
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 07 December 2023
Sec. Cardiovascular Epidemiology and Prevention
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1267906
 Chengui Zhuo1
Chengui Zhuo1 Lei Chen1
Lei Chen1 Qiqi Wang2
Qiqi Wang2 Haipeng Cai1
Haipeng Cai1 Zujin Lin1
Zujin Lin1 Huili Pan1
Huili Pan1 Meicui Wu1
Meicui Wu1 Yuxiang Jin1
Yuxiang Jin1 Hong Jin1
Hong Jin1 Liangrong Zheng3*
Liangrong Zheng3*
Background: Limited studies have explored the association between sexual factors [age at first sexual intercourse (AFS) and lifetime number of sexual partners (LNSP)] and cardiovascular diseases (CVDs), leaving the causality inconclusive.
Methods: We performed a bi-directional Mendelian randomization (MR) study to investigate the causality between sexual factors and CVDs, including coronary artery disease, myocardial infarction, atrial fibrillation (AF), heart failure (HF), and ischemic stroke (IS). Single-nucleotide polymorphisms (SNPs) for sexual factors were extracted from the UK Biobank. Statistics for each CVD were derived from two different databases. MR estimates were calculated per outcome database and were combined through meta-analysis. Several complementary sensitivity analyses were also performed.
Results: The primary analysis suggested that AFS was causally associated with the risk of CVDs; the odds ratios (ORs) ranged from 0.686 [95% confidence interval (CI), 0.611–0.770] for HF to 0.798 (95% CI, 0.719–0.886) for AF. However, the association between AFS and IS (OR, 0.844; 95% CI, 0.632–1.126) was not consistent in the meta-analysis after excluding SNPs related to confounders. Moreover, non-significant associations were found between LNSP and CVDs. Reverse direction MR analysis showed that CVDs were not associated with sexual factors.
Conclusions: Genetic evidence suggested that AFS was causally associated with the risk of CVDs except for IS, whereas non-significant association of LNSP with CVDs was detected. Further investigation into AFS could be warranted in preventing the progression of CVDs.
Globally, cardiovascular diseases (CVDs) remain the principal contributor to mortality, responsible for nearly 32% of all deaths (1–3). The heavy burden of CVDs underscores the importance of identifying risk factors for prevention. In recent decades, reproductive behaviors have been increasingly implicated as significant factors in cancers, respiratory disease, and CVDs.
Reproductive behaviors are heritable traits, encompassing age at menarche and menopause, age at birth, age at first sexual intercourse (AFS), lifetime number of sexual partners (LNSP), and more (4). To date, several of these reproductive behaviors have been demonstrated as risk factors for cardiovascular diseases (4–8). For instance, a younger age at menarche and menopause is positively associated with the risk of cardiovascular diseases (5–7). Compared with women experiencing menarche at 13 years and menopause at 45 years or older, the adjusted relative risk of coronary artery disease (CAD) for early menarche (≤10 years) and early menopause (<45 years) is 1.27 and 1.50, respectively (5, 7). Previous studies have also shown that the age at first birth is positively associated with CVDs (8, 9). However, limited studies have considered risky sexual factors (such as younger AFS and higher LNSP) in relation to CVDs. Ngueta and Ndjaboue first explored the relationship between early sexual activity and hypertension (10), but it remains unclear whether risky sexual factors are causally associated with other CVDs such as CAD and heart failure (HF).
Sexual activity increasingly occurs at an earlier age, with one-third of contemporary British adolescents having initial sexual intercourse by the age of 16. Early sexual activity could have long-term implications for adult health (11). As a result, numerous programs on adolescent sexuality education aim to delay the onset of sexual activity to improve health-related outcomes. However, to validate that this focus is warranted, it is crucial to demonstrate that risky sexual activity does indeed cause harmful health impacts.
Mendelian randomization (MR) is a novel epidemiological approach used to infer the causal association of exposures and outcomes by using genetic variants from genome-wide association studies (GWASs) as instrumental variables (IVs) (12, 13). MR studies had been successfully applied in the strategies of CVD prevention (14–16), such as the development of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, which significantly decreased the risk of CVD (16). Previous MR studies also provided considerable evidence that CVD was causally related to traditional risk factors, which had already been suggested by observational studies, including hypertension, adiposity, type 2 diabetes, and so on (17–19). However, few MR studies have evaluated the causality between risky sexual factors and CVDs. Therefore, in this study, bi-directional MR analysis is first conducted to explore the causal association between sexual factors (AFS and LNSP) and cardiovascular diseases, including CAD, myocardial infarction (MI), HF, atrial fibrillation (AF), and ischemic stroke (IS).
