- 1Department of Cardiology, Huangshi Central Hospital, Affiliated Hospital of Hubei Polytechnic University, Huang Shi, China
- 2School of Medicine, Wuhan University of Science and Technology, Wuhan, China
Background: The benefits and risks of aspirin verse clopidogrel monotherapy in patients with coronary artery disease (CAD) remain controversial. This meta-analysis evaluated the efficacy and safety of aspirin verse clopidogrel monotherapy for long-term treatment in patients with CAD.
Methods: Literature was searched in the Pubmed, the Cochrane Library, and the Embase databases until March 2023. The Cochrane Risk of Bias Tool was used to assess the risk of bias in included studies. Data were extracted from the included studies, heterogeneity analysis, and pooled analysis conducted by RevMan 5.3 software.
Results: A total of five trials were included, involving 11, 766 patients with CAD. Compared with the aspirin group, the clopidogrel group was associated with reduced risk of major adverse cardiac and cerebrovascular events (MACCE) [risk ratio (RR) = 0.68, P = 0.0007], myocardial infarction (MI, RR = 0.66, P = 0.01), stroke (RR = 0.58, P = 0.003), and BARC major bleeding (RR = 0.63, P = 0.02). There were no significant differences in death from any cause (RR = 1.06, P = 0.59) and vascular death (RR = 0.92, P = 0.62) between the two groups.
Conclusions: Patients with CAD use clopidogrel could further reduce the risk of MACCE, MI, stroke, and BARC major bleeding, compared with the use of aspirin. This finding supported the use of clopidogrel rather than aspirin in patients with CAD who required long-term antiplatelet monotherapy for preventing ischemic events.
1. Introduction
Cardiovascular diseases are the main cause of death and disability worldwide (1). Coronary artery disease (CAD) is the main cause of cardiovascular event, including myocardial infarction, heart failure, and cardiovascular death. Antiplatelet therapy is the most important for preventing the occurrence of adverse cardiovascular events for patients with CAD. Aspirin, the first-line antithrombotic drug, has been preferred use for primary and secondary prophylaxis in populations at high risk for ischemic events for decades (2). However, a clinical trial indicated the primary prevention of aspirin has little or no benefit for vascular adverse events in the healthy elderly population, instead, it increases the risks of bleeding (3). Therefore, the clinical benefit of aspirin in patients with CAD needs to be further examined. Clopidogrel is the adenosine diphosphate-receptor blocker, has been used as an alternative in patients who could not tolerant aspirin or used in combination with aspirin as a dual antiplatelet therapy (DAPT) to provide a stronger platelet inhibitory effect for patients with acute coronary syndrome or undergoing percutaneous coronary intervention (PCI) (4). Interestingly, both our previous meta-analysis and recent clinical guideline have emphasized that early discontinuation of aspirin and continuation of P2Y12 inhibitor monotherapy for some patients after PCI can effectively reduce the risk of ischemic events, and did not increase the risk of bleeding (5, 6). However, controversy remains on the use of aspirin or clopidogrel monotherapy for long-term antiplatelet therapy in patients with CAD. Therefore, we conducted this meta-analysis intent to systematically compared the efficacy and safety of aspirin verse clopidogrel monotherapy for patients with CAD.
2. Materials and methods
2.1. Search strategy
Literature in English was searched in the Pubmed, the Cochrane Library, and the Embase databases until March 2023. Search terms were used as followed: (1) Aspirin vs. clopidogrel monotherapy and coronary artery disease; (2) Aspirin vs. clopidogrel and percutaneous coronary intervention; (3) Aspirin monotherapy and coronary artery disease; (4) clopidogrel monotherapy and coronary artery disease.
2.2. Selection criteria
The irrelevant publications and duplicates were removed by reading the title and abstract, then screened the leftover articles by reading the full text. The clinical trials included in this study had the following characteristics: (1) Comparing the effects of aspirin and clopidogrel in patients with CAD; (2) Outcomes reported in these articles included ischemic events and bleeding events; (3) Published in English. Other studies were excluded because as followed: (1) other types of articles including reviews, meta-analysis, letter, and retrospective analysis; (2) incompatible comparison; (3) no data available.
2.3. Data extraction and quality assessment
Two investigators (Dr. Liu and Dr. Xu) independently read these articles and extracted relevant data and assessed the risk of bias. If there is a difference of opinion emerged during this process, the discrepancies were resolved by Mrs. Zhao. The Cochrane Risk of Bias Tool was used to assess the risk of bias in included studies.
