
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 19 October 2023
Sec. Cardiovascular Imaging
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1253440
This article is part of the Research TopicStress Echocardiography in Cardiovascular DiseasesView all 7 articles
 Yuyou Duan1,†
Yuyou Duan1,† Luwei Ye2,†
Luwei Ye2,† Qinglan Shu2
Qinglan Shu2 Yu Huang2
Yu Huang2 Hongmei Zhang2
Hongmei Zhang2 Qingfeng Zhang2
Qingfeng Zhang2 Geqi Ding2
Geqi Ding2 Yan Deng2
Yan Deng2 Chunmei Li2
Chunmei Li2 Lixue Yin2*
Lixue Yin2* Yi Wang1,2*
Yi Wang1,2*
Aims: Subclinical left ventricular (LV) dysfunction may occur in T2DM patients at the early asymptomatic stage, and LV reserve function is a sensitive index to detect subtle LV dysfunction. The purpose of our study is (1) to assess the LV reserve function using treadmill exercise stress echocardiography (ESE) in asymptomatic type 2 diabetes mellitus (T2DM) patients; (2) to explore the link of serum biological parameters and LV reserve function.
Methods: This study included 84 patients with asymptomatic T2DM from September 2021 to July 2022 and 41 sex- and age-matched healthy controls during the corresponding period. All subjects completed treadmill ESE, LV systolic function-related parameters such as global longitudinal strain (GLS) and systolic strain rate (SRs), as well as diastolic function-related parameters such as E wave (E), early diastolic velocity (e′), E/e′ ratio, early diastolic SR (SRe), and late diastolic SR (SRa) were compared at rest and immediately after exercise. The difference between LV functional parameters after treadmill exercise and its corresponding resting value was used to compute LV reserve function. In addition, the associations of LV reserve function and serum biological parameters were analyzed.
Results: Patients with T2DM did not significantly vary from the controls in terms of alterations in LV diastolic reserve measures, the changes of LVGLS and SRs (ΔGLS: 2.19 ± 2.72% vs. 4.13 ± 2.79%, P < 0.001 and ΔSRs:0.78 ± 0.33 s−1 vs. 1.02 ± 0.28 s−1, P < 0.001) in the T2DM group were both lower than those in the control group. Glycated hemoglobin (HbA1c), N-terminal pro-brain natriuretic peptide (NTproBNP), waist circumference, and high-sensitive C-reactive protein (hsCRP) were identified as independent predictors of LV systolic reserve by stepwise multiple linear regression analysis.
Conclusion: LV systolic reserve function, as measured by pre- and post-exercise differences in GLS and SRs were significantly impaired in patients with asymptomatic T2DM, whereas diastolic reserve remained normal during exercise and was comparable to that of the control group. This was different from previous findings. High levels of HbA1c, NTproBNP, hsCRP, and increasing waist circumference were independent predictors of LV systolic reserve.
Globally, the incidence of type 2 diabetes mellitus (T2DM) is increasing. Patients with T2DM have twice the risk of developing cardiovascular disease (CVD) compared with healthy individuals, resulting in an increased risk of death from cardiovascular complications (1). In patients with T2DM, the most common complication and the leading cause of death is diabetic cardiomyopathy (DMCM) (2). In 1972, the concept of DMCM was proposed by Rubler et al., defining that DMCM belonged to a kind of myocardial lesion independent of coronary atherosclerotic heart disease, hypertension, and valvular disease (3). The progression of DMCM eventually results in chronic heart failure (HF). Early notice of subclinical HF in T2DM patients can effectively identify the high-risk patients for cardiovascular complications (4).
Using conventional echocardiography, the first hallmark of DMCM was previously thought to be diastolic dysfunction and was described as typical HF with preserved ejection fraction (EF). However speckle-tracking imaging (STI) could detect subtle changes in LV systolic function earlier than the decrease in the LVEF (5). Consequently, the onset of DMCM is concealed, early LV dysfunction in asymptomatic T2DM patients is subtle, and in a subclinical state, LV function may remain unchanged at rest. Exercise can increase the load of blood flow and increase the work done by LV to maintain the increase of adaptive cardiac output. This can also affect LV reserve function, which is the ability to increase LV function as the body's metabolic requirements increase. Impaired LV reserve function is an early manifestation of various types of HF. Therefore, assessment of LV systolic and diastolic reserve during exercise in combination with STI in patients with asymptomatic T2DM may reveal subclinical myocardial dysfunction and identify early-stage HF.
The identification of biomarkers that accurately predict HF and cardiovascular events in T2DM could improve patient management, aid clinical trials, and highlight novel pathogenesis and therapeutic targets, leading to further improvements in clinical practice (6). Thus, investigating the correlation between LV reserve function and serum biological parameters might provide ideas for the management of T2DM to delay or prevent LV function impairment.
The aims of this study were (1) to explore the clinical application of treadmill ESE combined with STI in assessing LV reserve function, and (2) to analyze the association of serum biological parameters and LV reserve function, which may facilitate early identification of the changes of LV function in asymptomatic T2DM patients.
In a cross-sectional study design, we prospectively enrolled asymptomatic patients with T2DM and healthy controls during the period from September 2021 to July 2022. T2DM was diagnosed according to World Health Organization criteria (7). The exclusion criteria for both populations included LVEF ≤ 50%, any existing CVD, T2DM-related complications (such as kidney disease, neurological disorder and retinopathy), severe obesity and poor echogenicity. None of the subjects had epicardial coronary artery disease as evidenced by coronary angiography and no symptoms (such as difficulty breathing or chest pain during exercise). A total of 100 asymptomatic T2DM patients and 50 healthy control subjects entered the study. All subjects completed clinical examinations that included baseline 12-lead electrocardiography (ECG), and thorough conventional echocardiography at rest and immediately after treadmill exercise (images acquired within 90 s).
Obtaining the basic data for all subjects including gender, age, mass, waist circumference, neck circumference, the waist–hip circumference ratio (WHR), hip circumference, smoking history, drinking history, and resting blood pressure. Serum biological data were collected during fasting and included the measurement of glucose (Glu), glycated hemoglobin (HbA1c), total cholesterol (TC), triglycerides (TG), high- and low-density lipoprotein cholesterol (HDL-C, LDL-C), high-sensitive C-reactive protein (hsCRP), effective glomerular filtration rate (eGFR), homeostasis model assessment of insulin resistance (HOMA-IR), and N-terminal pro-brain natriuretic peptide (NTproBNP). HOMA-IR is the product of fasting serum glucose and fasting serum insulin, calculated as (8).
