- 1The Second Clinical Medical College, Lanzhou University, Lanzhou, China
- 2Department of Obstetrics, Lanzhou University Second Hospital, Lanzhou, China
Background: Pre-eclampsia (PE) is a severe pregnancy complication. Thrombocytopenia and platelet dysfunction are common hematology disorders in PE. Previous studies considered mean platelet volume (MPV), a functional marker of platelets, as a potentially useful predictor for the diagnosis of PE.
Methods: PubMed, China Biomedical Literature Database, Chinese National Knowledge Infrastructure, Embase, Wanfang, VIP, and Cochrane Library databases were searched to gather diagnostic trials evaluating the diagnosis of PE using MPV, from their inception to 13 March 2023. We also searched Google Scholar and Baidu.
Results: A total of 22 studies from 20 articles were found. The pooled diagnostic accuracy of the MPV for PE recognition was as follows: sensitivity (SEN) 0.676 [95% confidence interval (CI) (0.658–0.694)], specificity (SPE) 0.710 [95% CI (0.703–0.717)], and diagnostic odds ratio (DOR) 7.012 [95% CI (4.226–11.636)], and the SROC-AUC and Q* indices were 0.7889 and 0.7262, respectively. The pooled SEN, SPE, and DOR of the diagnostic accuracy of MPV for PE before 16 weeks of gestation were 0.707 [95% CI (0.670–0.743)], 0.639 [95% CI (0.611–0.667)], and 4.026 [95% CI (2.727–5.943)], and the SROC-AUC and Q* indices were 0.7278 and 0.6753, respectively. For the interval of truncation values between 9 and 10 fl, the SROC-AUC and Q* indices for MPV were 0.8856 and 0.8162, respectively.
Conclusions: Available evidence suggests that MPV has a moderate predictive and diagnostic value for PE, particularly in diagnosing after 20 weeks of gestation. The diagnostic accuracy is higher when the MPV cut-off falls between 9 and 10 fl. The sensitivity of MPV alone in diagnosing PE is not high, and the combination of other markers for predictive diagnosis may better differentiate PE.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023425154, identifier: CRD42023425154.
1. Introduction
Maternal mortality is a serious global problem. In 2019, WHO (World Health Organization) reported that almost 95% of maternal deaths occur in low- and lower–middle-income countries, in which pre-eclampsia (PE) and eclampsia are important causes (1). Each year, more than 500,000 fetal and neonatal deaths and more than 70,000 maternal deaths occur worldwide because of PE (2). PE manifests primarily as a progressive pregnancy disorder with new-onset hypertension occurring after 20 weeks of gestation with the simultaneous involvement of multiple organ systems (3, 4). Clinical studies have confirmed that PE increases the risk of developing chronic diseases in later life, including cardiovascular complications (5, 6), renal disease (7), and neurological disorders (8). At the same time, PE is associated with neonatal neurodevelopmental problems (9) and congenital heart disease (10).
The etiology of PE is unclear, and its pathophysiological mechanisms are associated with placental hypoperfusion, endothelial dysfunction, oxidative stress, inflammation, and immune abnormalities (4). Pregnancy changes the maternal hemostatic–fibrinolytic system, shifting the equilibrium toward a hypercoagulable state. However, PE exacerbates this change process (11, 12). Endothelial cell dysfunction leading to vasoconstriction and platelet adhesion aggregation, triggering coagulation, increased platelet activation leading to increased platelet consumption, and subsequent stimulation of the inflammatory response are key pathogenic steps in PE, causing thrombocytopenia, a prevalent hematological abnormality in PE (13–15).
Mean platelet volume (MPV), a platelet-related index, is a marker of platelet size, function, and activation. During platelet activation, the number and size of pseudopods will increase, while platelet depletion leads to the release of new and larger platelets, resulting in an increment in MPV (16, 17). MPV is a non-invasive biomarker. Compared with other plasma or serum-based biomarkers and various imaging modalities for prediction, MPV allows the use of complete blood count (CBC) tests in limited healthcare resources, which is simpler and less costly, reducing the healthcare burden on pregnant women in low- and middle-income areas. It is demonstrated that MPV can be used as a predictor of the severity and prognosis of cardiovascular disease (18, 19) and infectious diseases (20), while increased MPV is associated with the occurrence and severity of gestational diabetes (21) and intrahepatic cholestasis (22).
Drugs that significantly slow the progression of PE have not been identified, and the only option to prevent the disease is to deliver the fetus and placenta. Aspirin is the only preventive medication for PE strongly supported by research evidence (23). National guidelines recommend that women with high-risk factors can start taking aspirin before 16 weeks of gestation (24–26).
Aspirin operates by regulating vascular homeostasis and platelet function (26). Therefore, understanding the variation in MPV and its associated predictive value in PE is increasingly crucial. Predicting PE in early gestation, identifying women most suitable for aspirin prophylaxis, and eliminating the short- or long-term adverse consequences caused by PE remain a challenge, especially when considering the conditions of scarce medical resources in low- and middle-income areas. Evidence from cohort studies and meta-analyses indicates that PE is associated with elevated platelet function, and MPV, an important parameter of platelet activation, is elevated in PE (14–16, 27, 28). Further evaluation is needed to determine whether MPV can be used as a marker for the early diagnosis of PE. This meta-analysis aims to initially accumulate the available literature to assess the diagnostic efficacy of MPV as a predictive and diagnostic marker for PE.
2. Methods
This meta-analysis was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (29), and registration was completed with PROSPERO (CRD42023425154). We included diagnostic trials to investigate the predictive and diagnostic value of MPV for PE in pregnant women across all trimesters.
