- 1Department of Cardiothoracic Pathology, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania
- 2Department of Cardiology, Emergency Clinical Hospital Bucharest, Bucharest, Romania
- 3Department of Cardiology, Elias University Hospital, Bucharest, Romania
Background and aims: There is limited data concerning the effect of non-revascularized chronic total occlusions (NR-CTOs) after VT ablation. This study sought to evaluate the impact of NR-CTOs after ablation for electrical storm (ES).
Methods: Post-hoc retrospective analysis of data regarding 64 consecutive post-myocardial infarction patients (out of which 12 patients with NR-CTOs and 52 without NR-CTOs) undergoing substrate ablation for ES with an available median follow-up of 37.53 (7.25–64.65) months. Ablation result was assessed by inducibility of sustained monomorphic VT (SMVT) during final programmed ventricular stimulation (PVS). The primary endpoints were all-cause mortality and VT/VF recurrences after ablation, respectively, stratified by the presence of NR-CTOs. The secondary endpoint was to assess the predictive effect of NR-CTOs on all-cause mortality and VT/VF recurrences in relation to other relevant prognostic factors.
Results: At baseline, the presence of NR-CTOs was associated with higher bipolar BZ-to-total scar ratio (72.4% ± 17.9% vs. 52% ± 37.7%, p = 0.022) and more failure to eliminate the clinical VT (25% (3) vs. 0% (0), p < 0.001). During follow-up, overall all-cause mortality and recurrences were more frequent in the NR-CTO subgroup (75% (9) vs. 19.2% (10), log rank p = 0.003 and 58.3% vs. 23.1% (12), log rank p = 0.042 respectively). After adjusting for end-procedural residual SMVT inducibility, NR-CTOs predicted death during follow-up (HR 3.380, p = 0.009) however not recurrence (HR 1.986, p = 0.154).
Conclusions: NR-CTO patients treated by RFCA for drug-refractory ES demonstrated a higher ratio of BZ-to-total-scar area. In this analysis, NR-CTO was associated with worse acute procedural results and may as well impact long-term outcomes which should be further assessed in larger patient populations.
1. Introduction
Non-revascularized chronic total occlusions (NR-CTOs) increase long-term mortality and appropriate implantable cardioverter defibrillator (ICD) therapies in patients with ischemic cardiomyopathy (ICM) (1–4). This has been demonstrated in both primary and secondary sudden cardiac death prevention ICD recipients. Limited data has assessed the prognostic effect of NR-CTOs after ventricular tachycardia (VT) ablation (5–7). However, the impact of NR-CTOs after electrical storm (ES) ablation is unknown.
2. Methods
2.1. Study population
We performed a single-centre longitudinal retrospective analysis on available data regarding the baseline characteristics and post-procedural course of consecutive patients that fulfilled the following set of inclusion criteria.
- Post-myocardial infarction (post-MI) patients.
- At least three distinct episodes of sustained ventricular monomorphic tachycardia (SMVT) treated by adequate ICD therapies in a 24-h interval refractory to medical treatment and without reversible triggers (8–10).
- Treated by radiofrequency catheter ablation (RFCA) targeting ventricular arrhythmic substrate from January 2014 to June 2021.
Patients with ES induced by acute coronary syndromes, patients with no coronary angiography prior to ablation (two patients) and patients receiving surgical or percutaneous revascularization (one patient during index hospitalization) during the follow-up interval were excluded.
2.2. Imaging, electrophysiology study and ablation strategy
All patients underwent coronary angiography during the same hospitalization, prior to the moment of ablation or at the referring hospital, prior to transfer. With the exception of NR-CTOs, potentially significant lesions were defined by ≥70% luminal stenosis (50% for left main lesions), as assessed by two senior interventional cardiologists. Multivessel disease (MVD) was defined by at least one lesion ≥70% simultaneously present or previously treated in at least two epicardial coronary arteries. NR-CTOs were defined angiographically in an untreated [neither surgically nor percutaneously (PCI)] vessel based on the lesion morphology characteristics (as evaluated by two senior interventional cardiologists), irrespective of the degree of anterograde or retrograde collateral flow. NR-CTOs were considered to be incidentally diagnosed by pre-ablation angiography if the patient had no previously documented MI compatible with the NR-CTO localization; NR-CTOs were considered to be clinical if the patient had a previously documented MI compatible to the NR-CTO localization. Mitral regurgitation severity and biplane Simpson-based left ventricular ejection fraction (LVEF) were defined by transthoracic echocardiography formally-recommended criteria prior to ablation (11, 12).
All patients underwent electrophysiological study (EPS) and RFCA in a fasting state under conscious sedation and analgesia. EPS was performed using dedicated recording and analysis system (Boston Scientific Labsystem PRO EP Recording System v.2.7.0.16). High density electroanatomical mapping [>1,800 points, 70% of points focusing on scar and its border-zone (BZ)] was performed in sinus rhythm (SR) with 16–500 Hz signal filtering (CARTO-3™, Biosense Webster, Diamond Bar, California). Mapping/ablation catheter was placed into the RV via transfemoral approach or into the LV via transseptal or retrograde aortic approach. When required, epicardial access was obtained by fluoroscopy-guided anterior percutaneous subxiphoid puncture. Remote magnetic navigation (RMN) (Niobe II, Stereotaxis Inc., St. Louis, MO) and/or multielectrode catheter mapping (decapolar or duodecapolar) was used at the discretion of the electrophysiologist.