The study was approved by the local ethics committee and was conducted in accordance with institutional policies, national legal requirements, and the revised principles of the Declaration of Helsinki.
Using standard Bruce protocols, each subject completed a symptom-limited treadmill (TMX-425, Full Vision Inc, Kansas, USA) exercise test (9). Subjects were advised to exercise to exhaustion. Blood pressure and a 12-lead electrocardiogram were measured and monitored at rest and again at 2-min intervals throughout the exercise examination, at maximum effort, and after exercise. The exercise test was terminated if the following criteria were reached: symptoms or arrhythmias, hypertension (220/120 mmHg), symptomatic hypotension (> 40 mmHg reduction), and significant ST-segment change (ST-segment depression or elevation >1 mm in two consecutive leads).
All subjects underwent standard conventional echocardiography using an ultrasound system Vivid E95 equipped with an M5S 3.5-MHz transducer (GE Vivid E95, Vingmed Ultrasound, Horten, Norway) at rest and immediately after exercise in the left lateral decubitus position. Briefly, at rest and immediately after exercise, at least five periods of two-dimensional images(frame rate >70 frames/second) were obtained from the apical four-, three-, and two-chamber sections and digitally stored.
Both groups complete a comprehensive 2D echocardiographic assessment at rest, according to guidelines of the American Society of Echocardiography(ASE) and the European Association of Cardiovascular Imaging(EACVI), all conventional echocardiographic and Doppler measurements of LV function were analyzed (10). LVEF was calculated using the Simpson biplane method. In the apical four-chamber view, peak early (E wave), atrial (A wave) transmitral flow velocity, and myocardial systolic (s′), early diastolic (e′), and atrial (a′) velocity were obtained using pulsed-wave Doppler (PW) and tissue Doppler velocity imaging (TDI). Simultaneously, we recorded E wave deceleration time (EDT) and LV isovolumic relaxation time (IVRT). All parameters were averaged over 3 consecutive cardiac cycles.
Electrocardiogram-triggered echocardiographic data were collected for 2D strain analysis with EchoPac (EchoPAC version 204, GE Healthcare, Horten, Norway). Using Automated Function Imaging to analyze the LV longitudinal function in three apical views (four, three, and two chambers). For optimal tracking results, the speckle region of interest was carefully modified. Segments that did not track adequately were manually readjusted, and if this was ineffective, they were removed from future research. Peak global longitudinal strain (GLS), peak systolic SR (SRs), peak early diastolic SR (SRe), and peak late diastolic SR (SRa) for the LV myocardium were determined (Figure 1). The mean LV GLS/SR was calculated from the three individual apical GLS/SR curves.
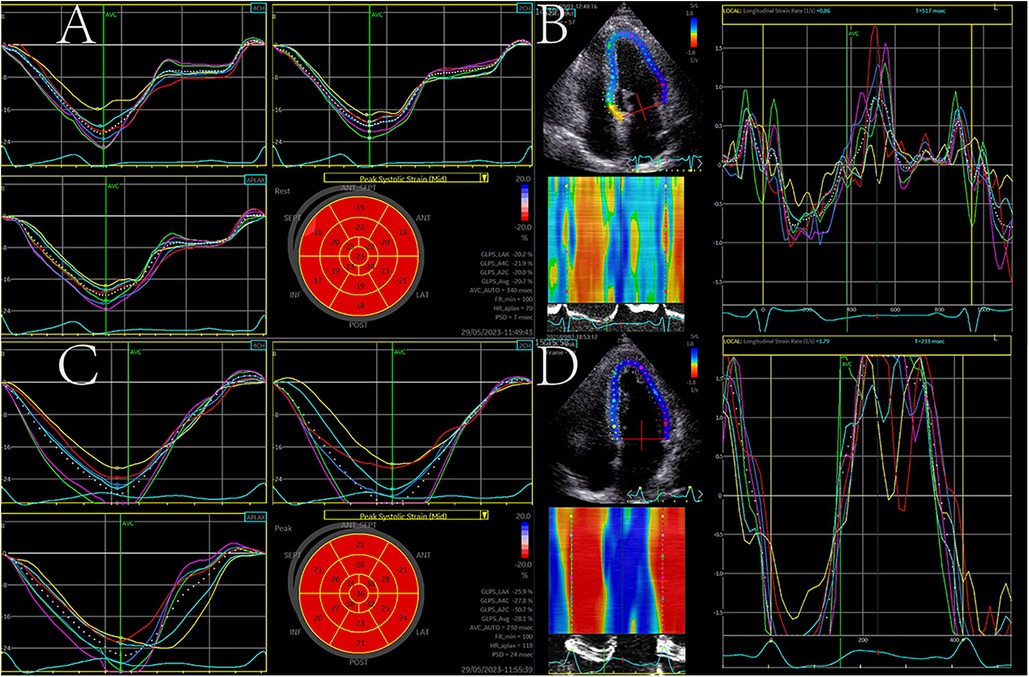
Figure 1. Left ventricular (LV) longitudinal strain (LS) and strain rate (SR) analysis at rest (A,B) and post-exercise (C,D). GLS and SR both increased after exercise.
The difference between resting and post-exercise LVEF, GLS, SRs, and s′ values were used to compute the LV systolic reserve function (expressed as ΔEF, ΔGLS, ΔSRs, and Δs′, respectively). The difference between resting and post-exercise E, A, EDT, IVRT, e′, a′, E/e′, SRe, and SRa was used to compute the LV diastolic reserve function (expressed as ΔE, ΔA, ΔEDT, ΔIVRT, Δe′, Δa′, ΔE/e′, ΔSRe, and ΔSRa, respectively).
Statistical analyses were conducted using SPSS for Windows (IBM SPSS Statistics, version 26), and all continuous variables in the study were presented as mean ± standard deviation or median and interquartile range. Categorical variables are expressed as frequencies and percentages. The normality of all continuous variables was evaluated by the Shapiro-Wilk test to satisfy the assumptions of subsequent tests. For data that conformed to normal distribution, an independent-sample t-test was adopted to perform the comparison between two groups, and a paired t-test was used for within-group comparisons at rest and after exercise; for data that did not conform to normal distribution, a Mann-Whitney U test was adopted to perform the comparison between two groups, and Wilcoxon rank sum test was adopted for comparisons within a group. The Chi-square test was used to evaluate categorical variables. For correlation analyses, Pearson correlation was used for data that conformed to normal distribution, and Spearman correlation was used for data that did not conform to normal distribution. Independent influence factors of LV reserve function were analyzed by stepwise multivariable regression. P < 0.05 was considered to be statistically significant.
