edema decompensation, hospitalization, and even emergency department visits. The decrease in left atrial function should alert the clinician that such patients require diuresis and afterload reduction. In addition, patients with heart failure showing significant reduction in left atrial strain should be evaluated by pulmonary ultrasonography or chest x-ray to rule out pulmonary edema.
In our study, we attempted to highlight the decrease in the LA pump function in patients with left heart failure with pulmonary edema. Ramkumar et al. found that LA pump function is independent of LV function (27). Different from conduit function, the LA pump is not only influenced by LV end-diastolic pressure (afterload), as the left atrium must pump blood across a higher pressure-gradient in patients with heart failure, but also influenced by atrial returned blood volume (preload) as atrial contraction adds an additional 38% filling volume during the last third of diastole in the impaired relaxation group (28). Pulmonary venous return is the predominant determinant of preload. In patients with left heart failure, occurrence of pulmonary edema further reduces the blood flow from the pulmonary circulation to the LA, further aggravating the decrease in pump function.
Limitations
First, this was a single-center study. The study population comprised exclusively of inpatients in the Department of Cardiology who could receive cardiopulmonary ultrasound evaluation. Second, sufficient image quality for strain analysis was needed, so 5 patients were excluded from our analysis. Third, we used noninvasive B-lines scores to evaluate the degree of pulmonary edema rather than invasive hemodynamic validation. Future studies should also employ invasive hemodynamic evaluation to better characterize the physiology of LA function in patients with pulmonary edema.
Conclusion
In patients with HF, those with pulmonary edema have worse left and right heart function. The degree of pulmonary edema increases with the decrease in left atrial function. We demonstrated that LA reservoir function is more closely related with pulmonary edema compared to the other echocardiographic parameters. Therefore, non-invasively obtained LA deformation parameters may be used to predict the occurrence of pulmonary edema.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Shenzhen Traditional Chinese Medicine Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HL: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. PH: Conceptualization, Project administration, Supervision, Writing – review & editing. JC: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by Sanming Project of Medicine in Shenzhen (No. SZSM201612027).
Acknowledgments
We thank Qiang Liu and Yi Wei of the Department of Cardiology who provided the cases of this study in Shenzhen Hospital of traditional Chinese Medicine Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Girerd N, Seronde MF, Coiro S, Chouihed T, Bilbault P, Braun F, et al. Integrative assessment of congestion in heart failure throughout the patient journey. JACC Heart Fail. (2018) 6:273–85. doi: 10.1016/j.jchf.2017.09.02329226815
2. Picano E, Pellikka PA. Ultrasound of extravascular lung water: a new standard for pulmonary congestion. Eur Heart J. (2016) 37:2097–104. doi: 10.1093/eurheartj/ehw16427174289
3. Li H, Li YD, Zhu WW, Kong LY, Ye XG, Cai QZ, et al. A simplified ultrasound comet tail grading scoring to assess pulmonary congestion in patients with heart failure. Biomed Res Int. (2018) 2018:8474839. doi: 10.1155/2018/847483929487872
4. Jambrik Z, Monti S, Coppola V, Agricola E, Mottola G, Miniati M, et al. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am J Cardiol. (2004) 93:1265–70. doi: 10.1016/j.amjcard.2004.02.01215135701
5. Malagoli A, Rossi L, Bursi F, Zanni A, Sticozzi C, Piepoli MF, et al. Left atrial function predicts cardiovascular events in patients with chronic heart failure with reduced ejection fraction. J Am Soc Echocardiogr. (2019) 32:248–56. doi: 10.1016/j.echo.2018.08.01230316541
6. Cameli M, Mandoli GE, Loiacono F, Dini FL, Henein M, Mondillo S. Left atrial strain: a new parameter for assessment of left ventricular filling pressure. Heart Fail Rev. (2016) 21:65–76. doi: 10.1007/s10741-015-9520-926687372
7. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2016) 18:891–975. doi: 10.1002/ejhf.59227207191
8. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14. doi: 10.1016/j.echo.2014.10.00325559473
9. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2016) 29:277–314. doi: 10.1016/j.echo.2016.01.01127037982
10. Gan GCH, Bhat A, Chen HHL, Gu KH, Fernandez F, Kadappu KK, et al. Left atrial reservoir strain by speckle tracking echocardiography: association with exercise capacity in chronic kidney disease. J Am Heart Assoc. (2021) 10:e017840. doi: 10.1161/jaha.120.01784033372523