Normal myocardium was electrically defined by endocardial bipolar signals amplitude >1.5 mV, LV unipolar signals amplitude >8.3 mV, RV unipolar signals amplitude >5.5 mV and epicardial bipolar signals amplitude >1 mV, while dense scar and borderzone (BZ) myocardium were defined by endocardial bipolar signals <0.5 mv and 0.5–1.5 mV, respectively. Area measurements (total scar area, dense scar area) were manually performed using the integrated CARTO-3 measuring software tool based on the end-procedural voltage electroanatomical map as defined above (BZ scar area and BZ to total scar ratio were derived from the directly measured values). The ablation strategy was based on a previously published scar-dechannelling protocol targeting conduction channel entrances (CCEs) (13) within the scar BZ using open-irrigated ablation catheters (35–50 W, 45°C). Activation/entrainment mapping were performed if VTs were spontaneously or mechanically induced during mapping and were hemodynamically tolerated by the patient.
After elimination of CCEs, a programmed ventricular stimulation (PVS) was routinely performed with at least 2 drive cycle lengths (CLs) and 4 extra stimuli (ESx) [3 ESx in patients with heart failure (HF) symptoms at rest or extreme frailty] (at a minimum of 200 ms or until ventricular refractoriness was reached) from 2 sites of the BZ area (usually medially and laterally to the scar) to assess for VT inducibility [as previously described (14)]. PVS could not be performed in three patients (4.68% of the entire population, all of them without NR-CTOs). If SMVTs [which were considered relevant if their cycle lengths (CLs) ≥250 ms] (15) were induced during PVS, scar reconnection was reassessed and the scar-dechannelling protocol was repeated. If the end-procedural post-ablation PVS induced any SMVT (CL ≥250 ms), the patient was considered to have residual SMVT PVS-inducibility. A SMVT was considered to be the clinical SMVT based on the 12-lead electrocardiogram QRS morphology or based on ICD-derived intracardiac electrograms with similar (±20 ms) CLs. Data regarding procedural characteristics was reported from each patient's last performed ablation procedure.
2.3. Follow-up protocol
All patients were monitored from the most recent ES ablation procedure and all the observed events were assessed in relation to the last procedure. Data was obtained from medical records and routine periodical 6 month-interval post-RFCA ICD interrogation. For patients not evaluated in our center, data was obtained from telephone interviews addressed to the referring physicians and patients and from ICD interrogations performed by the referring physicians. ICD interrogation was performed in all patients that were alive in January 2022.
Post-ablation recurrence was defined by SMVT or VF episodes which were adequately treated by either antitachycardia pacing and/or internal electrical shock. Post-ablation detection zones were programmed accordingly to allow detection of any ventricular arrhythmia which was previously spontaneously or PVS-induced (−20 bpm relative the slowest recorded VT). There were no monitoring zones below this VT rate threshold. All-cause mortality rates were retrospectively analyzed during the post-RFCA monitoring interval, irrespective of cardiovascular and non-cardiovascular causes of death.
The study protocol complied with the Declaration of Helsinki and it was approved by the human research committee of the Emergency Clinical Hospital of Bucharest Ethics Committee (12521-01/04/2022).
2.4. Endpoints
The primary endpoints were all-cause mortality and VT/VF recurrences, respectively, in the two subgroups (with NR-CTOs and without NR-CTOs, respectively). The secondary endpoint was to evaluate the predictive effect of NR-CTOs on primary endpoints in relation to other relevant prognostic factors.
2.5. Statistical analysis
Continuous data was expressed as mean ± standard deviation (SD) for normally distributed data and median (IQR) for non-normally distributed data. Categorical data was expressed as percentage (count). The normality of data was evaluated by Kolmogorov-Smirnov test. Categorical variables were compared using the Fisher's exact test/chi-square analysis and continuous variables were compared using Student t-test if normally distributed and non-parametric tests (Mann-Whitney U-Test).
Survival curves were plotted via Kaplan–Meier method and the statistical pairwise over strata comparison between curves was determined using the log-rank test. Univariate and multivariate Cox regression analyses were performed in order to determine the predictive factors. Variables with P < 0.2 in the univariate analysis or were then included in the multivariable Cox regression analysis for the determination of hazard ratio (HR) and its 95% confidence interval (CI). The number of predictors assessed in each multivariable model was adapted to the number of events observed during follow-up.
A 2-sided p-value < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 23 (IBM Corp., Armonk, NY) software and Prism 9 Version 9.5.0 (GraphPad Software, LLC).
3. Results
3.1. Baseline characteristics
Sixty-four consecutive patients were included and monitored for a median interval of 34.36 (7.25–63.65) months. The baseline characteristics are summarized in Table 1. The median interval between ES diagnosis and the ablation procedure was 0.8 (0.05–2) weeks.
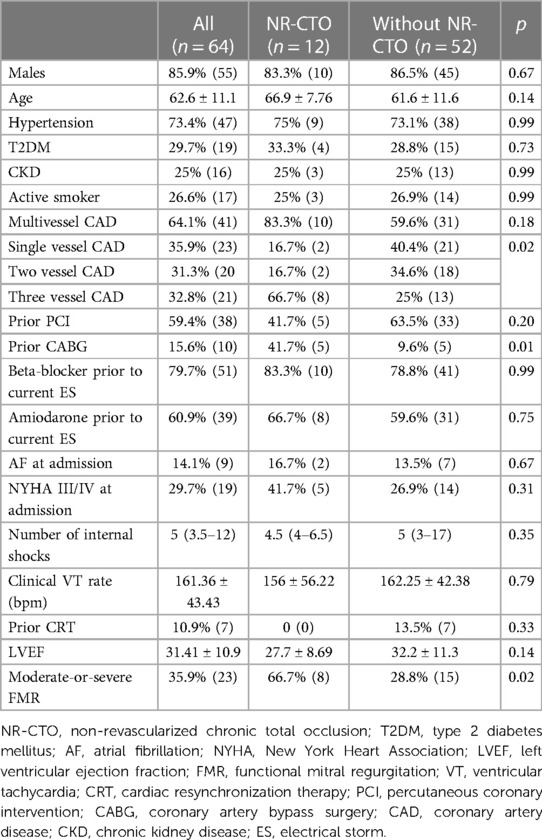
Table 1. Baseline characteristics of the electrical storm cohort stratified by the presence of NR-CTO.