2.4. Statistical analysis
Data analysis was performed by Review Manager 5.3 software (Cochrane Collaboration, UK). RR with 95% confidence intervals (CI) was used to count the effects of each study. Heterogeneity among studies was assessed using the Q test and I2 statistics. A cut-off of I2 < 50% and P > 0.1 indicates low heterogeneity, and the FEM (fixed-effect model) was used to pool analyze the RR value of each study. I2 > 50% and P < 0.1 indicated significant heterogeneity, the REM (random effect model) was used to pool analysis RR value, and heterogeneity analysis and sensitivity analysis were conducted. To evaluate the consistency of our findings, the sensitivity analysis was by removing every single trial in order from the pooled analysis. P < 0.05 were considered a statistically significant difference.
3. Results
3.1. Identification and selection of study
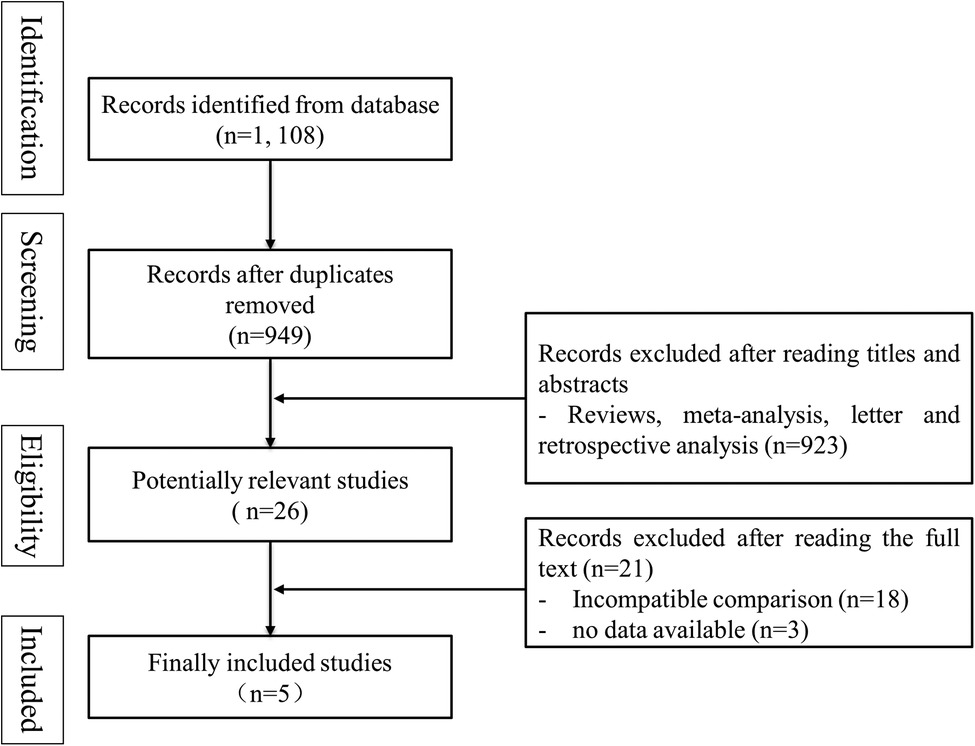
According to the search strategy, 1, 108 records were obtained from databases. 1, 082 records were excluded after reading titles and abstracts (159 articles were duplicates, and 923 articles were not clinical trials). A total of 26 articles were read in full, and finally, five trials were included for meta-analysis (7–11). The process of literature screening was shown in Figure 1.
3.2. Basic characteristics of included studies
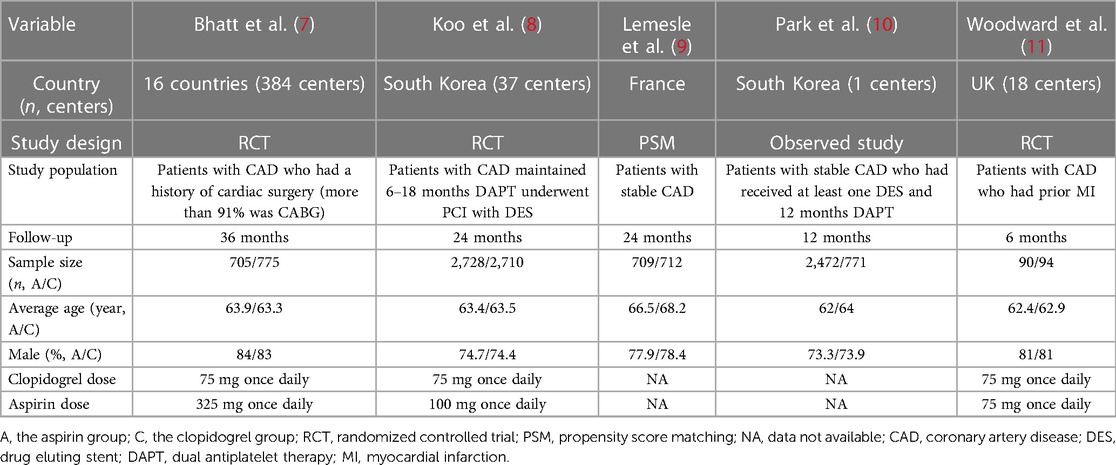
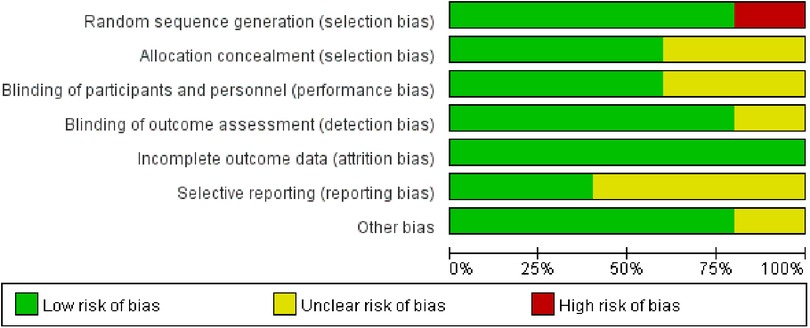