2.1. Search strategy
Two authors (DY and SL) independently performed the database search. The PubMed, Cochrane Library, Embase, Chinese National Knowledge Infrastructure (CNKI), Wanfang, China Biomedical Literature Database (CBM), and VIP databases were searched to gather publicly published articles from the establishment of the database to 13 March 2023. In addition, Google Scholar, Baidu, and the references of the included literature were also searched to supplement access to relevant literature. Ages, races, gestational weeks, and languages were not limited in the selection process. A medical subject headings (MeSH) thesaurus in conjunction with free word, in combination with Boolean operators (e.g., “OR” or “AND”), was used. The search terms included Mean Platelet Volume, Mean Platelet Volumes, Platelet Volume, Mean, Volume, Mean Platelet, MPV, blood indices, Pre-Eclampsia, Preeclampsia Hypertension, Pregnancy-Induced, hypertensive disorder complicating pregnancy, HDCP, gestational hypertension, PE, Hypertension, Pregnancy-Induced, etc. Supplementary Figure S1 shows a sample detailed search strategy for PubMed. Any discrepancies in the literature search were referred to a third party (RH) for resolution.
2.2. Literature screening and data extraction
The titles of the articles were read first. After excluding irrelevant literature, the abstract and full text were read to determine inclusion. If necessary, the authors of the original studies were contacted via email and telephone to obtain information not determined but essential for our review.
The inclusion criteria included (1) the diagnostic test for PE; (2) the index test of MPV; (3) guidelines (30, 31, 32), obstetrics and gynecology (33), and clinical diagnostic criteria as the reference standard; and (4) calculation of true positives (TP), false positives (FP), false negatives (FN), and true negatives (TN).
The exclusion criteria included (1) reviews, case reports, letters, conference abstracts, and editorials; (2) non-human studies; (3) unavailable critical information; (4) case or control group sample size less than 10; and (5) duplicate publications.
The data extracted included information on the first author, year of publication, study region, reference standard, study type, gestational week at sampling, sample size, sample source, MPV measurement method, MPV level, MPV cut-off, and TP, TN, FP, FN data. Two investigators (DY and SL) independently screened, extracted, and cross-checked the literature. Disagreements were resolved through discussion or consultation with a third party.
2.3. Quality assessment
The risk of bias was evaluated using RevMan 5.3 software. Two investigators (DY and YD) assessed the risk of bias by employing the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool and cross-checked the results. Any disagreement was submitted to a third party for negotiation.
2.4. Statistical analysis
This meta-analysis was performed utilizing RevMan 5.3, Meta Disc 1.4, and Stata 16.0 software. Heterogeneity among the studies was analyzed using the χ2 test (test level α = 0.1), combined with the I2 value to determine the magnitude of heterogeneity quantificationally. The I2 values of 25%, 50%, and 75% were considered low, medium, and high heterogeneities, respectively. If there was no statistical heterogeneity among the findings, a fixed effect model was used. If statistical heterogeneity was observed, the source of heterogeneity was analyzed, the effect of obvious clinical heterogeneities was excluded, and a random effect model was further applied to the meta-analysis.
The diagnostic value of MPV was calculated, including the pooled sensitivity (SEN), specificity (SPE), negative likelihood ratio (−LR), positive likelihood ratio (+LR), diagnostic odds ratio (DOR), and area under the summary receiver operating characteristic curve (SROC-AUC). Heterogeneity was categorized into threshold and non-threshold effects, and non-threshold effects were investigated using subgroup analysis. Spearman correlation analysis was employed to check for threshold effects, suggesting no threshold effect if P > 0.05 and heterogeneity because of threshold effect if P < 0.05. SEN analyses were conducted to estimate the effect of each study on the pooled measures. Publication bias was assessed by the funnel plot. P < 0.05 was considered statistically significant.
3. Results
3.1. Study selection
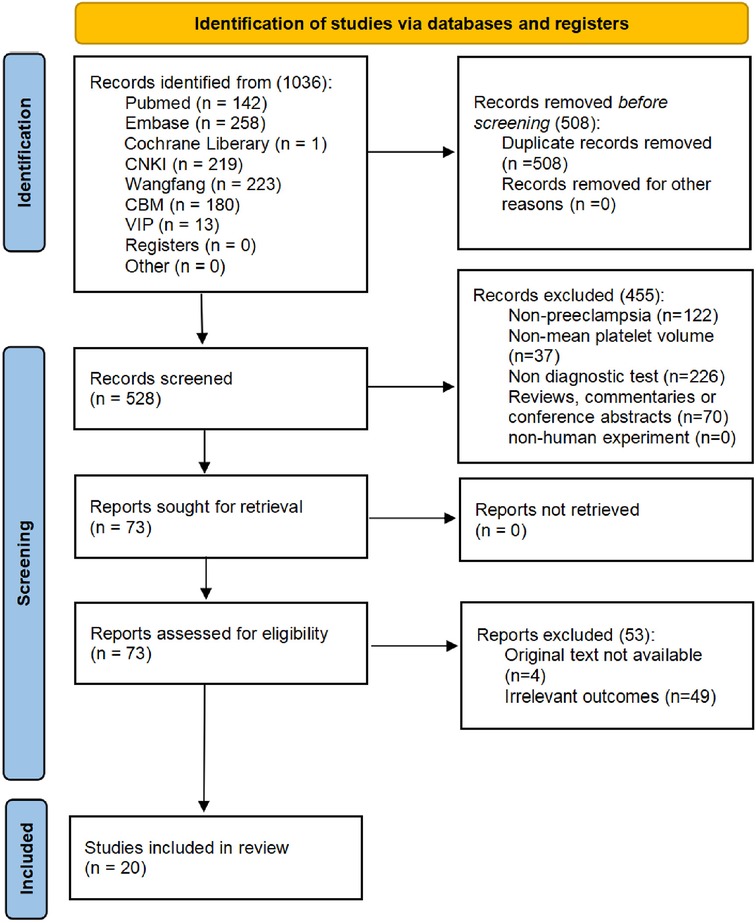
The works of literature were selected according to the PRISMA diagram. All literature that met the criteria up to 13 March 2023 was included, with a total of 1,036. After removing duplicates, a sum of 528 studies remained to be screened.