In the present study, we applied a bi-directional two-sample MR study to comprehensively explore the causality between two sexual factors and CVDs. In the first stage, we evaluated whether genetically predicted sexual factors were causally related to CVDs. In the second stage, we also assessed whether genetically predicted CVDs were causally associated with sexual factors (Supplementary Figure S1). Two-sample MR is based on three principal assumptions: First, the single-nucleotide polymorphisms (SNPs) selected as the IVs are strongly related to exposures. Second, IVs should be independent of any confounders. Third, IVs affect the outcome exclusively via the exposure.
All summary statistics for sexual factors and CVDs were sourced from public GWASs, as shown in Supplementary Table S1. For sexual factors, we obtained data from the GWAS meta-analysis of the UK Biobank, including 397,338 and 378,882 pooled individuals for AFS and LNSP, respectively. Summary statistics for CVDs were extracted from the Coronary Artery Disease Genome-wide Replication and Meta-analysis plus The Coronary Artery Disease Genetics (CARDIoGRAMplusC4D) consortium for CAD and MI (20), the GWAS by Nielsen et al. for AF (21), the GWAS by Shah et al. for HF (22), and the MEGASTROKE consortium for IS (23). We also applied summary statistics from the FinnGen study for replication purposes. The FinnGen study is a medical project that started in 2017, combining genomic information from Finnish biobanks and health data from Finnish health registries (24).
The present study identified independent SNPs significantly associated with exposure (p < 5 × 10−8) by applying the PLINK clumping method (r2 < 0.001 and window size = 10 Mb). If the identified SNPs for the exposures were absence in the GWAS of the outcome, proxy SNPs (linkage disequilibrium r2 > 0.8) would be searched using an online tool (https://ldlink.nci.nih.gov/). The F-statistic was computed to quantify the strength of selected SNPs (25). A larger F-statistic (>10) indicates stronger strength of SNPs. A priori statistical power was also conducted.
To rule out any potential confounders, we searched for each IV and its proxies in PhenoScanner to evaluate any previously demonstrated associations (p-value < 5 × 10−6) with plausible confounders or CVDs. In the present study, the confounders included tobacco use, alcohol use, body mass index, lipid profile, and diabetes. We would repeat the MR analysis after excluding the SNPs related to confounders.
After extracting and harmonizing the data, we applied the fixed-effects inverse variance-weighted (IVW) method as the primary analysis to assess the causal association between exposures and outcomes. The IVW method could combine the SNP-specific Wald ratio estimates and provide an unbiased estimate (26). Moreover, we further used Cochran's Q to evaluate the presence of heterogeneity. In cases with evidence of heterogeneity (p-value < 0.05), we conducted the random-effects IVW method.
In addition, we also conducted a complementary sensitivity analysis to test the robustness of our findings. First, the simple and weighted median methods were performed to validate the results. For the weighted median, it tends to give valid estimates when more than half of the weight is obtained from valid SNPs (27). Second, the results of MR might be biased due to the directional pleiotropy. Therefore, the MR-Egger method was applied, and a p-value >0.05 for the MR-Egger intercept indicated the absence of directional pleiotropy. In addition, I2GX was used to evaluate the suitability of MR-Egger, and an I2GX > 95% was desired (28). Scatter plots were also conducted to show the effects estimated by each method. Third, MR pleiotropy residual sum and outlier (MR-PRESSO) were conducted to evaluate potential pleiotropic effects (outlier IVs) and provided outlier-adjusted estimates by excluding outlier IVs (29). Next, the leave-one-out analysis was conducted to guarantee that causality was not influenced by the particular IV. Finally, replication MR analyses were conducted using different outcome GWAS datasets (the FinnGen study), and then all MR estimates were meta-analyzed to generate the pooled estimates for each exposure on outcomes. A two-sided p-value below 0.05 indicated a statistically significant causal association. All MR analyses were performed through R Version 3.6.3 with the “TwoSampleMR,” “MR-PRESSO,” and “meta” packages.
Summary information of selected SNPs is shown in Supplementary Tables S2–S5. In total, they explained nearly 2.2% and 0.1% of the phenotypic variability of AFS and LNSP, respectively. The F-statistic for all SNPs was above 10, suggesting that they were strong enough to minimize bias from weak instrument bias. SNPs that were detected to be related to confounders or CVDs are shown in Supplementary Tables S6,S7. Cochran's Q identified the presence of heterogeneity in several MR results (Supplementary Table S8), and as a result, random-effects IVW methods were performed. Given a type I error of 0.05, the power calculation results of two sexual factors are provided in Supplementary Table S9. Scatter plots are also presented in Supplementary Figures S2–S5.