Five trials involved a total of 11,766 participants compared the benefits and risks of aspirin vs. clopidogrel in patients with CAD (7–11). The characteristics of these studies were summarized in Table 1. Except for the population of one trial was patients with stable coronary heart disease after coronary artery bypass grafting, the populations of other studies were patients with coronary heart disease after PCI (7). Moreover, the quality of the literature was analyzed and visualized by the Cochrane Risk of Bias Tool (Figure 2).
3.3. Efficacy outcomes
3.3.1. Major adverse cardiac and cerebrovascular events (MACCE)
MACCE was defined as a composite of death of all causes, myocardial infarction (MI), stroke, and target vessel revascularization. Three trials reported the MACCE, involving 10, 102 patients (8–10). Heterogeneity among these studies was at a high level (I2 = 72%, P = 0.03), thus the REM was used to further analysis. The result of pool analysis showed that the aspirin group has a similar risk of MACCE to the clopidogrel group [Risk ratio (RR) = 0.84, 95% CI: 0.55–1.27, P = 0.41], as shown in Figure 3A. To eliminate bias due to heterogeneity, we performed a sensitivity analysis. It was found that the result of one study was the main source of heterogeneity (9). After removing this study, the heterogeneity became very low and the pooled results showed clopidogrel could significantly decrease the risk of MACCE compared with the use of aspirin (RR = 0.68, 95% CI: 0.55–0.85, P = 0.0007), as shown in Figure 3B. These results suggested that the use of clopidogrel in the prevention of MACCE in patients with CAD is significantly better than the use of aspirin.
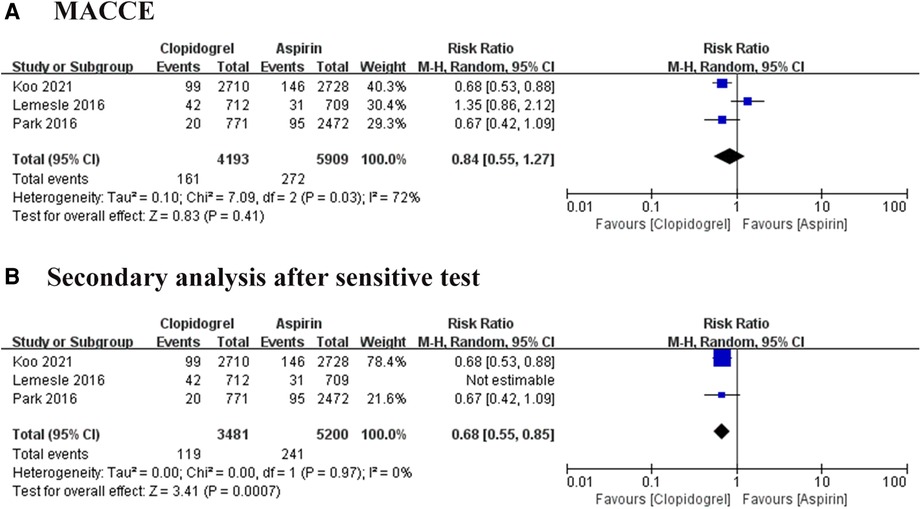
Figure 3. Forest plot of meta-analysis for MACCE. (A) Forest plot of meta-analysis for MACCE before heterogeneity analysis; (B) forest plot of meta-analysis for MACCE after heterogeneity analysis.
