
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med. , 22 September 2023
Sec. Cardio-Oncology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1257734
This article is part of the Research Topic Global Excellence in Cardiovascular Medicine: Asia and Australasia View all 11 articles
 Joseph Kassab1
Joseph Kassab1 Georges Gebrael2
Georges Gebrael2 Michel Chedid El Helou1,†
Michel Chedid El Helou1,† Joseph El Dahdah1,†
Joseph El Dahdah1,† Elio Haroun1
Elio Haroun1 Rebecca Kassab2
Rebecca Kassab2 Saad Abou Ali3
Saad Abou Ali3 Ziad Khabbaz4
Ziad Khabbaz4 Roland Kassab4*
Roland Kassab4*
A 64-year-old man presented with symptoms indicative of superior vena cava syndrome. Imaging work-up revealed an obstructing right atrial mass, which was subsequently excised and diagnosed as primary cardiac lymphoma. Post-surgery, the patient showed significant clinical improvement and was started on a chemotherapy regimen with complete remission at 1 year.
A previously healthy 64-year-old man, presented to the emergency department with 2 weeks of increasing exertional dyspnea, worsening orthopnea, facial and arm swelling, and a nonproductive cough. Vital signs on admission were within normal limits. On physical examination, the patient demonstrated marked facial and upper extremity edema, as well as distended neck veins. Additionally, signs of plethora and cyanosis of the face were noted, with an increase in jugular venous pressure on cardiovascular examination.
The patient had no significant past medical history.
Given the patient's symptoms suggestive of superior vena cava syndrome (SVC), a broad differential diagnosis were considered. Malignant causes included primary or secondary cardiac tumors, lung cancer, mediastinal tumors, or lymphoma, which may obstruct or compress the superior vena cava. Non-malignant etiologies encompassed SVC thrombosis, aortic aneurysm, or fibrosing mediastinitis. Cardiopulmonary conditions such as heart failure, pericardial disease, and pulmonary hypertension were also suggested, given the symptoms of dyspnea and orthopnea.
The patient underwent a chest CT-scan, followed by a confirmatory transesophageal echocardiogram (TEE) which revealed the presence of a prominent, heterogeneous, partially non-enhancing, right atrial mass, measuring 66 × 41 × 37 mm, partially disrupting inferior vena cava flow and obstructing the superior vena cava (Figures 1, 2, Supplementary Video S1). A filling defect was also noted in the proximal right pulmonary artery, suggesting emboli origination from the tumor. A whole-body positron emission tomography/computed tomography (PET/CT) showed pronounced uptake of fluorine-18 fluorodeoxyglucose in the right atrium, with no other metabolically active lesions (Figure 3, Supplementary Figure S1).
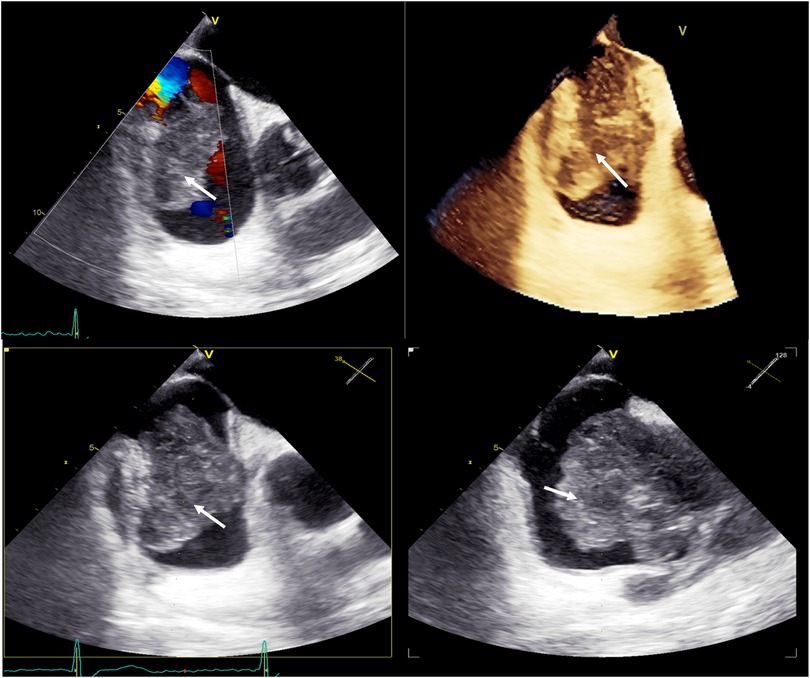
Figure 2. Baseline transesophageal echocardiography with 3D reconstruction, showing a large tumor arising from the lateral wall of the right atrium (white arrows).