After reading the title or abstract, 455 articles were excluded for not satisfying the inclusion criteria. A total of 49 articles were excluded because the data of the four-compartment table for diagnosing PE by MPV were inaccessible after reading the full text. The full texts of four articles were unavailable. Finally, a total of 20 literature studies (27, 34–52), including 22 studies, were employed in the analysis. Figure 1 shows the screening flow chart.
3.2. Study characteristics
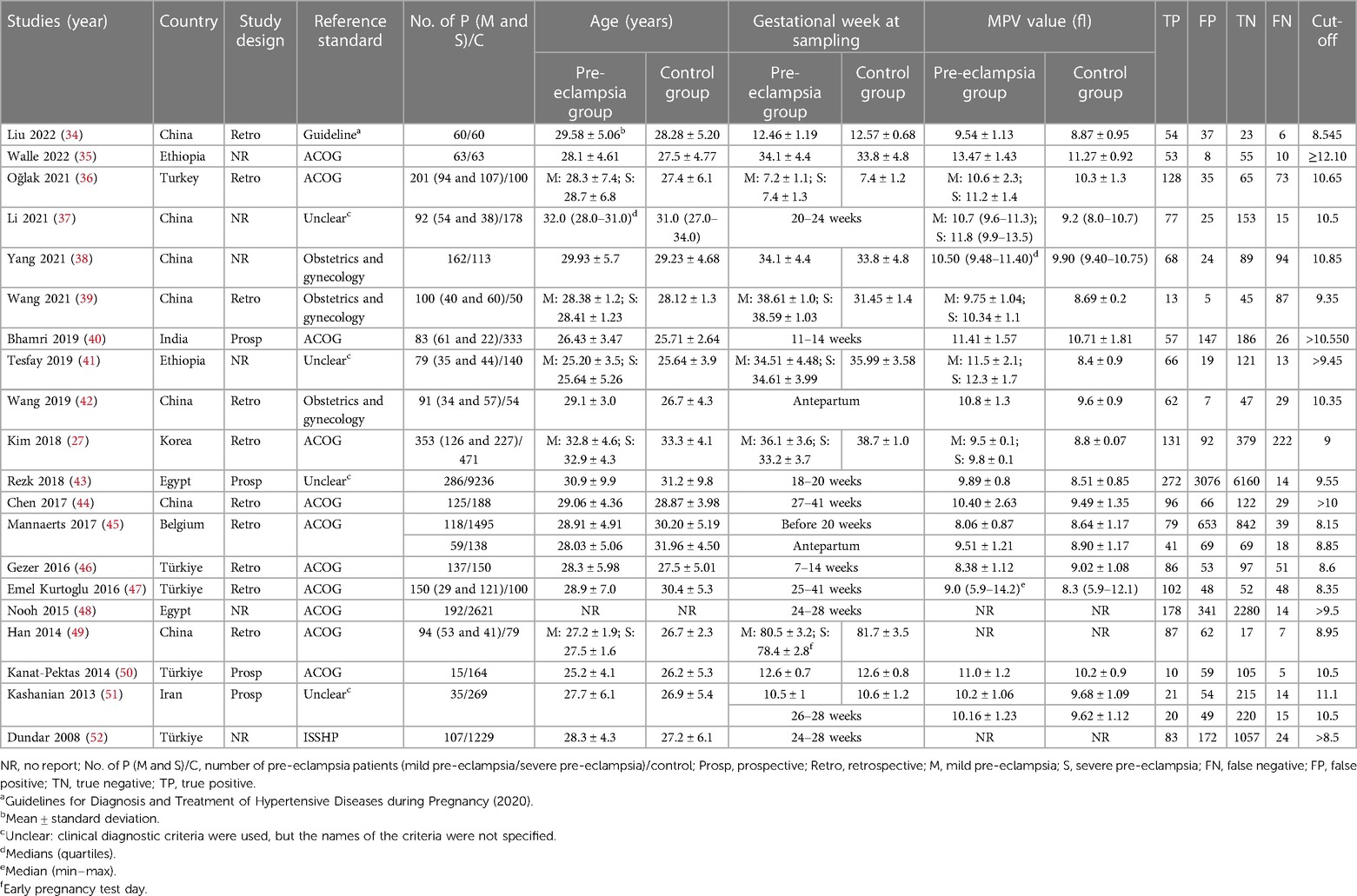
This systematic review included 20 publications containing 22 studies. A total of 2,637 cases of PE and 17,500 cases of control were included. Among the 20 literature studies included, seven were performed in China (34, 37–39, 42, 44, 49), five in Turkey (36, 46, 47, 50, 52), two in Ethiopia (35, 41), two in Egypt (43, 48), and four in India (40), Korea (27), Belgium (45), and Iran (51), respectively. Table 1 shows the basic characteristics of the included studies.