3.3.2. Death from any cause
All included trials reported the death of any cause, involving 11, 766 patients (7–11). We divided all studies into RCT subgroups or non-RCT subgroups according to the design of each study. Whatever the pooled analysis or subgroup analysis found a low heterogeneity among these trials, and no significant difference in death from any cause between the aspirin group and the clopidogrel group (RR = 1.06, 95% CI: 0.85–1.33, P = 0.59; Figure 4A). We also analyzed the publication bias, suggesting the effect calculated by meta-analysis was consistent with the effect of the intervention in included trials (Figure 4B).
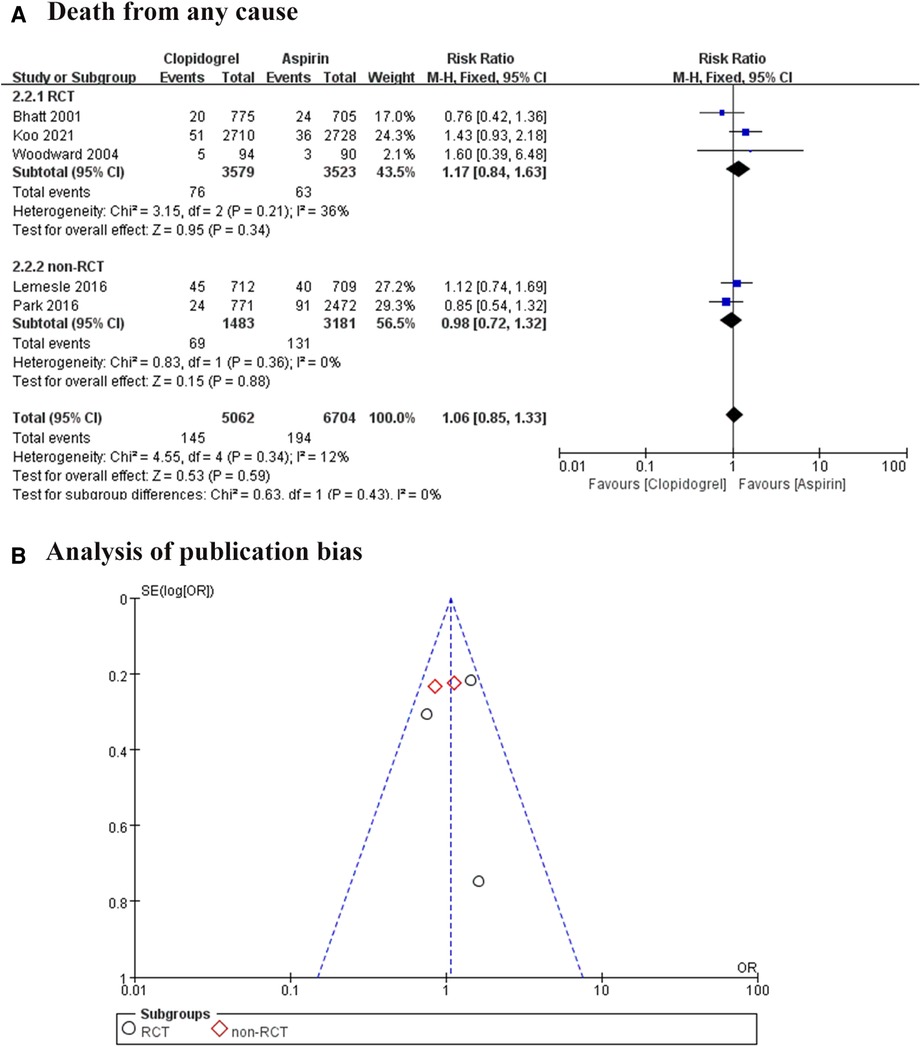
Figure 4. Forest plot and funnel plot of meta-analysis for death from any cause. (A) Forest plot of meta-analysis for death from any cause; (B) funnel plot of meta-analysis for death from any cause.
3.3.3. MI
Five studies followed 11, 766 patients, 107 times MI occurred in the aspirin group and 58 in the clopidogrel group (7–11). Heterogeneity analysis showed a low heterogeneity among the four trials (I2 = 0%, P = 0.50), suggesting a consistency of results. The FEM was used for pooled analysis, and the results significantly favor the clopidogrel group (RR = 0.66, 95% CI: 0.48–0.92, P = 0.01; Figure 5A).