Overall, the primary IVW analysis suggested significant inverse associations between age at first sexual intercourse and the risk of coronary artery disease [odds ratio (OR), 0.704; 95% confidence interval (CI), 0.610–0.812], myocardial infarction (OR, 0.727; 95% CI, 0.627–0.843), heart failure (OR, 0.686; 95% CI, 0.611–0.770), atrial fibrillation (OR, 0.798; 95% CI, 0.719–0.886), and ischemic stroke (OR, 0.737; 95% CI, 0.657–0.827), as shown in Figure 1. In the reverse direction MR analysis, CVDs showed non-significant associations with age at first sexual intercourse (Supplementary Table S10). Most OR estimates were consistent using different sensitivity analyses but for the MR-Egger method (Figure 3). Owing to the lower precision of the MR-Egger method, we noticed that its estimates differed from the other MR analyses and the CIs were wider than in other methods (30). The intercept of MR-Egger regression (Supplementary Tables S11,S12) detected no directional pleiotropy except for the analysis of age at first sexual intercourse on AF (intercept = 0.992; 95% CI, 0.985–0.999; p = 0.028). Although the MR-PRESSO method identified some outlier SNPs, the estimate did not change significantly after correction (Figure 3 and Supplementary Tables S13,S14). In addition, we further excluded the SNPs associated with any confounders, and the analysis of the remaining SNPs observed broadly similar results (Supplementary Figures S6,S8). Moreover, the results of the leave-one-out analyses are displayed in Supplementary Figures S9–S13,S19–S23.
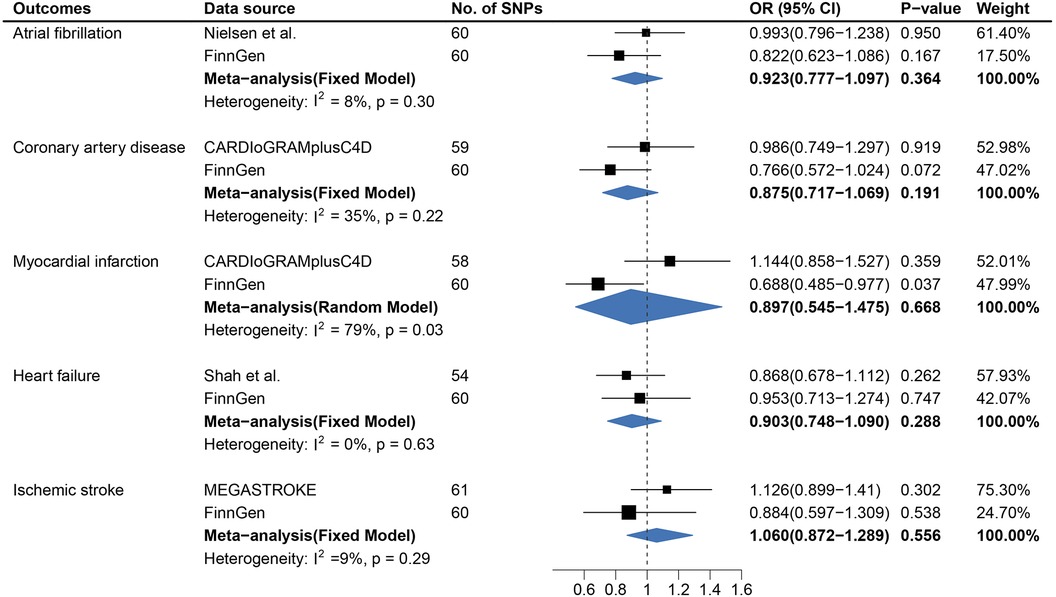
Figure 1. Causal association between age at first sexual intercourse and cardiovascular diseases. Estimated odds ratios of cardiovascular disease per one-unit increase in age at first sexual intercourse were determined from the primary IVW analysis. These estimations were done for each outcome data source separately, and then combined across the two data sources using a meta-analysis.
In a replication study based on the FinnGen data, there was significant evidence of causal associations between AFS and the risk of CAD, MI, AF, and HF, whereas statistically non-significant associations were detected between AFS and IS.
In the meta-analysis, the pooled OR before and after removing SNPs related to confounders is shown in Figure 1 and Supplementary Figure S6. The causal association for AFS and IS (OR, 0.844; 95% CI, 0.632–1.126) was not replicated in the meta-analysis after excluding the SNPs associated with confounders (Supplementary Figure S6).
In contrast to the findings from AFS, no causal association between genetically predicted LNSP and CVDs was observed except for MI (Figure 2 and Supplementary Figure S7). Genetically predicted LNSP was positively associated with MI (OR, 1.456; 95% CI: 1.101–1.926; p = 0.008) after excluding the SNPs related to confounders. However, the association was not consistent in sensitivity analyses and the pooled OR for MI was 1.147 (95% CI: 0.687–1.917). The details of sensitivity analysis results are shown in Figure 3 and Supplementary Figure S8. Reverse direction MR analysis identified that CVDs were not associated with LNSP (Supplementary Tables S10,S14). The results of the leave-one-out analyses are shown in Supplementary Figures S14–S18,S24–S28.
