- 1Mwanza Intervention Trials Unit/National Institute for Medical Research, Mwanza, Tanzania
- 2Weill Cornell Medicine – Cornell University, New York, NY, United States
- 3Department of Infectious Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, United Kingdom
- 4Catholic University of Health and Allied Sciences, School of Public Health, Mwanza, Tanzania
Introduction: Hypertension (HTN) among adolescents is common in high-income countries, and leads to increased premature cardiovascular diseases (CVD). In sub-Saharan Africa (SSA), the prevalence of HTN among adolescents, associated risk factors and CVD complications are not well-described. Such data is needed for planning public health programs to prevent premature CVD in SSA.
Methods: We systematically searched 5 databases (MEDLINE, Embase, Google Scholar, Web of Science, and African Index Medicus) from their establishment to December 2021. Key search terms were: adolescent, arterial hypertension, and names of the 48 countries in SSA. We used Covidence® to manage the search results. The review was registered in the Open Science Framework (OSF) https://osf.io/p5sbt/.
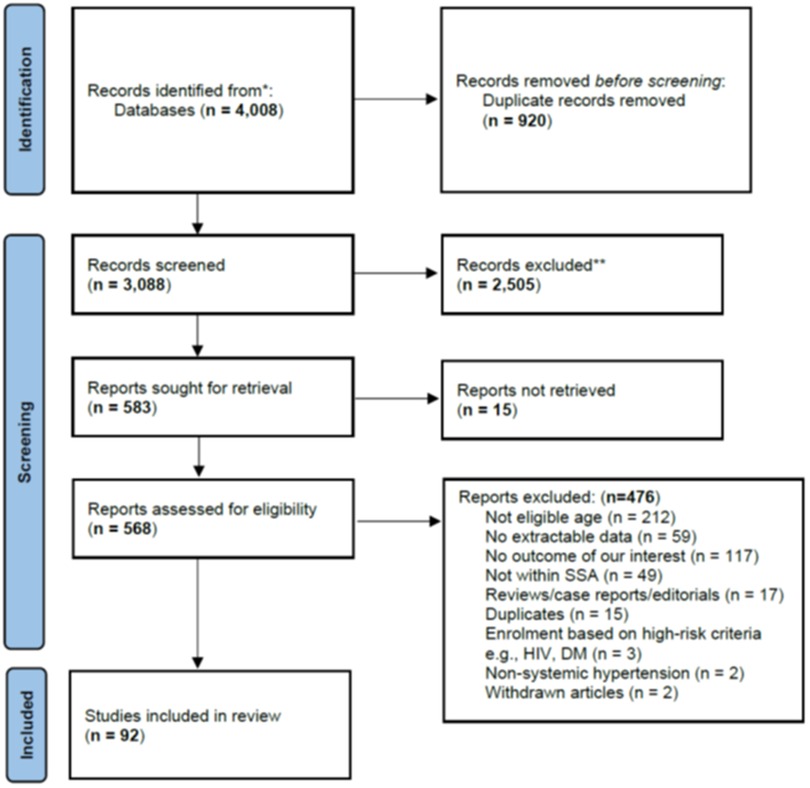
Results: We identified 4,008 articles out of which we screened 3,088 abstracts, and reviewed 583 full-text articles. We finally included 92 articles that were published between 1968 to December 2021. The majority were cross-sectional studies (80%) and conducted in school settings (78%). The risk of bias was low for 59 studies (64.1%), moderate for 29 studies (31.5%), and high for 4 studies (4.3%). Overall, the prevalence of HTN varied widely from 0.18% to 34.0% with a median (IQR) of 5.5% (3.1%, 11.1%). It was relatively higher in studies using automated blood pressure (BP) devices, and in studies defining HTN using thresholds based on percentile BP distribution for one's height, age, and sex. In addition, the prevalence of HTN was significantly higher in studies from Southern Africa region of SSA and positively correlated with the year of publication. Across studies, traditional risk factors such as age, sex, body mass index, and physical inactivity, were commonly found to be associated with HTN. In contrast, non-traditional risk factors related to poverty and tropical diseases were rarely assessed. Only three studies investigated the CVD complications related to HTN in the study population.
Conclusion: The prevalence of HTN among adolescents in SSA is high indicating that this is a major health problem. Data on non-traditional risk factors and complications are scarce. Longitudinal studies are needed to clearly define the rates, causes, and complications of HTN.
Systematic Review Registration: https://osf.io/p5sbt/, identifier (10.17605/OSF.IO/P5SBT).
Introduction
Hypertension (HTN) among adolescents is common globally, but most data come from high-income countries (1). Much less information is available from sub–Saharan Africa (SSA) where adolescents constitute about 18% of the population (2). In high-income countries, obesity is the major risk factor for HTN, and other traditional risk factors such as physical inactivity, unhealthy diet, and family history of HTN are also common (3, 4). In SSA, potential risk factors related to poverty and tropical diseases, also referred to as “non-traditional” risk factors, may be common. They include low birth weight, malnutrition, stunted growth, malaria, and/or other tropical infectious diseases. However, there is limited information describing the contribution of non-traditional risk factors for HTN among adolescents in Africa (3, 5–9).
Increasing evidence from long-term follow-up studies in high-income countries shows that HTN during adolescence is strongly associated with premature onset of cardiovascular diseases (CVD) including left ventricular hypertrophy, chronic kidney disease, decline in cognitive function, and type-2-diabetes mellitus (10–13). By comparison, little is known about blood pressure (BP) trajectory and its potential complications among adolescents in SSA (14, 15).
We are aware of three systematic reviews (and meta-analyses) describing the burden of HTN in children and adolescents in Africa (16–18). The reviews used published data from 1996 to 2017 and from 2017 to 2020, and from 2010 to 2021with the pooled estimates for prevalence of HTN of 5.5% and 7.5% and 9.9% respectively. The major limitations of two of these reviews were that they combined BP measurements for adolescents with those of children as young as 2 years. Also, neither of the reviews reported non-traditional risk factors and complications of HTN. Since the epidemiology of HTN varies between adolescents and children, studies that focus on adolescents only may provide useful information specific for this population.
Therefore, the current systematic review aims to describe the burden, risk factors, and complications of HTN among adolescents aged 10–19 years in SSA. Findings from this study will inform (1) programs promoting regular and proper BP measurements among adolescents; (2) primary CVD preventive interventions targeting adolescents; and (3) the design of new studies aimed to bridge existing gaps in the literature.
Methods
Databases and search strategy
We systematically searched for studies on HTN among adolescents aged 10–19 years in SSA, published in any language, from a range of databases from their inception until December 2021. Two investigators (MKN and RHA) were supported by a librarian (AH) to develop and conduct the search strategy. We searched MEDLINE, Embase, and African Index Medicus to identify relevant published literature. We searched Embase and Web of Science Core collection to identify studies that had been presented as conference abstracts but not yet published as full-length journal articles. We searched Google Scholar to identify potentially relevant information apart from the published research articles (19). The search strategy consisted of three concepts, combined with Boolean operators: adolescents, sub-Saharan Africa (names of the 48 countries in SSA), and hypertension. Both controlled vocabulary and free-text keywords were identified using the Yale MeSH Analyzer (20). Proximity operators were incorporated to retrieve variations of phrases. No limits were used for any of the databases. Terms used for HTN were “arterial hypertension”, “hypertension” and “blood pressure”. Terms for adolescent were “adolescent”, “teen”, and “youth”. The specific search strategies used for each of the databases are attached in Supplementary Appendix 1.
Selection criteria
Using Covidence (Melbourne, VIC 3000, Australia), two investigators (MKN and RHA) independently assessed the articles for eligibility in two steps. First, they independently screened the titles and abstracts of the retrieved articles. Then, they obtained full texts of potentially eligible articles and independently reviewed them for possible inclusion. A third reviewer (GAK) resolved any disagreements between the two investigators. Throughout this selection process, duplicates were removed both by Covidence and the two reviewers. We used Google Translate for studies published in languages other than English.
We included studies if they were: observational (cross-sectional, case-control, and cohort studies) or experimental studies, enrolled adolescents residing in SSA, reported at least one of the outcomes of interest (incidence/prevalence, risk factors, and complications of HTN), and reported data for a group or subgroup of participants within the ages of 10–19 years.
We excluded studies if they were animal studies, only reported non-systemic HTN (e.g., portal hypertension, pulmonary hypertension, and intracranial hypertension), case studies, case series, studies with sample size less than 5, qualitative studies and systematic reviews, studies including only participants with high BP, studies including only adolescents from a single high-risk group (e.g., those with HIV, sickle cell disease, malnutrition), and studies including adolescents with secondary HTN.
Data extraction procedures
The two investigators (MKN and RHA) independently extracted data using a pre-validated data extraction table. This process did not use any automation tools. Extracted data included: the author's name, year of publication, country of study, study design, participant's age range, average age, sample size, type of BP measurement device used (automated vs. manual), criteria used for defining HTN, prevalence and/or incidence estimates, risk factors and complication of HTN. We only extracted data for the eligible age range. We hand-calculated proportion of adolescents with HTN in the eligible age range if this information was not reported and where the numerator and the corresponding denominator were available. For cohort studies, we used prevalence estimates from baseline data and incidence from follow-up visits. We left blank any information which was either missing or not reported for the eligible age range. A third reviewer (GAK) resolved any discrepancies in the extracted data.