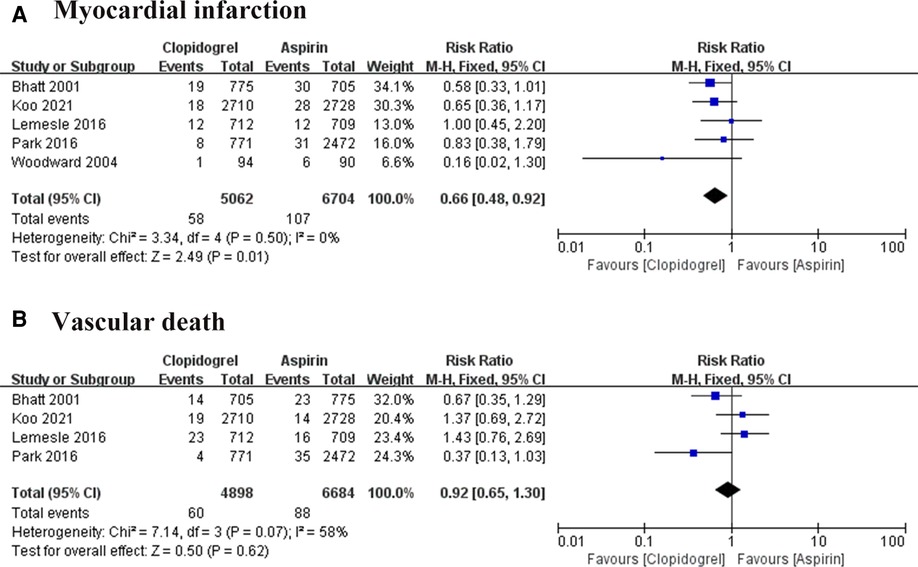
Figure 5. Forest plot of meat-analysis for MI and vascular death. (A) Forest plot of meta-analysis for MI; (B) forest plot of meta-analysis for vascular death.
3.3.4. Vascular death
Cardiac death was reported in four trials (7–10). Heterogeneity among these studies is I2 = 58%, P = 0.07, suggesting a higher heterogeneity. So, the effect sizes were combined using REM, and results in the forest plot showed there was no difference between the two groups in preventing cardiac death (RR = 0.92, 95% CI: 0.65–1.30, P = 0.62; Figure 5B).
3.3.5. Stroke
The stroke event was reported in four trials, involving 11, 582 patients (7–10). A high heterogeneity among these studies (I2 = 68%, P = 0.02), thus the REM was used for combined analysis. The result was shown in Figure 6A, there was no significant difference exist between the two groups, but one study showed a visible heterogeneity (9). A subsequent sensitivity analysis was conducted, confirming this study was the major source of heterogeneity (Figure 6B). After removing this article, the remarkable heterogeneity was eliminated (I2 = 8%, P = 0.34), and the results of the pooled analysis showed a significant difference between the aspirin group and the clopidogrel group (RR = 0.58, 95% CI: 0.40–0.84, P = 0.003).
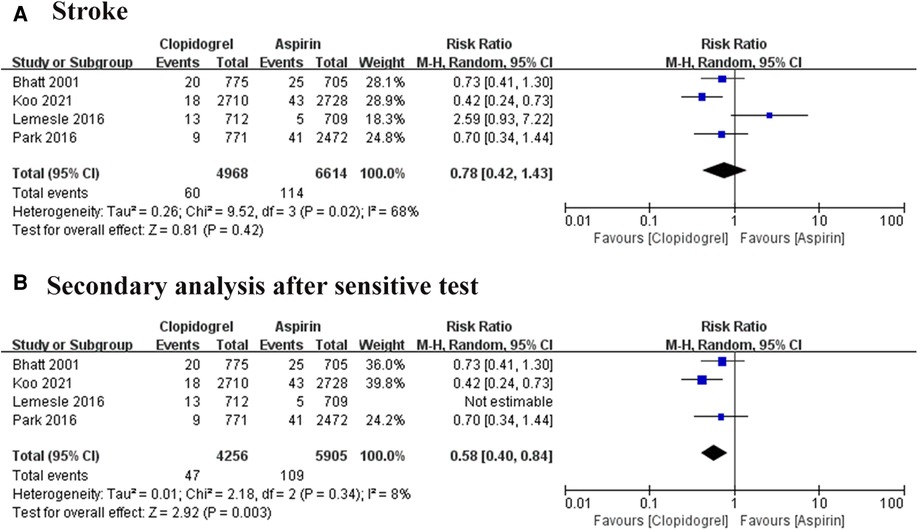
Figure 6. Forest plot of meta-analysis for stroke. (A) Forest plot of meta-analysis for stroke before heterogeneity analysis; (B) forest plot of meta-analysis for stroke after heterogeneity analysis.
3.4. Safety of outcomes: BARC major bleeding
Type 3–5 of BARC bleeding was defined as BARC major bleeding events. Three trials reported this event, involving 10, 102 participants, and a total of 152 positive events were reported (8–10). Heterogeneity among these trials is at a high level (I2 = 68%, P = 0.05), thus REM was used. The result showed no significant difference between the aspirin group and the clopidogrel group. However, the sensitive analysis found one article (10) is the major source of the heterogeneity, then remove this article. Interestingly, the second analysis showed a distinct result as shown in Figure 7 (RR = 0.63, 95% CI: 0.42–0.94, P = 0.02). This result favored the clopidogrel group and suggested aspirin could increase the 60% risk of BARC major bleeding compared with the use of clopidogrel.