To evaluate the reliability of STI-related parameters at rest and immediately after exercise, we randomly selected 10 subjects. The first and second researchers independently re-analyzed the images one week after the initial analysis, without knowledge of the original results. This study was done to assess inter- and intra-observer variability by intra-class correlation(ICC). The intra-observer and interobserver ICC of GLS at rest were 0.94 and 0.93, respectively. The intra-observer and interobserver ICC of GLS after exercise were 0.93 and 0.92, respectively.
This study involved 100 asymptomatic T2DM patients and 50 control subjects, after which 84 asymptomatic T2DM patients and 41 control subjects entered the analysis. The selection criteria are shown in the flowchart Figure 2. During the exercise, six asymptomatic T2DM patients experienced mild ST-segment depression. None of the subjects discontinued the testing due to contraindications.84 patients with T2DM (mean age: 58.02 ± 8.54 years; 54% male) and 41 healthy controls (mean age: 56.88 ± 8.95 years; 49% male). Age, gender, smoking and drinking in T2DM group were similar to the controls (P > 0.05). The T2DM group had larger neck, waist, and hip circumferences, as well as a higher WHR than the healthy controls (P < 0.05). For biological parameters, TC, TG, LDL-C, HDL-C, hsCRP, and eGFR in T2DM group were not different from the control group (P > 0.05). However, the T2DM group had higher levels of Glu, HbA1c, HOMA-IR, and NTproBNP compared with those in the control group (P < 0.05). Table 1 presents the specific clinical data, outcome measures, and medication usage for both groups.
Table 1 presents the parameters of conventional 2D echocardiographic in the two groups. The difference in LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) between the two groups were not statistically significant (P > 0.05). LVEF in the T2DM group was comparable to that in the control group (P > 0.05).
Table 2 summarizes the resting and post-exercise parameters of LV function for both groups. The difference in E and s′ between the two groups were not significant (P > 0.05) at rest, but the T2DM group had significantly higher levels of A, a′, and E/e′ (P < 0.05) at rest. Compared with the control group, e′ were markedly decreased in the patients with T2DM (P < 0.05) at rest, but these parameters were still within the normal range according to current guideline (10). During exercise, these parameters of diastolic function increased in both groups. After exercise, the differences in E, A, and E/e′ between the two groups were not significant (P > 0.05). ΔE, ΔA, ΔEDT, ΔIVRT, Δe′, Δa′, ΔE/e′, ΔSRe, and ΔSRa did not significantly differ between the two group (Table 3 and Figure 3), indicating that the diastolic reserve in the T2DM group was preserved as in the control group.
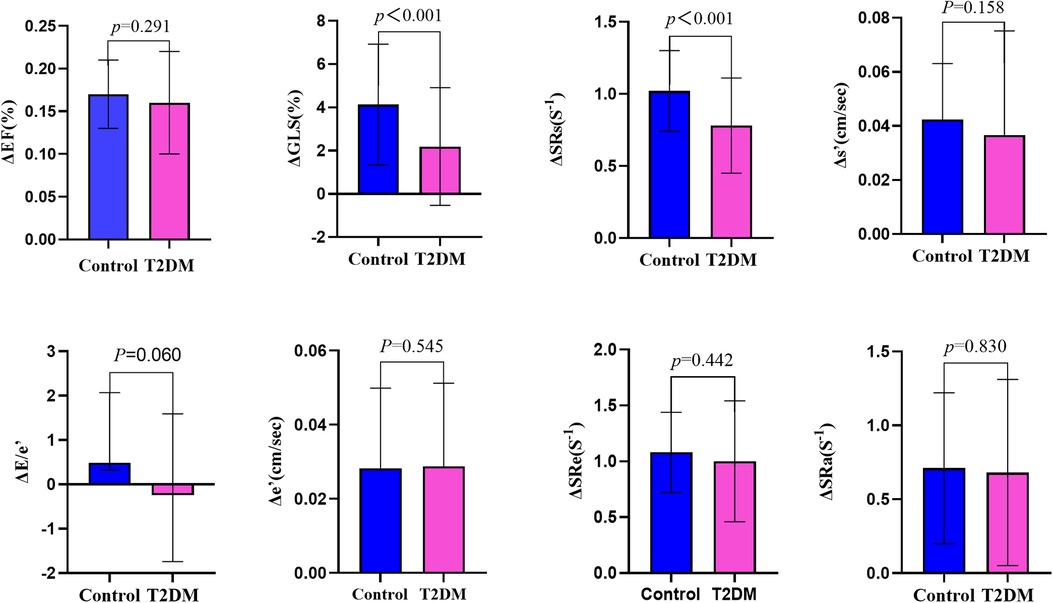
Figure 3. Systolic reserve and diastolic reserve parameters were compared between patients with asymptomatic type 2 diabetes mellitus and healthy controls. Δ, delta value; EF, ejection fraction; GLS, global longitudinal strain; SRs, peak systolic longitudinal strain rate; s′, systolic mitral annulus tissue velocity; e′, early diastolic mitral annulus tissue velocity; SRe, early diastolic longitudinal strain rate; SRa, late diastolic longitudinal strain rate.
At rest, LVGLS and SRs in asymptomaticT2DM patients were similar to those in healthy individuals (P > 0.05), but significant differences were revealed after exercise (P < 0.05). Although the differences in ΔEF and Δs′ were not significant between the two groups(P < 0.05), ΔGLS and ΔSRs were both lower in the T2DM group compared with those in the control group (Table 3 and Figure 3). GLS and SRs were increased in T2DM group during exercise, but compared with the control group, the increase was noticeably less than the control group, indicating a considerably decreased systolic reserve.
Table 4 and Figure 4 illustrate the alterations in parameters associated with systolic and diastolic function at rest and post-exercise, comparing the asymptomatic T2DM group with the control group, as well as within each group. In the control group, the parameters related to diastolic function (E, A, e′, a′, SRa, SRe) exhibited a significant increase, while EDT and IVRT were notably shortened after exercise compared to the resting state. However, there was no significant difference in E/e′ between rest and post-exercise. Additionally, the systolic function parameters (GLS, SRs, and s′) also showed a significant increase after exercise. These findings were similarly observed in the asymptomatic T2DM group. It is suggested that both diastolic and systolic functions responded adequately to exercise in both the control and the asymptomatic T2DM groups.