Evaluation of risk of bias
The two investigators (MKN and RHA) independently assessed each of the included studies for risk of bias using the Newcastle-Ottawa Scale (NOS) for assessment of the quality of non-randomized studies (21). The assessment was done on three domains: (1) selection of study participants, (2) comparability of participants in different outcome groups, and (3) ascertainment of study outcomes or exposure status. Across the three domains, a range of “high-quality” items could score a star (depending on the study design). Case-control and cohort studies have a total of 8 items with a maximum score of 9 stars. And cross-sectional studies have a total of 7 items with a maximum score of 10 stars. Studies with a total score of ≥7 stars were regarded as of high quality, 4–6 stars as of moderate quality, and 0–3 stars as of low quality (high risk of bias). We resolved any disagreements on the assessment of the risk of bias by consensus.
Data synthesis and analysis
We summarized the study selection process using a PRISMA flow diagram (22). We used tables to show characteristics of included studies, prevalence and/or incidence of HTN, and associated risk factors. We summarized our findings using proportions (for categorical data), and mean and/or median (for continuous data). We assessed the association between prevalence of HTN and each of the following study characteristics: year of publication, study settings (rural, rural/urban and urban), sub Saharan Africa region from which the studies came from (West, South, East, and Central), type of BP measurement device used (manual vs. automated), and criteria for defining HTN (cut offs based on a fixed vs. percentile BP values). We used meta-regression analysis to assess the adjusted association between prevalence of HTN and all the five study characteristics mentioned above. We did not perform pooled analysis due to heterogeneity in methods as well settings/context across the studies.
Ethical considerations
This study was exempted from ethical approval as it involved a review of published data. To our knowledge, all included studies obtained ethics clearance before data collection. The protocol for this review was registered in the Open Science Framework (OSF) https://osf.io/p5sbt/.
Role of funding organization
The funders had no role in the study design, data collection, data analysis, data interpretation, and writing of this manuscript.
Results
We identified 4,008 articles, and after removing 920 duplicates, we screened the title and abstract of the remaining 3,088 articles and selected 583 articles for full-text review. We could not retrieve the full texts of 15 publications (Supplementary Appendix 2). Of the remaining 568 articles, 476 were excluded after a full-text review (their exclusion reasons are summarized in the Figure 1 PRISMA flow diagram below. We finally included 92 articles in our systematic review.
Characteristics of the included studies
Table 1 shows the characteristics of the 92 included studies. The studies were published from 1968 to 2021, with the majority (75%) published between 2010 and 2021. The studies came from 14 (29%) out of the 48 countries in SSA. The majority of the studies came from the west (46.2%), followed by the south (29.7%), east (22.0%), and central (2.2%) African regions. Of the 92 studies, the majority were cross-sectional (80.0%), and conducted in schools (70.3%) and in urban settings (65.9%). They included a total of 89,599 subjects with sample sizes ranging from 53 to 7,746 (median = 670, IQR: 375, 1,080). The risk of bias was low for 59 studies (64.1%), moderate for 29 studies (31.5%), and high for 4 studies (4.3%).
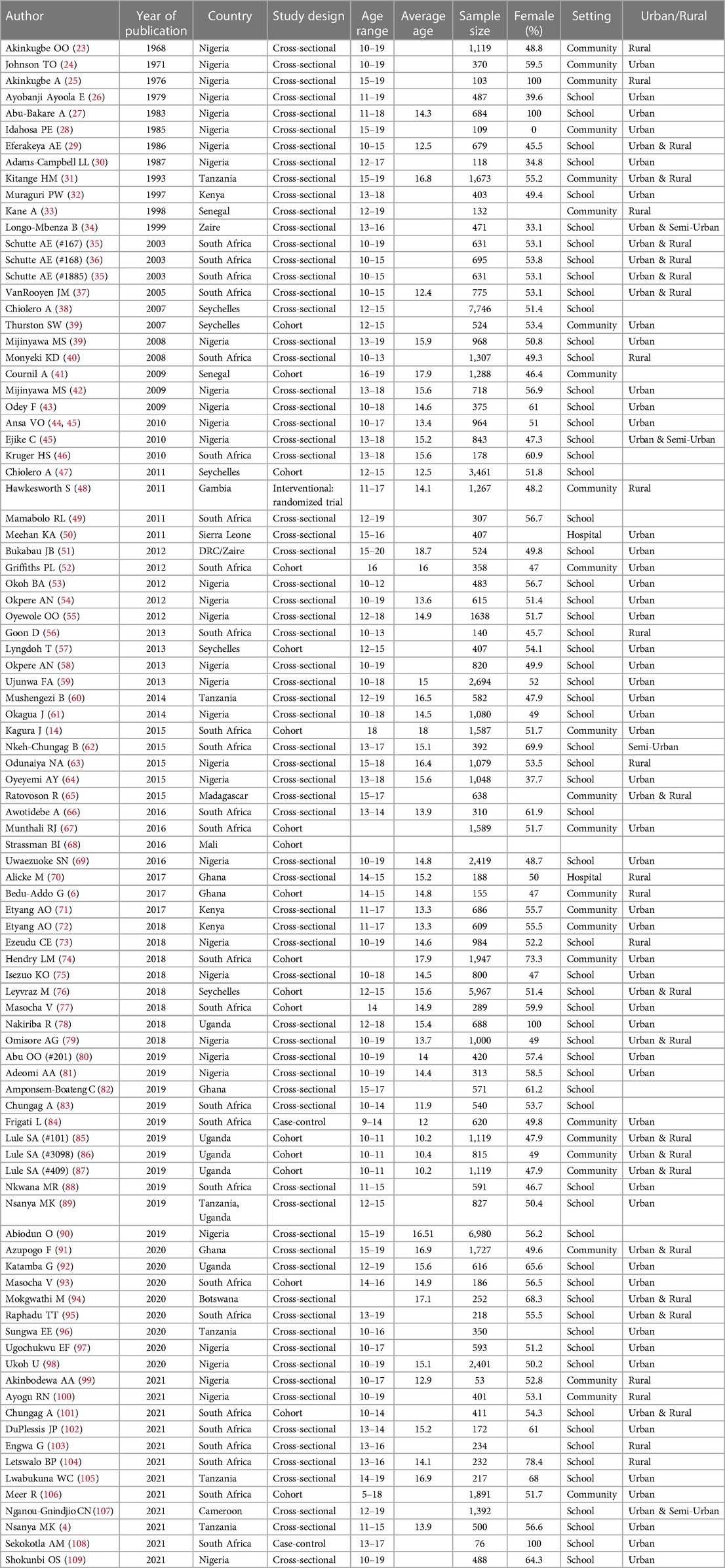
Table 1. Characteristics of studies with data on hypertension among adolescents aged 10–19 years in sub-Saharan Africa (N = 92).
Methods of BP measurements
The studies used different devices for BP measurements, and the definition of HTN was not consistent across studies (Table 2).
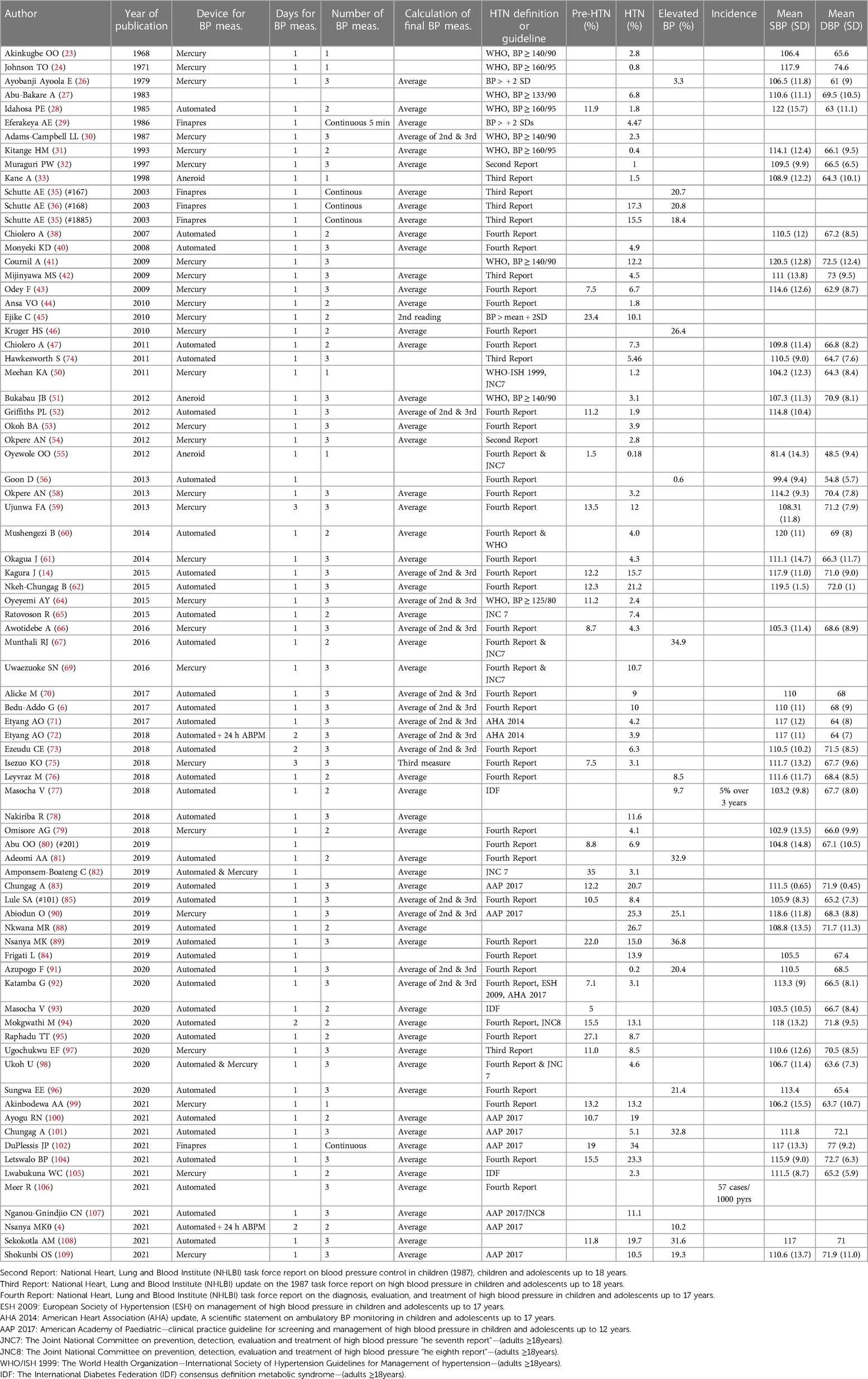
Table 2. Studies that reported prevalence and incidence of hypertension among adolescents aged 10–19 years in sub-Saharan Africa (N = 78).