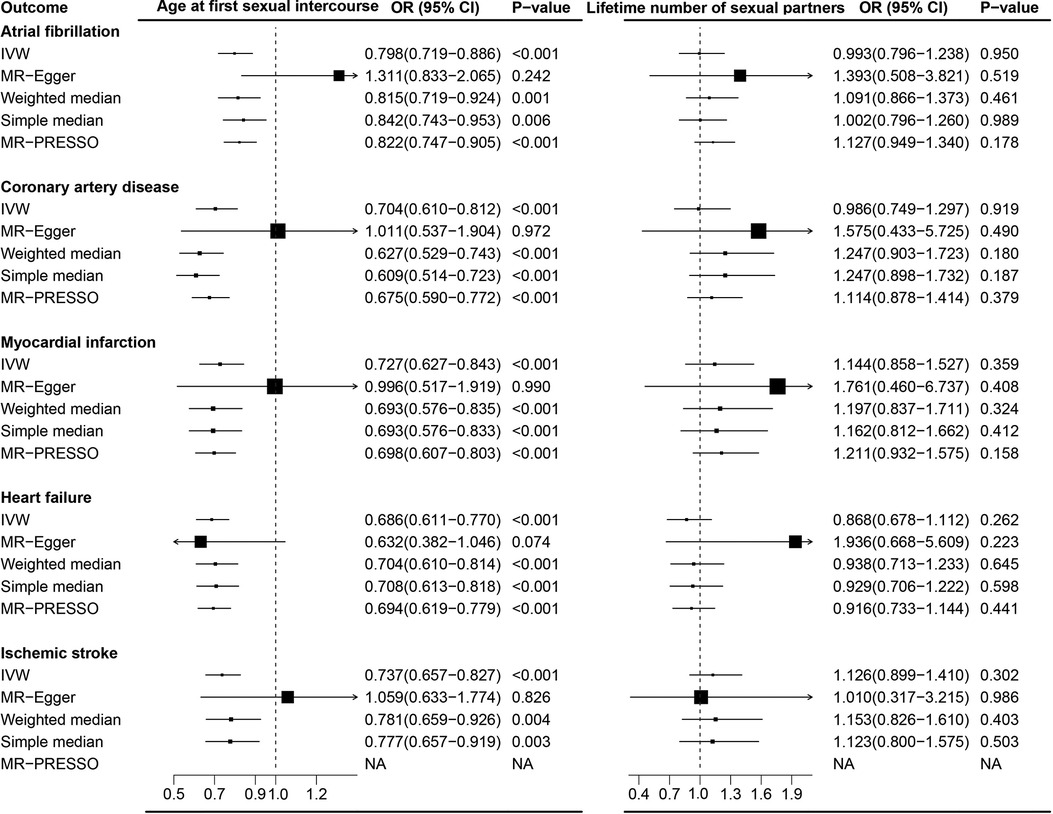
Figure 2. Causal association between lifetime number of sexual partners with cardiovascular diseases. Estimated odds ratios of cardiovascular disease per one-unit increase in lifetime number of sexual partners were determined from the primary IVW analysis. These estimations were done for each outcome data source separately, and then combined across the two data sources using a meta-analysis.
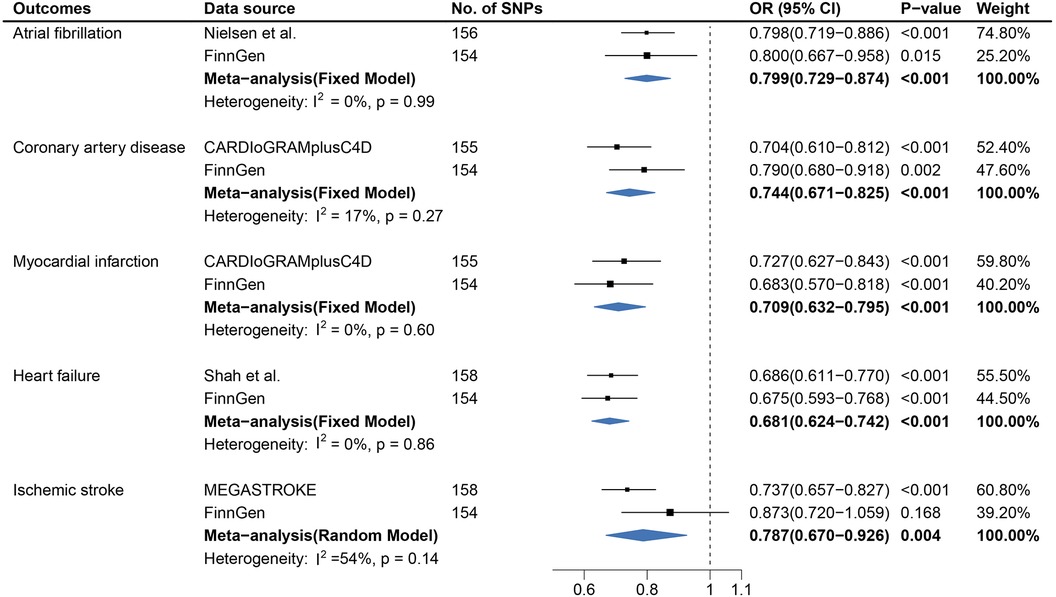
Figure 3. Complementary sensitivity analysis of the association between sexual factors and cardiovascular diseases.