In the NR-CTO group, there were seven (n = 7) patients with one NR-CTO and five (n = 5) patients with two NR-CTOs. The localization of the NR-CTOs was as following: LAD (41.7%, n = 5), LCX (25%, n = 3) and RCA (75%, n = 9). There were only two patients (16.7%) within the NR-CTO subgroup with incidentally diagnosed NR-CTOs and ten patients (83.3%) with NR-CTOs compatible to the localization of previously documented MIs. Additionally, at the moment of ablation there were five patients with residual potentially significant coronary stenoses [LCX (3.1%, n = 2) and RCA (4.7%, n = 3)], all of which only received medical treatment during the follow-up interval.
Hospitalization duration was almost two-fold higher in the NR-CTO subgroup (10 (23) vs. 5 (3) days), however without statistical significance (p = 0.06).
Overall, 35.9% (n = 23) patients had moderate-or-severe functional mitral regurgitation (FMR) (66.7% (n = 8) in the NR-CTO subgroup compared with 28.8% (n = 15) in the subgroup without NR-CTOs, p = 0.02).
3.2. Procedural characteristics
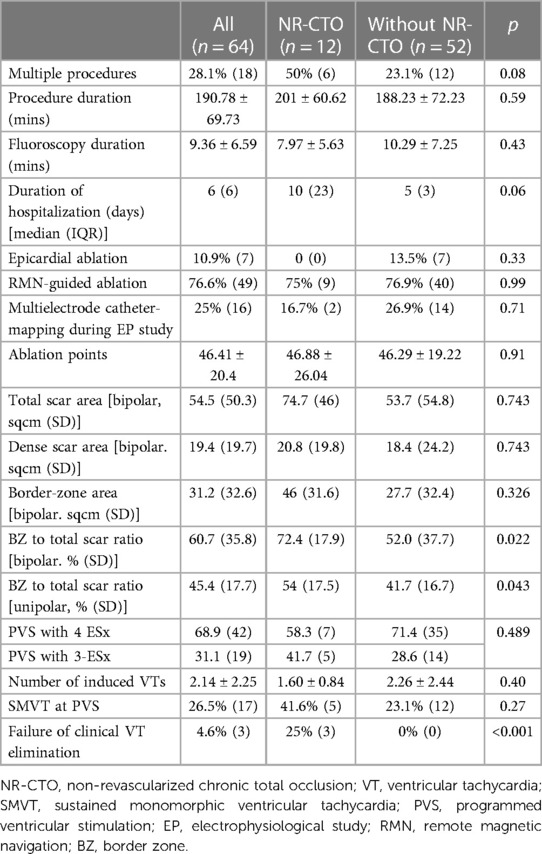
Procedural characteristics are summarized in Table 2. The number of procedures per patient were: one procedure (71.9%, n = 46), two (20.3%, n = 13), three (6.3%, n = 4) and one patient (1.6%) underwent four procedures. There were no significant differences between NR-CTO and without NR-CTO subgroups with the exception of failure of clinical VT elimination ablation result which was more frequent in the NR-CTO subgroup compared to those without (25% vs. 0%, p < 0.001). Out of the five (n = 5) NR-CTO patients with residual SMVT inducibility, three (n = 3) developed periprocedural progressive HF symptoms and were deferred from epicardial mapping. The other two patients (n = 2) underwent epicardial mapping with no targetable subepicardial substrate.
There were no significant differences in the number of ESx used during PVS in the cases with NR-CTOs vs. those without NR-CTOs (p = 0.489). There were three patients from the subgroup with no NR-CTOs which were deferred from post-ablation PVS testing.
The NR-CTO subgroup had a higher bipolar BZ-to-scar ratio (%) compared to the subgroup without NR-CTO (72.4 (17.9) vs. 52.0 (37.7), p = 0.022). There were no differences in bipolar BZ-to-scar ratio in the NR-CTO subgroup in the presence of incidental NR-CTOs (71.7% vs. 72.4% in those with localization-compatible MI history, p = 0.936). Moderate-or-severe FMR did not influence residual SMVT inducibility at PVS (OR 2.286, CI 95% 0.748–6.986, p = 0.147). However, BZ-to-scar ratio did not predict SMVT inducibility (OR 0.985, CI 95% 0.985–1.045, p = 0.329).
At final PVS, the NR-CTO subgroup had a significantly higher rate of residual clinical VT inducibility (25% (3) vs. 0% (0), p < 0.001) and a higher (yet non-significant) rate of residually inducible SMVTs compared to the subgroup without (41.6% (5) vs. 23.1% (12), p = 0.27).
3.3. Post-ablation outcomes
During the follow-up interval, Kaplan-Meier survival curves (Figure 1A) demonstrated that the NR-CTO subgroup had significantly lower survival compared to patients without NR-CTO (log-rank p = 0.003) and more frequent recurrences (log rank p = 0.042) (Figure 1B).