Of 79 studies shown in Table 2, 39 studies (49.4%) used automated BP machines, 33 studies (41.7%) used manual (mercury or aneroid) BP machines, 2 studies (2.6%) used both automated and manual BP machines, and 4 studies (5.1%) used Finapres (Finger artery pressure)—an automated device which records BP measurements “continuously” from a finger artery. Only 3 studies used 24-h ambulatory BP monitoring for confirming HTN (4, 71, 72).
Of the 79 studies, 71 (89.8%) had their BP measurements obtained in one day. Of those 71 studies, the number of BP measurement obtained in one day ranged from one in 6 studies (8.5%), two in 19 studies (26.8%) and three in 37 studies (52.1%). In addition, 5 studies (7.0%) collected more than 3 BP measurements (“continuous”), and this information was not reported in 4 studies (5.6%).
Of the 68 studies with more than one BP measurements, most studies 51(75.0%) used an average of all BP measurements to obtain final BP measure. In addition, 14 studies (20.5%) used an average of second and third BP measurements, 1 study (1.5%) only used second measurement, 1 study (1.5%) only used third measurement and this information was not reported in another 1 study (1.5%).
Definitions of hypertension
Of the 77 studies with information on standard guidelines used for defining HTN (Table 2), 58 (75.3%) used one of the six American guidelines for the diagnosis and management of HTN in children and adolescents. The 2004 “Fourth Report” was the most commonly used version in 39 studies (50.6%) (110). In addition, the fixed BP cut-offs for defining HTN in adults were used in 10 studies (13.0%), often in older adolescents aged 18 years and above. Fewer studies used the z-distribution (n = 3) and the International Diabetes Federation definitions (n = 3).
Guidelines for defining HTN among adolescents have evolved over the years (Supplementary Appendix 3) provides a summary of the guidelines that were used for diagnosing HTN in the included studies. Overall, the aim for guideline revisions over the years was to account for age, height, and sex differences in BP thresholds for HTN. Also, in particular to American guidelines, the reference population size has increased from about 9,000 in 1977 to nearly 50,000 adolescents with normal BMI and percentile thresholds for HTN have decreased in the most recent guidelines (11).
Prevalence and incidence of HTN
Of the 92 studies, 78 studies (84.6%) reported estimates for prevalence of pre-hypertension and/or elevated BP and/or HTN with only 2 studies reporting incidence of HTN (Table 2). The incidence of HTN was estimated at 5% over 2 years (77) and 57 cases per 1,000 person-years (106).
The prevalence of pre-hypertension was reported in 27 studies and ranged from 1.5% to 35%, with median (IQR) = 11.8% (8.7%, 15.5%). The prevalence of HTN was reported in 65 studies and ranged from 0.18% to 34.0%, with median (IQR) = 5.5% (3.1%, 11.1%); while the prevalence of elevated BP (combined pre-hypertension and HTN) was reported in 18 studies and ranged from 0.6% to 36.8%, and median (IQR) = 20.8% (10.2%, 31.6%).
Prevalence of HTN across the four African regions in sub-Saharan Africa
Of all 65 studies with information on prevalence of HTN, studies from south African region (n = 13) had higher HTN prevalence, median (IQR) of 13.9% (5.1% to 21.2%), followed by west African region with a median (IQR) of 4.5% (2.6% to 9.5%), east African region with a median (IQR) of 4.2% (3.1% to 8.4%), and central African region with a median (IQR) of 3.1% (3.1% to 3.1%).
Factors associated with prevalence of HTN
Overall, studies in which BP measurements were obtained using automated BP devices (n = 31) had significantly higher mean HTN prevalence (11.1% (95% CI: 8.2%–14.1%)), than those using manual devices—Mercury or Aneroid BP machines (n = 31) (5.4% (95% CI, 3.6%–7.2%)) (p = 0.0006) (Figure 2).
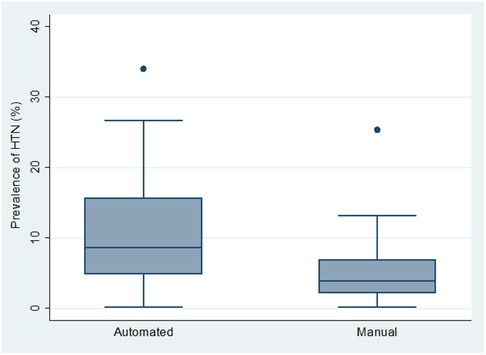
Figure 2. Box and whiskers plot showing the distribution of hypertension prevalence across the 2 types of BP measurement device.
Similarly, studies in which HTN was defined using cut offs based on a percentile BP distribution according to age, sex, and height (n = 43) had significantly higher mean HTN prevalence (8.7% (95% CI: 6.7%–10.7%)), than those using cut-offs based a fixed BP measure (n = 14), (3.8% (95% CI: 1.9%–5.6%)) (p = 0.004) (Figure 3).
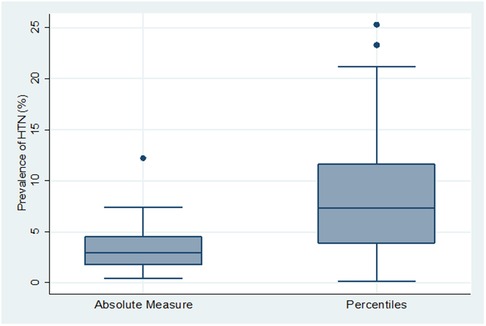
Figure 3. Box and whiskers plot showing the distribution of hypertension prevalence across 2 types of hypertension definition.
There were statistically significant differences between mean HTN prevalence across the four sub Saharan Africa regions (F = 7.49, p = 0.0002). However, there was no significant difference in mean HTN prevalence across study settings (rural, rural/urban and urban) (Figures 4A,B).
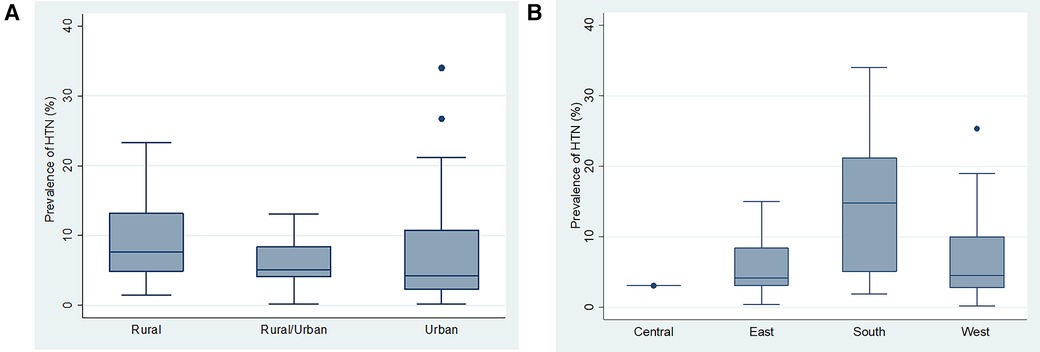
Figure 4. (A) Box and whiskers plot showing the distribution of hypertension prevalence across the study settings. (B) Box and whiskers plot showing the distribution of hypertension prevalence across the different SSA regions.
There was a significant positive correlation between HTN prevalence, and year of study publication (n = 64, Pearson Correlation coefficient “r” = 0.4, p = 0.0009) (Figure 5).
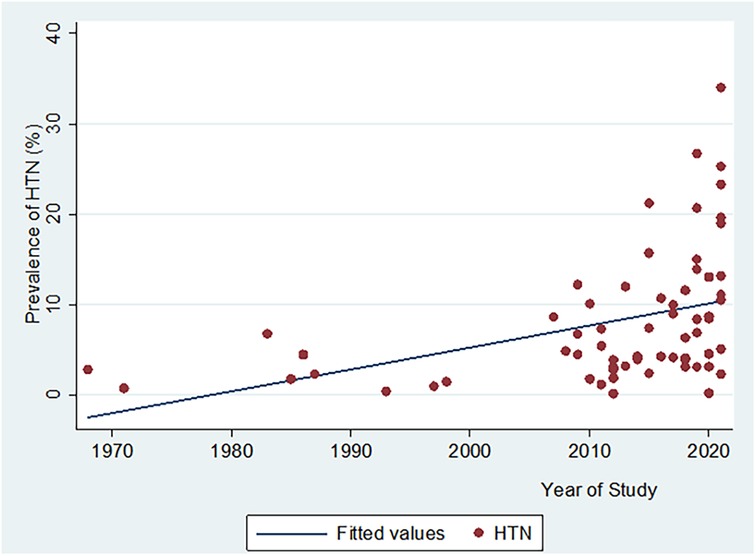
Figure 5. Scatter plot showing the relationship between hypertension prevalence and year of study publication.