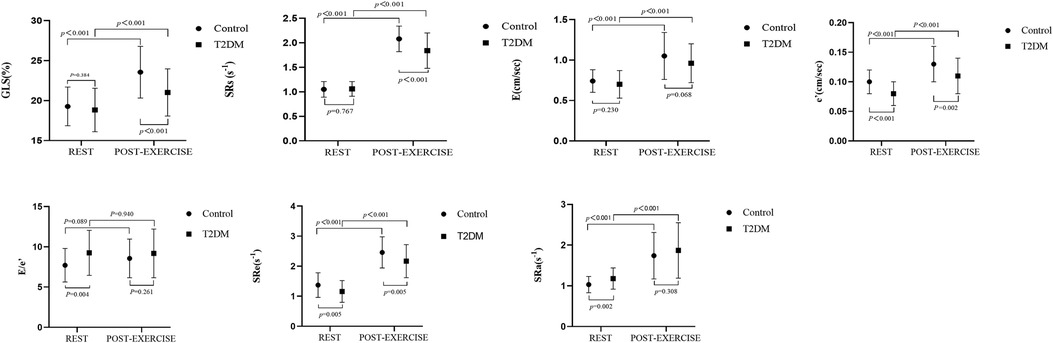
Figure 4. Systolic and diastolic reserve function parameters were compared between and within the two groups at rest and after exercise. In addition to the E/ e′ ratio, systolic and diastolic parameters increased significantly after exercise in both the control and asymptomatic T2DM groups.
In Table 5 and Figure 5, the outcomes of the univariate and multivariate regression analysis are shown. ΔGLS was good correlated with HbA1c and glucose, and ΔSRs was mildly correlated with waist circumference and WHR, and ΔSRs was weak correlated with NTproBNP. ΔGLS was significantly independently correlated with HbA1c (β = −0.614, P < 0.001), and ΔSRs was modest independently correlated with NTproBNP (β = −0.262, P = 0.027), waist circumference (β = −0.299, P = 0.013), and hsCRP (β = −0.245, P = 0.039) through the stepwise multiple linear regression analysis.
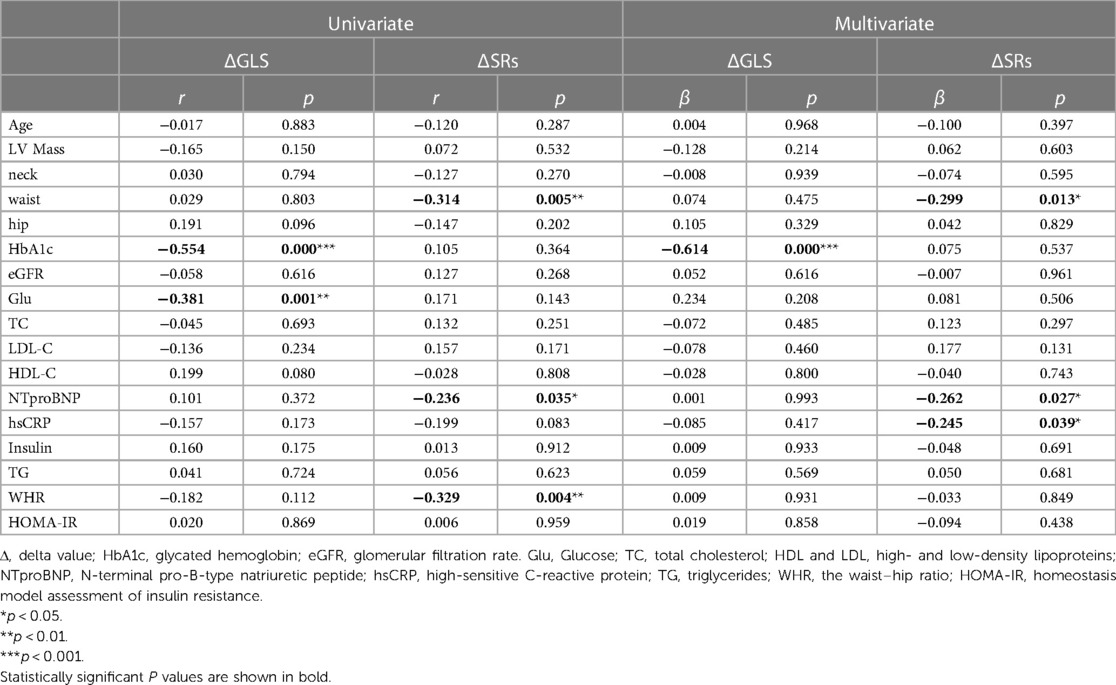
Table 5. Univariate and multivariate correlation analysis of systolic reserve parameters with clinical and biologic parameters.
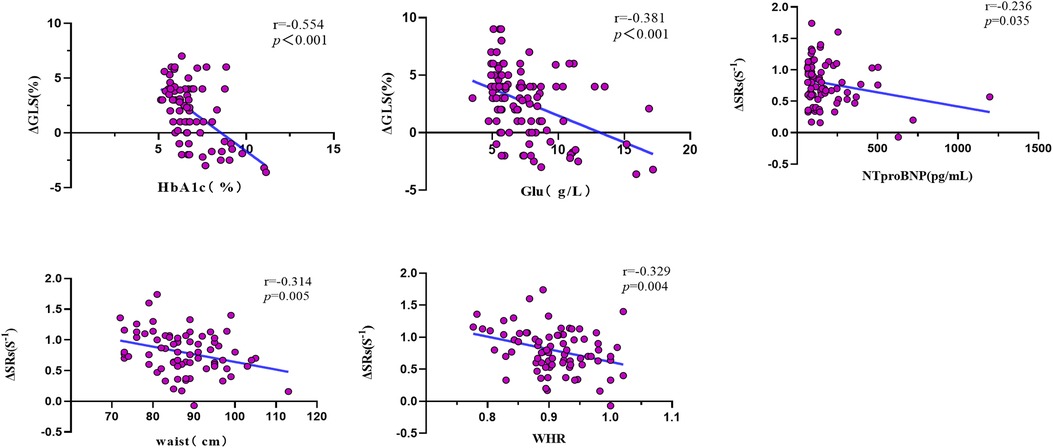
Figure 5. Univariate correlation analysis of LV systolic reserve function with clinical and biological parameters. Δ, delta value; GLS, global longitudinal strain; SRs, peak systolic longitudinal strain rate; HbA1c, glycated hemoglobin; Glu, Glucose; NTproBNP, N-terminal pro-B-type natriuretic peptide; WHR, the waist–hip ratio.
