
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 14 December 2023
Sec. Thrombosis and Haemostasis
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1260487
This article is part of the Research Topic Immune Thrombocytopenia (ITP) - Diagnosis and Treatment View all 8 articles
 Mohamed A. Yassin1*
Mohamed A. Yassin1* Mona Al-Rasheed2
Mona Al-Rasheed2 Murtadha Al-Khaboori3
Murtadha Al-Khaboori3 Mahmoud Marashi4
Mahmoud Marashi4 Hani Osman5
Hani Osman5 Yasser Wali6
Yasser Wali6 Salam Al Kindi3
Salam Al Kindi3 Faisal Alsayegh7
Faisal Alsayegh7 Drew Provan8
Drew Provan8
Introduction: Thrombopoietin-receptor agonist (TPO-RAs) currently represent the state of art for treating immune thrombocytopenia. Their different molecular structures contribute to the difference in their pharmacodynamics and pharmacokinetics. This narrative review aims to provide an overview of the current TPO-RAs approved for primary immune thrombocytopenia (romiplostim, eltrombopag, avatrombopag) and the effect of intermittent fasting in adult patients receiving TPO-RAs.
Areas covered: Literature was searched with no limits on date or language, using various combinations of keywords. Data on the pharmacokinetics, pharmacodynamics, efficacy, and safety of TPO-RAs and the effect of intermittent fasting were summarized.
Expert opinion: Switching between TPO-RAs is a useful strategy to tackle some associated limitations. Romiplostim and avatrombopag have an advantage over eltrombopag as they do not require any dietary restrictions. In cases where romiplostim and avatrombopag are unavailable, patients should be educated on the appropriate administration, possible interactions, and dietary restrictions before initiating eltrombopag.
Immune thrombocytopenia (ITP) is an autoimmune disease characterized by transient or persistent thrombocytopenia (platelet count <100 × 109/L) and increased bleeding tendency (1). The incidence of ITP among adults was estimated to be 3.3 per 100,000 adults per year (2). The clinical picture ranges from asymptomatic mild thrombocytopenia to severe life-threatening bleeding, including intracerebral hemorrhage (3). In addition, patients may present with petechiae (on the skin or mucus membranes), ecchymoses, and oral mucosal blood blisters (3). Moreover, patients with ITP often report fatigue and impaired health-related quality of life (HRQoL) (4).
The mechanisms of ITP are complicated and not well-understood (5). One proposed pathogenic mechanism is that the antibody-coated platelets are prematurely destroyed in the spleen, liver, or both (6). Another pathogenic mechanism suggests that autoantibodies can induce complement-mediated or desialylation-induced destruction of platelets (7, 8) and inhibit megakaryocyte function (9).
The ITP World Impact Survey (iWISh), an exploratory survey focusing on the impact of ITP on HRQoL from patient and physician perspectives, demonstrated that ITP has a substantial impact on HRQoL (10). Patients reported that ITP reduced their energy levels and productivity. In the same context, physicians defined three main treatment goals for ITP patients: reduced spontaneous bleeding, improved quality of life, and healthy blood counts (11). The goals of treatment of ITP recommended by the International Consensus Report on the investigation and management of ITP included preventing severe bleeding episodes, maintaining a target platelet level >20–30 × 109/L, minimizing treatment toxicity, and optimizing HRQoL (12). ITP management includes glucocorticoids, intravenous immune globulin, immunosuppressants, and occasionally platelet transfusions; withdrawal of anticoagulant and antiplatelet agents is advisable when the platelet count is low, <50 × 103/L.
Thrombopoietin-receptor agonists (TPO-RAs) are used for patients who fail to respond to glucocorticoids or have recurrent bleeds and decrease in platelet count after glucocorticoids are discontinued (13). The TPO-RAs romiplostim, eltrombopag, and avatrombopag are approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) to treat patients with insufficient response to prior treatment. The three TPO-RAs are also approved by and commercially available in the Gulf Cooperation Council countries. These agents have demonstrated a high response rate in clinical trials including patients with chronic ITP (14–16). Moreover, long-term treatment with TPO-RAs was associated with a significant improvement in HRQoL, particularly romiplostim (17–19) and eltrombopag (20, 21).
The different molecular structures of TPO-RAs contribute to the differences in their pharmacodynamics and pharmacokinetics. For example, romiplostim is administered subcutaneously; however, avatrombopag and eltrombopag are administered orally rendering them vulnerable to food-drug interaction. Interaction with food (particularly polyvalent cations) is attributed to the biphenyl hydrazone structure in eltrombopag; hence, dietary restriction is required (22). Food-drug interaction is one of the challenges in oral administration as food may impact drugs' release, absorption, distribution, and metabolism. On the other hand, pharmacodynamic drug-food interaction results in a particular pharmacological effect (23).
Fasting was found to be one of the factors that affect drugs' metabolism and response (24). Knowing the effects of fasting contributes to predicting drug response and to optimizing disease management. This paper aims to review the current TPO-RAs approved for primary ITP (romiplostim, eltrombopag, avatrombopag) and the effect of intermittent fasting in adult patients with primary ITP receiving TPO-RAs. Hetrombopag (a novel TPO-RA approved only in China for the treatment of ITP) was also included in our review. Lusutrombopag (approved for thrombocytopenia in patients with chronic liver disease) is outside the scope of our review.
The authors conducted a literature search, with no limits on date or language, using various combinations of keywords, including “immune thrombocytopenia”, “thrombopoietin receptor agonist”, “intermittent fasting”, “avatrombopag”, “eltrombopag”, “romiplostim”, and “hetrombopag”. Further references were identified by searching the reference lists of retrieved articles and from the authors' knowledge of the field. The authors concluded the manuscript with their expert opinion.
The pharmacokinetics and pharmacodynamics of romiplostim were evaluated in a double-blind, single-dose study. Healthy subjects received a single dose of placebo, subcutaneous romiplostim (a dose range of 0.1–2 µg/kg), or intravenous romiplostim (a dose range of 0.3–10 µg/kg). The achieved biologically active dose (defined as more than two-fold increase in platelet count) was 2 µg/kg subcutaneously and 1 µg/kg intravenously (25). There was a dose-dependent increase in the platelet count, which increased 1–3 days after intravenous administration, 4–9 days after subcutaneous administration, and peaking on days 12–16 (25). The half-life ranged from 1 to 34 days, with a median of 3.5 days (26). Romiplostim is eliminated primarily by the platelets and the mononuclear phagocytic cells, and has a biphasic distribution that was nonlinear with dose (25). Since the elimination of romiplostim is platelet-dependent, clearance and serum concentration are partly and inversely dependent on patient's platelet count (27, 28). Such a wide half-life range may interfere with dose-response predictability and dosing schedule.
Romiplostim, a peptibody (a structure composed of a biologically active peptide fused to the Fc region of an antibody) (29), binds to and stimulates TPO receptors found on cells such as megakaryocytes, platelets, stem cells, and all progenitor cells found in the bone marrow. This binding activates intracellular transcriptional pathways, ultimately stimulating megakaryopoiesis (26) (Figure 1).