In an adjusted meta regression analysis model (including year of publication, study settings, African region, type of BP measurement device used, and criteria for defining HTN), the year of publication (β = 0.14%, 95% CI: −0.003% to 0.28%, p = 0.05) and African regions (F = 3.95, p = 0.01) remained significantly associated with higher prevalence of hypertension. (Adjusted R2 = 0.38). Additionally, on average HTN prevalence estimates from Southern Africa were higher by 6.05%, 95% CI (1.97% to 10.13%, p = 0.005) than those from Western Africa.
Risk factors for hypertension
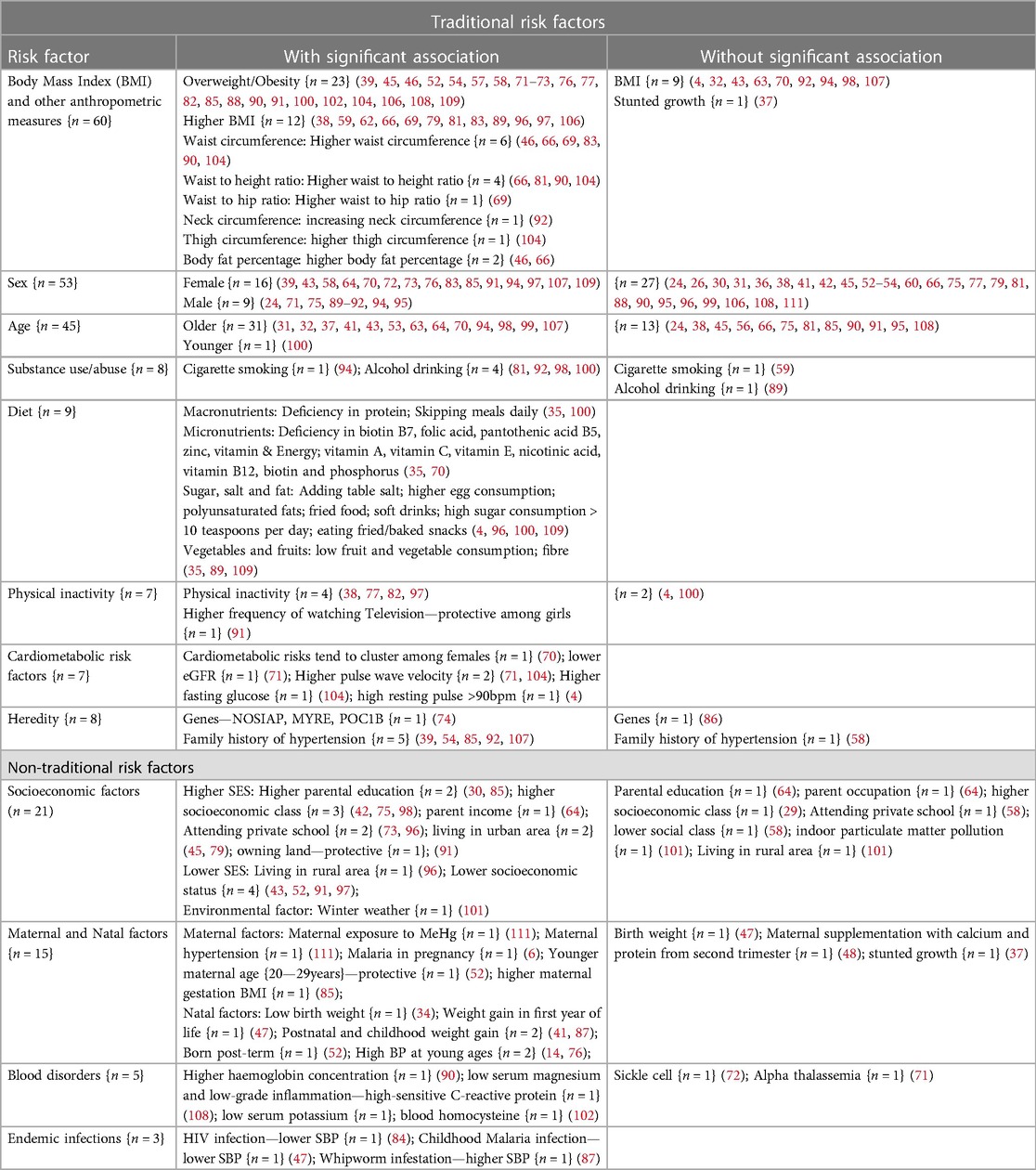
Table 3 summarizes common risk factors for HTN among adolescents in SSA. Of all 92 studies, 79 (85.9%) assessed at least one kind of traditional and/or non-traditional risk factor, and a majority (79.7%) were cross-sectional studies. Most of the reported risk factors were significantly associated with HTN although some of the associations were in an unexpected direction (82, 91, 100).
Traditional risk factors
Sex (n = 53), age (n = 45), body mass index (n = 45), and other anthropometric measures (n = 15) were the most commonly studied traditional risk factors for HTN. Other important traditional risk factors include alcohol/tobacco use (n = 8), diet (n = 9), level of physical activity (n = 7), genetic (n = 7), and cardiometabolic risk factors (n = 7)—Table 3.
Sex: Of 53 studies, nearly half 26 (49%) showed a significant association with HTN. Of those 26 studies, a slight majority 16 (62%) showed that females had a higher risk of HTN than males, and obesity and an unhealthy diet were the major factors to account for the gender differences.
Age: Of 45 studies, a majority 32 (71%) showed a significant association with HTN, and 97% of these 32 studies showed that older age was associated with HTN.
Body Mass Index (BMI) and other anthropometric measures: Of 59 studies, a majority 49 (83%) had a significant association with HTN. However, 10 (17%) studies did not find a significant association with HTN. These 10 studies were all cross-sectional, school-based, and the majority were conducted in urban settings.
Diet: All 9 studies reporting on various dietary factors found significant associations with HTN. The studies assessed a deficiency of macronutrients, micronutrients, and minerals; excessive consumption of sugar, salt, and fats; and low consumption of vegetables and fruits.
Physical inactivity: Of 7 studies, 4 (71%) found a significant association between physical inactivity and HTN. However, 1 study (14%) surprisingly showed that physical inactivity was associated with normal BP.
Cardiometabolic risk factors: All 7 studies reporting various cardiometabolic risks found significant associations with HTN. The identified cardiometabolic risk factors were lower eGFR, higher pulse wave velocity, high fasting glucose, high resting pulse, and higher blood homocysteine, and these risk factors tended to cluster in females.
Heredity: Of 8 studies, 6 (75%) showed a significant association with HTN. However, the majority 5 (83%) relied on self-reported family history of HTN. Importantly, one study using human genome data from the Birth to Twenty cohort (South Africa) showed that the NOSIAP, MYRE, and POCIB genes were associated with HTN in South African adolescents.
Non-traditional risk factors
These are factors related to socio-economic environmental and endemic diseases which are related to HTN.
Socioeconomic status and environmental factors (n = 21) as well as maternal and natal factors (n = 15) were the most commonly reported non-traditional risk factors. Other important non-traditional risks were chronic inflammation, blood composition, diseases of the blood (n = 5), and endemic infections (n = 3) (Table 3).
Socioeconomic and environmental factors: Of 21 studies, 17 (81%) found a significant association between socioeconomic and environmental factors with HTN. Of the 17 studies, 12 (71%) found higher socioeconomic status and 5 (29%) found lower socioeconomic as a significant risk for HTN.
Maternal and natal factors: Of 15 studies, majority 12 (80%) found a significant association between maternal and natal factors with HTN. Of the 12 studies, 5 (42%) assessed maternal factors, including events before or during pregnancy, including maternal exposure to mercury, maternal HTN, and malaria in pregnancy. Seven studies (58%) assessed the association between events occurring during or after birth, including low birth weight, weight gain after birth and during early childhood, and higher BP at a young age.
Blood disorders: Of 5 studies, 3 (60%) found a significant association with HTN. Of the 3 studies, the reported risks were higher hemoglobin concentration (90), lower serum magnesium (108), lower serum potassium, and higher C-reactive protein levels (102, 109).
Endemic infections: Of the 3 studies on HIV, malaria and whipworm infestation, only whipworm infestation was significantly associated with HTN (85).
Complications of hypertension
Only 3 studies assessed potential complications of HTN among adolescents in SSA. Two studies found no significant association between HTN and cognitive decline (57), proteinurias, left ventricular hypertrophy, and retinopathy (39). One study found a significant association between HTN and microalbuminuria (54) (Table 4).
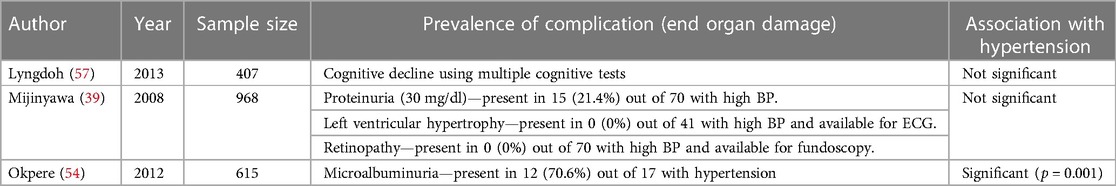
Table 4. Summary of studies on complication of hypertension among adolescents aged 10–19 years in sub-Saharan Africa.
Discussion
In this systematic review, we aimed to determine the burden, risk factors, and complications of HTN among adolescents in SSA.