This study provides a thorough analysis of LV reserve function in asymptomatic T2DM patients, which is crucial for understanding the development of HF. Furthermore, we looked into the link between biomarkers and myocardial function in T2DM, enabling early detection of changes in LV function. A first principal outcome of the study was that asymptomatic T2DM patients have the similar GLS and SRs at rest to the controls. In contrast, compared to the control group GLS and SRs rose less during exercise in the T2DM group, whereas the changes in E/e′, E, A, EDT, IVRT, a′, e′, SRe, and SRa during exercise were similar in two groups. This suggests that LV systolic reserve impairment might appear early before diastolic reserve impairment. Our second major finding was that the primary contributors to these patients' decreased systolic response to treadmill ESE are high levels of HbA1c, NTproBNP, hsCRP and increased waist circumference, especially increased HbA1c level.
T2DM is recognized as a separate CVD risk factor (11). T2DM and HF often coexist, and the presence of one enhances the incidence and severity of the other (12, 13). Left HF is particularly significant for patient prognosis, as the LV reserve function can indicate the progression from left HF to refractory or end-stage HF. Research has shown that the initial pathological change in HF patients is a decrease in cardiac reserve (14–16). Early detection of abnormal LV reserve function in patients with T2DM is beneficial for the early identification of high-risk individuals with HF, and combined with relevant biological markers, will facilitate active interventions to prevent HF.
The complex factors of DMCM can lead to structural changes in the myocardium. LV hypertrophy and myocardial fibrosis are the earliest morphological changes that occur in patients with early and asymptomatic diabetes and can lead to diastolic dysfunction. Abnormal ventricular reserve function indicates subclinical ventricular dysfunction early. The ability of the LV to strengthen its diastolic function to preserve normal filling pressure during exercise is referred to as LV diastolic reserve function. Several studies of diastolic reserve in T2DM patients, which showed the differences in e′ and E/e′ were not significant between the two groups at rest. e′ velocity rose significantly less and the E/e′ ratio increased significantly more in the T2DM group during exercise, suggesting impaired diastolic reserve in this population (17, 18). Hence, in the early phases of diastolic dysfunction, such as that found in DMCM, a reduced diastolic reserve may be observed (19). However, our study showed contrasting results. Although some diastolic function parameters in the asymptomatic T2DM group have been significantly different from those in the control group at rest, it is not yet possible to diagnose diastolic dysfunction according to current guidelines (10). Regardless of whether it was the control group or the asymptomatic T2DM group, both E and e′ increased after exercise. The increase in these two parameters was similar, indicating minimal changes in E/e′ before and after exercise in both groups. Additionally, our study revealed that E, e′, and E/e′ increases during exercise were comparable in asymptomatic T2DM patients to controls, and during exercise the SRe and SRa rises were comparable between the two groups. During exercise, increased sympathetic tone and an aggravated LV suction action result in a higher relaxation rate. This improves the E wave and sustains LV filling volumes despite a shorter diastolic filling time, with no appreciable pressure increase in the left atrium (20). Both E and e′ velocities increased correspondingly in patients with normal myocardial relaxation, resulting in little change in the E/e′ ratio. However, e′ velocity changed less than E velocity in patients with abnormal myocardial relaxation, causing an increased E/e′ ratio with exercise (21). In contrast to previous studies, our study found that patients with asymptomatic T2DM demonstrated normal myocardial relaxation and did not exhibit any noticeable impairment of diastolic reserve.
LV systolic function is instrumental in CVD. In our study, changes in LVEF were similar between the two groups both at rest and after exercise. Patients with asymptomatic T2DM showed similar results as the control group for LVGLS and SRs at rest. However, after exercise, LVGLS and SRs were significantly impaired in the T2DM group. Previous studies revealed that LVEF was not a sensitive index to identify subclinical LV systolic dysfunction, and a sensitive and feasible approach is to assess LV systolic function by GLS using STI (22). It is difficult to identify modest impairment of LV systolic function by resting deformation imaging (23), and stress echocardiography (SE) could be helpful in revealing subclinical abnormalities. SE is frequently employed to evaluate LV systolic reserve in patients with coronary artery disease and patients with conditions such valvular diseases (24, 25), hypertrophic cardiomyopathy (26), and hypertensive heart disease (27). Systolic reserve refers to the ventricle's capacity to respond to stress and serves as a disease outcome predictor (28). Decreased systolic reserve is considered an early sign of LV dysfunction (29). Previous studies have not specifically examined systolic reserve using STI in asymptomatic T2DM. We found significant lower increase in GLS and SRs during exercise in asymptomatic T2DM group than in the controls, indicating that their longitudinal contractile reserve is abnormal, GLS and SRs primarily represent the function of subendocardial myocardial fibers, which are more vulnerable to ischemia and increased wall stress, and whose function is impaired by interstitial fibrosis and abnormal microcirculation (30). Patients with T2DM may experience microvascular dysfunction, which is a common complication of T2DM and can occur in the diabetic heart before macrovascular disease. During exercise, the body requires more oxygen, and microvascular dysfunction can lead to myocardial hypoxia (31). This can affect myocardial behavior during a stress state and may explain the abnormal reserve of systolic function observed in our patients. After loading conditions, the lower deformation measurements in T2DM patients after exercise strongly suggest that there are changes in cardiac intrinsic contraction.
For the prediction of incident HF and cardiovascular events in patients with T2DM and in those with existing HF and T2DM, we looked at how clinical features and serum biological markers affected LV systolic reserve. Based on univariate and multivariate correlation analyses, we identified the independent factors associated with LV systolic reserve. Parameters related to LV systolic reserve function are associated with the markers of diabetes metabolism, visceral adiposity, and the cardiovascular system. In particular, HbA1c, an indicator of glycemic control, showed an independent and significant correlation with ΔGLS.
