
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Cardiovasc. Med. , 15 September 2023
Sec. Cardio-Oncology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1259620
This article is part of the Research Topic Case Reports in Cardio-Oncology: 2022 View all 39 articles
 Martina Iengo1,†
Martina Iengo1,† Ester Topa1,†
Ester Topa1,† Alessandra Cuomo1
Alessandra Cuomo1 Giancarlo Marone2
Giancarlo Marone2 Remo Poto1,3
Remo Poto1,3 Gilda Varricchi1,3,4,5
Gilda Varricchi1,3,4,5 Leonardo Cristinziano1
Leonardo Cristinziano1 Maria Rosaria Galdiero1,3,4
Maria Rosaria Galdiero1,3,4 Anne Lise Ferrara1,5
Anne Lise Ferrara1,5 Stefania Loffredo1,3,4,5
Stefania Loffredo1,3,4,5 Luigi Formisano6,7
Luigi Formisano6,7 Teresa Troiani8
Teresa Troiani8 Valentina Mercurio1,7,9
Valentina Mercurio1,7,9 Carlo Gabriele Tocchetti1,4,7,9*
Carlo Gabriele Tocchetti1,4,7,9*
Cancer immunotherapies have revolutionized antineoplastic treatments. CTLA-4, PD-1, and PD-L1 are crucial regulators of the immune response and play a central role in the maintenance of self-tolerance (1). Monoclonal antibodies directed against CTLA-4, PD-1, and PD-L1 block these immune checkpoints and unleash anti-tumor immunity, leading to tumor cell death through cytolytic molecules. Unfortunately, these immune checkpoint inhibitors (ICIs) either alone or in combination, can lead to imbalances in immunologic tolerance resulting in a broad spectrum of immune-related adverse events (irAEs) (2–4). The true incidence of cardiac irAEs due to ICIs is unknown; current estimates suggest less than 1% of patients (2).
The largest case series of 122 subjects with ICI-associated myocarditis had early symptoms (median of 30 days after initial exposure to ICI), and up to 50% died (5). Late cardiovascular (CV) events (>90 days) are not well characterized but usually show higher risk of non-inflammatory heart failure (HF), progressive atherosclerosis, hypertension, and mortality rates (6). Other CV toxicities described during ICI therapy are MI, Atrio-Ventricular (AV) block, supraventricular and ventricular arrhythmias, sudden death, Takotsubo-like syndrome (TTS), hypercholesterolaemia, pericarditis, pericardial effusion, ischaemic stroke, and VTE (7, 8). Conditions related with high baseline ICI-related CV toxicity risk include dual ICI therapy (e.g., ipilimumab and nivolumab), combination ICI therapy with other cardiotoxic therapies, and patients with ICI-related non-CV events or prior cancer therapy-related cardiac dysfunction (CTRCD) or cardiovascular disease (CVD) (Figure 1) (9–11).
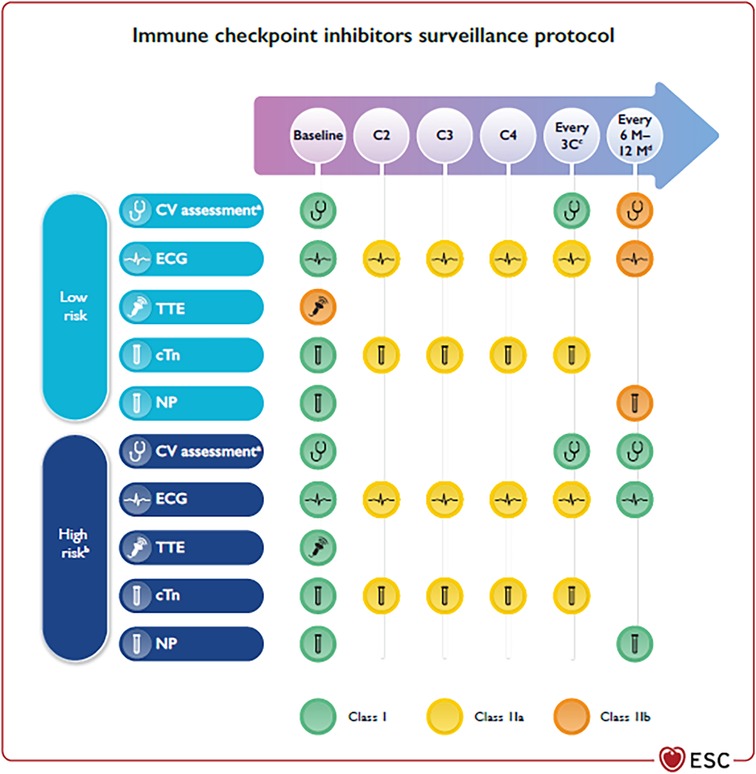
Figure 1. (reproduced with permission from 9). Cardiovascular surveillance in patients treated with immune checkpoint inhibitors. BNP, B-type natriuretic peptide; BP, blood pressure; C, chemotherapy cycle; cTn, cardiac troponin; CV, cardiovascular; CVD, cardiovascular disease; CTRCD, cancer therapy-related cardiac dysfunction; CTR-CVT, cancer therapy-related cardiovascular toxicity; CVRF, cardiovascular risk factors; ECG, electrocardiogram; HbA1c, glycated haemoglobin; ICI, immune checkpoint inhibitors; M, months; NP, natriuretic