To the best of our knowledge, the present MR study is the first to systematically explore the bi-directional association between sexual factors (including AFS and LNSP) and the risk of CVDs. Our results identified that AFS could be causally associated with CVDs, with the exception of IS, while LNSP showed no association. The results from complementary sensitivity analyses were generally consistent. Moreover, reverse direction MR analysis suggested that genetically predicted CVDs were unlikely to be causal determinants of sexual factors.
Our study suggested that genetic predisposition to AFS was causally associated with the risk of CAD, MI, AF, and HF, with the findings remaining robust in replication and meta-analysis. Few epidemiological studies have considered AFS in relation to CVDs (10, 31). Nikpay et al. conducted a phenome-wide search and suggested that AFS was negatively associated with CAD in both males and females (31). In addition, another cohort study involving 39,788 individuals initially confirmed that a younger age at first sexual intercourse was significantly linked to hypertension (10). The exact mechanisms underpinning this association are unclear but may involve both environmental and genetic factors. Previous research has shown that early sexual debut correlates with adverse environmental conditions, including lower levels of education, as well as greater exposure to smoking, drinking, and illicit drug use (32). Furthermore, early sexual debut typically contributes to poorer quality partner relationships (33). People dissatisfied with their relationships tend to have an increased risk of CVDs (34, 35). In addition, studies have already explored the influence of exercise on adverse cardiac remodeling, and intense exercise may even cause exercise-induced cardiomyopathy (36, 37). Early sexual activity (<16-year-old boys) is associated with more physical activity, which may potentially induce the development of cardiomyopathy and ultimately lead to cardiovascular disease (38). Beyond environmental factors, the potential role of genetics should also be emphasized. The dopamine D4 receptor gene has been linked to younger age at first sexual intercourse (39, 40) and elevated blood pressure, which directly contributes to the development of CVDs (41).
The current results on the causal association of AFS with IS are still controversial and incomplete. A previous MR study (42) reported that genetically predicted AFS was significantly associated with IS. However, another MR study showed no causality (43). This divergence might be attributed to pleiotropic bias, statistical analysis, and different datasets. In our study, we found that age at first sexual intercourse was negatively associated with IS in the primary analysis. However, after excluding SNPs associated with relevant confounders, the results were inconsistent. Several MR studies have already demonstrated the causal association between poor environmental conditions (such as tobacco, drug, and alcohol use) and IS (44, 45). Early initiation of sexual intercourse typically occurs in adolescence and is often linked to poor environmental conditions, which may directly impact IS rather than through age at first sexual intercourse, thereby generating bias in MR analysis (32).
No previous study has directly investigated the causal association between LNSP and CVDs using bi-directional MR, making it difficult to compare our present results with those of former MR studies. Lifetime number of sexual partners has previously been applied as an indicator of reproductive behaviors, as the number of sexual partners reflects mating success and thus potential reproductive success (46–48). Prior epidemiological and MR studies have provided evidence and confirmed that reproductive behaviors could play a crucial role in the development of CVDs (4–8). However, our study did not find a clear causal association between LNSP and CVDs. It is worth considering that contraception, commonly used in developed countries for population control, may influence reproductive success and affect the results (49). Notably, contraception allows for the decoupling of sexual and reproductive partners (47). Therefore, the causal association between the lifetime number of reproductive partners and CVDs needs further exploration.
Our study has several remarkable strengths. First, the MR analysis is less susceptible to being influenced by confounders and other biases than standard regression analysis, thereby strengthening the reliability of causal inference (50, 51). Furthermore, we conducted a complementary sensitivity analysis to confirm the robustness of our results. Second, to avoid potential confounders that could impact the causal association, we repeated the analysis after excluding potentially pleiotropic SNPs related to body mass index, lipid profile, diabetes, and tobacco, drug, and alcohol use. Third, we meta-analyzed the potential causal effects in the current study using different databases, and the results were generally consistent.