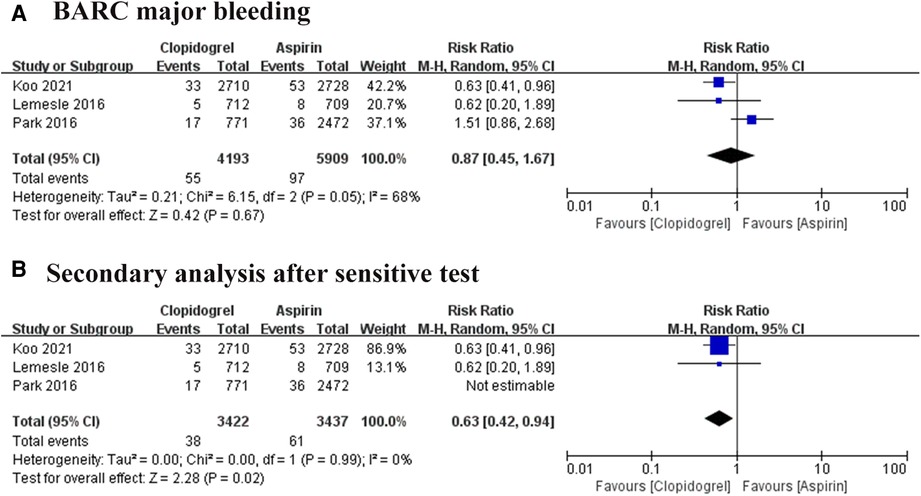
Figure 7. Forest plot of meta-analysis for BARC major bleeding. (A) Forest plot of meta-analysis for BARC major bleeding before heterogeneity analysis; (B) forest plot of meta-analysis for BARC major bleeding after heterogeneity analysis.
4. Discussion
In this meta-analysis, we included five studies involving 11, 766 participants with CAD who used aspirin or clopidogrel monotherapy for preventing ischemic events. We compared the efficacy and safety between the aspirin group and the clopidogrel group by heterogeneity analysis, sensitive analysis, and pooled analysis. The efficacy outcomes suggested that the use of clopidogrel is associated with lower risks of MACCE, MI, and stroke in patients with CAD compared with the use of aspirin. The safety outcomes suggested clopidogrel could drop the risk of BARC major bleeding down, compared with the aspirin group. Moreover, using aspirin or clopidogrel had a similar risk of death from any cause and vascular reason in patients with CAD. Given to the benefits of efficacy and safety of clopidogrel for patients with CAD is superior to that of aspirin, we recommend that clopidogrel be preferred as a sustained monotherapy antiplatelet therapy in the clinical scenario.
Guidelines recommend continual treatment with aspirin for preventing ischemic events in patients with CAD. After patients are treated with an adequate course of DAPT, they will be advised to discontinue the P2Y12 receptor inhibitor and continue taking aspirin. However, the ASPREE trial reported an unexpected result that 100 mg of aspirin daily as primary prevention for ischemic events in older healthy adults had a significantly higher risk of hemorrhage but did not reduce a significant risk of MACE (3). People cannot help but doubt the role of aspirin in the primary prevention of CAD in high-risk groups. Recently, the HOST-EXAM trial (8) found that clopidogrel, compared with aspirin, could reduce the risk of ischemic endpoint, all-cause death, BARC major bleeding, and any gastrointestinal complications in patients during the chronic maintenance period after PCI with DES implantation, suggesting that clopidogrel was superior to aspirin for secondary preventing in patients with CAD. Therefore, our study further included related studies on the use of aspirin or clopidogrel in patients with CAD to investigate the efficacy and safety of these two drugs in monotherapy for secondary prevention.