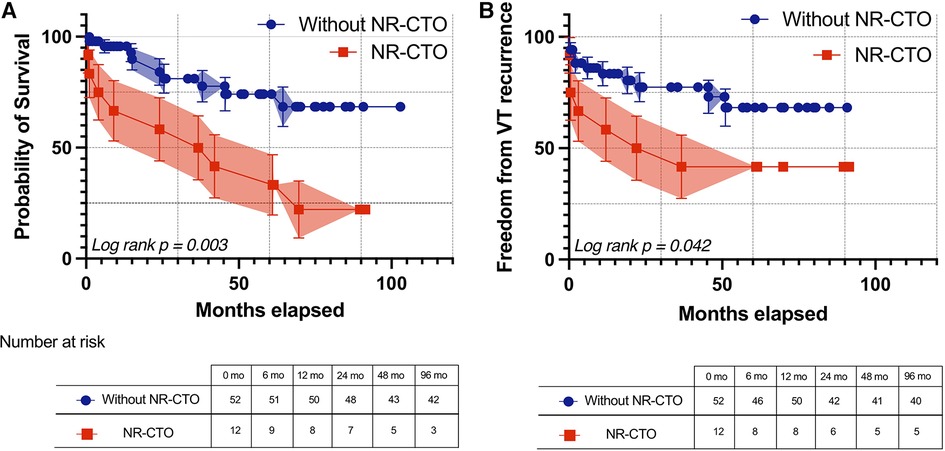
Figure 1. Kaplan-Meier survival curves with 95% confidence intervals of all-cause mortality (A) and freedom from ventricular arrhythmia recurrence (B) during follow-up in patients with at least one NR-CTO (red line) and without NR-CTOs (blue line). NR-CTO, non-revascularized chronic total occlusion; VT, ventricular tachycardia.
The overall rate of all-cause mortality during follow-up was 29.7% (n = 19). All-cause mortality was higher in patients with inducible SMVT at PVS (55.6%, n = 10) vs. those without inducible SMVT at PVS (19.6%, n = 9), p = 0.007. The overall rate of recurrence during follow-up was 29.7% (n = 19). Recurrences were higher in patients with inducible SMVT at PVS (66.7%, n = 12) vs. those without inducible SMVT at PVS (15.2%, n = 7), p < 0.001.
Overall post-ablation, 84.4% (n = 54) patients received beta-blockers (NR-CTO 91.7% (n = 11) vs. without NR-CTO 82.7% (n = 43), p = 0.672) and amiodarone in 65.5% (n = 42) (NR-CTO 83.3% (n = 10) vs. without NR-CTO 61.5% (n = 32), p = 0.193). Neither post-ablation beta-blockers (p = 0.99 for mortality, p = 0.99 for recurrences) nor post-ablation amiodarone (p = 0.16 for mortality, p = 0.56 for recurrences) influenced mortality or recurrences.
3.4. Predictive risk factors for clinical endpoints
Cox regression analysis results are summarized in Table 3 and are further detailed in Supplementary Table S1. Residual SMVT at PVS predicted all-cause mortality (HR 5.384, CI 95% 2.155–13.446, p < 0.001) and recurrences (HR 7.185, CI 95% 2.666–19.365, p < 0.001). In multivariable analysis, residual SMVT at PVS independently predicted all-cause mortality when adjusted by age aHR 4.965, CI 95% 1.847–11.933, p < 0.001) and recurrences when adjusted by NYHA III or IV symptoms at admission (aHR 5.214, CI 95% 2.056–13.219, p = 0.001).
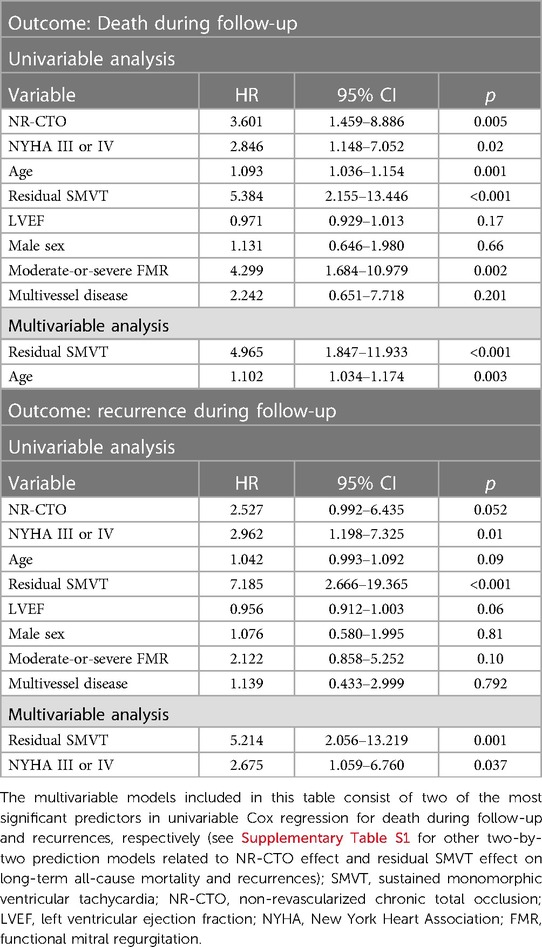
Table 3. Cox regression univariable and multivariable proportional hazards model for prediction of death during follow-up and recurrence during follow-up.
Particularly, univariable Cox regression showed that NR-CTO predicted all-cause mortality (HR 3.601, CI 95% 1.459–8.886, p = 0.005). In multivariable Cox regression, although NR-CTO remained an independent predictor for all-cause mortality during follow-up when adjusting for residual SMVT at PVS [adjusted HR (aHR) for NR-CTO 5.605, p = 0.001], age (aHR for NR-CTO 2.76, p = 0.03) and NYHA III or IV symptoms at admission (aHR for NR-CTO 2.09, p = 0.003), respectively, it was not significant when adjusting for the presence of moderate-or-severe FMR (p = 0.15) (Table 3 and Supplementary Table S1). NR-CTO did not predict recurrence (HR 2.252, CI 95% 0.992–6.435, p = 0.052) in univariable Cox regression.