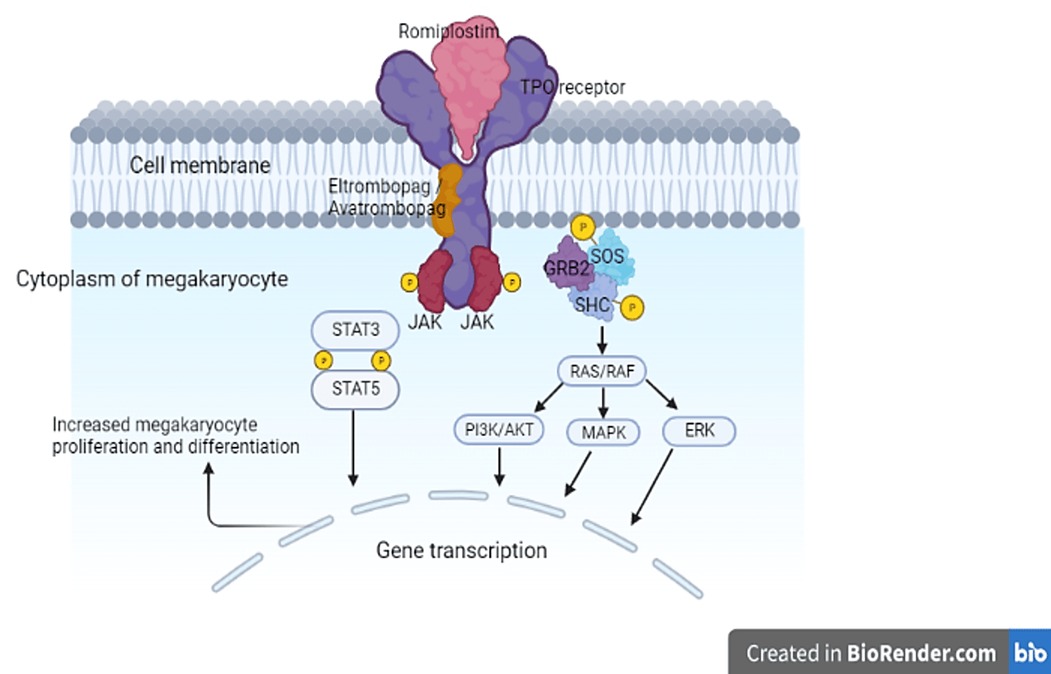
Figure 1. Mechanism of action of TPO-RAs. Binding of the TPO-RAs to the TPO receptor induces a conformational change in the receptor and the phosphorylation (activation) of the Janus kinases. Consequently, various signaling pathways are activated which result in increased platelet production. AKT, protein kinase B; ERK, extracellular-signal-regulated kinase; GRB2, growth factor receptor-binding protein 2; JAK, Janus kinase; MAPK, mitogen-activated protein kinase; P, phosphorylation; PI3K, phosphatidylinositol 3-kinase; RAF, rapidly accelerated fibrosarcoma kinase; RAS, rat sarcoma guanosine triphosphatase; SHC, Src homology collagen protein; SOS, son of sevenless; STAT, signal transducer and activator of transcription; TPO, thrombopoietin; TPO-RA, thrombopoietin-receptor agonist.
A large body of evidence supports the efficacy and safety of romiplostim in treating ITP. Several clinical trials of different phases showed that romiplostim increased platelet count sustainably and reduced the rate of bleeding events in adults with ITP (16, 30–36). In addition, results from observational and real-world studies were similar to those of clinical trials; data showed that romiplostim improved platelet count and reduced bleeding events and hospitalization (37, 38). Interestingly, some studies reported that patients had maintained a treatment-free response for a considerable duration (33, 39–42).
In a phase 2 trial, median time from first dose to peak platelet count was 18 days (range, 8–43) for 1 μg/kg dose, 19 days (range, 8–36) for 3 μg/kg, and 63 days (range, 7–78) for placebo (30). In a phase 3 trial including both splenectomized and nonsplenectomized adult patients, a target platelet count of ≥50 × 109/L was achieved by 25% of patients after 1 week and by 50% within 2–3 weeks of romiplostim administration (16). In another phase 3 trial, 76% of adults who received romiplostim responded (platelet count ≥50 × 109/L) at Week 2 (31).
A pooled analysis reported that 68% of splenectomized patients and 80% of nonsplenectomized patients attained a sustained platelet response (defined as platelet counts ≥50 × 109/L for 9 out of 12 weeks, with no use of rescue medication during the 4 weeks prior to each qualifying platelet count) (36).
In a prospective multicenter interventional study including adults with persistent or chronic primary ITP and complete response on TPO-RAs (romiplostim or eltrombopag), 27 of 48 patients achieved sustained response off treatment (platelet count >30 × 109/L and no bleeding), and 15 of 48 patients achieved sustained complete response off treatment (platelet count >100 × 109/L and no bleeding) at Week 24 (43). Moreover, in a multicenter observational study of 121 adult patients with ITP, 51.3% of patients receiving romiplostim achieved therapy-free response (platelet counts ≥50 × 109/L for at least 6 months in the absence of any therapies meant to increase platelet counts) (42).
Analysis of data integrated from nine studies conducted between 2002 and 2014 demonstrated that romiplostim increased platelet counts in patients with either ITP ≤1 year or ITP >1 year, with more treatment-free remission in those with ITP ≤1 year. Both romiplostim and placebo/standard of care had comparable safety profiles (44).
ITP-patient assessment questionnaire was used to investigate the impact of romiplostim therapy on HRQoL in two, placebo-controlled, phase 3 clinical trials of splenectomized and non-splenectomized patients. Data pooled from these clinical trials showed that romiplostim significantly improved HRQoL in adult patients with chronic ITP (18).
Romiplostim was well-tolerated and showed mild to moderate adverse events (AEs) in clinical trials. Short-term AEs were headache, fatigue, arthralgia, dizziness, insomnia, myalgia, pain in the extremity, abdominal pain, shoulder pain, dyspepsia, and paresthesia (16, 31, 34, 35, 45). The reported serious AEs were bleeding, thrombosis, increased bone marrow reticulin, and hematologic cancer or myelodysplastic syndromes (31, 35, 46). Bleeding was not deemed to be treatment-related (35, 46). Although there was no increased risk of thromboembolic complications associated with romiplostim, it should be used cautiously in patients with ITP who have a history of, or are at increased risk of, thromboembolic complications (36, 45, 47–50).
Few studies reported that some patients developed neutralizing antibodies to romiplostim; however, these antibodies were not associated with a clinical complication or reduction in platelet count (51, 52).
The pharmacokinetics of eltrombopag were dose-dependent and linear (53). Its absorption is reduced when administered with products containing polyvalent cations (e.g., antacids), calcium-rich foods, and mineral supplements (iron, calcium, aluminum, magnesium, selenium, zinc), high-fat meals (54, 55). Therefore, it is recommended to administer eltrombopag under fasting conditions, with low-calcium foods only, or four hours after and two hours before food and products containing polyvalent cations (e.g., antacids, mineral supplements and dairy products) (56). Eltrombopag is mainly metabolized in the liver and no clinically significant interactions were detected when it is co-administered with cytochrome P450 (CYP) substrates, inducers, or inhibitors (55).
Eltrombopag is a small non-peptide molecule that binds to the transmembrane domain of the TPO receptor which is expressed on the surface of stem cells and megakaryocytes (57, 58). Binding to the TPO receptor activates intracellular signal transduction pathways that increase the proliferation and differentiation of human bone marrow progenitor cells leading ultimately to the production of normal platelets (59–61) (Figure 1). Eltrombopag interacts synergistically with endogenous TPO rather than competing for its binding site (62). In a phase 1 clinical trial, eltrombopag showed a consistent increase in platelet count starting after 8 days of repeated dosing with a peak platelet count achieved at day 16 (53).