The prevalence of HTN among adolescents in SSA was found to be high with varying estimates across studies. Overall, prevalence estimates from recently published studies were higher than those published in the past. This finding persisted even after taking into account of other possible reasons including differences in the devices used to measure BP and guidelines for defining HTN. In addition, studies with significantly higher prevalence estimates came from Southern Africa region, and overweight/obesity among girls was the major reported risk factor (62, 83, 88, 103, 104). Similar findings were observed in the previous systematic reviews (17, 18). The finding suggests that the burden of HTN is increasing and could explain the rising burden of CVD among young adults (17, 18, 112–114).
Nearly 90% of the included studies had their BP measurements obtained in one day contrary to the standard recommendations which require taking multiple BP measurements on at least 2 separate occasions (days) (11, 110). This raises concern about the accuracy of the HTN prevalence estimates and a potential possibility of an overestimated HTN burden (3). Adolescents are more likely than adults to experience white coat HTN (4, 115, 116). Moreover, majority of adolescents in SSA have never had their BP measured and are therefore more prone to the “white coat effect”, particularly when the BP measurements are taken on one occasion (4). This observation underscores the importance of following the standard BP measurement procedures particularly in this population to ensure accurate estimates.
Non-traditional risk factors related to poverty and tropical diseases may be important drivers of HTN among adolescents in SSA despite being under-reported and/or their role being less acknowledged (6, 15). We found relatively fewer studies that assessed/reported non-traditional risk factors, likely reflecting low awareness of their potential role on HTN in this population. In addition, the scarcity of long-term cohort studies among adolescents in SSA could partly explain this finding since most of the non-traditional risks involve tracking long term exposures occurring in one's lifetime (5, 15, 85, 87). However, it is worth noting that majority of studies assessing non-traditional risk factors often reported significant associations with HTN. This observation underscores the importance of the non-traditional risk factors although we cannot rule out publication bias.
The majority of the included studies were conducted in school settings among apparently healthy adolescents. This finding may reflect the willingness of schools and/or students to participate in research and health related interventions. It highlights the importance of schools as a potentially suitable platform for CVD prevention interventions targeting adolescents (117). Such interventions could be integrated into existing school health programs, and address multiple aspects of cardiovascular health including a healthy diet, physical exercise, and body weight control (117). BP measurement in schools might also help to raise awareness of the relevance of cardiovascular health for adolescents among teachers, parents, and entire communities. In addition, this finding points to the scarcity of hospital-based studies among adolescents in SSA where the epidemiology of HTN could be different.
Only three studies assessed/reported potential complications of HTN among adolescents in SSA (39, 54, 57), and mostly they found little or no complications. This may be due to using less-sensitive tools for the detection of HTN-related complications. For instance, in one of the three studies, left ventricular hypertrophy was assessed by electrocardiography which is known to have a sensitivity of less than 35% (39, 118). Tools with higher sensitivity and specificity to increase our awareness and knowledge of complications of HTN will help to determine the public health importance of HTN among adolescents in SSA.
Our study should be viewed in the context of the following strengths and limitations. A strength of our study is that we searched from multiple databases since their inception (from 1946). The wider review time frame allowed us to retrieve more studies published at widely ranging time points, hence giving a wider scope on how HTN estimates and their determinants have evolved over time. Our review focused on studies involving adolescents, and our findings provide useful information specific to this population. This is the first review to look at potential role of non-traditional risk factors and complications of hypertension among adolescents in SSA.
Our study has limitations. First, the retrieved studies came from only 14 (29%) of all 48 countries in SSA. The majority of studies came from Nigeria and South Africa, which are the two most advanced economies in SSA. Further, there were significant differences in prevalence estimates by African regions from which the studies came from. In this regard, our findings may not be generalizable to all countries in SSA. Secondly, a majority of studies were cross-sectional in design and therefore it is difficult to determine the temporal association with the reported risk factors. Thirdly, we cannot rule out publication bias, particularly for studies assessing/reporting non-traditional risk factors. And lastly, we did not conduct meta-analysis due to heterogeneity in study methods and settings/geography among the included studies.
Added value and implications of these findings
To our understanding, this is the second systematic review including adolescent only data in SSA region (16). Our review is updating the previous three reviews and has mapped available literature in SSA and highlighted existing knowledge/data gaps (16–18). These findings underscore the importance of accurate BP measurement and diagnosis of HTN in adolescents, both in research and clinical settings. Since HTN during adolescence is associated with the growing HTN/CVD epidemic among adults, broadly these findings will help us to design a CVD prevention intervention targeting adolescents in SSA, particularly in school settings.
Conclusion
HTN among adolescents in SSA is high, and non-traditional risk factors may be an important driver. Longitudinal observational and interventional studies are needed to clearly define the causes and complications of HTN in adolescents in SSA. In addition, governments and healthcare systems should provide the resources and accountability necessary for regular and proper BP measurements for adolescents in health facilities.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
All authors had access to data extraction sheets and data analysis outputs. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1251817/full#supplementary-material
SUPPLEMENTARY DATA SHEET 1
Search methods for rates, risk factors, and consequences of high blood pressure among adolescents in sub-Saharan Africa.
SUPPLEMENTARY DATA SHEET 2
List of publications whose full texts were not immediately available for review.
SUPPLEMENTARY DATA SHEET 3
Summary table of hypertension guidelines used in published studies among adolescents aged 10–19 years in sub-Saharan Africa.
References
1. Song P, Zhang Y, Yu J, Zha M, Zhu Y, Rahimi K, et al. Global prevalence of hypertension in children: a systematic review and meta-analysis. JAMA Pediatr. (2019) 173(12):1154–63. doi: 10.1001/jamapediatrics.2019.3310
2. UNICEF. The state of World’s Children—Investing in adolescents for breaking the cycles of poverty and inequity (2011). Available at: https://www.unicef.org/reports/state-worlds-children-2011
3. Falkner B. Hypertension in children and adolescents: epidemiology and natural history. Pediatr Nephrol. (2010) 25:1219–24. doi: 10.1007/s00467-009-1200-3
4. Nsanya MK, Ayieko P, Hashim R, Mgema E, Fitzgerald D, Kapiga S, et al. Sustained high blood pressure and 24-h ambulatory blood pressure monitoring in Tanzanian adolescents. Sci Rep. (2021) 11:8397. doi: 10.1038/s41598-021-87996-0
5. Alissa EM, Ferns GA. Heavy metal poisoning and cardiovascular disease. J Toxicol. (2011) 2011:870125–46. doi: 10.1155/2011/870125
6. Bedu-Addo G, Alicke M, Boakye-Appiah JK, Abdul-Jalil I, van der Giet M, Schulze MB, et al. In utero exposure to malaria is associated with metabolic traits in adolescence: the agogo 2000 birth cohort study. J Infect. (2017) 75(5):455–63. doi: 10.1016/j.jinf.2017.08.010
7. Brook RD, Weder AB, Rajagopalan S. “Environmental hypertensionology” the effects of environmental factors on blood pressure in clinical practice and research. J Clin Hypertens. (2011) 13(11):836–42. doi: 10.1111/j.1751-7176.2011.00543.x
8. Mandy M, Nyirenda M. Developmental origins of health and disease: the relevance to developing nations. Int Health. (2018) 10(2):66–70. doi: 10.1093/inthealth/ihy006
9. Rajagopalan S, Landrigan PJ. Pollution and the heart. N Engl J Med. (2021) 385(20):1881–92. doi: 10.1056/nejmra2030281
10. Fishman B, Grossman E, Zucker I, Orr O, Lutski M, Bardugo A, et al. Adolescent hypertension and risk for early-onset type 2 diabetes: a nationwide study of 1.9 million Israeli adolescents. Diabetes Care. (2020) 44(1):e6–8. doi: 10.2337/dc20-1752
11. Flynn JT, Falkner BE. New clinical practice guideline for the management of high blood pressure in children and adolescents. Hypertension. (2017) 70(4):683–6. doi: 10.1161/HYPERTENSIONAHA.117.10050
12. Leiba A, Fishman B, Twig G, Gilad D, Derazne E, Shamiss A, et al. Association of adolescent hypertension with future end-stage renal disease. JAMA Intern Med. (2019) 179(4):517–23. doi: 10.1001/jamainternmed.2018.7632
13. Urbina EM, Mendizábal B, Becker RC, Daniels SR, Falkner BE, Hamdani G, et al. Association of blood pressure level with left ventricular mass in adolescents SHIP AHOY. Hypertension. (2019) 74(3):590–6. doi: 10.1161/HYPERTENSIONAHA.119.13027
14. Kagura J, Adair LS, Musa MG, Pettifor JM, Norris SA. Blood pressure tracking in urban black South African children: birth to twenty cohort. BMC Pediatr. (2015) 15(1):78–85. doi: 10.1186/s12887-015-0402-z
15. Naidoo S, Kagura J, Fabian J, Norris SA, Commentary SE. Early life factors and longitudinal blood pressure trajectories are associated with elevated blood pressure in early adulthood. Hypertension. (2019) 73(2):301–9. doi: 10.1161/HYPERTENSIONAHA.118.11992
16. Chen A, Waite L, Mocumbi AO, Chan YK, Beilby J, Ojji DB, et al. Elevated blood pressure among adolescents in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. (2023) 11(8):e1238–48. doi: 10.1016/S2214-109X(23)00218-8
17. Crouch SH, Soepnel LM, Kolkenbeck-Ruh A, Maposa I, Naidoo S, Davies J, et al. Paediatric hypertension in Africa: a systematic review and meta-analysis. EClinicalMedicine. (2022) 43:101229. doi: 10.1016/j.eclinm.2021.101229
18. Noubiap JJ, Essouma M, Bigna JJ, Jingi AM, Aminde LN, Nansseu JR. Prevalence of elevated blood pressure in children and adolescents in Africa: a systematic review and meta-analysis. Lancet Public Health. (2017) 2(8):e375–86. doi: 10.1016/S2468-2667(17)30123-8
19. Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Syst Rev. (2017) 6(1):1–12. doi: 10.1186/s13643-017-0644-y
20. Grossetta Nardini HK, Wang L. The Yale MeSH Analyzer (2018). http://mesh.med.yale.edu/
21. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. Newcastle-Ottawa Quality Assesment Scale Cohort Studies (2021). Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
22. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
23. Akinkube OO, Ojo AO. The systemic blood pressure in a rural Nigerian population. Trop Geogr Med. (1968) 20:347–56.5707221
24. Johnson TO. Arterial blood pressures and hypertension in an urban African population sample. Br J Prev Soc Med. (1971) 25(1):26–33. doi: 10.1136/jech.25.1.26
25. Akinkugbe A. Arterial pressures in non-pregnant women of child-bearing age in Ile-Ife, Nigeria. Br J Obstet Gynaecol. (1976) 83:545–9. doi: 10.1111/j.1471-0528.1976.tb00883.x