4. Discussions
4.1. Acute ablation results
In our study, five of twelve NR-CTO patients had residual post-ablation SMVT inducibility compared to the lower rates of positive PVS observed in those without NR-CTOs (23.1%). Moreover, ablation was not able to eliminate the clinical VT in three out of twelve NR-CTO patients (which was however successfully abolished in all those without NR-CTO). This is highly significant as failure to eliminate the clinical VT during ES ablation is strongly associated with short-term very high mortality (16). Furthermore, we emphasize that the PVS protocol was similarly aggressive and there were no significant differences of medical treatment in NR-CTO subjects compared to those without NR-CTOs.
Our results contrast with those of the only three analysed post-VT ablation NR-CTO cohorts in which ablation results were not affected by NR-CTOs (5–7). However, this difference may be influenced firstly by the lower ratio of ES patients included in their analysis (less than one third in the former and ≈60% in the latter) and the relatively lower LVEF observed in our NR-CTO subgroup. Moreover, only three-ESx based PVS was used in Lurz et al.'s protocol which may impact its sensitivity (5).
We hypothesize that our observations can be explained by more complex substrate which limits RFCA efficiency, especially in ES acute settings. In the presence of CTOs, scar border-zone (BZ) area is usually expected to be larger and more heterogenous (6) which strongly correlates with both spontaneous incident VTs (17) and VT inducibility at PVS (18). Most myocardial segments which are supplied by NR-CTOs have less than 50% scar transmurality (19). Even more, recanalization of CTOs may promote reverse remodelling particularly within the BZ (20). Hence, the presence of infarct-related CTOs is known to double the risk of ES development (21). Our dataset showed that although the total scar area was not significantly higher in the NR-CTO subgroup (74.7 sqcm vs. 53.7 sqcm), there was however a significantly higher proportion of BZ myocardium within the total scar identified at electroanatomical mapping (72.4% compared to 52% in those without NR-CTOs) (Figure 2). Additionally, we considered the lack of epicardial ablation in the NR-CTO subgroup is most likely a result of the reduced analyzed sample. Out of the five NR-CTO patients with residual SMVT inducibility, three developed periprocedural progressive HF symptoms and were deferred from epicardial mapping. The other two patients underwent epicardial mapping with no targetable subepicardial substrate.
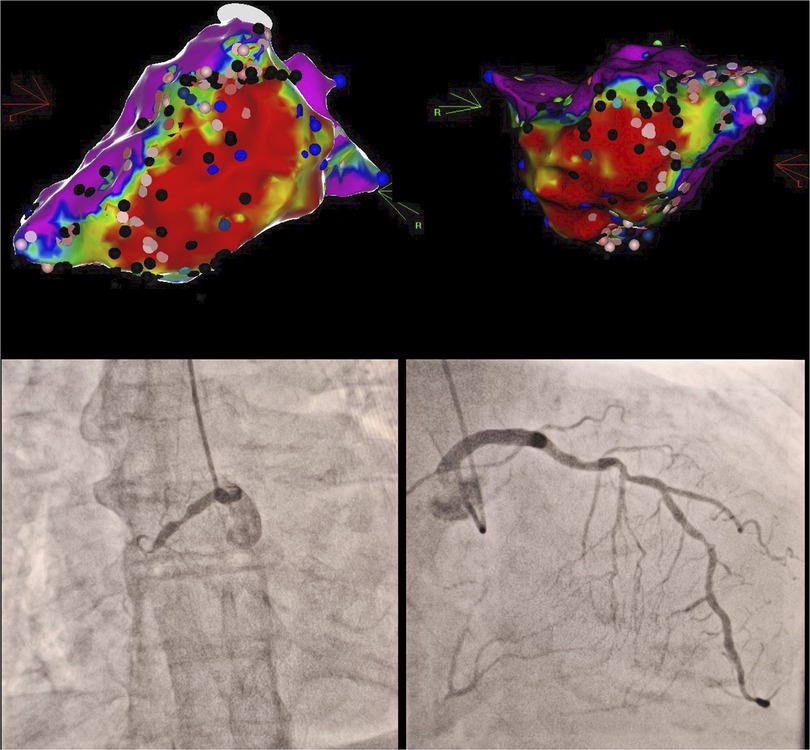
Figure 2. Clinical vignette in a case of proximal RCA chronic total occlusion with contralateral LAD septal collaterals (bottom) and extensive inferior wall to apical segment scar tissue in electroanatomical bipolar voltage mapping (top) with multiple conduction channel entries (black marks), intra-scar late potentials (blue marks).Red areas correspond to dense scar bipolar voltages (<0.5 mV), purple areas correspond to normal bipolar voltages (>1.5 mV), whereas intermediate colours (yellow-green-blue) correspond to border zone myocardial bipolar voltages (0.5–1.5 mV). RCA, right coronary artery; LAD, left anterior descending.
Considering that almost all CTOs generate myocardial ischemia even at rest, as proven by abnormal fractional flow-reserve (FFR), irrespective of collateral flow (22), it may be speculated that variable coronary flow can lead to changes in relevant electrical substrate which hinders its complete characterization during EPS (which can alter ablation results and, importantly, subsequent recurrences).
In addition, NR-CTO patients exhibited significantly more extensive CAD and significantly more frequent moderate or severe FMR. All of these may contribute to the trend of more severe HF symptoms (five out of twelve NYHA III/IV) in the NR-CTOs subgroup. Furthermore, more extensive CAD, systolic dysfunction and, interestingly enough, better developed collateralization have all predicted development of ischemic FMR after primary PCI in ST-elevation MIs (23).
Therefore, the presence of NR-CTOs may impact ES ablation outcomes due to substrate complexity and disease severity.