3.3. Quality assessment
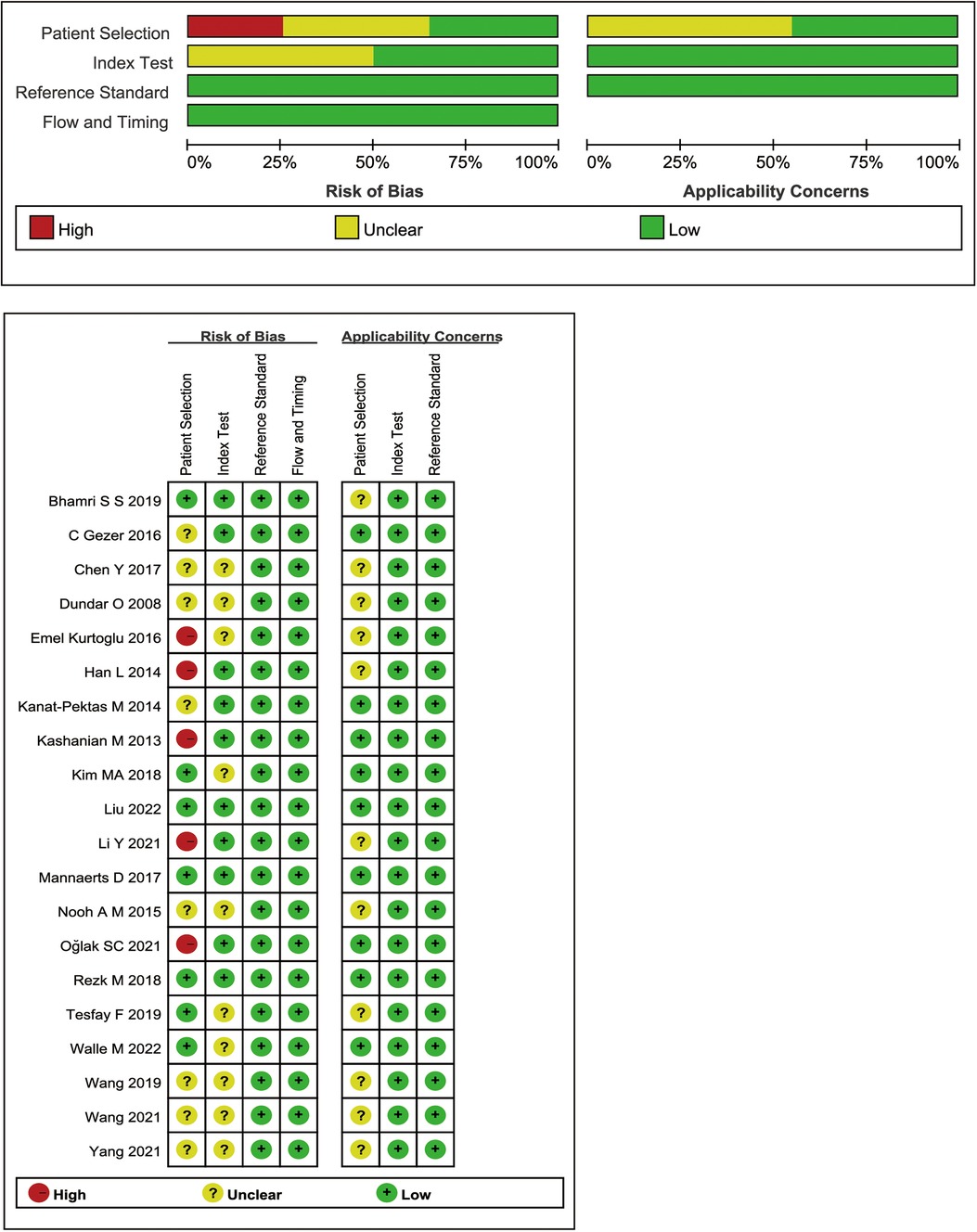
Figure 2 summarizes the QUADAS-2 quality evaluation of the 20 publications included. 14 studies had (unclear) risk of bias in patient selection, which was due to the lack of (not explicitly reported) whether all females tested by MPV were consecutively or randomly entered, the study applied a case-control design, or was not clearly reported. The bias in the index test and reference standard were attributed to the unclear timing of the MPV threshold setting and the lack of reporting on the use of blinding. The results indicated that the quality of the identified studies was generally satisfactory (Figure 2). In addition, SEN analyses were performed to estimate the impact of each study on the overall, omitting one study at a time, and no outliers were found (Supplementary Figure S2).
3.4. Meta-analysis
The Spearman correlation analysis of 22 studies within 20 literature sources used the SEN logarithm with the (1-SPE) logarithm, with a value of 0.178, P = 0.428. The I2 statistics value for SEN and SPE of the pooled studies were 96.6% and 98.2%, respectively, indicating heterogeneity from non-threshold effects.
By performing a meta-analysis using the random effect model, the overall diagnostic accuracy of MPV for PE was as follows: SEN was 0.707 [95% confidence interval (CI) (0.670–0.743)], SPE was 0.639 [95% CI (0.611–0.667)], +LR was 1.844 [95% CI (1.389–2.447)], −LR was 0.533 [95% CI (0.466–0.610)], and DOR was 4.026 [95% CI (2.727–5.943)] (Figures 3A–E). Figure 3F shows the SROC curve for the diagnostic accuracy of MPV for PE. Based on the SROC, AUC, and Q* indices, the diagnostic accuracy was calculated, and the outcomes showed that the AUC and Q* indices were 0.7889 [standard error (SE) of 0.0300] and 0.7262 (SE of 0.0258).