The efficacy and safety of eltrombopag were evaluated in several trials which are summarized in Table 1.
A dose-finding phase 2 trial was conducted to determine the optimal dose of eltrombopag. Patients with chronic ITP were randomized into four groups receiving 30, 50, and 75 mg of eltrombopag, or placebo daily for 6 weeks. The primary efficacy endpoint was a platelet count greater than 50 × 109/L or more on day 43. The response rate was 70% and 81% in groups receiving 50 and 75 mg of eltrombopag respectively. It was significantly greater than that observed in the placebo group (11%) and sustained over the entire duration of treatment. The response rate in the 30 mg group (28%) did not differ significantly from that of the placebo group. Both the 50 and 75 mg groups showed a reduction in bleeding symptoms (15).
A phase 3 randomized, double-blind clinical trial was conducted where patients received either 50 mg of eltrombopag or a placebo for up to 6 weeks. The primary endpoint was similar to that used in the dose-finding study. The response rate in the eltrombopag group (59%) was significantly greater than that of the placebo group (16%). In addition, patients in the eltrombopag group showed fewer bleeding symptoms during the treatment duration. After treatment discontinuation, platelet counts returned to baseline in the following weeks and the percentage of patients presenting bleeding symptoms increased (63).
The RAISE trial was a double-blind phase 3 study evaluating the safety and efficacy of eltrombopag for a prolonged treatment duration (6 months) (20). The primary endpoint was the odds of reaching a platelet count of 50–400 × 109/L. It was significantly greater in the eltrombopag group than in the placebo group throughout the 6-month treatment period. In addition, the rates of bleeding and clinically significant bleeding were significantly lower in the eltrombopag group. However, platelet counts returned to baseline after discontinuation of eltrombopag.
The REPEAT trial (an open-label, single-arm, repeat-dose phase 2 study) investigated the effectiveness of repeated short-term cycles of eltrombopag (64). Recruited patients received 50 mg of eltrombopag daily over 3 cycles, each consisting of up to 6 weeks on-therapy followed by up to 4 weeks off therapy. A response to treatment was defined as a platelet count of more than 50 × 109/L and at least twice the baseline count at Day 43 of the treatment cycle, and the primary endpoint was the proportion of patients with a response in Cycle 1 who subsequently responded in Cycles 2 or 3. In Cycle 1, 80% of patients responded of whom 87% responded in Cycles 2 or 3% and 71% in Cycles 2 and 3.
The open-label EXTEND study evaluated long-term safety and efficacy of eltrombopag in adults with ITP who had completed a previous eltrombopag study (65). The primary endpoints were safety and tolerability parameters, including clinical laboratory tests, ocular examinations, and frequency of all AEs. The secondary endpoints included the proportion of patients who achieved platelet count of at least 50 × 109/L at least once during treatment. The median duration of eltrombopag treatment was 2.4 years and the study follow up patients for around 8 years. Around 86% of patients achieved a platelet count of at least 50 × 109/L at any time during the study period; more than half (52%) of patients achieving a count higher than 50 × 109/L maintained a continuous platelet count of 50 × 109/L or more for at least 25 weeks in the absence of rescue therapy.
A phase 2 trial explored the sustained remission off treatment achieved by adult patients with newly diagnosed or persistent primary ITP treated with eltrombopag. The primary endpoint was the proportion of subjects who achieved sustained remission off treatment defined as the proportion of responders that were able to taper and discontinue eltrombopag maintaining a platelet count of at least 30 × 109/L during a period of observation of 24 weeks with no bleeding nor administration of other ITP medications. The rate of sustained remission off treatment was 25% in patients who started eltrombopag (66).
Overall, eltrombopag was well-tolerated and safe, and important AEs (thrombosis, hepatobiliary AEs, and bone marrow fibrosis) were infrequent. Headache was the most frequent AE in all eltrombopag trials. All studies showed no significant changes in blood coagulation and platelet aggregation, and electrocardiographic findings (QT prolongation) (55, 67). Eltrombopag use is associated with elevations in alanine aminotransferase and bilirubin which may resolve despite ongoing treatment (20). The safety profile of eltrombopag is satisfactory. However, patients should be closely monitored for thromboembolic events or hepatic damage (68).
Avatrombopag is administered orally. After a single-dose administration, the increase in platelet count was dose-dependent, the maximum concentration was achieved within 6–8 h, and the half-life was 16–19 h (69). Avatrombopag is metabolized mainly via the hepatic enzymes CYP2C9 and CYP3A4 (70). It is mainly excreted through feces (88%) while only 6% is excreted in urine (69). Unlike eltrombopag (dietary restriction is required around the dose), dietary fat and divalent cations (e.g., calcium) do not impact the absorption of avatrombopag (22, 69). The administration of avatrombopag with meals reduces pharmacokinetic variability and does not affect the rate or extent of its absorption (69).
In vitro and in vivo studies showed that avatrombopag mimics, to a certain extent, the effect of endogenous TPO through stimulating megakaryocyte development and megakaryocyte colony-formation from human CD34 + hematopoietic cells. Further studies of avatrombopag in humans have shown that a single dose of the drug leads to a dose-dependent increase in platelet count, which reached its maximum from baseline in 8–11 days, then returned to baseline at approximately 4 weeks after the dose (69). Avatrombopag works synergistically with endogenous TPO. For example, combining avatrombopag in with recombinant human TPO increased platelet count by up to 200% more than TPO alone (71).
A phase 2 double-blind, randomized dose-ranging, placebo-controlled parallel-group study recruited patients to randomly receive either a once-daily fixed-dose of avatrombopag (2.5 mg, 5 mg, 10 mg, or 20 mg) or placebo and received treatment for 28 days (72). The primary endpoint was a platelet count response defined as the proportion of patients who achieved platelet count ≥50 × 109/L and a minimum increase of 20 × 109/L above baseline at day 28. The response rates of the avatrombopag group were 13%, 53%, 50%, and 80% in the 2.5, 5, 10, and 20 mg groups, respectively, compared to a 0% response rate in the placebo group. The objective of the extension part of the study was to assess the safety and tolerability of avatrombopag. Results of the extension part showed that 52% and 76% had a durable (platelet count response at ≥75% of their platelet assessments over the last 14 weeks) and overall (stable response or response at any ≥2 consecutive visits) response, respectively.
A second study was conducted to evaluate the efficacy and safety of avatrombopag in chronic ITP patients (14). It was a 6-month multicenter phase 3 trial; adult patients were randomly assigned to receive either 20 mg of avatrombopag daily or a placebo. The primary endpoint was the cumulative number of weeks with a platelet count of at least 50 × 109/L without rescue therapy. It was significantly longer in the avatrombopag group (mean of 12 weeks) compared to the placebo group (mean of 0.1 weeks). Efficacy was sustained in the long-term extension part of the study. A post hoc analysis was conducted to evaluate the durability of platelet count response to avatrombopag in different subgroups of patients who were enrolled in this phase 3 multicenter trial. The analysis showed that response to avatrombopag was stable and durable despite having some variation in patients' characteristics (73).
In the two studies, avatrombopag appeared to be safe and tolerable. The most frequent AEs reported in the phase 2 trial were epistaxis, headache, fatigue, and confusion (72). On the other hand, the most common treatment-emergent AE reported in the phase 3 trial were headache, contusion, upper respiratory tract infection, arthralgia, epistaxis, fatigue, gingival bleeding, and petechiae (14).