4.2. Long-term clinical course after ES ablation
In our cohort, post-ablation ES patients demonstrated high all-cause mortality (29.7% overall & 9.4% in the first year) and recurrences (29.7% overall & 20.3% in the first year). We observed that the most prominent driver of mortality in univariable analysis (5-fold higher risk) and recurrences (7-fold more probable) is residual SMVT inducibility at post-ablation PVS which is in line with previous publications (5, 6). Furthermore, residual SMVT maintained predictive effects on death and recurrences when adjusted by the presence of NR-CTOs and all other included factors. There are, however, multivariable models which have shown that other predictors (such as disease severity, comorbidities and procedural complications) may outweigh the effect of persistent inducibility at PVS (24).
In the presence of NR-CTOs, overall all-cause mortality (75%) and recurrences (58.3%) were significantly higher. However, these observations stem from a limited number of included patients (particularly with NR-CTOs) and should be further evaluated in larger samples. The excess mortality and incident VT episodes associated with NR-CTOs has been demonstrated in ICM patients, particularly in primary and secondary prevention ICD recipients (1–4, 25, 26). In addition, previous studies suggest that outcomes may be improved after NR-CTO revascularization (27). There is however, very limited published experience with NR-CTO patients after VT ablation (5–7).
Our analysis suggests that NR-CTOs independently predicted death after ES ablation when adjusted by SMVT inducibility at PVS, severe HF symptoms at admission, age and LVEF, respectively. Existing data suggests this effect was attenuated by confounding factors during a shorter interval of monitoring (≤20 months) predominantly after VT ablation (not ES) (5). One possible explanation of this difference could be the significantly longer monitored interval in our study. As previously emphasized, ES development in ICD recipients can be both a cause and an effect of HF progression, especially in certain HFrEF subgroups of patients (28). We believe it is highly valuable to distinguish previously stable patients which are more likely to respond well to ablation as opposed to end-stage HF cases which should rather receive specific advanced HF treatment such as cardiac transplant or mechanical devices.
In our cohort, NR-CTOs did not predict recurrences, which contrasts Di Marco et al.'s observations (6). This was especially evident when adjusting for PVS inducibility, which was not previously included in any prediction models. PVS (especially aggressive protocol with 4-extrastimuli) unmasks residual arrhythmogenic substrate which may become relevant for subsequent VT episodes which may explain why it drives recurrence prediction during follow-up.
Although significant coronary stenoses should be revascularized prior to ES ablation, addressing NR-CTOs before ablation may not be a reasonable option (especially if J-CTO scores are high). However, the NR-CTO effect on long-term mortality suggest that this decision should be revisited after ES treatment, as selective revascularization may improve outcomes, particularly guided by viability (27). Considering the ongoing ischemia attributed to NR-CTO even at rest, tackling HF-inducing mechanisms by targeted therapies (as shown by the anti-arrhythmic effect of CRT vs. ICD in propensity-matched previous registries) may deny the manifestation (or recurrence) of ES or non-clustered VTs (29).
In summary, our data suggests there may be incremental mortality attributable to the presence of NR-CTOs in ablated ES patients. However, it seems not to be driven by recurrences, but by other mechanisms (most likely due to progressive HF deterioration). Consequently, identifying NR-CTOs in ES patients may warrant close monitoring after ablation due to the higher risk of unfavourable outcomes.
Last but not least, advanced age and severe HF symptoms at admission maintained independent prediction of death after ES ablation when adjusted by NR-CTO and positive PVS, respectively. This is in line with previous dedicated prognostic scores such as PAAINESD and I-VT (30–32). Furthermore, moderate-or-severe FMR is known to independently aggravate outcomes in HF patients (25, 33) and attenuated the effect of NR-CTOs on all-cause mortality. In our dataset, moderate-or-severe FMR did not influence recurrences.
5. Limitations
1. Data regarding NR-CTOs and CAD complexity were retrospectively acquired on a limited number of patients which were included in a post-hoc analysis. However, our cohort solely consisted of ES patients as opposed to previous cohorts which also included isolated VT cases.
2. The small number of events during follow-up (19 deaths and 19 recurrences) limited the maximum number of variables to be included in multivariable Cox regression models. Two-by-two Cox regression models have been included in Supplementary data section (Supplementary Tables S1 and S2). This may hinder the complete understanding of each factor's effect in ES long-term clinical course.
3. There was no data available regarding Rentrop collateral grading and/or myocardial viability or ischemia inducibility. The potentially significant coronary lesions were not evaluated by FFR (as defined in the Methods section). There were five patients with potentially significant lesions during ablation (however none with previous documented ischemia).
4. Monitoring zones were not uniformly applied below the previously described ICD programmed zones which may influence recurrence rates. However, if patients became symptomatic or if VT was diagnosed during follow-up, ICD zones were reprogrammed accordingly.
5. Long-term mortality endpoints were only based on all-cause mortality (irrespective of cardiovascular vs. non-cardiovascular mortality).
6. There was limited data concerning beta-blocker or amiodarone doses (or HF-directed treatment) prescribed prior to or post-ablation. However, there was no effect caused by post-ablation beta-blocker or amiodarone presence on the evaluated end-points.