In diabetic patients, HbA1c offers an indication of glycemic control over a period of two to three months, the standard for good glycemic control is HbA1c < 7% (32). Hyperglycemia is considered as an initiating factor, poor glycemic control can cause a range of negative effects on myocardial metabolism, including disruptions to the excitation–contraction relationship, increased oxidative stress and metabolic substrates. These metabolic disturbances can also activate inflammatory pathways in diabetes. Ultimately, disturbances in glucose and lipid metabolism can lead to cardiac hypertrophy, fibrosis, increased stiffness, and loss of cardiomyocytes (33). Such changes result in significant impairment of LV reserve function. Numerous studies have verified the link between HbA1c and incident cardiac events (34). Each 20 mg/dl rise in blood glucose increases the risk of HF by 25%, and each 1% increase in HbA1c increases the risk by 8% (35,36). According to recent studies found that the greatest decrease of HbA1c levels were beneficial to the improvement of diastolic and systolic functions in patients (37–43). Therefore, in diabetic patients, maintaining appropriate blood glucose control may help lower their risk of myocardial damage and HF.
One of the measures of abdominal adiposity is waist circumference which was found to be independently and mildly associated with LV systolic reserve function in our study. Previous studies have found that increased abdominal obesity is independently associated with impaired left ventricular systolic function, regardless of the presence of systemic obesity (44). The mechanism may be that abdominal obesity negatively affects myocardial function due to its association with inflammatory cytokines, insulin resistance and hyperinsulinemia, as well as lipotoxicity resulting from lipid accumulation in cardiac tissue (45). Several studies have highlighted that weight loss plays an important role in the reversibility of impaired systolic and diastolic function in diabetic patients (37, 46–48). NTproBNP can be utilized to assess diabetic patients for subclinical LV impairment (49–51). NTproBNP is secreted by ventricular myocytes due to increased wall tension. Therefore, an important factor determinant of NTproBNP is wall stress, which is determined by LV diastolic filling pressure, wall thickness and LV diastolic diameter. This may explain the independent but weak association of NTproBNP with LV systolic reserve function in our study. In addition, our study found that hsCRP, an important biomarker of inflammation, was only independently associated with LV systolic reserve. Evidence from previous studies has suggested that hsCRP is associated with metabolic syndrome and DM, and can be used as a clinical tool to assess cardiovascular risk, showing strong predictive power in patients known to have CVD (52). However, current studies using indicators of inflammatory that have exclusively considered atherosclerotic cardiovascular events without taking heart failure into account have failed to successfully predict risk (6).
The collective findings of these studies emphasize the significance of effectively managing HbA1c, waist circumference, NTproBNP and hsCRP in the treatment of DMCM and in preventing the progression of DMCM from subclinical to overt stages. Particularly, maintaining good glycemic control is crucial.
The limitations of this study are as follows:
1. This study was a single-center study with a small sample size resulting in a high standard deviation of the measurement parameters. In addition, it is important to note that the results of this study are based on hypotheses.
2. LV function is the result of multi-directional deformation (longitudinal, circumferential, and radial direction) interacting with torsional mechanics. This study solely looked at longitudinal strain, and circumferential/radial strain and LV torsion angle (twist) need to be taken into consideration in future work. Furthermore, since the heart has a three-dimensional (3D) structure, its motion is also 3D. However, some of this information is inevitably lost because this research is based on 2D STI, resulting in a less comprehensive and less accurate assessment of myocardial strain.
In this study, we found that asymptomatic patients with T2DM exhibited impaired systolic reserve function, while diastolic reserve function remained preserved. This finding contradicts previous suggestions that diastolic function is impaired first in DMCM. These results suggest that combining treadmill ESE with STI might be valuable in detecting subtle myocardial injury in asymptomatic T2DM patients. Furthermore, HbA1c, waist circumference, NTproBNP and hsCRP are independent predictors of the blunted systolic function which results in an inadequate response to treadmill ESE in these patients. Good control of these parameters, particularly HbA1c levels, might be beneficial for LV systolic reserve function.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Ethics Committee of Sichuan Provincial Peoples' hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
YD: Data curation, Formal Analysis, Writing – original draft. LY: Conceptualization, Resources, Formal Analysis. QS: Visualization, Funding acquisition. YH: Investigation, Methodology. HZ: Investigation, Methodology. QZ: Investigation, Methodology. GD: Investigation, Methodology. YD: Writing – review & editing. CL: Writing – review & editing. LY: Validation, Supervision. YW: Resources, Project administration, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by a research grant from the Natural Science Foundation of Sichuan Province (grant number: 2022NSFSC0662).
We would like to thank all participants for their support and contributions to this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fcvm.2023.1253440/full#supplementary-material
1. Preis SR, Hwang SJ, Coady S, Pencina MJ, D'Agostino RB Sr, Savage PJ, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the framingham heart study, 1950 to 2005. Circulation. (2009) 119:1728–35. doi: 10.1161/CIRCULATIONAHA.108.829176
2. Waddingham MT, Edgley AJ, Tsuchimochi H, Kelly DJ, Shirai M, Pearson JT. Contractile apparatus dysfunction early in the pathophysiology of diabetic cardiomyopathy. World J Diabetes. (2015) 6:943–60. doi: 10.4239/wjd.v6.i7.943
3. Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. (1972) 30:595–602. doi: 10.1016/0002-9149(72)90595-4
4. Nishi T, Kobayashi Y, Christle JW, Cauwenberghs N, Boralkar K, Moneghetti K, et al. Incremental value of diastolic stress test in identifying subclinical heart failure in patients with diabetes mellitus. Eur Heart J Cardiovasc Imaging. (2020) 21(8):876–84. doi: 10.1093/ehjci/jeaa070
5. Minciună IA, Hilda Orășan O, Minciună I, Lazar AL, Sitar Tăut AV, Oltean M, et al. Assessment of subclinical diabetic cardiomyopathy by speckle-tracking imaging. Eur J Clin Invest. (2021) 51:e13475. doi: 10.1111/eci.13475
6. Seferović P, Farmakis D, Bayes Genis A, Ben Gal T, Böhm M, Chioncel O, et al. Biomarkers for the prediction of heart failure and cardiovascular events in patients with type 2 diabetes: a position statement from the heart failure association of the European society of cardiology. Eur J Heart Fail. (2022) 24:1162–70. doi: 10.1002/ejhf.2575
7. Alberti KGMM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med. (1998) 15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7%3C539::AID-DIA668%3E3.0.CO;2-S
8. Gustavsson S, Fagerberg B, Sallsten G, Andersson EM. Regression models for log-normal data: comparing different methods for quantifying the association between abdominal adiposity and biomarkers of inflammation and insulin resistance. Int J Environ Res Public Health. (2014) 11:3521–39. doi: 10.3390/ijerph110403521
9. Pellikka PA, Arruda-Olson A, Chaudhry FA, Chen MH, Marshall JE, Porter TR, et al. Guidelines for performance, interpretation, and application of stress echocardiography in ischemic heart disease: from the American society of echocardiography. J Am Soc Echocardiogr. (2020) 33(1):1–41. doi: 10.1016/j.echo.2019.07.001
10. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2016) 29:277–314. doi: 10.1016/j.echo.2016.01.011
11. Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. (2004) 27:699–703. doi: 10.2337/diacare.27.3.699
12. Seferović PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, et al. Type 2 diabetes mellitus and heart failure: a position statement from the heart failure association of the European society of cardiology. Eur J Heart Fail. (2018) 20:853–72. doi: 10.1002/ejhf.1170
13. Maack C, Lehrke M, Backs J, Heinzel FR, Hulot JS, Marx N, et al. Heart failure and diabetes: metabolic alterations and therapeutic interventions: a state-of-the-art review from the translational research committee of the heart failure association-European society of cardiology. Eur Heart J. (2018) 39:4243–54. doi: 10.1093/eurheartj/ehy596
14. Tan LB. Evaluation of cardiac dysfunction, cardiac reserve and inotropic response. Postgrad Med J. (1991) 67:S10–20.1924075
15. Tan LB, Littler WA. Measurement of cardiac reserve in cardiogenic shock: implications for prognosis and management. Br Heart J. (1990) 64:121–8. doi: 10.1136/hrt.64.2.121
16. Timmins AC, Hayes M, Yau E, Watson JD, Hinds CJ. The relationship between cardiac reserve and survival in critically ill patients receiving treatment aimed at achieving supranormal oxygen delivery and consumption. Postgrad Med J. (1992) 68:S34–40.1461869
17. Leung M, Phan V, Whatmough M, Heritier S, Wong VW, Leung DY. Left ventricular diastolic reserve in patients with type 2 diabetes mellitus. Open Heart. (2015) 2(1):e000214. doi: 10.1136/openhrt-2014-000214
18. Al-Gburi AJJ. Left ventricular diastolic reserve by exercise stress echocardiography in prediabetes. Tzu Chi Med J. (2022) 35(2):188–92. doi: 10.4103/tcmj.tcmj_151_22
19. Leung M, Phan V, Leung DY. Endothelial function and left ventricular diastolic functional reserve in type 2 diabetes mellitus. Open Heart. (2014) 1(1):e000113. doi: 10.1136/openhrt-2014-000113
20. Ha JW, Ahn JA, Kim JM, Choi EY, Kang SM, Rim SJ, et al. Abnormal longitudinal myocardial functional reserve assessed by exercise tissue Doppler echocardiography in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. (2006) 19(11):1314–9. doi: 10.1016/j.echo.2006.05.022
21. Ha JW, Andersen OS, Smiseth OA. Diastolic stress test: invasive and noninvasive testing. JACC Cardiovasc Imaging. (2020) 13(1):272–82. doi: 10.1016/j.jcmg.2019.01.037
22. Manganaro R, Marchetta S, Dulgheru R, Ilardi F, Sugimoto T, Robinet S, et al. Echocardiographic reference ranges for normal non-invasive myocardial work indices: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. (2019) 20:582–90. doi: 10.1093/ehjci/jey188
23. Philouze C, Obert P, Nottin S, Benamor A, Barthez O, Aboukhoudir F. Dobutamine stress echocardiography unmasks early left ventricular dysfunction in asymptomatic patients with uncomplicated type 2 diabetes: a comprehensive two-dimensional speckle-tracking imaging study. J Am Soc Echocardiogr. (2018) 31(5):587–97. doi: 10.1016/j.echo.2017.12.006
24. Magne J, Mahjoub H, Dulgheru R, Pibarot P, Pierard LA, Lancellotti P. Left ventricular contractile reserve in asymptomatic primary mitral regurgitation. Eur Heart J. (2014) 35(24):1608–16. doi: 10.1093/eurheartj/eht345
25. D’Andrea A, Sperlongano S, Formisano T, Tocci G, Cameli M, Tusa M, et al. Stress echocardiography and strain in aortic regurgitation (SESAR protocol): left ventricular contractile reserve and myocardial work in asymptomatic patients with severe aortic regurgitation. Echocardiography. (2020) 37(8):1213–21. doi: 10.1111/echo.14804
26. Morris DA, Otani K, Bekfani T, Takigiku K, Izumi C, Yuda S, et al. Multidirectional global left ventricular systolic function in normal subjects and patients with hypertension: multicenter evaluation. J Am Soc Echocardiogr. (2014) 27(5):493–500. doi: 10.1016/j.echo.2014.01.017
27. Marghany KA, Abu Rahhal AEI, Shawkat AEA. Assessment of left ventricular function and contractile reserve in patients with hypertension. Egypt J Hosp Med. (2019) 77(1):4720–6. doi: 10.21608/ejhm.2019.46750
28. Tayal U, Wage R, Newsome S, Manivarmane R, Izgi C, Muthumala A, et al. Predictors of left ventricular remodelling in patients with dilated cardiomyopathy–a cardiovascular magnetic resonance study. Eur J Heart Fail. (2020) 22(7):1160–70. doi: 10.1002/ejhf.1734