The clinical characteristics and outcomes following avatrombopag initiation in adult ITP patients were described in a retrospective study conducted in the USA. In this recent real-world study, ITP patients achieved clinically meaningful platelet count with avatrombopag without the need of rescue medication; many were able to discontinue baseline concomitant steroid or immunosuppressants (74).
Oral hetrombopag demonstrated a high inter-individual pharmacokinetic variability in both healthy subjects and ITP patients. In healthy subjects, hetrombopag plasma concentration showed two peaks, and bioavailability was markedly reduced by high-fat and high-calorie food. Consequently, hetrombopag should be administered on an empty stomach to avoid drug-food interaction which ultimately decrease pharmacodynamic activity. The administration of hetrombopag in ITP patients showed a prolonged absorption and steady state was achieved around Day 10. The elimination half-life ranged between 23.2 and 39.8 h. Hetrombopag is extensively metabolized and the predominant route of excretion is via feces (89.05%) (75–78).
Hetrombopag is an oral non-peptide TPO-RA. Similar to eltrombopag and avatrombopag, hetrombopag binds to the transmembrane domain of human TPO receptor on progenitor cells and induces megakaryopoiesis. Hence, hetrombopag does not compete with the action of native thrombopoietin. The interaction between hetrombopag and the transmembrane domain of human TPO receptor activates intracellular thrombopoietin signaling pathways which ultimately induce platelet proliferation (78, 79).
The efficacy and safety of hetrombopag in ITP patients were evaluated in a randomized, multicenter, placebo-controlled phase 3 study. Patients were randomized to receive 2.5 mg or 5 mg (to a maximum dose of 7.5 mg) hetrombopag once daily, or placebo for 10 weeks. The primary endpoint was the proportion of responders defined as patients achieving a platelet count of ≥50 × 109/L after 8 weeks of treatment. The primary endpoint was significantly achieved in the 2.5 mg hetrombopag group (58.9%) and the 5 mg hetrombopag group (64.3%) vs. the placebo group (5.9%) (p < 0.0001). Platelet response to hetrombopag was durable and maintained after the 14-week extension period (80).
After the 10-week treatment period, the most common adverse events were upper respiratory tract infection, urinary tract infection, elevated platelet counts, hematuria, increased blood level of lactate dehydrogenase, and diarrhea. The reported serious adverse events were thrombocytopenia, gastrointestinal hemorrhage, and cerebral hemorrhage. However, none of the hemorrhagic episodes in the hetrombopag group were treatment-related (80).
Hetrombopag is currently approved in China for the treatment of primary ITP in adult patients who have failed to respond to other lines of treatment such as glucocorticoids and immunoglobulins (81).
Intermittent fasting is the voluntary abstinence from food and drinks for an extended period (e.g., 16–48 h) with intervening periods of regular food intake, on a recurring basis (82, 83). Different types of intermittent fasting were shown to impact health outcomes such as complete alternate-day fasting, time-restricted feeding, and religious fasting (e.g., Ramadan Fasting) (82).
Fasting affects the four processes of pharmacokinetics: absorption, distribution, metabolism, and excretion (84). A fast-break meal rich in carbohydrates, fats, and proteins may impact the rate and extent of drug absorption and elimination (84). For instance, the ability of eltrombopag to chelate with polyvalent cations, reduced plasma eltrombopag exposure by 70% when co-administered with an antacid containing metal cations (aluminum hydroxide and magnesium carbonate) (22).
The administration of romiplostim is considered convenient for ITP patients since it is given as a once-weekly subcutaneous injection with no dietary or time restriction (85). In addition, romiplostim has no drug-food interaction (3) and can be considered for ITP patients during Ramadan fasting, particularly if home self-injections are available (86). On the other hand, romiplostim may be inconvenient for some patients since, upon treatment initiation, more frequent clinic visits are required until platelet counts are stabilized. In addition, some patients may prefer oral route over injections (86).
Eltrombopag is an oral TPO-RA. However, it showed significant drug-food interaction; its bioavailability was reduced by 70% with aluminum hydroxide and magnesium carbonate antacid tablets compared to the fasting state (22). The bioavailability of eltrombopag was also reduced by 60% when administered with high-calcium dairy products, irrespective of fat content. The reduced bioavailability of eltrombopag will diminish its effect on platelet count. Therefore, patients should be aware of products containing metal cations (such as calcium, aluminum, iron, magnesium, selenium, or zinc) which should be taken at a different time of the day spaced from eltrombopag (22).
Eltrombopag must be administered on an empty stomach; it should be taken four hours after and two hours before food or products containing polyvalent cations (56). Some studies recommended a 4- to 6-hour fasting window around the administration of eltrombopag to separate it from polyvalent cations (e.g., antacids, dairy products, multivitamins) (22, 87). A retrospective study evaluating the effect of Ramadan fasting on patients with ITP receiving eltrombopag showed that platelet count dropped significantly during fasting and returned back to normal after Ramadan ended (88). In daily practice, patients may consider administering eltrombopag before bedtime during Ramadan fasting. Hence, healthcare providers must be aware of such practice as it may affect platelet count and increase bleeding risk (88).
Avatrombopag is another orally administered TPO-RA. Unlike eltrombopag, avatrombopag has no drug-food interaction and does not require dietary restriction around its administration (12, 89). Different studies showed that the administration of avatrombopag with food did not affect the rate or extent of its absorption; however, administration with food reduced intra- and inter-subject variability (69, 70). Therefore, it is recommended to administer avatrombopag with food (90). Interestingly, a study showed that around 51% of patients had switched from eltrombopag to avatrombopag due to its convenient administration without any dietary restrictions (89).
In general, TPO-RAs (either romiplostim or eltrombopag) are considered a second-line therapy for steroid-unresponsive chronic ITP patients (91). In the absence of head-to-head comparisons, there is no rationale to rank among these TPO-RAs. However, for fasting ITP patients in particular, we suggest that eltrombopag is not the preferred choice because drug-food interaction affects its bioavailability. In addition, the consequent dietary restriction creates a challenge for eltrombopag patients regarding adherence and quality of life. For instance, after being fasted for several hours (e.g., Ramadan fasting), it may not be convenient to administer eltrombopag with the fast-break meal.
Switching between TPO-RAs is a useful strategy to tackle some associated limitations including route of administration and dietary restriction. Romiplostim and avatrombopag have an advantage over eltrombopag as they do not require any dietary restrictions. We advise our patients with intermittent fasting to switch to romiplostim or avatrombopag if available. Since avatrombopag is administered orally, it may be preferred over romiplostim, injected subcutaneously. On the other hand, unlike romiplostim, avatrombopag is administered daily and not weekly. The choice of either drug will be based eventually on the patient's convenience and adherence.
In addition to fasting, many patients have erratic lifestyles because of shift working patterns, international travel and changes in time zones, and teenagers eating late at night; for these individuals, a treatment where strict timing of administration is required will often fail and compliance issues arise. These groups would also benefit from a convenient medication with no dietary restriction or refrigeration such as avatrombopag.
Since the three TPO-RAs have comparable safety and efficacy profiles, drug cost and availability could interfere in drug selection. In cases where romiplostim and avatrombopag are unavailable, patients should be educated on the appropriate administration, possible interactions, and dietary restrictions before initiating eltrombopag. Eltrombopag should be administered with a 4-hour fasting window to achieve optimal disease outcomes (Figure 2).