Nonetheless, a number of limitations need consideration. First, in our study, MR analysis was conducted under the linear assumption to evaluate the causality between sexual factors and CVDs. We were unable, however, to test the non-linear effect with the present summary-level data. Future work should focus on non-linear MR analysis using individual-level data. Second, the partial overlap between sexual factors and CVD samples may create a weak instrument bias in our results. We applied the F-statistics to quantify weak IVs, and F-statistics for all IVs were above 10, thus avoiding weak instrument bias. Third, IVs for lifetime number of sexual partners explained only a small fraction of the phenotypic variance, with an R2 of 0.6%. Larger GWAS of lifetime number of sexual partners will facilitate MR studies with higher statistical power to examine CVD risks. Fourth, our study subjects were largely restricted to European ancestry, which restricts the generalizability of our results to other ancestry. It is necessary to validate our results in different ancestries. Finally, age at first sexual intercourse was causally associated with CVDs except for IS, but we could not absolutely exclude the possibility that the causal association between AFS and IS was not big enough to be detected even within the large sample. However, such a potential association would be exceedingly small and is unlikely to lead to a clinically relevant reduction of IS risk, as achieved by other strategies, such as smoking cessation, blood glucose, and lipid lowering.
According to the present study, age at first sexual intercourse could be causally associated with the risk of CVDs, except for IS, while no significant causal effect of CVDs on AFS was detected. Furthermore, LNSP showed no causal association with CVDs in bi-directional MR analysis. These conclusions can serve as a reference for adolescent sexuality education to aid sexually active adolescents in preventing the progression of CVDs.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
CZ: Conceptualization, Formal analysis, Supervision, Writing – review & editing. LC: Data curation, Writing – original draft. QW: Formal analysis, Writing – original draft. HC: Data curation, Writing – original draft. ZL: Data curation, Writing – original draft. HP: Writing – original draft. MW: Writing – original draft. YJ: Writing – review & editing. HJ: Data curation, Writing – original draft. LZ: Conceptualization, Supervision, Writing – review & editing.
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
We sincerely appreciate all the study authors for the summary data on GWASs for sexual factors and cardiovascular diseases.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1267906/full#supplementary-material
AFS, age at first sexual intercourse; LNSP, lifetime number of sexual partners; CVDs, cardiovascular diseases, MR, Mendelian randomization; CAD, coronary artery disease; MI, myocardial infarction; AF, atrial fibrillation; HF, heart failure; IS, ischemic stroke; ORs, odds ratios; CI, confidence interval; GWASs, genome-wide association studies; IVs, instrumental variables; SNPs, single-nucleotide polymorphisms; CARDIoGRAMplusC4D, Coronary Artery Disease Genome-wide Replication and Meta-analysis plus The Coronary Artery Disease Genetics; MR-PRESSO, MR pleiotropy residual sum and outlier
1. Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J. (2014) 35(42):2929. doi: 10.1093/eurheartj/ehu378
2. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. (2019) 139(10):e56–528. doi: 10.1161/CIR.0000000000000659
3. Lennon RP, Claussen KA, Kuersteiner KA. State of the heart: an overview of the disease burden of cardiovascular disease from an epidemiologic perspective. Prim Care. (2018) 45(1):1–15. doi: 10.1016/j.pop.2017.11.001
4. Rich-Edwards JW. Reproductive health as a sentinel of chronic disease in women. Womens Health. (2009) 5(2):101–5. doi: 10.2217/17455057.5.2.101
5. Yang L, Li L, Millwood IY, Peters SAE, Chen Y, Guo Y, et al. Age at menarche and risk of major cardiovascular diseases: evidence of birth cohort effects from a prospective study of 300,000 Chinese women. Int J Cardiol. (2017) 227:497–502. doi: 10.1016/j.ijcard.2016.10.115
6. Canoy D, Beral V, Balkwill A, Wright FL, Kroll ME, Reeves GK, et al. Age at menarche and risks of coronary heart and other vascular diseases in a large UK cohort. Circulation. (2015) 131(3):237–44. doi: 10.1161/CIRCULATIONAHA.114.010070