26. Ayobanji Ayoola E. Prevalence of adolescent hypertension in Nigeria. Niger J Paediatr. (1979) 6(1):18–26.
27. Abu-Bakare A, Oyaide SM. Blood pressure levels in Nigerian school girls. J Trop Pediatr. (1983) 29:225–9. doi: 10.1093/tropej/29.4.225
28. Idahosa PE. Blood pressure pattern in urban edos. J Hypertens. (1985) 3(Suppl. 3):S379–81. PMID: 2856744.
29. Eferakeya AE. Arterial blood pressures in Benin city (Nigeria) children. Public Health. (1986) 100(3):174–9. doi: 10.1016/S0033-3506(86)80031-2
30. Adams-Campbell LL, Ukoli F, Young MP, Omene J, Nwankwo M, Haile GT, et al. An epidemiological assesment of blood pressure determinants in an adolescent population of Nigerians. J Hypertens. (1987) 5:575–80. doi: 10.1097/00004872-198710000-00011
31. Kitange HM, Swai ABM, Masuki G, Kilima PM, Alberti KGMM, McLarty DG. Coronary heart disease risk factors in sub-Saharan Africa: studies in Tanzanian adolescents. J Epidemiol Community Health. (1993) 47(4):303–7. doi: 10.1136/jech.47.4.303
32. Muraguri PW, McLigeyo SO, Kayima JK. Proteinuria, other slected urinary abnormalities and hypertension among teenage secondary school students in Nairobi, Kenya. East Afr Med J. (1997) 74(8):467–73. PMID: 9487409.9487409
33. Kane A, Diof N. Arterial pressure and body mass index of children and adolescents in a rural area of Thiadiaye, Senegal. Dakar Med. (1998) 43:83–9. PMID: 9827163.9827163
34. Longo-Mbenza B, Ngiyulu R, Bayekula M, Vita EK, Nkiabungu FB, Seghers KV, et al. Low birth weight and risk of hypertension in African school children. J Cardiovasc Risk. (1999) 6(5):311–4. doi: 10.1177/204748739900600507
35. Schutte AE, Van Rooyen JM, Huisman HW, Kruger HS, Malan NT, De Ridder JH. Dietary risk markers that contribute to the aetiology of hypertension in black South African children: the THUSA BANA study. J Hum Hypertens. (2003) 17(1):29–35. doi: 10.1038/sj.jhh.1001508
36. Schutte AE, Van Rooyen JM, Huisman HW, Kruger HS, De Ridder JH. Factor analysis of possible risks for hypertension in a black South African population. J Hum Hypertens. (2003) 17(5):339–48. doi: 10.1038/sj.jhh.1001553
37. Van Rooyen JM, Kruger HS, Huisman HW, Schutte AE, Malan NT, Schutte R. Early cardiovascular changes in 10- to 15-year-old stunted children: the transition and health during urbanization in South Africa in children study. Nutrition. (2005) 21(7–8):808–14. doi: 10.1016/j.nut.2004.12.007
38. Chiolero A, Madeleine G, Gabriel A, Burnier M, Paccaud F, Bovet P. Prevalence of elevated blood pressure and association with overweight in children of a rapidly developing country. J Hum Hypertens. (2007) 21(2):120–7. doi: 10.1038/sj.jhh.1002125
39. Mijinyawa MS, Iliyasu Z, Borodo MM. Prevalence of hypertension among teenage students in Kano, Nigeria. Niger J Med. (2008) 17(2):173–8. doi: 10.4314/njm.v17i2.37378
40. Monyeki KD, Kemper HCG, Makgae PJ. Relationship between fat patterns, physical fitness and blood pressure of rural South African children: ellisras longitudinal growth and health study. J Hum Hypertens. (2008) 22(5):311–9. doi: 10.1038/jhh.2008.3
41. Cournil A, Coly AN, Diallo A, Simondon KB. Enhanced post-natal growth is associated with elevated blood pressure in young Senegalese adults. Int J Epidemiol. (2009) 38(5):1401–10. doi: 10.1093/ije/dyp255
42. Mijinyawa MS, Abdu A, Habib A. Pattern of blood pressure in adolescents. Sahel Med J. (2009) 12(4):159–64. doi: 10.4314/smj2.v12i4.55694
43. Odey F, Anah M, Ansa V, Ogbeche J, Meremikwu M, Ekanem E. Pre-hypertension and hypertension in apparently healthy adolescents in Calabar, Nigeria. Global J Commun Med. (2009) 2(1–2):13–20. doi: 10.4314/gjcm.v2i1-2.47924
44. Ansa V, Anah M, Odey F, Mbu P, Agbor E. Relationship between parental socio-economic Status and casual blood pressure in coastal Nigerian adolescents. West Afr J Med. (2011) 29(3):146–52. doi: 10.4314/wajm.v29i3.68211
45. Ejike CECC, Ugwu CE, Ezeanyika LUS. Variations in the prevalence of point (pre)hypertension in a Nigerian school-going adolescent population living in a semi-urban and an urban area. BMC Pediatr. (2010) 10:13. doi: 10.1186/1471-2431-10-13
46. Kruger HS, Pretorius R, Schutte AE. Stunting, adiposity, and low-grade inflammation in African adolescents from a township high school. Nutrition. (2010) 26(1):90–9. doi: 10.1016/j.nut.2009.10.004
47. Chiolero A, Paradis G, Madeleine G, Hanley JA, Paccaud F, Bovet P. Birth weight, weight change, and blood pressure during childhood and adolescence: a school-based multiple cohort study. J Hypertens. (2011) 29(10):1871–9. doi: 10.1097/HJH.0b013e32834ae396
48. Hawkesworth S, Walker CG, Sawo Y, Fulford AJC, Jarjou LMA, Goldberg GR, et al. Nutritional supplementation during pregnancy and offspring cardiovascular disease risk in the Gambia. Am J Clin Nutr. (2011) 94(6):1853. doi: 10.3945/ajcn.110.000877
49. Mamabolo R, Van Rooyen J, Schutte A, Monyeki M, Kruger H. Association between blood pressure, measures of body composition and lifestyle factors in township adolescents, North-West Province, South Africa. Afr J Phys Health Educ Recreat Dance. (2011) 17(1):51–68. doi: 10.4314/ajpherd.v17i1.65245
50. Meehan KA, Bankoski AJ, Tejan E, Ansumana R, Bangura U, Stenger DA, et al. Hypertension in Bo, Sierra Leone. Ethn Dis. (2011) 21(2):237–42. PMID: 21749030.21749030
51. Bukabau JB, Makulo JRR, Pakasa NM, Cohen EP, Lepira FB, Kayembe PK, et al. Chronic kidney disease among high school students of Kinshasa. BMC Nephrol. (2012) 13:24–9. doi: 10.1186/1471-2369-13-24
52. Griffiths PL, Sheppard ZA, Johnson W, Cameron N, Pettifor JM, Norris SA. Associations between household and neighbourhood socioeconomic status and systolic blood pressure among urban South African adolescents. J Biosoc Sci. (2012) 44(4):433–58. doi: 10.1017/S0021932012000107
53. Okoh BA, Alikor EA, Akani N. Prevalence of hypertension in primary schoolchildren in Port Harcourt, Nigeria. Paediatr Int Child Health. (2012) 32(4):208–12. doi: 10.1179/2046905512Y.0000000039
54. Okpere AN, Anochie IC, Eke FU. Prevalence of microalbuminuria among secondary school children. Afr Health Sci. (2012) 12(2):140–7. doi: 10.4314/ahs.v12i2.10
55. Oyewole OO, Oritogun KS. Pre-hypertension and hypertension in adolescence: how much does it occur in a Nigerian community? West Afr J Med. (2012) 31(2):71–5. http://www.ncbi.nlm.nih.gov/pubmed/2320847323208473
56. Goon D, Amusa L, Mhlongo D, Khoza L, Any-Anwu F. Elevated blood pressure among rural South African children in Thohoyandou, South Africa. Iran J Public Health. (2013) 42(5):489–96. http://ijph.tums.ac.ir23802106
57. Lyngdoh T, Viswanathan B, Kobrosly R, Van Wijngaarden E, Huber B, Davidson PW, et al. Blood pressure and cognitive function: a prospective analysis among adolescents in Seychelles. J Hypertens. (2013) 31(6):1175–82. doi: 10.1097/HJH.0b013e3283604176
58. Okpere AN, Anochie IC, Eke FU. Pattern of blood pressure and hypertension in adolescents in Port Harcourt, Nigeria. West Afr J Med. (2013) 32(2):93–8. PMID: 23913495.23913495
59. Ujunwa FA, Ikefuna AN, Nwokocha AR, Chinawa JM. Hypertension and prehypertension among adolescents in secondary schools in Enugu, South East Nigeria. Ital J Pediatr. (2013) 39:1–6. doi: 10.1186/1824-7288-39-70
60. Mushengezi B, Chillo P. Association between body fat composition and blood pressure level among secondary school adolescents in Dar es Salaam, Tanzania. Pan Afr Med J. (2014) 19:327–38. doi: 10.11604/pamj.2014.19.327.5222
61. Okagua J, Akani N. Prevalence of hypertension in school going adolescents in rural areas of Rivers State, South-South Nigeria. Niger Health J. (2014) 14(4):157–64.