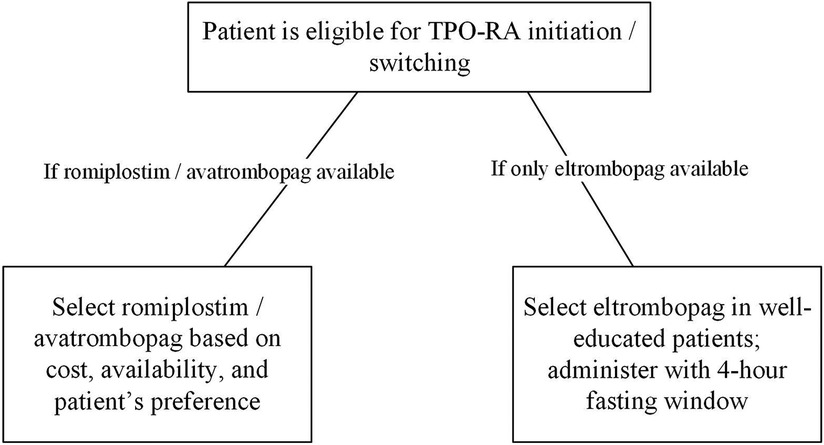
Figure 2. TPO-RAs selection in ITP patients. ITP, immune thrombocytopenia; TPO-RA, thrombopoietin-receptor agonist.
MY: Conceptualization, Project administration, Writing – original draft, Writing – review & editing. MA-R: Writing – original draft, Writing – review & editing. MA-K: Writing – original draft, Writing – review & editing. MM: Writing – original draft, Writing – review & editing. HO: Writing – original draft, Writing – review & editing. YW: Writing – original draft, Writing – review & editing. SA: Writing – original draft, Writing – review & editing. FA: Writing – original draft, Writing – review & editing. DP: Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
The authors wish to thank Mohammad Yassine and Racha Aaraj from Phoenix Clinical Research for the support in the preparation of this manuscript.
MY declares having received research grants from SOBI. MA-R declares having received fees for consultancies from SOBI and Amgen; lectures from SOBI, Amgen, and Novartis; clinical studies from SOBI and Novartis. MA-K declares having received fees for consultancies and lectures from SOBI, Novartis, Pfizer, and Amgen; and clinical studies from Pfizer and Novartis. MM declares having received fees for consultancies and lectures from Amgen, Novartis and Sobi. HO declares having received fees for consultancies from SOBI, Amgen, Janssen, and Takeda; lectures from SOBI, Roche, Novo Nordisk, Janssen, Novartis, and Takeda; and clinical studies from Amgen and Takeda. YW declares having received fees for consultancies from Novartis; lectures from Novartis, Novo Nordisk, Pfizer, Amgen, SOBI, and Takeda; clinical studies and research grants from Novartis, GBT, and Pfizer; and travel support to conferences from Pfizer, SOBI, and Emmaus. SA declares having received fees for consultancies from Novartis, Pfizer, GBT, Emmaus, and BMS. FA declares having received fees for consultancies from Amgen, SOBI, Sanofi, Bayer, Boehringer Ingelheim, Pfizer, Novo Nordisk, Novartis, and Bristol Myers Squibb; lectures from Sanofi, SOBI, Amgen, Bayer, Boehringer Ingelheim, and Novo Nordisk; and clinical studies and research grants from Sanofi, Amgen, SOBI, Novo Nordisk. DP declares having received fees for consultancies from UCB, MedImmune, ONO, SOBI, Argenx, and Takeda; lectures from Amgen, Novartis, SOBI, Grifols, and Argenx; and research grants from Amgen, Novartis, and Rigel. This study received funding from SOBI. The funder had the following involvement in the study: editorial and medical writing assistance for the preparation of this manuscript based on the Good Publication Practice (GPP 2022) and the ICMJE requirements.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The views and opinions expressed are those of the authors. SOBI has had no influence over the content of the paper.
1. Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. (2009) 113(11):2386–93. doi: 10.1182/blood-2008-07-162503
2. Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. (2010) 85(3):174–80. doi: 10.1002/ajh.21616
3. Al-Samkari H, Kuter DJ. Immune thrombocytopenia in adults: modern approaches to diagnosis and treatment. Semin Thromb Hemost. (2020) 46(3):275–88. doi: 10.1055/s-0039-1700512
4. Efficace F, Mandelli F, Fazi P, Santoro C, Gaidano G, Cottone F, et al. Health-related quality of life and burden of fatigue in patients with primary immune thrombocytopenia by phase of disease. Am J Hematol. (2016) 91(10):995–1001. doi: 10.1002/ajh.24463
5. Cooper N, Ghanima W. Immune thrombocytopenia. N Engl J Med. (2019) 381(10):945–55. doi: 10.1056/NEJMcp1810479
6. Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J Clin Med. (2017) 6(2):16. doi: 10.3390/jcm6020016
7. Stasi R, Cooper N, Del Poeta G, Stipa E, Laura Evangelista M, Abruzzese E, et al. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood. (2008) 112(4):1147–50. doi: 10.1182/blood-2007-12-129262
8. Najaoui A, Bakchoul T, Stoy J, Bein G, Rummel MJ, Santoso S, et al. Autoantibody-mediated complement activation on platelets is a common finding in patients with immune thrombocytopenic purpura (ITP). Eur J Haematol. (2012) 88(2):167–74. doi: 10.1111/j.1600-0609.2011.01718.x
9. McMillan R, Wang L, Tomer A, Nichol J, Pistillo J. Suppression of in vitro megakaryocyte production by antiplatelet autoantibodies from adult patients with chronic ITP. Blood. (2004) 103(4):1364–9. doi: 10.1182/blood-2003-08-2672
10. Cooper N, Kruse A, Kruse C, Watson S, Morgan M, Provan D, et al. Immune thrombocytopenia (ITP) world impact survey (I-WISh): impact of ITP on health-related quality of life. Am J Hematol. (2021) 96(2):199–207. doi: 10.1002/ajh.26036
11. Cooper N, Kruse A, Kruse C, Watson S, Morgan M, Provan D, et al. Immune thrombocytopenia (ITP) world impact survey (iWISh): patient and physician perceptions of diagnosis, signs and symptoms, and treatment. Am J Hematol. (2021) 96(2):188–98. doi: 10.1002/ajh.26045
12. Provan D, Arnold DM, Bussel JB, Chong BH, Cooper N, Gernsheimer T, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. (2019) 3(22):3780–817. doi: 10.1182/bloodadvances.2019000812
13. Ghanima W, Godeau B, Cines DB, Bussel JB. How I treat immune thrombocytopenia: the choice between splenectomy or a medical therapy as a second-line treatment. Blood. (2012) 120(5):960–9. doi: 10.1182/blood-2011-12-309153
14. Jurczak W, Chojnowski K, Mayer J, Krawczyk K, Jamieson BD, Tian W, et al. Phase 3 randomised study of avatrombopag, a novel thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia. Br J Haematol. (2018) 183(3):479–90. doi: 10.1111/bjh.15573
15. Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, Meddeb B, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. (2007) 357(22):2237–47. doi: 10.1056/NEJMoa073275
16. Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet Lond Engl. (2008) 371(9610):395–403. doi: 10.1016/S0140-6736(08)60203-2
17. Sanz MA, Aledort L, Mathias SD, Wang X, Isitt JJ. Analysis of EQ-5D scores from two phase 3 clinical trials of romiplostim in the treatment of immune thrombocytopenia (ITP). Value Health J Int Soc Pharmacoeconomics Outcomes Res. (2011) 14(1):90–6. doi: 10.1016/j.jval.2010.10.017