7. Muka T, Oliver-Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. (2016) 1(7):767–76. doi: 10.1001/jamacardio.2016.2415
8. Rosendaal NTA, Pirkle CM. Age at first birth and risk of later-life cardiovascular disease: a systematic review of the literature, its limitation, and recommendations for future research. BMC Public Health. (2017) 17(1):627. doi: 10.1186/s12889-017-4519-x
9. Peters SA, Woodward M. Women’s reproductive factors and incident cardiovascular disease in the UK Biobank. Heart. (2018) 104(13):1069–75. doi: 10.1136/heartjnl-2017-312289
10. Ngueta G, Ndjaboue R. Early sexual experience and hypertension in US adults: results from the National Health and Nutrition Examination Survey 2001–2016. J Hypertens. (2018) 36(12):2414–9. doi: 10.1097/HJH.0000000000001821
11. Fernandez V, Kramer T, Fong G, Doig A, Garralda ME. Depressive symptoms and behavioural health risks in young women attending an urban sexual health clinic. Child Care Health Dev. (2009) 35(6):799–806. doi: 10.1111/j.1365-2214.2009.00982.x
12. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27(8):1133–63. doi: 10.1002/sim.3034
13. Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. (2016) 27(11):3253–65. doi: 10.1681/ASN.2016010098
14. Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. (2006) 354(12):1264–72. doi: 10.1056/NEJMoa054013
15. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. (2017) 376(18):1713–22. doi: 10.1056/NEJMoa1615664
16. Li J, Guasch-Ferré M, Chung W, Ruiz-Canela M, Toledo E, Corella D, et al. The Mediterranean diet, plasma metabolome, and cardiovascular disease risk. Eur Heart J. (2020) 41(28):2645–56. doi: 10.1093/eurheartj/ehaa209
17. van de Luitgaarden IAT, van Oort S, Bouman EJ, Schoonmade LJ, Schrieks IC, Grobbee DE, et al. Alcohol consumption in relation to cardiovascular diseases and mortality: a systematic review of Mendelian randomization studies. Eur J Epidemiol. (2022) 37(7):655–69. doi: 10.1007/s10654-021-00799-5
18. Zanetti D, Tikkanen E, Gustafsson S, Priest JR, Burgess S, Birthweight IE. Type 2 diabetes mellitus, and cardiovascular disease: addressing the barker hypothesis with Mendelian randomization. Circ Genom Precis Med. (2018) 11(6):e002054. doi: 10.1161/CIRCGEN.117.002054
19. Wan EYF, Fung WT, Schooling CM, Au Yeung SL, Kwok MK, Yu EYT, et al. Blood pressure and risk of cardiovascular disease in UK biobank: a Mendelian randomization study. Hypertension. (2021) 77(2):367–75. doi: 10.1161/HYPERTENSIONAHA.120.16138
20. Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, et al. A comprehensive 1,000 genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. (2015) 47(10):1121–30. doi: 10.1038/ng.3396
21. Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. (2018) 50(9):1234–9. doi: 10.1038/s41588-018-0171-3
22. Shah S, Henry A, Roselli C, Lin H, Sveinbjörnsson G, Fatemifar G, et al. Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat Commun. (2020) 11(1):163. doi: 10.1038/s41467-019-13690-5
23. Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. (2018) 50(4):524–37. doi: 10.1038/s41588-018-0058-3
24. FinnGen_Consortium. FinnGen data freeze 5. Available at: https://www.finngen.fi/ (Accessed May 10, 2023).
25. Burgess S, Thompson SG, CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. (2011) 40(3):755–64. doi: 10.1093/ije/dyr036
26. Staley JR, Burgess S. Semiparametric methods for estimation of a nonlinear exposure-outcome relationship using instrumental variables with application to Mendelian randomization. Genet Epidemiol. (2017) 41(4):341–52. doi: 10.1002/gepi.22041
27. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40(4):304–14. doi: 10.1002/gepi.21965
28. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-egger regression: the role of the I2 statistic. Int J Epidemiol. (2016) 45(6):1961–74. doi: 10.1093/ije/dyw220
29. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50(5):693–8. doi: 10.1038/s41588-018-0099-7
30. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44(2):512–25. doi: 10.1093/ije/dyv080
31. Nikpay M, Mohammadzadeh S. Phenome-wide screening for traits causally associated with the risk of coronary artery disease. J Hum Genet. (2020) 65(4):371–80. doi: 10.1038/s10038-019-0716-z
32. Umberson D, Crosnoe R, Reczek C. Social relationships and health behavior across life course. Annu Rev Sociol. (2010) 36:139–57. doi: 10.1146/annurev-soc-070308-120011
33. Harden KP. True love waits? A sibling-comparison study of age at first sexual intercourse and romantic relationships in young adulthood. Psychol Sci. (2012) 23(11):1324–36. doi: 10.1177/0956797612442550