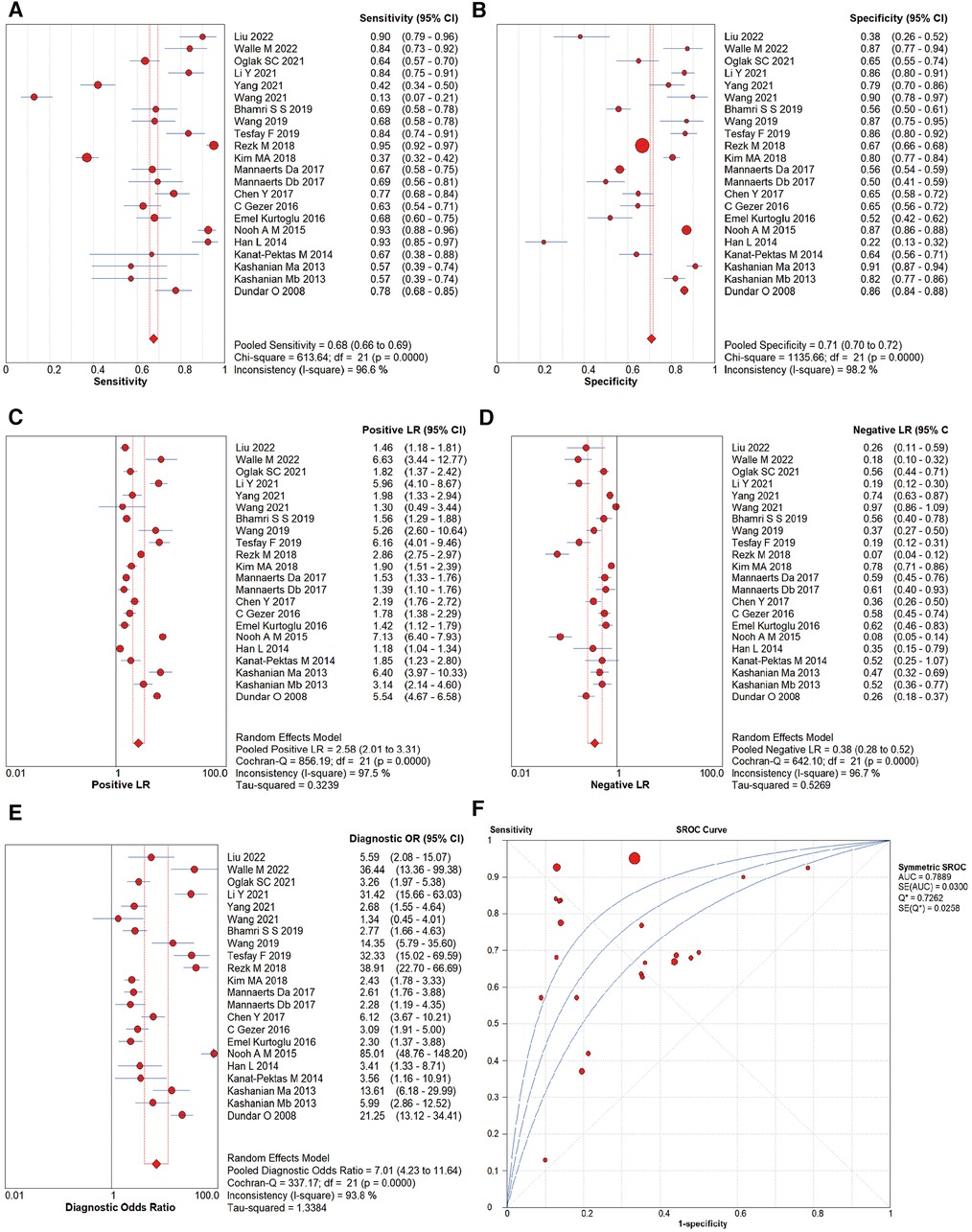
Figure 3. Forest plots of diagnostic performance of MPV for PE: (A) sensitivity, (B) specificity, (C) negative likelihood ratio, (D) positive likelihood ratio, (E) DOR, and (F) area under the SROC curve. The first author's name and year of each study are listed.
Studies that sampled MPV measurements before 16 weeks of gestation were selected from 22 studies for the meta-analysis. Seven studies (34, 36, 40, 46, 49, 50, 51) were entered, showing a pooled SEN of 0.707 [95% CI (0.670–0.743)], SPE of 0.639 [95% CI (0.611–0.667)], +LR of 1.844 [95% CI (1.389–2.447)], −LR of 0.533 [95% CI (0.466–0.610)], DOR of 4.026 [95% CI (2.727–5.943)], SROC-AUC of 0.7278 (SE of 0.0294), and Q* indices of 0.6753 (SE of 0.0239).
The cut-off values for MPV in the included literature studies were categorized for analysis. Seven studies (34, 45–47, 49, 52) used the 8–9 fl range as the cut-off, five studies (39, 41, 27, 43, 48) used the 9–10 fl range, eight studies (36–38, 40, 42, 44, 50, 51) used the 10–11 fl range, and two studies used the 11–12 fl (51) and >12 fl (35) ranges as the cut-off, respectively. Since only two reports had a cut-off value of 11–12 and >12 fl, the SROC analysis was performed for studies with a cut-off range of 8–9, 9–10, or 10–11 fl.
In the cut-off range of 8–9 fl for MPV, the pooled SEN, SPE, AUC, and Q* indices for the diagnosis of PE were 0.734 [95% CI (0.700–0.766)], 0.663 [95% CI (0.647–0.680)], 0.7363, and 0.6822, respectively. The pooled SEN, SPE, AUC, and Q* indices for the 9–10 fl range were 0.653 [95% CI (0.623–0.683)], 0.718 [95% CI (0.710–0.726)], 0.8856, and 08162. In 10–11 fl, the pooled SEN was 0.644 [95% CI (0.610–0.677)], SPE was 0.706 [95% CI (0.681–0.729)], AUC was 0.7623, and Q* index was 0.7037.