29. Wu XP, Li YD, Zhang M, Zhu W, Cai QZ, Jiang W, et al. Impaired left ventricular mechanics and functional reserve are associated with reduced exercise capacity in patients with hypertrophic cardiomyopathy. Echocardiography. (2019) 36:266–75. doi: 10.1111/echo.14241
30. Kosmala W, Przewlocka-Kosmala M, Rojek A, Mysiak A, Dabrowski A, Marwick TH. Association of abnormal left ventricular functional reserve with outcome in heart failure with preserved ejection fraction. JACC: Cardiovasc Imaging. (2018) 11(12):1737–46. doi: 10.1016/j.jcmg.2017.07.028
31. Cortigiani L, Rigo F, Gherardi S, Galderisi M, Bovenzi F, Sicari R. Prognostic meaning of coronary microvascular disease in type 2 diabetes mellitus: a transthoracic Doppler echocardiographic study. J Am Soc Echocardiogr. (2014) 27:742–8. doi: 10.1016/j.echo.2014.02.010
32. American Diabetes Association. Standards of medical care in diabetes–2012. Diabetes Care. (2012) 35(Suppl 1):S11–63. doi: 10.2337/dc12-s011
33. Tan Y, Zhang Z, Zheng C, Wintergerst KA, Keller BB, Cai L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat Rev Cardiol. (2020) 17:585–607. doi: 10.1038/s41569-020-0339-2
34. Rubin J, Matsushita K, Ballantyne CM, Hoogeveen R, Coresh J, Selvin E. Chronic hyperglycemia and subclinical myocardial injury. J Am Coll Cardiol. (2012) 59:484–9. doi: 10.1016/j.jacc.2011.10.875
35. Aune D, Schlesinger S, Neuenschwander M, Feng T, Janszky I, Norat T, et al. Diabetes mellitus, blood glucose and the risk of heart failure: a systematic review and meta-analysis of prospective studies. Nutr Metab Cardiovasc Dis. (2018) 28:1081–91. doi: 10.1016/j.numecd.2018.07.005
36. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br Med J. (2000) 321:405–12. doi: 10.1136/bmj.321.7258.405
37. Leung M, Wong VW, Durmush E, Phan V, Xie M, Leung DY. Cardiac dysfunction in type II diabetes: a bittersweet, weighty problem, or both? Acta Diabetol. (2017) 54:91–100. doi: 10.1007/s00592-016-0911-8
38. Negishi K. Echocardiographic feature of diabetic cardiomyopathy: where are we now? Cardiovasc Diagn Ther. (2018) 8:47–56. doi: 10.21037/cdt.2018.01.03
39. Tadic M, Cuspidi C, Vukomanovic V, Ilic S, Obert P, Kocijancic V, et al. Layer-specific deformation of the left ventricle in uncomplicated patients with type 2 diabetes and arterial hypertension. Arch Cardiovasc Dis. (2018) 111:17–24. doi: 10.1016/j.acvd.2017.01.014
40. Leung M, Wong VW, Hudson M, Leung DY. Impact of improved glycemic control on cardiac function in type 2 diabetes mellitus. Circ Cardiovasc Imaging. (2016) 9:e003643. doi: 10.1161/CIRCIMAGING.115.003643
41. Chen X, Guo H, Yang Q, Fang J, Kang X. Quantitative evaluation of subclinical left ventricular dysfunction in patients with type 2 diabetes mellitus by three-dimensional echocardiography. Int J Cardiovasc Imaging. (2020) 36:1311–9. doi: 10.1007/s10554-020-01833-5
42. Wang Y, Yang H, Huynh Q, Nolan M, Negishi K, Marwick TH. Diagnosis of nonischemic stage B heart failure in type 2 diabetes mellitus: optimal parameters for prediction of heart failure. JACC Cardiovasc Imaging. (2018) 11:1390–400. doi: 10.1016/j.jcmg.2018.03.015
43. Armstrong AC, Ambale-Venkatesh B, Turkbey E, Donekal S, Chamera E, Backlund JY, et al. Association of cardiovascular risk factors and myocardial fibrosis with early cardiac dysfunction in type 1 diabetes: the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. (2017) 40:405–11. doi: 10.2337/dc16-1889
44. Russo C, Sera F, Jin Z, Palmieri V, Homma S, Rundek T, et al. Abdominal adiposity, general obesity, and subclinical systolic dysfunction in the elderly: a population-based cohort study. Eur J Heart Fail. (2016) 18(5):537–44. doi: 10.1002/ejhf.521
45. Fernandes-Silva MM, Shah AM, Claggett B, Cheng S, Tanaka H, Silvestre OM, et al. Adiposity, body composition and ventricular–arterial stiffness in the elderly: the atherosclerosis risk in communities study. Eur J Heart Fail. (2018) 20(8):1191–201. doi: 10.1002/ejhf.1188
46. Levelt E, Pavlides M, Banerjee R, Mahmod M, Kelly C, Sellwood J, et al. Ectopic and visceral fat deposition in lean and obese patients with type 2 diabetes. J Am Coll Cardiol. (2016) 68:53–63. doi: 10.1016/j.jacc.2016.03.597
47. Lin JL, Sung KT, Su CH, Chou TH, Lo CI, Tsai JP, et al. Cardiac structural remodeling, longitudinal systolic strain, and torsional mechanics in lean and nonlean dysglycemic Chinese adults. Circ Cardiovasc Imaging. (2018) 11:e007047. doi: 10.1161/CIRCIMAGING.117.007047
48. Mueller C, Mcdonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, et al. Heart failure association of the European society of cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. (2019) 21:715–31. doi: 10.1002/ejhf.1494
49. Kim JY, Lee EY, Jee JH, Lee BW, Chung JH, Jeun ES, et al. N-terminal pro-brain natriuretic peptide (NT-proBNP) in type 2 diabetes with left ventricular dysfunction. Diabetes Res Clin Pract. (2007) 77(3):S238–42. doi: 10.1016/j.diabres.2007.01.064
50. Magnusson M, Melander O, Israelsson B, Grubb A, Groop L, Jovinge S. Elevated plasma levels of nt-proBNP in patients with type 2 diabetes without overt cardiovascular disease. Diabetes Care. (2004) 27(8):1929–35. doi: 10.2337/diacare.27.8.1929
51. Grewal J, McKelvie R, Lonn E, Tait P, Carlsson J, Gianni M, et al. BNP and NT-proBNP predict echocardiographic severity of diastolic dysfunction. Eur J Heart Fail. (2008) 10(3):252–9. doi: 10.1016/j.ejheart.2008.01.017
Keywords: asymptomatic type 2 diabetes mellitus, treadmill exercise stress echocardiography, two-dimensional speckle-tracking imaging, left ventricular reserve function, serum biological parameters
Citation: Duan Y, Ye L, Shu Q, Huang Y, Zhang H, Zhang Q, Ding G, Deng Y, Li C, Yin L and Wang Y (2023) Abnormal left ventricular systolic reserve function detected by treadmill exercise stress echocardiography in asymptomatic type 2 diabetes. Front. Cardiovasc. Med. 10:1253440. doi: 10.3389/fcvm.2023.1253440
Received: 5 July 2023; Accepted: 9 October 2023;
Published: 19 October 2023.
Edited by:
Attila Kardos, Milton Keynes University Hospital, United KingdomReviewed by:
Attila Oláh, Semmelweis University, Hungary© 2023 Duan, Ye, Shu, Huang, Zhang, Zhang, Ding, Deng, Li, Yin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lixue Yin eWlubGl4dWVfY2FyZGlhY0AxNjMuY29t Yi Wang d2FuZ3lpaGRsQDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.