peptides (including BNP and NT-proBNP); NT-proBNP, N-terminal pro-B-type natriuretic peptide; TTE, transthoracic echocardiography; AF, atrial fibrillation. aIncluding physical examination, BP, lipid profile, and HbA1c. bDual ICI, combination ICI-cardiotoxic therapy, ICI-related non-CV events, prior CTRCD or CVD. cEvery three cycles until completion of therapy to detect subclinical ICI-related CV toxicity. dIn patients who require long-term (>12 months) ICI treatment.
More recently, engineered T cells with chimeric antigen receptors (CAR-T cells) are being used for acute lymphocytic leukaemia and aggressive B-cell lymphomas (12). There is a growing recognition of the association between CAR-T therapy and cancer therapy-related cardiovascular toxicity (CTR-CVT), including left ventricular dysfunction (LVD), HF, cardiac arrhythmias, pericardial effusion, TTS, and cardiac arrest (13–18). The majority of the described CV toxicities have been shown to be associated with cytokine release syndrome (CRS) (12, 19), a systemic inflammatory response due to the widespread release of cytokines.
Acknowledging the revolutionary advances in cancer obtained with immunotherapies (and unfortunately also their toxicities), it is noteworthy that this Special Issue contains several papers that describe toxicities from immunologic therapies. Su and Colleagues (20) report a case of head and neck squamous cell carcinoma treated with pembrolizumab, a humanized monoclonal IgG4 antibody, that binds to programmed death receptor-1 (PD-1) and blocks its interaction with programmed death ligand-1 (PD-L1). The authors report that pembrolizumab-induced atrioventricular block complicated by myocarditis, successfully treated with glucocorticoids within 24 h after initial symptoms. Although left ventricular ejection fraction (LVEF) was normal, speckle tracking echocardiography revealed a slightly decreased left ventricular global longitudinal strain (GLS).
Nivolumab (anti-PD-1) and Pembrolizumab were probably the cause of immune related pericarditis, pericardial effusion and tamponade in two cases described by Chye and coworkers, who underlined the importance of monitoring and follow-up of selected patients for rechallenge with ICI after full recovery from immune-related pericardial disease (21) The two patients were suffering from advanced non-small cell lung cancer (NSCLC).
Sintilimab is a humanized monoclonal IgG4 antibody approved for the treatment of hematological cancers and several advanced solid tumors in China. Lin and collaborators presented a sintilimab related decrease of LVEF at echocardiography (22) in a patient with advanced lung adenocarcinoma. Echocardiography showed severely impaired heart function with a LVEF of 35% on admission. A significant improvement of LVEF to 52% was noted several days after treatment with methylprednisolone and immunoglobulin. However, cardiac magnetic resonance (CMR) showed extensive myocardium fibrosis. Therefore, longer follow-up is warranted to determine whether myocardial fibrosis can fully regress and to observe the long-term prognosis of the patient (Lin et al.).