3.5. Subgroup analysis
To examine the heterogeneity of the 22 studies identified, six subgroups were distinguished. The diagnostic accuracy was similar between study areas and sample sizes, with significant differences between gestational week at sampling, cut-off, reference standard, and study design, as detailed in Table 2. (i) In the group of gestational weeks at sampling, the diagnostic accuracy of MPV for PE after 20 weeks of gestation [DOR = 8.661, 95% CI (4.156–18.048)] was higher than the predictive value before 20 weeks of gestation [DOR = 5.165, 95% CI (2.582–10.332)] (Supplementary Figures S3, S4). (ii) Compared with the seven studies employing MPV <9 fl as the cut-off [DOR = 4.021, 95% CI (2.034–7.951), AUC (SE) = 0.7363 (0.0543)], the 15 studies with MPV cut-off ≥9 fl had a DOR = 9.129, 95% CI (4.614–18.059) along with an AUC (SE) = 0.8421 (0.0294), showing a better overall accuracy, suggesting that the capacity to distinguish between pregnant women with PE is stronger when the cut-off is ≥9 fl than when the MPV < 9 fl. (iii) When a different reference standard was used to diagnose PE in pregnant women, the pooled diagnostic precision was lower in 12 studies using ACOG [DOR = 4.843, 95% CI (2.645–8.867)] than in five studies using the clinical criteria [DOR = 20.367, 95% CI (9.970–41.608)]. (iv) The diagnostic value of prospective studies had a higher AUC (SE) = 0.8053 (0.0589) than that of retrospective studies AUC (SE) = 0.6889 (0.0228) at the time of differentiating between study designs.
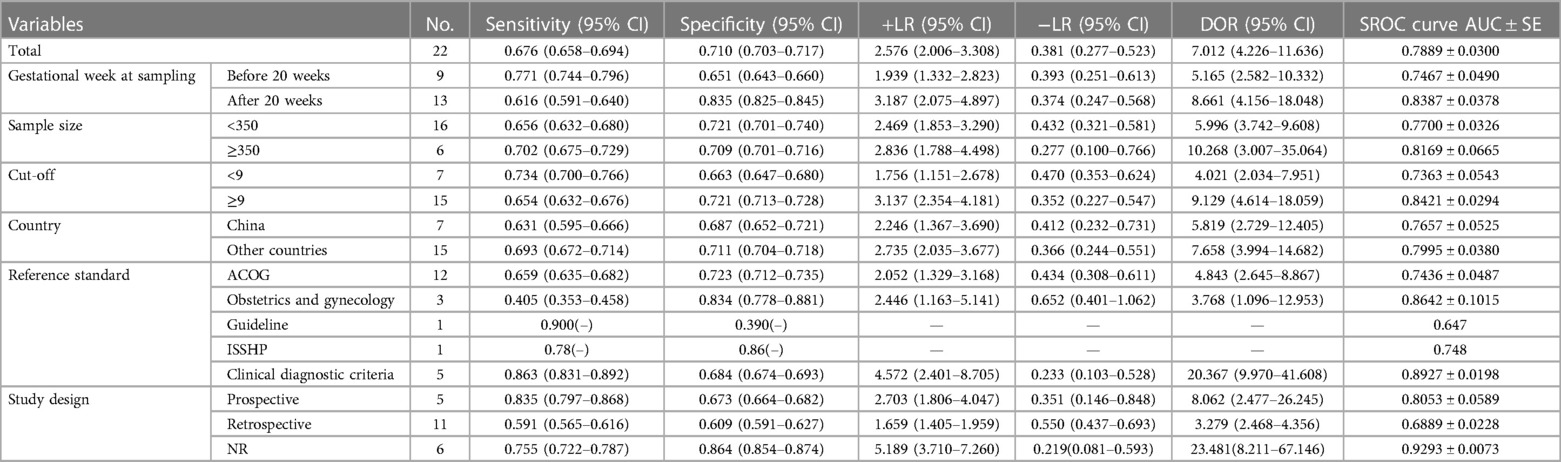
Table 2. Summary estimates of sensitivity, specificity, +LR, −LR, DOR, and SROC curve (AUC) of MPV for the identification of PE in different subgroups.
3.6. Publication bias
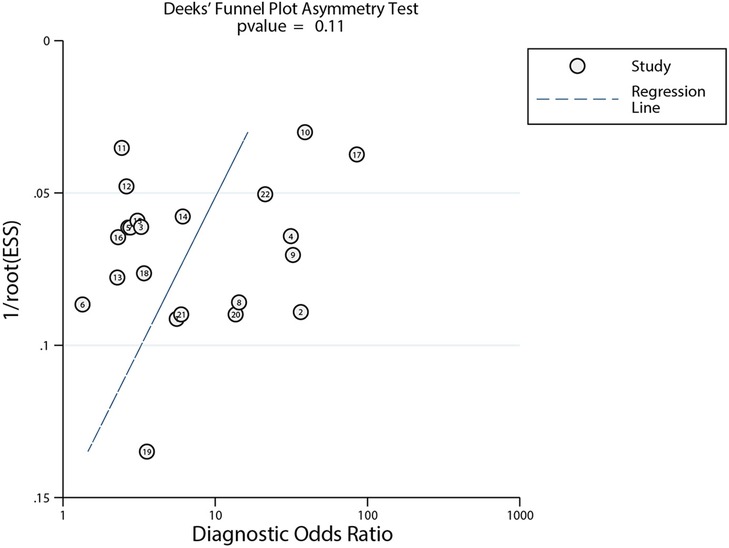
After plotting Deek's funnel plot to test for publication bias, the results showed that the left–right distribution of study points was generally symmetrical, with P = 0.11, indicating that the likelihood of publication bias in our review was low (Figure 4).