CARPRIE trial was the first RCT to investigate the effects of aspirin and clopidogrel in patients who had a recent ischemic stroke, recent myocardial infarction, or symptomatic peripheral arterial disease (12). The results indicated that long-term administration of clopidogrel for patients at higher ischemic risk is more effective than aspirin in reducing thrombotic composite endpoint. Moreover, antiplatelet therapy is associated with reduced perioperative myocardial infarction and bypass patency in patients undergoing coronary artery bypass grafting. Bhatt and his colleagues compared the use of aspirin or clopidogrel in patients after coronary artery bypass grafting, suggesting clopidogrel can reduce the risk of ischemic composite endpoint, along with a decreased risk of bleeding, compared with aspirin (11). In our study, we found that clopidogrel monotherapy could further reduce the risk of MACCE, MI, and stroke in patients with CAD compared with aspirin, showing the advantage of clopidogrel over aspirin in anti-ischemic events. These results are consistent with the findings of the CAPRIE trial (12) and the HOST-EXAM trial (8). The antiplatelet superiority of clopidogrel to aspirin could be explained by the results of the I-LOVE-MONO trial (13), which showed that clopidogrel could lead to better endothelial function, greater platelet inhibition, and lower coagulation activity. Given these studies and our results, we prefer the use of clopidogrel rather than aspirin in patients requiring long-term antiplatelet monotherapy for preventing ischemic events.
Studies have proven that extending the duration of DAPT failed to offer better anti-ischemic risk than 12-month DAPT, while potentially increasing bleeding events (14). But shortening the duration of DAPT to 1–3 months is associated with a similar ischemic risk to standard DAPT with a significantly lower risk of bleeding (15). It was suggested that more thrombotic adverse events occur within 3 months after PCI, but the bleeding risk is always associated with the use of antiplatelet drugs. In the current era of second-generation drug-eluting stents implantation and intensive statin, we need to pay more attention to the bleeding risks of patients using antiplatelet drugs in the long term. The COMPASS trial (16) reported noteworthy results that gastrointestinal bleeding events during antithrombotic therapy were associated with a nearly 20-fold increased risk of newly diagnosed gastrointestinal cancers (7.4% vs. 0.5%, HR = 20.6) and a 70% increased risk of other cancers (3.8% vs. 3.1%, HR = 1.70). A meta-analysis of 22 trials for aspirin vs. clopidogrel, suggested that aspirin increases the risk of gastrointestinal bleeding but no other bleeding (17). Although the number of studies reporting gastrointestinal bleeding events in our study was too small to be pooled for analysis, results from the real-world trial showed that patients taking aspirin had significantly higher gastrointestinal bleeding events than clopidogrel, which may potentially increase the risk of gastrointestinal tumors. Moreover, in our meta-analysis, the safety outcomes showed that clopidogrel could potentially decrease the BARC major bleeding event, although this difference becomes significant after removing sources of heterogeneity. Interestingly, our results are inconsistent with the safety analysis of Yuan et al. (18), because we included the latest study by Koo et al. (8) and performed a sensitivity analysis. More studies are needed to further compare the bleeding risks of aspirin and clopidogrel monotherapy in patients with CAD.
Our meta-analysis had some limitations. First, due to the few trials available to be included in this study, the heterogeneity of some results was high. Although we did a second analysis after heterogeneity and sensitivity analysis, our results may have a bias to some degree. Second, the population in this study was a mixed cohort of patients with CAD, including patients with stable CAD who did not receive revascularization, those who completed a standard duration of DAPT after PCI, and those who had undergone CABG, thus we could not perform subgroup analyses for this diversity due to our lack of direct access to patients' data. Third, data on minor bleeding and gastrointestinal bleeding were sufficient for pooled analyses because of differences in the observed outcomes of the included studies in this study. Therefore, more eligible studies were required to assess the efficacy and safety of aspirin and clopidogrel for personalized medicine in patients with CAD.
5. Conclusions
In conclusion, our results showed that clopidogrel could further reduce the risk of MACCE, MI, stroke, and BARC major bleeding in patients with CAD, compared with the use of aspirin. This finding supported the use of clopidogrel rather than aspirin in patients with CAD who required long-term antiplatelet monotherapy for preventing ischemic events.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
DJ: Writing – original draft, Writing – review & editing, Supervision. LZ: Writing – original draft, Writing – review & editing, Supervision. DL: Data curation, Software, Writing – review & editing. WX: Data curation, Investigation, Writing – review & editing. HX: Data curation, Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This research was funded by Research project of Natural Science Foundation of Hubei Province, grant number 2022CFB159; Foundation of Health Commission of Hubei Province, grant number WJ2023F061; Hubei Polytechnic University, grant number 22xjz06Q.