6. Conclusions
NR-CTO patients treated by RFCA for drug-refractory ES demonstrated a higher ratio of BZ-to-total-scar area. In this analysis, NR-CTO was associated with worse acute procedural results and may as well impact long-term outcomes which should be further assessed in larger patient populations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Emergency Clinical Hospital of Bucharest Ethics Committee (12521-01/04/2022). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CC: Investigation, Methodology, Conceptualization, Data curation, Formal Analysis, Software, Visualization, Writing – original draft, Writing – review & editing. AN: Writing – review & editing, Data curation, Investigation, Resources. SB: Writing – review & editing, Conceptualization, Methodology, Project administration. CI: Writing – review & editing, Investigation, Methodology, Supervision. AD: Writing – review & editing, Investigation, Supervision. SO: Writing – review & editing, Conceptualization, Methodology, Supervision. RV: Validation, Visualization, Writing – review & editing, Writing – original draft, Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The publishing fee of this paper was supported by the University of Medicine and Pharmacy Carol Davila, through the institutional program Publish not Perish.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1258373/full#supplementary-material
References
1. Nombela-Franco L, Iannaccone M, Anguera I, Amat-Santos IJ, Sanchez-Garcia M, Bautista D, et al. Impact of chronic total coronary occlusion on recurrence of ventricular arrhythmias in ischemic secondary prevention implantable cardioverter-defibrillator recipients (VACTO secondary study): insights from coronary angiogram and electrogram analysis. JACC Cardiovasc Interv. (2017) 10:879–88. doi: 10.1016/j.jcin.2017.02.008
2. Nombela-Franco L, Mitroi CD, Fernández-Lozano I, García-Touchard A, Toquero J, Castro-Urda V, et al. Ventricular arrhythmias among implantable cardioverter-defibrillator recipients for primary prevention. Circ Arrhythmia Electrophysiol. (2012) 5:147–54. doi: 10.1161/CIRCEP.111.968008
3. van Dongen IM, Yilmaz D, Elias J, Claessen BEPM, Delewi R, Knops RE, et al. Evaluation of the impact of a chronic total coronary occlusion on ventricular arrhythmias and long-term mortality in patients with ischemic cardiomyopathy and an implantable cardioverter-defibrillator (the eCTOpy-in-ICD study). J Am Heart Assoc. (2023) 7:e008609. doi: 10.1161/JAHA.118.008609
4. Chi WK, Gong M, Bazoukis G, Yan BP, Letsas KP, Liu T, et al. Impact of coronary artery chronic total occlusion on arrhythmic and mortality outcomes: a systematic review and meta-analysis. JACC Clin Electrophysiol. (2018) 4:1214–23. doi: 10.1016/j.jacep.2018.06.011
5. Lurz JA, Schmidt E, Kresoja K-P, Torri F, König S, Darma A, et al. Relevance of chronic total occlusion for outcome of ventricular tachycardia ablation in ischemic cardiomyopathy. J Interv Cardiol. (2022) 2022:6829725. doi: 10.1155/2022/6829725
6. Di Marco A, Paglino G, Oloriz T, Maccabelli G, Baratto F, Vergara P, et al. Impact of a chronic total occlusion in an infarct-related artery on the long-term outcome of ventricular tachycardia ablation. J Cardiovasc Electrophysiol. (2015) 26:532–9. doi: 10.1111/jce.12622
7. Narducci ML, Niccoli G, Flore F, Perna F, Bencardino G, Montone RA, et al. Mid-term outcome of ventricular arrhythmias catheter ablation in patients with chronic coronary total occlusion compared to ischemic and non-ischemic patients. (2022).
8. Könemann H, Dagres N, Merino JL, Sticherling C, Zeppenfeld K, Tfelt-Hansen J, et al. Spotlight on the 2022 ESC guideline management of ventricular arrhythmias and prevention of sudden cardiac death: 10 novel key aspects. Europace. (2023) 25(5):euad091. doi: 10.1093/europace/euad091
9. Baldi E, Conte G, Zeppenfeld K, Lenarczyk R, Guerra JM, Farkowski MM, et al. Contemporary management of ventricular electrical storm in Europe: results of a European heart rhythm association survey. Europace. (2023) 25:1277–83. doi: 10.1093/europace/euac151
10. Kowlgi GN, Cha Y-M. Management of ventricular electrical storm: a contemporary appraisal. Europace. (2020) 22:1768–80. doi: 10.1093/europace/euaa232
11. Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J Am Soc Echocardiogr. (2017) 30:303–71. doi: 10.1016/j.echo.2017.01.007
12. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14. doi: 10.1016/j.echo.2014.10.003
13. Berruezo A, Fernández-Armenta J, Andreu D, Penela D, Herczku C, Evertz R, et al. Scar dechanneling: new method for scar-related left ventricular tachycardia substrate ablation. Circ Arrhythm Electrophysiol. (2015) 8:326–36. doi: 10.1161/CIRCEP.114.002386
14. Vătășescu R, Cojocaru C, Năstasă A, Popescu S, Iorgulescu C, Bogdan Ș, et al. Monomorphic VT non-inducibility after electrical storm ablation reduces mortality and recurrences. J Clin Med. (2022) 11(13):3887. doi: 10.3390/jcm11133887
15. Deyell MW, Doucette S, Parkash R, Nault I, Gula L, Gray C, et al. Ventricular tachycardia characteristics and outcomes with catheter ablation vs. Antiarrhythmic therapy: insights from the VANISH trial. Europace. (2022) 24:1112–8. doi: 10.1093/europace/euab328
16. Vergara P, Tung R, Vaseghi M, Brombin C, Frankel D, Biase D, et al. Successful ventricular tachycardia ablation in patients with electrical storm reduces recurrences and improves survival. Hear Rhythm. (2017) 15:48–55. doi: 10.1016/j.hrthm.2017.08.022