18. George JN, Mathias SD, Go RS, Guo M, Henry DH, Lyons R, et al. Improved quality of life for romiplostim-treated patients with chronic immune thrombocytopenic purpura: results from two randomized, placebo-controlled trials. Br J Haematol. (2009) 144(3):409–15. doi: 10.1111/j.1365-2141.2008.07464.x
19. Kuter DJ, Mathias SD, Rummel M, Mandanas R, Giagounidis AA, Wang X, et al. Health-related quality of life in nonsplenectomized immune thrombocytopenia patients receiving romiplostim or medical standard of care. Am J Hematol. (2012) 87(5):558–61. doi: 10.1002/ajh.23163
20. Cheng G, Saleh MN, Marcher C, Vasey S, Mayer B, Aivado M, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet Lond Engl. (2011) 377(9763):393–402. doi: 10.1016/S0140-6736(10)60959-2
21. Saleh MN, Bussel JB, Cheng G, Meyer O, Bailey CK, Arning M, et al. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: results of the long-term, open-label EXTEND study. Blood. (2013) 121(3):537–45. doi: 10.1182/blood-2012-04-425512
22. Williams DD, Peng B, Bailey CK, Wire MB, Deng Y, Park JW, et al. Effects of food and antacids on the pharmacokinetics of eltrombopag in healthy adult subjects: two single-dose, open-label, randomized-sequence, crossover studies. Clin Ther. (2009) 31(4):764–76. doi: 10.1016/j.clinthera.2009.04.010
23. Koziolek M, Alcaro S, Augustijns P, Basit AW, Grimm M, Hens B, et al. The mechanisms of pharmacokinetic food-drug interactions—a perspective from the UNGAP group. Eur J Pharm Sci Off J Eur Fed Pharm Sci. (2019) 134:31–59. doi: 10.1016/j.ejps.2019.04.003
24. Lammers LA, Achterbergh R, Mathôt RAA, Romijn JA. The effects of fasting on drug metabolism. Expert Opin Drug Metab Toxicol. (2020) 16(1):79–85. doi: 10.1080/17425255.2020.1706728
25. Wang B, Nichol JL, Sullivan JT. Pharmacodynamics and pharmacokinetics of AMG 531, a novel thrombopoietin receptor ligand. Clin Pharmacol Ther. (2004) 76(6):628–38. doi: 10.1016/j.clpt.2004.08.010
26. Ipema HJ, Jung MY, Lodolce AE. Romiplostim management of immune thrombocytopenic purpura. Ann Pharmacother. (2009) 43(5):914–9. doi: 10.1345/aph.1L643
27. Al-Samkari H, Grace RF, Kuter DJ. The role of romiplostim for pediatric patients with immune thrombocytopenia. Ther Adv Hematol. (2020) 11:2040620720912992. doi: 10.1177/2040620720912992
28. Neunert CE, Rose MJ. Romiplostim for the management of pediatric immune thrombocytopenia: drug development and current practice. Blood Adv. (2019) 3(12):1907. doi: 10.1182/bloodadvances.2019000279
29. Shimamoto G, Gegg C, Boone T, Quéva C. Peptibodies. MAbs. (2012) 4(5):586–91. doi: 10.4161/mabs.21024
30. Bussel JB, Kuter DJ, George JN, McMillan R, Aledort LM, Conklin GT, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med. (2006) 355(16):1672–81. doi: 10.1056/NEJMoa054626
31. Kuter DJ, Rummel M, Boccia R, Macik BG, Pabinger I, Selleslag D, et al. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. (2010) 363(20):1889–99. doi: 10.1056/NEJMoa1002625
32. Newland A, Caulier MT, Kappers-Klunne M, Schipperus MR, Lefrere F, Zwaginga JJ, et al. An open-label, unit dose-finding study of AMG 531, a novel thrombopoiesis-stimulating peptibody, in patients with immune thrombocytopenic purpura. Br J Haematol. (2006) 135(4):547–53. doi: 10.1111/j.1365-2141.2006.06339.x
33. Newland A, Godeau B, Priego V, Viallard JF, López Fernández MF, Orejudos A, et al. Remission and platelet responses with romiplostim in primary immune thrombocytopenia: final results from a phase 2 study. Br J Haematol. (2016) 172(2):262–73. doi: 10.1111/bjh.13827
34. Kuter DJ, Bussel JB, Newland A, Baker RI, Lyons RM, Wasser J, et al. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Br J Haematol. (2013) 161(3):411–23. doi: 10.1111/bjh.12260
35. Bussel JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood. (2009) 113(10):2161–71. doi: 10.1182/blood-2008-04-150078
36. Cines DB, Wasser J, Rodeghiero F, Chong BH, Steurer M, Provan D, et al. Safety and efficacy of romiplostim in splenectomized and nonsplenectomized patients with primary immune thrombocytopenia. Haematologica. (2017) 102(8):1342–51. doi: 10.3324/haematol.2016.161968
37. Steurer M, Quittet P, Papadaki HA, Selleslag D, Viallard JF, Kaiafa G, et al. A large observational study of patients with primary immune thrombocytopenia receiving romiplostim in European clinical practice. Eur J Haematol. (2017) 98(2):112–20. doi: 10.1111/ejh.12807
38. Doobaree IU, Newland A, McDonald V, Nandigam R, Mensah L, Leroy S, et al. Primary immune thrombocytopenia (ITP) treated with romiplostim in routine clinical practice: retrospective study from the United Kingdom ITP registry. Eur J Haematol. (2019) 102(5):416. doi: 10.1111/ejh.13221
39. Červinek L, Mayer J, Doubek M. Sustained remission of chronic immune thrombocytopenia after discontinuation of treatment with thrombopoietin-receptor agonists in adults. Int J Hematol. (2015) 102(1):7–11. doi: 10.1007/s12185-015-1793-1
40. Carpenedo M, Cantoni S, Coccini V, Fedele M, Morra E, Pogliani EM. Feasibility of romiplostim discontinuation in adult thrombopoietin-receptor agonist responsive patients with primary immune thrombocytopenia: an observational retrospective report in real life clinical practice. Hematol Rep. (2015) 7(1). doi: 10.4081/hr.2015.5673
41. Ghadaki B, Nazi I, Kelton JG, Arnold DM. Sustained remissions of immune thrombocytopenia associated with the use of thrombopoietin receptor agonists. Transfusion (Paris). (2013) 53(11):2807–12. doi: 10.1111/trf.12139
42. Lozano ML, Mingot-Castellano ME, Perera MM, Jarque I, Campos-Alvarez RM, González-López TJ, et al. Deciphering predictive factors for choice of thrombopoietin receptor agonist, treatment free responses, and thrombotic events in immune thrombocytopenia. Sci Rep. (2019) 9(1):16680. doi: 10.1038/s41598-019-53209-y
43. Guillet S, Crickx E, Azzaoui I, Chappert P, Boutin E, Viallard JF, et al. Prolonged response after TPO-RA discontinuation in primary ITP: results of a prospective multicenter study. Blood. (2023) 141(23):2867–77. doi: 10.1182/blood.2022018665