11. Hasan AA, Makhlouf HA. B-lines: transthoracic chest ultrasound signs useful in assessment of interstitial lung diseases. Ann Thorac Med. (2014) 9:99–103. doi: 10.4103/1817-1737.12885624791173
12. Sperandeo M, Varriale A, Sperandeo G, Polverino E, Feragalli B, Piattelli ML, et al. Assessment of ultrasound acoustic artifacts in patients with acute dyspnea: a multicenter study. Acta Radiol. (2012) 53:885–92. doi: 10.1258/ar.2012.12034022919052
13. Li H, Li YD, Zhu WW, Sun LL, Ye XG, Kong LY, et al. High-resolution transthoracic ultrasonography for assessment of pleural lines in patients with dyspnea with CT comparison: an observational study. J Ultrasound Med. (2017) 36:707–16. doi: 10.7863/ultra.16.0403028127786
14. Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest. (2015) 147:1659–70. doi: 10.1378/chest.14-131326033127
15. Nakabachi M, Yamada S, Iwano H, Hayashi T, Okada K, Kusunose K, et al. Left ventricular mass influences relationship between filling pressure and early-diastolic ratio of inflow velocity to mitral annular velocity (E/e′). Circ J. (2018) 82:732–8. doi: 10.1253/circj.CJ-17-101929311519
16. Santos M, Rivero J, McCullough SD, West E, Opotowsky AR, Waxman AB, et al. E/e′ ratio in patients with unexplained dyspnea: lack of accuracy in estimating left ventricular filling pressure. Circ Heart Fail. (2015) 8:749–56. doi: 10.1161/circheartfailure.115.00216126067855
17. Rinot E, Carasso S, Kinany W, Yarkoni M, Amir O, Greener GE. Left atrial phasic echocardiographic functional analysis in relation to diastolic left ventricular hemodynamic parameters acquired during right heart catheterization. Int J Cardiol Heart Vasc. (2022) 39:100957. doi: 10.1016/j.ijcha.2022.10095735402687
18. Haines PG, Dickey JB, Chambers AB, Ogunsua A, Wu WC, Aurigemma GP. Evaluating a decision tool for diagnosing diastolic dysfunction and estimation of left ventricular filling pressures in the presence of mitral annular calcium. Echocardiography. (2020) 37:1757–65. doi: 10.1111/echo.1487833021343
19. Cho GY, Hwang IC. Left atrial strain measurement: a new normal for diastolic assessment? JACC Cardiovasc Imaging. (2020) 13:2327–9. doi: 10.1016/j.jcmg.2020.05.01432771582
20. Gan GCH, Ferkh A, Boyd A, Thomas L. Left atrial function: evaluation by strain analysis. Cardiovasc Diagn Ther. (2018) 8:29–46. doi: 10.21037/cdt.2017.06.0829541609
21. Cameli M, Sparla S, Losito M, Righini FM, Menci D, Lisi M, et al. Correlation of left atrial strain and Doppler measurements with invasive measurement of left ventricular end-diastolic pressure in patients stratified for different values of ejection fraction. Echocardiography. (2016) 33:398–405. doi: 10.1111/echo.1309426493278
22. Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73:1961–77. doi: 10.1016/j.jacc.2019.01.05931000000
23. Ma CS, Liao YP, Fan JL, Zhao X, Su B, Zhou BY. The novel left atrial strain parameters in diagnosing of heart failure with preserved ejection fraction. Echocardiography. (2022) 39:416–25. doi: 10.1111/echo.1530435076951
24. Guyton AC, Lindsey AW. Effect of elevated left atrial pressure and decreased plasma protein concentration on the development of pulmonary edema. Circ Res. (1959) 7:649–57. doi: 10.1161/01.res.7.4.64913663218
25. Ayyat KS, Okamoto T, Niikawa H, Itoda Y, Dugar S, Latifi SQ, et al. Direct Lung Ultrasound Evaluation (CLUE): a novel technique for monitoring extravascular lung water in donor lungs. J Heart Lung Transplant. (2019) 38:757–66. doi: 10.1016/j.healun.2019.03.00531000373
26. Cameli M, Lisi M, Mondillo S, Padeletti M, Ballo P, Tsioulpas C, et al. Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc Ultrasound. (2010) 8:14. doi: 10.1186/1476-7120-8-1420409332
27. Ramkumar S, Yang H, Wang Y, Nolan M, Negishi T, Negishi K, et al. Association of the active and passive components of left atrial deformation with left ventricular function. J Am Soc Echocardiogr. (2017) 30:659–66. doi: 10.1016/j.echo.2017.03.01428476315
Keywords: pulmonary ultrasound, echocardiography, heart failure, pulmonary edema, left atrial function
Citation: Li H, Hu P-X and Chen J (2023) Correlation of left atrial function and pulmonary edema in patients with left heart failure on cardiopulmonary ultrasonography. Front. Cardiovasc. Med. 10:1274443. doi: 10.3389/fcvm.2023.1274443
Received: 8 August 2023; Accepted: 13 October 2023;
Published: 27 October 2023.
Edited by:
Tasneem Naqvi, Mayo Clinic Arizona, United StatesReviewed by:
Songnan Wen, Mayo Clinic Arizona, United StatesMasaki Izumo, St. Marianna University School of Medicine, Japan
© 2023 Li, Hu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Chen Y2hlbmppYW45ODIxQGhvdG1haWwuY29t Ping-Xiang Hu aHVweDY0MDhAMTYzLmNvbQ==
 Hong Li
Hong Li Ping-Xiang Hu1*
Ping-Xiang Hu1*