17. Thomsen AF, Bertelsen L, Jøns C, Jabbari R, Lønborg J, Kyhl K, et al. Scar border zone mass and presence of border zone channels assessed with cardiac magnetic resonance imaging are associated with ventricular arrhythmia in patients with ST-segment elevation myocardial infarction. Europace. (2023) 25(3):978–88. doi: 10.1093/europace/euac256
18. Sonoda K, Okumura Y, Watanabe I, Nagashima K, Mano H, Kogawa R, et al. Scar characteristics derived from two- and three-dimensional reconstructions of cardiac contrast-enhanced magnetic resonance images: relationship to ventricular tachycardia inducibility and ablation success. J Arrhythmia. (2017) 33:447–54. doi: 10.1016/j.joa.2016.11.001
19. Nakachi T, Kato S, Kirigaya H, Iinuma N, Fukui K, Saito N, et al. Prediction of functional recovery after percutaneous coronary revascularization for chronic total occlusion using late gadolinium enhanced magnetic resonance imaging. J Cardiol. (2017) 69:836–42. doi: 10.1016/j.jjcc.2017.01.002
20. Yamashita K, Igawa W, Ono M, Kido T, Okabe T, Isomura N, et al. Impact of recanalization of chronic total occlusion on left ventricular electrical remodeling. Pacing Clin Electrophysiol. (2019) 42:712–21. doi: 10.1111/pace.13691
21. Faga V, Anguera I, Oloriz T, Nombela-Franco L, Teruel L, Dallaglio P, et al. Improved prediction of electrical storm in patients with prior myocardial infarction and implantable cardioverter defibrillator. Int J Cardiol. (2022) 355:9–14. doi: 10.1016/j.ijcard.2022.02.016
22. Sachdeva R, Agrawal M, Flynn SE, Werner GS, Uretsky BF. The myocardium supplied by a chronic total occlusion is a persistently ischemic zone. Catheter Cardiovasc Interv. (2014) 83:9–16. doi: 10.1002/ccd.25001
23. Valuckiene Z, Budrys P, Jurkevicius R. Predicting ischemic mitral regurgitation in patients with acute ST-elevation myocardial infarction: does time to reperfusion really matter and what is the role of collateral circulation? Int J Cardiol. (2016) 203:667–71. doi: 10.1016/j.ijcard.2015.10.225
24. Darma A, Bertagnolli L, Dinov B, Torri F, Shamloo AS, Lurz JA, et al. Predictors of long-term mortality after catheter ablation of ventricular tachycardia in a contemporary cohort of patients with structural heart disease. Europace. (2020) 22:1672–9. doi: 10.1093/europace/euaa189
25. Tajstra M, Pyka Ł, Gorol J, Pres D, Gierlotka M, Gadula-Gacek E, et al. Impact of chronic total occlusion of the coronary artery on long-term prognosis in patients with ischemic systolic heart failure. JACC Cardiovasc Interv. (2016) 9(17):1790–7. doi: 10.1016/j.jcin.2016.06.007
26. Raja V, Wiegn P, Obel O, Christakopoulos G, Christopoulos G, Rangan B V, et al. Impact of chronic total occlusions and coronary revascularization on all-cause mortality and the incidence of ventricular arrhythmias in patients with ischemic cardiomyopathy. Am J Cardiol. (2015) 116:1358–62. doi: 10.1016/j.amjcard.2015.07.057
27. Iannaccone M, Nombela-Franco L, Gallone G, Annone U, Di Marco A, Giannini F, et al. Impact of successful chronic coronary total occlusion recanalization on recurrence of ventricular arrhythmias in implantable cardioverter-defibrillator recipients for ischemic cardiomyopathy (VACTO PCI study). Cardiovasc Revasc Med. (2022) 43:104–11. doi: 10.1016/j.carrev.2022.03.029
28. Guerra F, Shkoza M, Scappini L, Flori M, Capucci A. Role of electrical storm as a mortality and morbidity risk factor and its clinical predictors: a meta-analysis. Europace. (2014) 16(3):347–53. doi: 10.1093/europace/eut304
29. Guerra F, Palmisano P, Dell’Era G, Ziacchi M, Ammendola E, Pongetti G, et al. Cardiac resynchronization therapy and electrical storm: results of the OBSERVational registry on long-term outcome of ICD patients (OBSERVO-ICD). Europace. (2018) 20:979–85. doi: 10.1093/europace/eux166
30. Tzou WS, Tung R, Frankel DS, Vaseghi M, Bunch TJ, Di Biase L, et al. Ventricular tachycardia ablation in severe heart failure. Circ Arrhythmia Electrophysiol. (2017) 10:e004494. doi: 10.1161/CIRCEP.116.004494
31. Santangeli P, Rame JE, Birati EY, Marchlinski FE. Management of ventricular arrhythmias in patients with advanced heart failure. J Am Coll Cardiol. (2017) 69:1842–60. doi: 10.1016/j.jacc.2017.01.047
32. Vergara P, Tzou WS, Tung R, Brombin C, Nonis A, Vaseghi M, et al. Predictive score for identifying survival and recurrence risk profiles in patients undergoing ventricular tachycardia ablation. Circ Arrhythmia Electrophysiol. (2018) 11:e006730. doi: 10.1161/CIRCEP.118.006730
Keywords: chronic total occlusion, electrical storm, catheter ablation, risk stratification, ventricular tachycardia, ischemic cardiomyopathy
Citation: Cojocaru C, Nastasa A, Bogdan S, Iorgulescu C, Deaconu A, Onciul S and Vatasescu R (2023) Non-revascularized chronic total occlusions impact on substrate and post-ablation results in drug-refractory electrical storm. Front. Cardiovasc. Med. 10:1258373. doi: 10.3389/fcvm.2023.1258373
Received: 13 July 2023; Accepted: 7 September 2023;
Published: 21 September 2023.
Edited by:
Simone Savastano, San Matteo Hospital Foundation (IRCCS), ItalyReviewed by:
Luca Rosario Limite, Hopital privèe Les Franciscaines, FranceDominik Buckert, Ulm University Medical Center, Germany
© 2023 Cojocaru, Nastasa, Bogdan, Iorgulescu, Deaconu, Onciul and Vatasescu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Radu Vatasescu cmFkdV92YXRhc2VzY3VAeWFob28uY29t
 Cosmin Cojocaru
Cosmin Cojocaru Alexandrina Nastasa
Alexandrina Nastasa Stefan Bogdan1,3
Stefan Bogdan1,3 Alexandru Deaconu
Alexandru Deaconu Radu Vatasescu
Radu Vatasescu