62. Nkeh-Chungag B, Sekokotla AM. Prevalence of hypertension in 13–17 years old in MTHATHA South Africa. Cent Eur J Public Health. (2015) 23(1):59–64. doi: 10.21101/cejph.a3922
63. Odunaiya NA, Louw QA, Grimmer KA. Are lifestyle cardiovascular disease risk factors associated with pre-hypertension in 15–18 years rural Nigerian youth? A cross sectional study. BMC Cardiovasc Disord. (2015) 15(1):144–54. doi: 10.1186/s12872-015-0134-x
64. Oyeyemi AY, Usman MA, Oyeyemi AL, Jaiyeola OA. Casual blood pressure of adolescents attending public secondary schools in Maiduguri, Nigeria. Clin Hypertens. (2015) 21(3):16–21. doi: 10.1186/s40885-015-0026-5
65. Ratovoson R, Rasetarinera OR, Andrianantenaina I, Rogier C, Piola P, Pacaud P. Hypertension, a neglected disease in rural and urban areas in Moramanga, Madagascar. PLoS One. (2015) 10:9. doi: 10.1371/journal.pone.0137408
66. Awotidebe A, Monyeki MA, Moss SJ, Strydom GL, Amstrong M, Kemper HCG. Relationship of adiposity and cardiorespiratory fitness with resting blood pressure of South African adolescents: the PAHL study. J Hum Hypertens. (2016) 30(4):245–51. doi: 10.1038/jhh.2015.81
67. Munthali RJ, Kagura J, Lombard Z, Norris SA. Childhood adiposity trajectories are associated with late adolescent blood pressure: birth to twenty cohort. BMC Public Health. (2016) 16(1):665–75. doi: 10.1186/s12889-016-3337-x
68. Strassman BI, Smith CS, Vincenz C. Does puberty influence systolic blood pressure independent of the effects of adolescent growth and body size? Am J Phys Anthropol. (2016) 159:305. doi: 10.1002/ajpa.22955
69. Uwaezuoke S, Okoli C, Ubesie A, Ikefuna A. Primary hypertension among a population of Nigerian secondary school adolescents: prevalence and correlation with anthropometric indices: a cross-sectional study. Niger J Clin Pract. (2016) 19(5):649–54. doi: 10.4103/1119-3077.188706
70. Alicke M, Boakye-Appiah JK, Abdul-Jalil I, Henze A, Van Der Giet M, Schulze MB, et al. Adolescent health in rural Ghana: a cross-sectional study on the co-occurrence of infectious diseases, malnutrition and cardio-metabolic risk factors. PLoS One. (2017) 12(7):e0180436–51. doi: 10.1371/journal.pone.0180436
71. Etyang AO, Khayeka-Wandabwa C, Kapesa S, Muthumbi E, Odipo E, Wamukoya M, et al. Blood pressure and arterial stiffness in Kenyan adolescents with α+thalassemia. J Am Heart Assoc. (2017) 6(4):e005613–20. doi: 10.1161/JAHA.117.005613
72. Etyang AO, Wandabwa CK, Kapesa S, Muthumbi E, Odipo E, Wamukoya M, et al. Blood pressure and arterial stiffness in Kenyan adolescents with the sickle cell trait. Am J Epidemiol. (2018) 187(2):199–205. doi: 10.1093/aje/kwx232
73. Ezeudu CE, Chukwuka JO, Ebenebe JC, Igwe WC, Egbuonu I. Hypertension and prehypertension among adolescents attending secondary schools in urban area of South-east, Nigeria. Pan Afr Med J. (2018) 31:145–54. doi: 10.11604/pamj.2018.31.145.15994
74. Hendry LM, Sahibdeen V, Choudhury A, Norris SA, Ramsay M, Lombard Z. Insights into the genetics of blood pressure in black South African individuals: the birth to twenty cohort. BMC Med Genom. (2018) 11:2. doi: 10.1186/s12920-018-0321-6
75. Isezuo KO, Jiya NM, Audu LI, Ibitoye PK, Sani UM, Yusuf T, et al. Blood pressure pattern and the relationship with body mass index among apparently healthy secondary—school students in Sokoto Metropolis, Nigeria. SAJCH S Afr J Child Health. (2018) 12(3):105–10. doi: 10.7196/SAJCH.2018.v12i3.1475
76. Leyvraz M, Wahlen R, Bloetzer C, Paradis G, Bovet P, Chiolero A. Persistence of elevated blood pressure during childhood and adolescence: a school-based multiple cohorts study. J Hypertens. (2018) 36(6):1306–10. doi: 10.1097/HJH.0000000000001699
77. Masocha V, Czyż SH, Moss SJ, Monyeki AM. Two-year changes in body composition, physical activity and selected metabolic risk factors among adolescents living in tlokwe municipality area, north west province, South Africa: the PAHL study. S Afr J Res Sport Phys Educ Recreation. (2018) 40(2):99–114. doi: 10.1123/jpah.2018-0535
78. Nakiriba R, Mayega RW, Piloya T, Nabukeera-Barungi N, Idro R. Prevalence and factors associated with dysglycemia among girls in selected boarding secondary schools in Wakiso district, Uganda. Adolesc Health Med Ther. (2018) 9:167–76. doi: 10.2147/ahmt.s178746
79. Omisore AG, Omisore B, Abioye-Kuteyi EA, Bello IS, Olowookere SA. In-school adolescents’ weight status and blood pressure profile in south-western Nigeria: urban-rural comparison. BMC Obes. (2018) 5:2 (2018). doi: 10.1186/s40608-018-0179-3
80. Abu OO, Raji YR, Amodu OK. Risk factors for chronic kidney disease among in-school adolescents in Ibadan, Southwest, Nigeria. Sahel Med J. (2019) 22(2):64. doi: 10.4103/smj.smj_21_18
81. Adeomi AA, Adelusi IO, Adedeji PO, Awofeso AE, Oroleye OO, Gbadegesin DL. Nutritional status and cardiometabolic health among adolescents; findings from southwestern Nigeria. BMC Nutr. (2019) 5:45. doi: 10.1186/s40795-019-0308-5
82. Amponsem-Boateng C, Zhang W, Oppong TB, Opolot G, Kyere EKD. A cross-sectional study of risk factors and hypertension among adolescent senior high school students. Diabetes Metab Syndr Obes Targets Ther. (2019) 12:1173–80. doi: 10.2147/DMSO.S213552
83. Chungag A, Tata CM, Sewani-Rusike CR, Nel W, Nkeh-Chungag BN. Ellisras longitudinal study 2017: association of hypertension with increasing levels of adiposity in 10- to 14-year-old boys and girls in the eastern cape (ELS 31). Cardiovasc J Afr. (2019) 30(5):258–61. doi: 10.5830/CVJA-2019-017
84. Frigati L, Mahtab S, Nourse P, Ray P, Perrazzo S, Machemedze T, et al. Prevalence of risk factors for chronic kidney disease in South African youth with perinatally acquired HIV. Pediatr Nephrol. (2019) 34(2):313–8. doi: 10.1007/s00467-018-4080-6
85. Lule SA, Namara B, Akurut H, Lubyayi L, Nampijja M, Akello F, et al. Blood pressure risk factors in early adolescents: results from a Ugandan birth cohort. J Hum Hypertens. (2019) 33(9):679–92. doi: 10.1038/s41371-019-0178-y
86. Lule SA, Mentzer AJ, Namara B, Muwenzi AG, Nassanga B, Kizito D, et al. A genome-wide association and replication study of blood pressure in Ugandan early adolescents. Mol Genet Genomic Med. (2019) 7(10):e950. doi: 10.1002/mgg3.950
87. Lule SA, Namara B, Akurut H, Muhangi L, Lubyayi L, Nampijja M, et al. Are birthweight and postnatal weight gain in childhood associated with blood pressure in early adolescence? Results from a Ugandan birth cohort. Int J Epidemiol. (2019) 48(1):148–56. doi: 10.1093/ije/dyy118
88. Nkwana MR, Monyeki KD, Monyeki SM, Makata TT, Monyeki JM. Ellisras lingitudinal study 2017: the association of fat patterning with blood pressure in polokwane private school children aged five to 15 years (ELS 22). Cardiovasc J Afr. (2019) 30(3):142–5. doi: 10.5830/CVJA-2018-058
89. Nsanya MK, Kavishe BB, Katende D, Mosha N, Hansen C, Nsubuga RN, et al. Prevalence of high blood pressure and associated factors among adolescents and young people in Tanzania and Uganda. J Clin Hypertens. (2019) 21(4):470–8. doi: 10.1111/jch.13502
90. Abiodun O, Ladele A, Olu-Abiodun O, Ashipa T. Hypertension among adolescents in Nigeria: a retrospective study of adolescent university freshmen. Int J Adolesc Med Health. (2021) 33(5):20180287. doi: 10.1515/ijamh-2018-0287
91. Azupogo F, Abizari AR, Aurino E, Gelli A, Osendarp SJM, Bras H, et al. Malnutrition, hypertension risk, and correlates: an analysis of the 2014 Ghana demographic and health survey data for 15–19 years adolescent boys and girls. Nutrients. (2020) 12(9):1–23. doi: 10.3390/nu12092737