Toripalimab is a PD-1 monoclonal antibody approved by the National Medical Products Administration of China in 2018. A phase I trial registered with U.S. National Library of Medicine (identifier NCT03474640) is underway in the USA. Luo and coworkers describe a case presenting with polymyositis, myocarditis, and myasthenia gravis after toripalimab used for treatment of metastatic thymoma. Toripalimab can provoke diffuse inflammation of myocytes from striated muscles to Myocardial cells, worsened in case of thymic epithelial tumors, because of the alteration of T cells immune tolerance (23).
Another manuscript shows acute pericardial effusion with cardiac tamponade with CAR-T therapy (24) in a patient with diffuse large B-cell lymphoma. Specifically, the Authors evidenced that a rapid introduction of immunosuppressive therapy can reduce the need of pericardiocentesis.
The conditions and toxicities described in the above-mentioned papers of this Special Issue are well recognized by the first ESC 2022 Cardio-Oncology Guidelines, which recommend that all patients on ICI treatment should have an ECG and troponin assay at baseline (Figure 1) (9). In addition, high-risk patients should undergo trans-thoracic echocardiography (TTE). Once started on therapy, ECG, cTn, and NP should be checked (9). In high-risk patients, and in those with high baseline cTn levels, TTE monitoring may be considered. Subjects with new ECG abnormalities, biomarker changes, or cardiac symptoms at any time, need to be promptly evaluated by cardio-oncologists, including a TTE for the assessment of LVEF and GLS, and CMR if myocarditis is suspected (9). Cessation of ICI treatment is recommended with ICI-associated myocarditis; patients should be admitted to hospital with continuous ECG monitoring. CV complications should be treated as per specific ESC Guidelines (HF (25), tachyarrhythmias (26), AV block (27, 28) or pericardial effusion (29)). Methylprednisolone 500–1,000 mg i.v. once daily for the first 3–5 days should be started as soon as possible (9).
Baseline CV evaluation including ECG, NP, and cTn is also recommended in patients who are to be treated with CAR-T. Baseline TTE should also be considered, especially in subjects with pre-existing cardiovascular risk factors (CVRF) and CVD. CRS should be suspected if a subject develops fever, with or without tachypnoea, tachycardia, hypotension, hypoxia, and/or other end-organ dysfunction hours to days after treatment. A high index of suspicion is necessary to diagnose CRS and to distinguish it from other conditions that occur in these settings (infections, HF, drug reactions, and PE) (9). A rise in cTn can be frequently observed in subjects with CRS and is linked to a higher risk for subsequent CV events (13). CAR-T was associated with tachyarrhythmias (atrial fibrillation, AF, the most common, followed by ventricular arrhythmias), cardiomyopathy, and pleural and pericardial diseases (16). Globally, the fatality rate of CV and pulmonary adverse events was 30.9% (30). Early cardiac evaluation in patients with cTn increase should include NP, ECG, and TTE (9). When suspected, a resting 12-lead ECG, continuous ECG monitoring, TTE, and cTn and NP are recommended. In severe cases admission to ICU is recommended because of the risk of malignant cardiac arrhythmias, circulatory collapse, and multiorgan system failure (9).
The above-mentioned surveillance strategies of acute fulminant myocarditis brought to the recognition of more subtle forms of heart inflammation, spanning from smouldering myocarditis to asymptomatic rises in serum troponin I (31–34). Hence, chronic (lasting for >12 weeks after ICI discontinuation) irAEs are increasingly detected, and can affect up to 40% of patients (35). Chronic irAEs are mostly endocrine or rheumatological, but may also affect other organs and systems (31). Extrapolating from non-ICI-associated myocarditis, it may be expected that subjects who recover from ICI-associated myocarditis would also experience chronic consequences related to residual cardiomyopathy as a long-term sequela (36). This brings to a fundamental long-term implication of irAEs, whether patients who experienced some benefit but also severe toxicities from ICI treatment should be rechallenged upon resolution of the irAE. Retrospective studies suggest that recurrence of irAEs occurs in approximately 25%–50% of patients rechallenged with ICIs (31, 37, 38). De-escalation of therapy (such as de-escalation from combination anti-PD-1–anti-CTLA4 antibodies to anti-PD-1 monotherapy) appears to be linked with a lower risk of irAE recurrence (18% in one series, 38). Determining which patients bring the highest risk of recurrent irAEs is challenging; colitis, pneumonitis and hepatitis seem to recur more frequently on rechallenge than do other irAEs, with older age also being associated with irAE recurrence (37). It is not fully understood whether a longer delay between discontinuation and rechallenge would also decrease the risk of recurrent toxicities. When evaluating the reintroduction of an ICI-based therapy, both the type and severity of the irAE, as well as the clinical need for rechallenge should be taken into account. If rechallenge is undertaken, patients should undergo close clinical and/or laboratory monitoring should in order to assess for possible irAE recurrence (31).