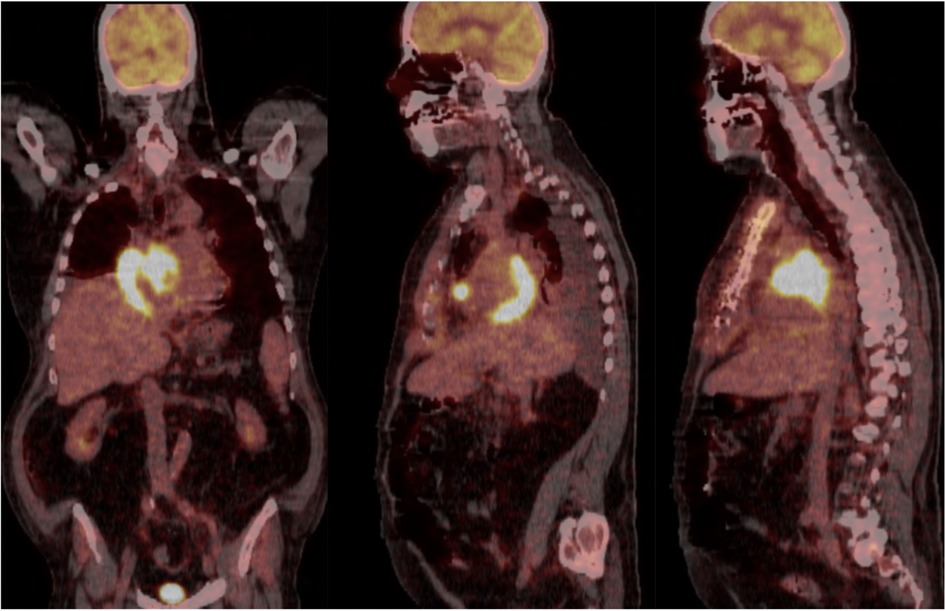
Figure 3. Whole-body positron emission tomography/computed tomography (PET/CT) in coronal and sagittal views showing pronounced uptake of fluorine-18 fluorodeoxyglucose in the right atrium, with no other metabolically active lesions.
Given the high risk of deteriorating clinical condition, surgical removal of the mass was planned and successfully executed. The surgical specimen (Supplementary Figure S2) was then sent to the pathology lab for microscopic examination. This examination showed a diffuse proliferation of large atypical lymphocytes exhibiting mitotic activity and apoptosis along with extensive areas of necrosis. Immunohistochemistry staining tested positive for CD20 (Figure 4), leading to a diagnosis of right atrial diffuse large B cell lymphoma, non-germinal center (activated) type. This confirmed the diagnosis of primary cardiac lymphoma (PCL). Following surgery, the patient's clinical condition improved significantly, and he was subsequently started on a R-CHOP chemotherapy regimen (intravenous rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone).
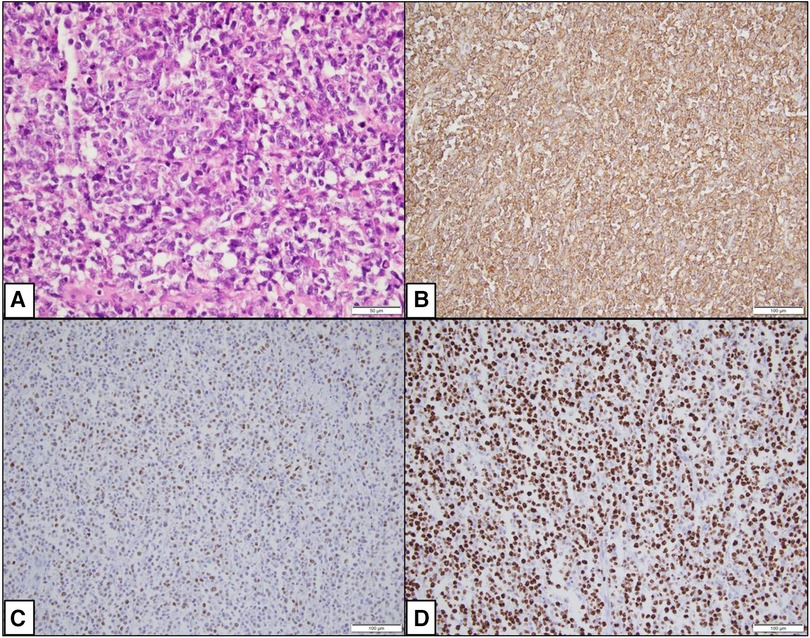
Figure 4. Photomicrographs showing histopathological changes. (A) (H&E × 400): Microscopy shows a diffuse proliferation of large atypical cells. (B) (IHC × 200): CD20 is positive in the large lymphocytes. (C) (IHC × 200): MUM1 is positive in the large lymphocytes. (D) (IHC × 200): Ki67 is approximately 95%.