4. Discussion
PE remains one of the primary contributors to maternal mortality globally, particularly in underdeveloped regions and countries. The only option to address the disease is to terminate the pregnancy. A substantial amount of evidence shows that taking aspirin before 16 weeks of pregnancy in women with high-risk factors can prevent PE (23). An early predictive diagnosis of PE has not yet become widespread, although guidelines suggest that clinical risk factors, blood pressure, uterine artery pulsatility index, and PlGF can be used as risk markers for screening for PE (2). A meta-analysis suggests that the sFlt-1/PlGF ratio can be used to predict the development of future PE or exclude PE in high-risk pregnancies (53). In fact, there are no reliable predictors or co-predictors of the development of PE in early or mid-pregnancy. In addition, testing methods can be financially prohibitive in less developed areas, making it difficult to detect and focus on the disease until severe clinical symptoms and multi-organ dysfunction are present, hindering the clinicians from preventing the disease early and providing timely medical intervention. Using convenient and cost-effective methods for predicting and diagnosing PE early remains a clinical challenge. To forecast PE and enable serial prevention and earlier treatment, more attention should be paid to readily available risk markers. Clinical reports of the diagnostic accuracy of MPV for PE cut-off vary, and no systematic reviews evaluated the accuracy of MPV in predicting and diagnosing PE, which is why we undertook this study.
One possible pathogenesis of PE is the placental release of pro-inflammatory mediators through the activation of immune and coagulation mechanisms, leading to endothelial dysfunction and increased platelet activation (4, 54). MPV is an indicator of platelet function activation and a routine parameter of CBC, which is easy and inexpensive to measure. It has been suggested that MPV rises progressively during pregnancy, with higher MPV values in PE than in normotension. MPV will increase as the severity of the disease increases (52, 55, 56). However, the diagnostic utility of MPV has been inconclusive. Our study is the first systematic review to explore the diagnostic value of MPV in PE. Based on a large number of original studies, including a sample of 2,637 patients with PE, the results of the meta-analysis revealed an acceptable SEN and moderate SPE, while the AUC and Q* indices were 0.7889 and 0.7262, which indicated that MPV has a certain diagnostic value for PE.
Based on recommendations on using aspirin to prevent PE, predicting PE before 16 weeks of gestation or even earlier and screening pregnancies at high risk of PE should be focused. A large sample of studies hinted that MPV has the potential to predict PE before the onset of the disease (57). Our review analyzed the predictive value of MPV for PE before 16 weeks of gestation, showing that MPV has moderate predictive power for PE in early pregnancy.
As different cut-off ranges were used to diagnose PE using MPV in our selected studies, this study analyzed the cut-off in various sections, proposing to ascertain the cut-off with the greatest diagnostic value. Results showed that SEN, SPE, AUC, and Q* indices for PE diagnosis were higher when the cut-off of MPV was in the range of 9–10 fl compared with other intervals. Three of the five studies in that range were around 9.5 fl. We infer that an optimal cut-off for the diagnostic accuracy of MPV for PE is 9.5 fl.
For the quality of the study, a large portion of the included literature studies used a case–control design, so selection bias cannot be eliminated. In addition, as the use of blinding is not adequately reported, the potential bias in the index test should be evaluated.
Heterogeneity is a potential problem for almost every meta-analysis, as its presence may partially reduce the stability of the study. The Spearman correlation analysis implied that heterogeneity in our review was attributable to non-threshold effects, so we conducted subgroup analyses of all 22 studies to explore the sources of heterogeneity. According to the original inclusion and exclusion criteria, no particular population was excluded because of limitations in the number of studies. Based on the diagnostic criteria for PE, we divided the groups using a threshold of 20 weeks of gestation. The subgroup analysis showed a superior diagnostic value of MPV for PE when sampled at >20 weeks of gestation, MPV ≥ 9 fl, using the clinical criteria as the reference standard and prospective design. Since the original study did not report information on ethnicity, race, education, economic status, treatment or not before enrollment, and other factors that might influence the diagnostic value of MPV for PE, these variables were not analyzed. In future studies, considering the impact of these risk factors on the diagnostic value of MPV may define the most appropriate diagnostic range for MPV. There was no publication bias among the enrolled studies.
Based on clinical features, PE can be classified into non-severe and severe. A prospective cohort study testing MPV at 24 weeks of gestation revealed an excellent predictive accuracy for mild PE, with outcomes suggesting a SEN of 0.78, SPE of 1.0, and AUC of 0.936 when MPV > 9.7 fl (58). As for the diagnostic value of MPV for severe PE, the study by Freitas et al. (59) in late pregnancy indicated a SEN and SPE of 0.5172 and 0.8276, respectively, with an AUC of 0.72, which did not accurately screen for severe PE. When the MPV was used to differentiate the severity of PE, it had a SEN of 0.875 and a SPE of 0.853, implying that it could distinguish disease severity better (60). However, the ability of MPV to differentiate the severity of PE could not be analyzed using the systematic review approach because of the limited number of studies. Furthermore, the limited number of studies dividing PE into early-onset and late-onset makes it impossible to obtain data on the diagnostic value of MPV in these two clinical subtypes.