MI: Writing – original draft. ET: Writing – original draft. AC: Writing – original draft. GM: Writing – original draft. RP: Writing – original draft. GV: Writing – review & editing. LC: Writing – original draft. MG: Writing – review & editing. AF: Writing – original draft. SL: Writing – review & editing. LF: Writing – review & editing. TT: Writing – review & editing. VM: Writing – review & editing. CT: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
CGT is supported by two grants from the Italian Ministry of Health (PNRR-MAD-2022-12376632 and RF-2016-02362988). ALF and LF are supported by a grant from the Italian Ministry of Health (PNRR-MAD-2022-12376632). LF is supported by MFAG 21505 – 2018 grant. MRG is supported by MIUR-PRIN 2017M8YMR8_005 and AIRC under MFAG 2020 (grant number 25123).
CT reports honoraria or consultation fees from VivaLyfe, Univers Formazione, Solaris, Summeet, Astra Zeneca, Myocardial Solutions; funding from Amgen and MSD, outside the submitted work; and is listed as an inventor of two patents related to HF. GV reports research support from AstraZeneca International. LF reports support from Lilly. TT reports support from Novartis BMS, Pfizer, Amgen, Merck, Sanofi, MSD.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer AG declared past co-authorships with the authors CGT, VM, AC, GV, and, RM to the handling editor.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hu JR, Florido R, Lipson EJ, Naidoo J, Ardehali R, Tocchetti CG, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res. (2019) 115(5):854–68. doi: 10.1093/cvr/cvz026
2. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. (2016) 375(18):1749–55. doi: 10.1056/nejmoa1609214
3. Tocchetti CG, Ameri P, de Boer RA, D’Alessandra Y, Russo M, Sorriento D, et al. Cardiac dysfunction in cancer patients: beyond direct cardiomyocyte damage of anticancer drugs: novel cardio-oncology insights from the joint 2019 meeting of the ESC working groups of myocardial function and cellular biology of the heart. Cardiovasc Res. (2020) 116(11):1820–34. doi: 10.1093/cvr/cvaa222
4. Zimmer L, Goldinger SM, Hofmann L, Loquai C, Ugurel S, Thomas I, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer. (2016) 60:210–25. doi: 10.1016/j.ejca.2016.02.024
5. Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Spectrum of cardiovascular toxicities of immune checkpoint inhibitors: a pharmacovigilance study. Lancet Oncol. (2018) 19(12):1579–89. doi: 10.1016/S1470-2045(18)30608-9
6. Dolladille C, Ederhy S, Allouche S, Dupas Q, Gervais R, Madelaine J, et al. Late cardiac adverse events in patients with cancer treated with immune checkpoint inhibitors. J Immunother Cancer. (2020) 8(1):e000261. doi: 10.1136/jitc-2019-000261
7. D’Souza M, Nielsen D, Svane IM, Iversen K, Rasmussen PV, Madelaire C, et al. The risk of cardiac events in patients receiving immune checkpoint inhibitors: a nationwide Danish study. Eur Heart J. (2021) 42(16):1621–31. doi: 10.1093/eurheartj/ehaa884
8. Kondapalli L, Neilan TG. Immune checkpoint inhibitors and cardiovascular events among patients with cancer: a window into the critical role of the immune system in cardiovascular biology. Eur Heart J. (2021) 42(48):4978–80. doi: 10.1093/eurheartj/ehab708
9. Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European hematology association (EHA), the European society for therapeutic radiology and oncology (ESTRO) and the international cardio-oncology society (IC-OS). Eur Heart J Cardiovasc Imaging. (2022) 23(10):e333–465. doi: 10.1093/ehjci/jeac106