While secondary cardiac lymphomas are relatively frequent and can contribute to 20% of extra nodal non Hodgkin lymphomas (NHL), primary cardiac lymphoma (PCL) is extremely rare and represents only 1%–2% of all heart tumors (1), since the cardiac tissue contains little to no lymphocytes. Almost all of PCLs appear to be derived from B-cell lineage with diffuse large B cell lymphoma (DLBCL) being the most common type, whereas other types such as Burkitt lymphoma or chronic lymphocytic leukemia/small lymphocytic lymphoma are less likely to be reported (1). Although immunosuppression (e.g., transplant recipients, HIV patients, immunosuppressant drugs) could be a risk factor for developing PCL, it usually occurs in immunocompetent adults with a median age of presentation of 55–65 years and 65%–85% of all cases in males (2)—features compatible with our patient. PCL may present with a wide range of symptoms, generally depending on the site of involvement of the heart, size and growth rate. These symptoms may encompass signs of heart failure, such as shortness of breath and pedal edema, as well as chest pain, arrhythmia (particularly atrial fibrillation and AV block), constitutional symptoms (e.g., fever, chills, night sweats, weight loss), and SVC syndrome as was seen in our patient (2, 3). All heart chambers as well as the interatrial and the interventricular septum may be involved; however, the tumor tends to arise in the wall of the right heart, specifically in the right atrium, with epicardial and pericardial involvement in about 50% of cases (4).
Differential diagnoses include benign myxoma (the most common type of cardiac tumor), intracardiac thrombi (in the setting of hypokinetic dilated cardiomyopathy) and angiosarcoma, (the most common malignant heart tumor, usually found in the left cavities) (5). Initial evaluation of cardiac masses often involves noninvasive multimodality imaging, including 2D or 3D echocardiography, MRI, and contrast CT scan, depending on diagnostic suspicion (6). As was done with our patient, transesophageal echocardiography is a key initial diagnostic tool for differentiating diagnosis and guiding further imaging and management. Cardiac CT scan and MRI are used to determine the extent of heart involvement and guide biopsy and/or surgical approach. PET scan may also be performed to assess for primary tumors or extracardiac involvement—which was negative in this case confirming the diagnosis of PCL. Histopathological confirmation is required for definitive diagnosis, and cytological examination is sometimes helpful in the presence of pericardial effusion.
The mainstay of PCL treatment relies on chemotherapy, using the combination of Rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP regimen), which has shown a response rate of 79%–87% (5). Prompt initiation of chemotherapy often provides symptomatic relief as well as complete remission. Side effects include tumor lysis syndrome and sepsis in around 10% of cases. In addition, chemotherapy can lead to massive thromboembolism, cardiac wall perforation, ventricular septal rupture, life threatening arrhythmias and pericardial effusion. For these reasons, some reports suggest that reduced doses of chemotherapy “R-mini-CHOP” in the initial course of treatment could reduce the risk of sudden cardiac death (7). Radiation therapy may be used in combination with chemotherapy, particularly in refractory cases, and reports have shown improved survival. However, it has some limitations given the risk of radiation-induced heart disease including pericarditis, cardiomyopathy, coronary artery disease, conduction defects and diastolic dysfunction (8). While surgical management is not the mainstay of treatment, prompt surgical debulking is indicated in patients with acute and severe presentations, including SVC syndrome (as in our patient) (9) and rapidly progressive heart failure (10). The overall prognosis of PCL is generally favorable, with a remission rate of 61% following treatment with chemotherapy alone (10).
The patient is currently in remission at 1-year follow-up.