92. Katamba G, Agaba DC, Migisha R, Namaganda A, Namayanja R, Turyakira E. Prevalence of hypertension in relation to anthropometric indices among secondary adolescents in Mbarara, Southwestern Uganda. Ital J Pediatr. (2020) 46(1):76–83. doi: 10.1186/s13052-020-00841-4
93. Masocha V, Monyeki MA, Czyż SH. Longitudinal relationships between changes in body composition and changes in selected metabolic risk factors (abdominal obesity and blood pressure) among South African adolescents. PeerJ. (2020) 2020(6):e9331. doi: 10.7717/peerj.9331
94. Mokgwathi M, Mwita JC. Prevalence of hypertension and selected cardiovascular risk factors among adolescents in selected rural and urban secondary schools in Botswana. S Afr J Diabetes Vasc Dis. (2020) 7(1):23–28. doi: 10.10520/EJC-1edab1a94e
95. Raphadu TT, Van Staden M, Dibakwane WM, Monyeki KD. A non-invasive investigation into the prevalence of higher than normal blood pressure, hypertension and the association between blood pressure and body weight in male and female adolescents in the Polokwane Local Municipality, Limpopo-South Africa: a cros. Children. (2020) 7(3):18. doi: 10.3390/children7030018
96. Sungwa EE, Kibona SE, Dika HI, Laisser RM, Gemuhay HM, Kabalimu TK, et al. Prevalence and factors that are associated with elevated blood pressure among primary school children in Mwanza Region, Tanzania. Pan Afr Med J. (2020) 37:283. doi: 10.11604/pamj.2020.37.283.21119
97. Ugochukwu EF, Onubogu CU, Ofora VC, Okeke KN, Uju CM. Blood pressure profiles and determinants of hypertension among public secondary school students in Nnewi, Southeast Nigeria. Eur J Med Health Sci. (2020) 2:3. doi: 10.24018/ejmed.2020.2.3.298
98. Ukoh U, Ujunwa F, Muoneke U, Manyike P, Okike C, Ibe B. Oscillometric blood pressure profile of adolescent secondary school students in Abakaliki metropolis. Ann Afr Med. (2020) 19(1):31–9. doi: 10.4103/aam.aam_21_19
99. Akinbodewa AA, Adejumo AO, Lamidi OA, Adeyemi O. Clustering of cardiometabolic risk factors among children and adolescents in a rural community in Ondo, Southwest Nigeria. J Trop Pediatr. (2021) 66(4):366–76. doi: 10.1093/TROPEJ/FMZ075
100. Ayogu RNB, Nwodo CJ. Epidemiological characteristics of hypertension, impaired fasting capillary glucose and their comorbidity: a retrospective cross-sectional population-based study of rural adolescents in Southeast Nigeria. BMJ Open. (2021) 11(5):e041481. doi: 10.1136/bmjopen-2020-041481
101. Chungag A, Engwa GA, Sewani-Rusike CR, Nkeh-Chungag BN. Effect of seasonal variation on the relationship of indoor air particulate matter with measures of obesity and blood pressure in children. J Health Pollution. (2021) 11:30. doi: 10.5696/2156-9614-11.30.210610
102. du Plessis JP, Nienaber-Rousseau C, Lammertyn L, Schutte AE, Pieters M, Kruger HS. The relationship of circulating homocysteine with fibrinogen, blood pressure, and other cardiovascular measures in African adolescents. J Pediatr. (2021) 234:158–63.e2. doi: 10.1016/j.jpeds.2021.03.034
103. Engwa G, Letswalo P, Nkeh-Chungag B. Obesity, hypertriglycaemia and endothelial dysfunction are risk factors of hypertension in South African adolescents. J Hypertens. (2021) 39(March):2021. doi: 10.1097/01.hjh.0000746524.27921.7e
104. Letswalo BP, Schmid-Zalaudek K, Brix B, Matjuda EN, Klosz F, Obernhumer N, et al. Cardiometabolic risk factors and early indicators of vascular dysfunction: a cross-sectional cohort study in South African adolescents. BMJ Open. (2021) 11(3):e042955. doi: 10.1136/bmjopen-2020-042955
105. Lwabukuna WC, Mgonda Y. Early clinical markers of metabolic syndrome among secondary school adolescents in Dar es Salaam, Tanzania. Tanzan J Health Res. (2021) 22(1):1–7. doi: 10.4314/thrb.v22i1.3
106. Meer R, Boateng D, Klipstein-Grobusch K, Norris SA, Kagura J. Incidence and correlates of high blood pressure from childhood to adulthood: the birth to twenty study. J Hypertens. (2022) 40(2):274–82. doi: 10.1097/HJH.0000000000003004
107. Nganou-Gnindjio CN, Essama DB, Nkeck JR, Tchebegna PY, Tchatchouang KM, Tankeu A, et al. Prevalence and factors associated with hypertension among school children and adolescents in urban and semi-urban areas in Cameroon. J Clin Hypertens. (2021) 23(8):1490–7. doi: 10.1111/jch.14309
108. Sekokotla AM, Iputo JE, Sewani-Rusike CR, Malema IM, Adeniyi OV, Goon DT, et al. Serum magnesium and high-sensitive c-reactive proteins in hypertensive, obese female school learners. West Indian Med J. (2021) 69(1):32–7. doi: 10.7727/wimj.2015.292
109. Shokunbi OS, Ukangwa NA. Relationship of blood pressure status, dietary factors and serum electrolytes of in-school adolescents in Ilishan-Remo, Ogun State, Nigeria. Afr Health Sci. (2021) 21(4):1754–63. doi: 10.4314/ahs.v21i4.32
110. NHLBI. The fourth report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescents. Pediatrics. (2004) 114(2):555–76. doi: 10.1542/peds.114.2.S2.555
111. Thurston SW, Bovet P, Myers GJ, Davidson PW, Georger LA, Shamlaye C, et al. Does prenatal methylmercury exposure from fish consumption affect blood pressure in childhood? NeuroToxicology. (2007) 28(5):924–30. doi: 10.1016/j.neuro.2007.06.002
112. Kavishe B, Biraro S, Baisley K, Vanobberghen F, Kapiga S, Munderi P, et al. High prevalence of hypertension and of risk factors for non-communicable diseases (NCDs): a population based cross-sectional survey of NCDS and HIV infection in northwestern Tanzania and Southern Uganda. BMC Med. (2015) 13:126–47. doi: 10.1186/s12916-015-0357-9
113. Peck R, Green E, Mtabaji J, Majinge C, Smart L, Downs J, et al. Hypertension related diseases as a common cause of hospital mortality in Tanzania. A 3 year prospective study. J Hypertens. (2014) 31(9):1806–11. doi: 10.1097/HJH.0b013e328362bad7.HYPERTENSION-RELATED
114. Peck R, Baisley K, Kavishe B, Were J, Mghamba J, Smeeth L, et al. Decreased renal function and associated factors in cities, towns and rural areas of Tanzania: a community-based population survey. Trop Med Int Health. (2016) 21(3):393–404. doi: 10.1111/tmi.12651
115. Myers MG, McInnis NH, Fodor GJ, Leenen FHH. Comparison between an automated and manual sphygmomanometer in a population survey. Am J Hypertens. (2008) 21(3):280–3. doi: 10.1038/ajh.2007.54
116. Stergiou GS, Boubouchairopoulou N, Kollias A. Accuracy of automated blood pressure measurement in children evidence, issues, and perspectives. Hypertension. (2017) 69(6):1000–6. doi: 10.1161/HYPERTENSIONAHA.116.08553
117. Steinberger J, Daniels SR, Hagberg N, Isasi RC, Kellly AS, Lloyd-Jones D, et al. Cardiovascular health promotion in children: challenges and opportunities for 2020 and beyond. Circulation. (2016) 134:e236–55. doi: 10.1161/CIR.0000000000000441
Keywords: hypertension, blood pressure, risk factors, adolescents, sub-Saharan Africa, cardiovascular complications
Citation: Nsanya MK, Abramson R, Kisigo GA, Hickner A, Nyanza EC, Peck RN and Kapiga SH (2023) Hypertension among adolescents in sub-Saharan Africa: a systematic review. Front. Cardiovasc. Med. 10:1251817. doi: 10.3389/fcvm.2023.1251817
Received: 2 July 2023; Accepted: 18 October 2023;
Published: 7 December 2023.
Edited by:
Okechukwu Ogah, University of Ibadan, NigeriaReviewed by:
Bert-Jan Van Den Born, Amsterdam University Medical Center, NetherlandsMaria Grazia Modena, University of Modena and Reggio Emilia, Italy Paolo Alberto Gasparini, University Hospital of Modena, Italy, in collaboration with reviewer [MGM]
© 2023 Nsanya, Abramson, Kisigo, Hickner, Nyanza, Peck and Kapiga. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mussa K. Nsanya bXVzc2EubnNhbnlhQG1pdHUub3IudHo=
 Mussa K. Nsanya
Mussa K. Nsanya Rachel Abramson2
Rachel Abramson2 Godfrey A. Kisigo
Godfrey A. Kisigo Elias C. Nyanza
Elias C. Nyanza Saidi H. Kapiga
Saidi H. Kapiga