10. Zamami Y, Niimura T, Okada N, Koyama T, Fukushima K, Izawa-Ishizawa Y, et al. Factors associated with immune checkpoint inhibitor-related myocarditis. JAMA Oncol. (2019) 5(11):1635–7. doi: 10.1001/jamaoncol.2019.3113
11. Zhang L, Reynolds KL, Lyon AR, Palaskas N, Neilan TG. The evolving immunotherapy landscape and the epidemiology, diagnosis, and management of cardiotoxicity: JACC: cardioOncology primer. Cardio Oncol. (2021) 3(1):35–47. doi: 10.1016/j.jaccao.2020.11.012
12. Frey N, Porter D. Cytokine release syndrome with chimeric antigen receptor T cell therapy. Biol Blood Marrow Transplant. (2019) 25(4):e123–7. doi: 10.1016/j.bbmt.2018.12.756
13. Alvi RM, Frigault MJ, Fradley MG, Jain MD, Mahmood SS, Awadalla M, et al. Cardiovascular events among adults treated with chimeric antigen receptor T-cells (CAR-T). J Am Coll Cardiol. (2019) 74(25):3099–108. doi: 10.1016/j.jacc.2019.10.038
14. Fradley MG, Damrongwatanasuk R, Chandrasekhar S, Alomar M, Kip KE, Sarnaik AA. Cardiovascular toxicity and mortality associated with adoptive cell therapy and tumor-infiltrating lymphocytes for advanced stage melanoma. J Immunother. (2021) 44(2):86–9. doi: 10.1097/CJI.0000000000000341
15. Ghosh AK, Chen DH, Guha A, Mackenzie S, Walker JM, Roddie C. CAR T cell therapy–related cardiovascular outcomes and management: systemic disease or direct cardiotoxicity? Cardio Oncol. (2020) 2(1):97–109. doi: 10.1016/j.jaccao.2020.02.011
16. Goldman A, Maor E, Bomze D, Liu JE, Herrmann J, Fein J, et al. Adverse cardiovascular and pulmonary events associated with chimeric antigen receptor T-cell therapy. J Am Coll Cardiol. (2021) 78(18):1800–13. doi: 10.1016/j.jacc.2021.08.044
17. Lefebvre B, Kang Y, Smith AM, Frey NV, Carver JR, Scherrer-Crosbie M. Cardiovascular effects of CAR T cell therapy: a retrospective study. Cardio Oncol. (2020) 2(2):193–203. doi: 10.1016/j.jaccao.2020.04.012
18. Salem JE, Ederhy S, Lebrun-Vignes B, Moslehi JJ. Cardiac events associated with chimeric antigen receptor T-cells (CAR-T): a VigiBase perspective. J Am Coll Cardiol. (2020) 75(19):2521–3. doi: 10.1016/j.jacc.2020.02.070
19. Ganatra S, Redd R, Hayek SS, Parikh R, Azam T, Yanik GA, et al. Chimeric antigen receptor T-cell therapy-associated cardiomyopathy in patients with refractory or relapsed non-hodgkin lymphoma. Circulation. (2020) 142(17):1687–90. doi: 10.1161/CIRCULATIONAHA.120.048100
20. Su L, Liu C, Wu W, Cui Y, Wu M, Chen H. Successful therapy for myocarditis concomitant with complete heart block after pembrolizumab treatment for head and neck squamous cell carcinoma: A Case Report with literature review. Front Cardiovasc Med. (2022) 9:898756. doi: 10.3389/fcvm.2022.898756
21. Chye AM, Nordman IIC, Sverdlov AL. Successful immune checkpoint inhibitor rechallenge after immune-related pericarditis: Clinical case series. Front Cardiovasc Med. (2022) 9:964324. doi: 10.3389/fcvm.2022.964324
22. Lin Y, Yuan X, Chen L. Immune myocarditis related to sintilimab treatment in a patient with advanced lung adenocarcinoma: A case report. Front Cardiovasc Med. (2022) 9:955527. doi: 10.3389/fcvm.2022.955527
23. Luo YB, Tang W, Zeng Q, Duan W, Li S, Yang X, et al. Case Report: The neuromusclar triad of immune checkpoint inhibitors: a Case Report of myositis, myocarditis, and myasthenia gravis overlap following toripalimab treatment. Front Cardiovasc Med. (2021) 8:714460. doi: 10.3389/fcvm.2021.714460
24. Moriyama S, Fukata M, Yokoyama T, Ueno S, Nunomura T, Mori Y, et al. Case Report: Cardiac tamponade in association with cytokine release syndrome following CAR-T cell therapy. Front Cardiovasc Med. (2022) 9:848091. doi: 10.3389/fcvm.2022.848091