PCL is an extremely rare neoplasm. While chemotherapy remains the mainstay of treatment, surgery could be considered for urgent symptomatic relief.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JK: Writing – original draft, Writing – review & editing. GG: Writing – original draft, Writing – review & editing. MC: Writing – original draft, Writing – review & editing. JE: Writing – original draft, Writing – review & editing. EH: Writing – original draft, Writing – review & editing. SA: Writing – review & editing. ZK: Writing – review & editing. RK: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1257734/full#supplementary-material
Supplementary Figure 1
Whole-body positron emission tomography/computed tomography (PET/CT) in axial view showing pronounced uptake of fluorine-18 fluorodeoxyglucose in the right atrium, with no other metabolically active lesions.
Supplementary Figure 2
Surgical specimen of the right atrial mass (white arrow).
Supplementary Video 1
Baseline transesophageal echocardiography, showing a large tumor arising from the lateral wall of the right atrium invading and causing significant obstruction of the superior vena cava.
1. Miguel CE, Bestetti RB. Primary cardiac lymphoma. Int J Cardiol. (2011) 149:358–63. doi: 10.1016/j.ijcard.2010.02.016
2. Csizmar CM, Sachs Z, Cayci Z, Bu L, Linden MA. Primary cardiac lymphoma: three case reports and a review of the literature. Open J Blood Dis. (2021) 11:120–32. doi: 10.4236/ojbd.2021.114012
3. Ikeda H, Nakamura S, Nishimaki H, Masuda K, Takeo T, Kasai K, et al. Primary lymphoma of the heart: case report and literature review. Pathol Int. (2004) 54:187–95. doi: 10.1111/j.1440-1827.2003.01606.x
4. Carras S, Berger F, Chalabreysse L, Callet-Bauchut E, Cordier JF, Salles G, et al. Primary cardiac lymphoma: diagnosis, treatment and outcome in a modern series. Hematol Oncol. (2017) 35:510–9. doi: 10.1002/hon.2301
5. Petrich A, Cho SI, Billett H. Primary cardiac lymphoma. Cancer. (2011) 117:581–9. doi: 10.1002/cncr.25444
6. Tyebally S, Chen D, Bhattacharyya S, Mughrabi A, Hussain Z, Manisty C, et al. Cardiac tumors. JACC CardioOncol. (2020) 2:293–311. doi: 10.1016/j.jaccao.2020.05.009
7. Linschoten M, Kamphuis JAM, van Rhenen A, Bosman LP, Cramer MJ, Doevendans PA, et al. Cardiovascular adverse events in patients with non-hodgkin lymphoma treated with first-line cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP with rituximab (R-CHOP): a systematic review and meta-analysis. Lancet Haematol. (2020) 7:e295–308. doi: 10.1016/S2352-3026(20)30031-4
8. Al Mawed M, Brockmeier J, Haertel D, Ellermeier M, Hartmann F, Gielen S. From inoperable to back to life: a case report of successfully treated obstructive right ventricular primary cardiac lymphoma. Eur Heart J Case Rep. (2022) 6:ytac051. doi: 10.1093/ehjcr/ytac051
9. Johri A, Baetz T, Isotalo PA, Nolan RL, Sanfilippo AJ, Ropchan G. Primary cardiac diffuse large B cell lymphoma presenting with superior vena cava syndrome. Can J Cardiol. (2009) 25:e210–2. doi: 10.1016/S0828-282X(09)70110-2
Keywords: lymphoma, cardiac lymphoma, primary cardiac lymphoma, superior vena cava syndrome, diffuse large B cell lymphoma
Citation: Kassab J, Gebrael G, Chedid El Helou M, El Dahdah J, Haroun E, Kassab R, Abou Ali S, Khabbaz Z and Kassab R (2023) Case report: Primary cardiac lymphoma manifesting as superior vena cava syndrome. Front. Cardiovasc. Med. 10:1257734. doi: 10.3389/fcvm.2023.1257734
Received: 12 July 2023; Accepted: 11 September 2023;
Published: 22 September 2023.
Edited by:
Zhonghua Sun, Curtin University, AustraliaReviewed by:
Mohammed Faisaluddin, Rochester General Hospital, United States© 2023 Kassab, Gebrael, Chedid El Helou, El Dahdah, Haroun, Kassab, Abou Ali, Khabbaz and Kassab. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roland Kassab cm9sYW5kb2thQHlhaG9vLmZy
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.