44. Kuter DJ, Newland A, Chong BH, Rodeghiero F, Romero MT, Pabinger I, et al. Romiplostim in adult patients with newly diagnosed or persistent immune thrombocytopenia (ITP) for up to 1 year and in those with chronic ITP for more than 1 year: a subgroup analysis of integrated data from completed romiplostim studies. Br J Haematol. (2019) 185(3):503–13. doi: 10.1111/bjh.15803
45. Janssens A, Tarantino M, Bird RJ, Mazzucconi MG, Boccia RV, Fernández MFL, et al. Romiplostim treatment in adults with immune thrombocytopenia of varying duration and severity. Acta Haematol. (2015) 134(4):215–28. doi: 10.1159/000381657
46. Bussel JB, Buchanan GR, Nugent DJ, Gnarra DJ, Bomgaars LR, Blanchette VS, et al. A randomized, double-blind study of romiplostim to determine its safety and efficacy in children with immune thrombocytopenia. Blood. (2011) 118(1):28–36. doi: 10.1182/blood-2010-10-313908
47. Tarantino MD, Bussel JB, Blanchette VS, Beam D, Roy J, Despotovic J, et al. Long-term treatment with romiplostim and treatment-free platelet responses in children with chronic immune thrombocytopenia. Haematologica. (2019) 104(11):2283–91. doi: 10.3324/haematol.2018.202283
48. Cines DB, Gernsheimer T, Wasser J, Godeau B, Provan D, Lyons R, et al. Integrated analysis of long-term safety in patients with chronic immune thrombocytopaenia (ITP) treated with the thrombopoietin (TPO) receptor agonist romiplostim. Int J Hematol. (2015) 102(3):259–70. doi: 10.1007/s12185-015-1837-6
49. Rodeghiero F, Stasi R, Giagounidis A, Viallard JF, Godeau B, Pabinger I, et al. Long-term safety and tolerability of romiplostim in patients with primary immune thrombocytopenia: a pooled analysis of 13 clinical trials. Eur J Haematol. (2013) 91(5):423–36. doi: 10.1111/ejh.12181
50. Hollenhorst MA, Al-Samkari H, Kuter DJ. Markers of autoimmunity in immune thrombocytopenia: prevalence and prognostic significance. Blood Adv. (2019) 3(22):3515–21. doi: 10.1182/bloodadvances.2019000400
51. Mytych DT, Park JK, Kim J, Barger TE, Boshier A, Jawa V, et al. Assessment of romiplostim immunogenicity in adult patients in clinical trials and in a global postmarketing registry. Br J Haematol. (2020) 190(6):923–32. doi: 10.1111/bjh.16658
52. Pamma R, Deshpande M, Hokom M, Gupta S, Zhuang Y, Chirmule N, et al. Impact assessment of immunogenicity of romiplostim in subjects with immune thrombocytopenic Purpura (ITP). Blood. (2010) 116(21):2517. doi: 10.1182/blood.V116.21.2517.2517
53. Jenkins JM, Williams D, Deng Y, Uhl J, Kitchen V, Collins D, et al. Phase 1 clinical study of eltrombopag, an oral, nonpeptide thrombopoietin receptor agonist. Blood. (2007) 109(11):4739–41. doi: 10.1182/blood-2006-11-057968
54. Gilreath J, Lo M, Bubalo J. Thrombopoietin receptor agonists (TPO-RAs): drug class considerations for pharmacists. Drugs. (2021) 81(11):1285–305. doi: 10.1007/s40265-021-01553-7
55. Stasi R. Eltrombopag for the treatment of idiopathic thrombocytopenic purpura. Expert Rev Hematol. (2008) 1(2):145–52. doi: 10.1586/17474086.1.2.145
56. Ghanima W, Cooper N, Rodeghiero F, Godeau B, Bussel JB. Thrombopoietin receptor agonists: ten years later. Haematologica. (2019) 104(6):1112–23. doi: 10.3324/haematol.2018.212845
57. Kuter DJ, Begley CG. Recombinant human thrombopoietin: basic biology and evaluation of clinical studies. Blood. (2002) 100(10):3457–69. doi: 10.1182/blood.V100.10.3457
58. Kaushansky K, Drachman JG. The molecular and cellular biology of thrombopoietin: the primary regulator of platelet production. Oncogene. (2002) 21(21):3359–67. doi: 10.1038/sj.onc.1205323
59. Ezumi Y, Takayama H, Okuma M. Thrombopoietin, c-mpl ligand, induces tyrosine phosphorylation of Tyk2, JAK2, and STAT3, and enhances agonists-induced aggregation in platelets in vitro. FEBS Lett. (1995) 374(1):48–52. doi: 10.1016/0014-5793(95)01072-M
60. Rojnuckarin P, Drachman JG, Kaushansky K. Thrombopoietin-induced activation of the mitogen-activated protein kinase (MAPK) pathway in normal megakaryocytes: role in endomitosis. Blood. (1999) 94(4):1273–82. doi: 10.1182/blood.V94.4.1273
61. Drachman JG, Millett KM, Kaushansky K. Thrombopoietin signal transduction requires functional JAK2, not TYK2. J Biol Chem. (1999) 274(19):13480–4. doi: 10.1074/jbc.274.19.13480
62. Kühne T, Imbach P. Eltrombopag: an update on the novel, non-peptide thrombopoietin receptor agonist for the treatment of immune thrombocytopenia. Ann Hematol. (2010) 89(Suppl 1):67–74. doi: 10.1007/s00277-010-0953-x
63. Bussel JB, Provan D, Shamsi T, Cheng G, Psaila B, Kovaleva L, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet Lond Engl. (2009) 373(9664):641–8. doi: 10.1016/S0140-6736(09)60402-5
64. Bussel JB, Saleh MN, Vasey SY, Mayer B, Arning M, Stone NL. Repeated short-term use of eltrombopag in patients with chronic immune thrombocytopenia (ITP). Br J Haematol. (2013) 160(4):538–46. doi: 10.1111/bjh.12169
65. Wong RSM, Saleh MN, Khelif A, Salama A, Portella MSO, Burgess P, et al. Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: final results of the EXTEND study. Blood. (2017) 130(23):2527–36. doi: 10.1182/blood-2017-04-748707
66. Lucchini E, Palandri F, Volpetti S, Vianelli N, Auteri G, Rossi E, et al. Eltrombopag second-line therapy in adult patients with primary immune thrombocytopenia in an attempt to achieve sustained remission off-treatment: results of a phase II, multicentre, prospective study. Br J Haematol. (2021) 193(2):386–96. doi: 10.1111/bjh.17334
67. Matthys G, Park JW, McGuire S, Wire MB, Zhang J, Bowen C, et al. Eltrombopag does not affect cardiac repolarization: results from a definitive QTc study in healthy subjects. Br J Clin Pharmacol. (2010) 70(1):24–33. doi: 10.1111/j.1365-2125.2010.03646.x
68. Cheng G. Eltrombopag, a thrombopoietin- receptor agonist in the treatment of adult chronic immune thrombocytopenia: a review of the efficacy and safety profile. Ther Adv Hematol. (2012) 3(3):155–64. doi: 10.1177/2040620712442525
69. Nomoto M, Pastino G, Rege B, Aluri J, Ferry J, Han D. Pharmacokinetics, pharmacodynamics, pharmacogenomics, safety, and tolerability of avatrombopag in healthy Japanese and white subjects. Clin Pharmacol Drug Dev. (2018) 7(2):188–95. doi: 10.1002/cpdd.349