25. McDonagh TA, Metra M, Adamo M, Baumbach A, Böhm M, Burri H, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42(36):3599–726. doi: 10.1093/eurheartj/ehab368
26. Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomstrom-Lundqvist C, et al. 2019 ESC guidelines for themanagement of patients with supraventricular tachycardia. Eur Heart J. (2020) 41(5):655–720. doi: 10.1093/eurheartj/ehz467
27. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, et al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. (2021) 42(35):3427–520. doi: 10.1093/eurheartj/ehab364
28. Zeppenfeld K, Tfelt-Hansen J, De Riva M, Winkel BG, Behr ER, Blom NA, et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. (2022) 43(40):3997–4126. doi: 10.1093/eurheartj/ehac262
29. Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases. Eur Heart J. (2015) 36(42):2921–64. doi: 10.1093/eurheartj/ehv318
30. Ragoonanan D, Khazal SJ, Abdel-Azim H, McCall D, Cuglievan B, Tambaro FP, et al. Diagnosis, grading and management of toxicities from immunotherapies in children, adolescents and young adults with cancer. Nat Rev Clin Oncol. (2021) 18(7):435–53. doi: 10.1038/s41571-021-00474-4
31. Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. (2022) 19(4):254–67. doi: 10.1038/s41571-022-00600-w
32. Bonaca MP, Olenchock BA, Salem J-E, Wiviott SD, Ederhy S, Cohen A, et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. (2019) 140(2):80–91. doi: 10.1161/CIRCULATIONAHA.118.034497
33. Norwood TG, Westbrook BC, Johnson DB, Litovsky SH, Terry NL, McKee SB, et al. Smoldering myocarditis following immune checkpoint blockade. J Immunother Cancer. (2017) 5(1):91. doi: 10.1186/s40425-017-0296-4
34. Waliany S, Neal JW, Reddy S, Wakelee H, Shah SA, Srinivas S, et al. Myocarditis surveillance with high-sensitivity troponin I during cancer treatment with immune checkpoint inhibitors. Cardio Oncol. (2021) 3(1):137–9. doi: 10.1016/j.jaccao.2021.01.004
35. Patrinely JR Jr, Johnson R, Lawless AR, Bhave P, Sawyers A, Dimitrova M, et al. Chronic immune-related adverse events following adjuvant anti-PD-1 therapy for high-risk resected melanoma. JAMA Oncol. (2021) 7(5):744–8. doi: 10.1001/jamaoncol.2021.0051
36. Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail. (2020) 13(11):e007405. doi: 10.1161/CIRCHEARTFAILURE.120.007405
37. Dolladille C, Ederhy S, Sassier M, Cautela J, Thuny F, Cohen AA, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. (2020) 6(6):865–71. doi: 10.1001/jamaoncol.2020.0726
38. Santini FC, Rizvi H, Plodkowski AJ, Ni A, Lacouture ME, Gambarin-Gelwan M, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol. (2018) 29(1):250–5. doi: 10.1093/annonc/mdx642
Keywords: cardio-oncology, immunotherapy, cardiotoxicity, detection, management
Citation: Iengo M, Topa E, Cuomo A, Marone G, Poto R, Varricchi G, Cristinziano L, Galdiero MR, Ferrara AL, Loffredo S, Formisano L, Troiani T, Mercurio V and Tocchetti CG (2023) The broad spectrum of cardiotoxicities from immunotherapies. Front. Cardiovasc. Med. 10:1259620. doi: 10.3389/fcvm.2023.1259620
Received: 16 July 2023; Accepted: 4 September 2023;
Published: 15 September 2023.
Edited by:
Luigi Tarantini, IRCCS Local Health Authority of Reggio Emilia, ItalyReviewed by:
Alessandra Ghigo, University of Turin, Italy© 2023 Iengo, Topa, Cuomo, Marone, Poto, Varricchi, Cristinziano, Galdiero, Ferrara, Loffredo, Formisano, Troiani, Mercurio and Tocchetti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlo Gabriele Tocchetti Y2d0b2NjaGV0dGlAZ21haWwuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.