Acknowledgment
Thanks to all the peer reviewers for their opinions and suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bauersachs R, Zeymer U, Brière JB, Marre C, Bowrin K, Huelsebeck M. Burden of coronary artery disease and peripheral artery disease: a literature review. Cardiovasc Ther. (2019) 2019:8295054. doi: 10.1155/2019/8295054
2. Sabouret P, Savage MP, Fischman D, Costa F. Complexity of antiplatelet therapy in coronary artery disease patients. Am J Cardiovasc Drugs. (2021) 21:21–34. doi: 10.1007/s40256-020-00414-0
3. McNeil JJ M, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. (2018) 379:1509–18. doi: 10.1056/NEJMoa1805819
4. Chen H. Integrative medicine on optimizing clopidogrel and aspirin therapy. Chin J Integr Med. (2019) 25:395–400. doi: 10.1007/s11655-017-2551-4
5. Liu D, Li YZ, Wu H, Yang J, Yang J, Ding JW, et al. Duration of dual antiplatelet therapy after percutaneous coronary intervention with implantation of second-generation drug-eluting stent: a meta-analysis of randomized controlled trials. Pharmazie. (2020) 75:113–7. doi: 10.1691/ph.2020.0301
6. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 79: 197–215. doi: 10.1016/j.jacc.2021.09.005
7. Bhatt DL, Chew DP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Superiority of clopidogrel versus aspirin in patients with prior cardiac surgery. Circulation. (2001) 103:363–8. doi: 10.1161/01.cir.103.3.363
8. Koo BK, Kang J, Park KW, Rhee TM, Yang HM, Won KB, et al. Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): an investigator-initiated, prospective, randomised, open-label, multicentre trial. Lancet. (2021) 397:2487–96. doi: 10.1016/S0140-6736(21)01063-1
9. Lemesle G, Schurtz G, Meurice T, Tricot O, Lemaire N, Caudmont S, et al. Clopidogrel use as single antiplatelet therapy in outpatients with stable coronary artery disease: prevalence, correlates and association with prognosis (from the CORONOR study). Cardiology. (2016) 134:11–8. doi: 10.1159/000442706
10. Park TK, Song YB, Ahn J, Carriere KC, Hahn JY, Yang JH, et al. Clopidogrel versus aspirin as an antiplatelet monotherapy after 12-month dual-antiplatelet therapy in the era of drug-eluting stents. Circ Cardiovasc Interv. (2016) 9:e002816. doi: 10.1161/CIRCINTERVENTIONS.115.002816
11. Woodward M, Lowe GD, Francis LM, Rumley A, Cobbe SM, Study Investigators CADET. A randomized comparison of the effects of aspirin and clopidogrel on thrombotic risk factors and C-reactive protein following myocardial infarction: the CADET trial. J Thromb Haemost. (2004) 2:1934–40. doi: 10.1111/j.1538-7836.2004.01017.x
12. Steering Committee CAPRIE. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE steering committee. Lancet. (1996) 348:1329–39. doi: 10.1016/s0140-6736(96)09457-3
13. Park HW, Kang MG, Ahn JH, Bae JS, Tantry US, Gurbel PA, et al. Effects of monotherapy with clopidogrel vs. Aspirin on vascular function and hemostatic measurements in patients with coronary artery disease: the prospective, crossover I-LOVE-MONO trial. J Clin Med. (2021) 10:2720. doi: 10.3390/jcm10122720
14. Sim DS, Jeong MH, Kim HS, Gwon HC, Seung KB, Rha SW, et al. Dual antiplatelet therapy beyond 12 months versus for 12 months after drug-eluting stents for acute myocardial infarction. J Cardiol. (2020) 75:66–73. doi: 10.1016/j.jjcc.2019.06.006
15. Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med. (2019) 381:2032–42. doi: 10.1056/NEJMoa1908419
16. Eikelboom JW, Connolly SJ, Bosch J, Shestakovska O, Aboyans V, Alings M, et al. Bleeding and new cancer diagnosis in patients with atherosclerosis. Circulation. (2019) 140:1451–9. doi: 10.1161/CIRCULATIONAHA.119.041949
17. Mcquaid KR, Laine L. Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomized controlled trials. Am J Med. (2006) 119:624–38. doi: 10.1016/j.amjmed.2005.10.039
Keywords: coronary artery disease, antiplatelet monotherapy, aspirin, clopidogrel, efficacy, safety
Citation: Liu D, Xu WP, Xu H, Zhao L and Jin DQ (2023) Efficacy and safety of clopidogrel versus aspirin monotherapy for secondary prevention in patients with coronary artery disease: a meta-analysis. Front. Cardiovasc. Med. 10:1265983. doi: 10.3389/fcvm.2023.1265983
Received: 24 July 2023; Accepted: 3 October 2023;
Published: 17 October 2023.
Edited by:
Fabio Mangiacapra, Campus Bio-Medico University, ItalyReviewed by:
Abhinav Grover, Medical College of Wisconsin, United StatesRafal Adam Januszek, University Hospital in Krakow, Poland
© 2023 Liu, Xu, Xu, Zhao and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Zhao NDk5MzczOTUwQHFxLmNvbQ== Dao Qun Jin amluZGFvcXVuaHNAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Di Liu1,†
Di Liu1,† Wei Pan Xu
Wei Pan Xu Dao Qun Jin
Dao Qun Jin