70. Nomoto M, Zamora CA, Schuck E, Boyd P, Chang M, Aluri J, et al. Pharmacokinetic/pharmacodynamic drug–drug interactions of avatrombopag when coadministered with dual or selective CYP2C9 and CYP3A interacting drugs. Br J Clin Pharmacol. (2018) 84(5):952–60. doi: 10.1111/bcp.13517
71. Fukushima-Shintani M, Suzuki KI, Iwatsuki Y, Abe M, Sugasawa K, Hirayama F, et al. AKR-501 (YM477) in combination with thrombopoietin enhances human megakaryocytopoiesis. Exp Hematol. (2008) 36(10):1337–42. doi: 10.1016/j.exphem.2008.04.020
72. Bussel JB, Kuter DJ, Aledort LM, Kessler CM, Cuker A, Pendergrass KB, et al. A randomized trial of avatrombopag, an investigational thrombopoietin-receptor agonist, in persistent and chronic immune thrombocytopenia. Blood. (2014) 123(25):3887–94. doi: 10.1182/blood-2013-07-514398
73. Jain S, Gernsheimer T, Kolodny S, Bernheisel C, Vredenburg M, Panch SR. Additional efficacy analysis of avatrombopag phase III data for the treatment of adults with immune thrombocytopenia. Platelets. (2023) 34(1):2195016. doi: 10.1080/09537104.2023.2195016
74. Oladapo A, Kolodny S, Vredenburg M, Swallow E, Goldschmidt D, Sarathy K, et al. Avatrombopag treatment response in patients with immune thrombocytopenia: the REAL-AVA 1.0 study. Ther Adv Hematol. (2023) 14:20406207231179856. doi: 10.1177/20406207231179856
75. Wang Z, Chen X, Li A, Chen L, Wang Y, Zheng L. Effect of food on the pharmacokinetic and pharmacodynamic profiles of hetrombopag in healthy volunteers. Clin Ther. (2020) 42(12):2280–8. doi: 10.1016/j.clinthera.2020.10.002
76. Wang Z, Chen L, Zhang F, Lu H, Chen X, Wen A, et al. First-in-patient study of hetrombopag in patients with chronic idiopathic thrombocytopenic purpura. J Thromb Haemost JTH. (2020) 18(11):3053–60. doi: 10.1111/jth.15078
77. Yang G, Huang R, Yang S, Zhang X, Yang X, Chen H, et al. Effect of postdose fasting duration on hetrombopag olamine pharmacokinetics and pharmacodynamics in healthy volunteers. Br J Clin Pharmacol. (2020) 86(8):1528–36. doi: 10.1111/bcp.14259
78. Zheng L, Liang MZ, Zeng XL, Li CZ, Zhang YF, Chen XY, et al. Safety, pharmacokinetics and pharmacodynamics of hetrombopag olamine, a novel TPO-R agonist, in healthy individuals. Basic Clin Pharmacol Toxicol. (2017) 121(5):414–22. doi: 10.1111/bcpt.12815
79. Xie C, Zhao H, Bao X, Fu H, Lou L. Pharmacological characterization of hetrombopag, a novel orally active human thrombopoietin receptor agonist. J Cell Mol Med. (2018) 22(11):5367–77. doi: 10.1111/jcmm.13809
80. Mei H, Liu X, Li Y, Zhou H, Feng Y, Gao G, et al. A multicenter, randomized phase III trial of hetrombopag: a novel thrombopoietin receptor agonist for the treatment of immune thrombocytopenia. J Hematol OncolJ Hematol Oncol. (2021) 14(1):37. doi: 10.1186/s13045-021-01047-9
81. Syed YY. Hetrombopag: first approval. Drugs. (2021) 81(13):1581–5. doi: 10.1007/s40265-021-01575-1
82. Patterson RE, Sears DD. Metabolic effects of intermittent fasting. Annu Rev Nutr. (2017) 37:371–93. doi: 10.1146/annurev-nutr-071816-064634
83. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. (2017) 39:46–58. doi: 10.1016/j.arr.2016.10.005
84. Karimi R, Cleven A, Elbarbry F, Hoang H. The impact of fasting on major metabolic pathways of macronutrients and pharmacokinetics steps of drugs. Eur J Drug Metab Pharmacokinet. (2021) 46(1):25–39. doi: 10.1007/s13318-020-00656-y
85. Chen F, McDonald V, Newland A. Experts’ review: the emerging roles of romiplostim in immune thrombocytopenia (ITP). Expert Opin Biol Ther. (2021) 21(11):1383–93. doi: 10.1080/14712598.2021.1960979
86. Bussel JB, Soff G, Balduzzi A, Cooper N, Lawrence T, Semple JW. A review of romiplostim mechanism of action and clinical applicability. Drug Des Devel Ther. (2021) 15:2243–68. doi: 10.2147/DDDT.S299591
87. Wire MB, Bruce J, Gauvin J, Pendry CJ, McGuire S, Qian Y, et al. A randomized, open-label, 5-period, balanced crossover study to evaluate the relative bioavailability of eltrombopag powder for oral suspension (PfOS) and tablet formulations and the effect of a high-calcium meal on eltrombopag pharmacokinetics when administered with or 2h before or after PfOS. Clin Ther. (2012) 34(3):699–709. doi: 10.1016/j.clinthera.2012.01.011
88. Yassin MA, Alasmar A, Chandra P, Ismail O, Nashwan AJ, Sideeg D, et al. Effects of Ramadan fasting on patients with immune thrombocytopenia (ITP) receiving eltrombopag. Blood. (2020) 136:8–9. doi: 10.1182/blood-2020-134421
89. Al-Samkari H, Jiang D, Gernsheimer T, Liebman H, Lee S, Wojdyla M, et al. Adults with immune thrombocytopenia who switched to avatrombopag following prior treatment with eltrombopag or romiplostim: a multicentre US study. Br J Haematol. (2022) 197(3):359–66. doi: 10.1111/bjh.18081
90. Tsykunova G, Ghanima W. Avatrombopag for the treatment of adult patients with chronic immune thrombocytopenia (cITP): focus on patient selection and perspectives. Ther Clin Risk Manag. (2022) 18:273–86. doi: 10.2147/TCRM.S251672
Keywords: thrombocytopenia, thrombopoietin-receptor agonist, avatrombopag, eltrombopag, romiplostim
Citation: Yassin MA, Al-Rasheed M, Al-Khaboori M, Marashi M, Osman H, Wali Y, Al Kindi S, Alsayegh F and Provan D (2023) Thrombopoietin-receptor agonists for adult patients with immune thrombocytopenia: a narrative review and an approach for managing patients fasting intermittently. Front. Cardiovasc. Med. 10:1260487. doi: 10.3389/fcvm.2023.1260487
Received: 20 July 2023; Accepted: 17 November 2023;
Published: 14 December 2023.
Edited by:
Nora V. Butta, University Hospital La Paz Research Institute (IdiPAZ), SpainReviewed by:
Thomas Pierre Lecompte, Université de Lorraine, France© 2023 Yassin, Al-Rasheed, Al-Khaboori, Marashi, Osman, Wali, Al Kindi, Alsayegh and Provan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed A. Yassin eWFzc2lubW9oYUBnbWFpbC5jb20=; eWFzc2luQGhhbWFkLnFh
Abbreviations AE, adverse event; EMA, European medicines agency; FDA, Food and Drug Administration; ITP, immune thrombocytopenia; TPO-RA, thrombopoietin-receptor agonist.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.