34. Uchino BN, Smith TW, Berg CA. Spousal relationship quality and cardiovascular risk: dyadic perceptions of relationship ambivalence are associated with coronary-artery calcification. Psychol Sci. (2014) 25(4):1037–42. doi: 10.1177/0956797613520015
35. Holt-Lunstad J, Birmingham W, Jones BQ. Is there something unique about marriage? The relative impact of marital status, relationship quality, and network social support on ambulatory blood pressure and mental health. Ann Behav Med. (2008) 35(2):239–44. doi: 10.1007/s12160-008-9018-y
36. De Bosscher R, Claeys M, Dausin C, Goetschalckx K, Claus P, Herbots L, et al. Three-dimensional echocardiography of the athlete’s heart: a comparison with cardiac magnetic resonance imaging. Int J Cardiovasc Imaging. (2023) 39(2):295–306. doi: 10.1007/s10554-022-02726-5
37. Mascia G, Arbelo E, Porto I, Brugada R, Brugada J. The arrhythmogenic right ventricular cardiomyopathy in comparison to the athletic heart. J Cardiovasc Electrophysiol. (2020) 31(7):1836–43. doi: 10.1111/jce.14526
38. Makenzius M, Larsson M. Early onset of sexual intercourse is an indicator for hazardous lifestyle and problematic life situation. Scand J Caring Sci. (2013) 27(1):20–6. doi: 10.1111/j.1471-6712.2012.00989.x
39. Guo G, Tong Y. Age at first sexual intercourse, genes, and social context: evidence from twins and the dopamine D4 receptor gene. Demography. (2006) 43(4):747–69. doi: 10.1353/dem.2006.0029
40. Rondou P, Haegeman G, Van Craenenbroeck K. The dopamine D4 receptor: biochemical and signalling properties. Cell Mol Life Sci. (2010) 67(12):1971–86. doi: 10.1007/s00018-010-0293-y
41. Sen S, Nesse R, Sheng L, Stoltenberg SF, Gleiberman L, Burmeister M, et al. Association between a dopamine-4 receptor polymorphism and blood pressure. Am J Hypertens. (2005) 18(9 Pt 1):1206–10. doi: 10.1016/j.amjhyper.2005.04.010
42. Chen M, Wang Z, Xu H, Chen X, Teng P, Ma L. Genetic liability to age at first sex and birth in relation to cardiovascular diseases: a Mendelian randomization study. BMC Med Genomics. (2023) 16(1):75. doi: 10.1186/s12920-023-01496-w
43. Wang Z, Lu J, Weng W, Zhang J. Women’s reproductive traits and cerebral small-vessel disease: a two-sample Mendelian randomization study. Front Neurol. (2023) 14:1064081. doi: 10.3389/fneur.2023.1064081
44. Larsson SC, Burgess S, Michaëlsson K. Smoking and stroke: a Mendelian randomization study. Ann Neurol. (2019) 86(3):468–71. doi: 10.1002/ana.25534
45. Jia Y, Wang R, Guo D, Sun L, Shi M, Zhang K, et al. Contribution of metabolic risk factors and lifestyle behaviors to cardiovascular disease: a Mendelian randomization study. Nutr Metab Cardiovasc Dis. (2022) 32(8):1972–81. doi: 10.1016/j.numecd.2022.04.019
46. Mullins N, Ingason A, Porter H, Euesden J, Gillett A, Ólafsson S, et al. Reproductive fitness and genetic risk of psychiatric disorders in the general population. Nat Commun. (2017) 8:15833. doi: 10.1038/ncomms15833
47. Nettle D, Clegg H. Schizotypy, creativity and mating success in humans. Proc Biol Sci. (2006) 273(1586):611–5. doi: 10.1098/rspb.2005.3349
48. Lawn RB, Sallis HM, Taylor AE, Wootton RE, Smith GD, Davies NM, et al. Schizophrenia risk and reproductive success: a Mendelian randomization study. R Soc Open Sci. (2019) 6(3):181049. doi: 10.1098/rsos.181049
49. Tropf FC, Stulp G, Barban N, Visscher PM, Yang J, Snieder H, et al. Human fertility, molecular genetics, and natural selection in modern societies. PLoS One. (2015) 10(6):e0126821. doi: 10.1371/journal.pone.0126821
50. Smith G D, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23(R1):R89–98. doi: 10.1093/hmg/ddu328
Keywords: age at first sexual intercourse, lifetime number of sexual partners, cardiovascular diseases, causal association, Mendelian randomization
Citation: Zhuo C, Chen L, Wang Q, Cai H, Lin Z, Pan H, Wu M, Jin Y, Jin H and Zheng L (2023) Association of age at first sexual intercourse and lifetime number of sexual partners with cardiovascular diseases: a bi-directional Mendelian randomization study. Front. Cardiovasc. Med. 10:1267906. doi: 10.3389/fcvm.2023.1267906
Received: 27 July 2023; Accepted: 3 November 2023;
Published: 7 December 2023.
Edited by:
Martin Bahls, Universitätsmedizin Greifswald, Germany© 2023 Zhuo, Chen, Wang, Cai, Lin, Pan, Wu, Jin, Jin and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liangrong Zheng MTE5MTA2NkB6anUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.