Currently, CBC has been emphasized in studies for the forecasting and detection of PE because of its simplicity; however, its availability still requires exploration. A systematic review by Walle et al. (16) showed that platelet count (PC) was dramatically decreased in PE. A recent comparative cross-sectional study (61) showed that PC has a strong diagnostic value for pregnancy-induced hypertension (PIH), with a SEN of 96.7%, SPE of 90%, and AUC of 0.995. Meanwhile, its AUC for distinguishing between severe and mild PIH was 0.947, which implies that PC is valuable for predicting the development of PIH and determining its severity. As a helpful marker of systemic inflammatory response, red cell distribution width (RDW) had markedly higher levels in PE and was notably higher in SPE than MPE (62). A prospective case–control study by Sachan et al. (63) revealed that the RDW had a SEN of 85.3%, SPE of 49.0%, and AUC of 0.751 in differentiating healthy normotensive pregnant women from non-severe PE. The neutrophil-to-lymphocyte ratio (NLR) is an inflammation-related indicator that can be calculated from the CBC. A meta-analysis reported that NLR is elevated in PE, especially in SPE, with an AUC of 0.82 (64, 65). Similar to NLR, the platelet-to-lymphocyte ratio (PLR) is also potentially predictive of PE, with a meta-analysis showing that PLR declines with PE severity and has an AUC of 0.7296 for the diagnosis (66). The above laboratory indices, together with MPV, are derived from CBC, and all indices have acceptable individual diagnostic values for PE, However, clinical studies on the predictive ability of PE are scarce. More high-quality studies are needed for validation.
The limitations of our study include the following: (i) Most of the included studies were diagnosed with confirmed cases and hospital populations, which may produce selective bias for specific regions, ethnicities, languages, or populations. (ii) There was a large heterogeneity between studies; however, our study only analyzed six aspects in subgroups, while differences in ethnicity, race, severity of disease, treatment or not before enrollment, type of instrumentation, and source of reagents may also have contributed to the heterogeneity, but an in-depth analysis was not conducted because of the limitations of the number of studies. (iii) The categories of PE were not differentiated for assessing the diagnostic value.
Our findings support that MPV is a promising biomarker with a good predictive diagnostic value for PE, especially after 20 weeks of gestation. Thus, in the absence of specific laboratory tests, MPV may be used as a predictive diagnostic tool for PE and as an adjunct to the management of PE by administering aspirin to prevent the onset of PE, pumped magnesium sulfate to prevent convulsions, anti-hypertensive medication to decrease blood pressure, and timely intervention for premature delivery. However, to fully elucidate its application in clinical practice and accurately evaluate the role of MPV in early pregnancy, larger prospective, multicenter, cohort studies are needed. Moreover, the onset and severity of PE should be distinguished, and the diagnostic accuracy in the different types of PE should be evaluated. Finally, MPV should be jointly estimated with other markers of PE, particularly those that can be extracted in CBC such as PC and NLR, and a combined predictive diagnostic model should be established to provide the most efficient predictive diagnosis of the disease.
5. Conclusion
The results of this meta-analysis suggest that MPV has an acceptable value as a promising, convenient, and affordable marker for the prediction and diagnosis of PE. For MPV, the optimal sampling gestational weeks and a more precise cut-off for its diagnosis of PE should be determined and investigated in depth. In addition, combining MPV parameters with other platelet parameters and measurable factors in CBC may be more important than using a single indicator alone to diagnose PE. Therefore, it is essential to explore the SEN and SPE of MPV in combination with other indicators. In the future, multicenter prospective studies are also needed to assess the role of MPV in different subtypes of PE.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
DY and RH planned and wrote the research protocol of the study. DY, SL, YD, and ZM screened the studies, extracted data from included studies, analyzed the data, and performed the quality assessment of studies. DY drafted the manuscript. All authors contributed to the discussion section of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Lanzhou University Education Development Fund (No. 071100146) and Long Yuan Youth Innovation and Entrepreneurship Talent Individual Project (No. 2022LQGR47).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1251304/full#supplementary-material
References
1. WHO. Maternal mortality (2019). Available at: https://www.who.int/news-room/fact-sheets/detail/maternal-mortality (Accessed June 28, 2020).
2. Magee LA, Brown MA, Hall DR, Gupte S, Hennessy A, Karumanchi SA, et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. (2022) 27:148–69. doi: 10.1016/j.preghy.2021.09.008
3. Espinoza J, Vidaeff A, Pettker CM, Simhan H. Gestational hypertension and preeclampsia: ACOG practice bulletin, number 222. Obstet Gynecol. (2020) 135(6):e237–60. doi: 10.1097/AOG.0000000000003891
4. Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. (2021) 398(10297):341–54. doi: 10.1016/S0140-6736(20)32335-7
5. Theilen LH, Meeks H, Fraser A, Esplin MS, Smith KR, Varner MW. Long-term mortality risk and life expectancy following recurrent hypertensive disease of pregnancy. Am J Obstet Gynecol. (2018) 219(1):107.e1–6. doi: 10.1016/j.ajog.2018.04.002
6. Thilaganathan B, Kalafat E. Cardiovascular system in preeclampsia and beyond. Hypertension. (2019) 73(3):522–31. doi: 10.1161/HYPERTENSIONAHA.118.11191
7. Kristensen JH, Basit S, Wohlfahrt J, Damholt MB, Boyd HA. Pre-eclampsia and risk of later kidney disease: nationwide cohort study. Br Med J. (2019) 365:l1516. doi: 10.1136/bmj.l1516
8. Basit S, Wohlfahrt J, Boyd HA. Pregnancy loss and risk of later dementia: a nationwide cohort study, Denmark, 1977–2017. Alzheimers Dement (N Y). (2019) 5:146–53. doi: 10.1016/j.trci.2019.02.006
9. Kong L, Chen X, Liang Y, Forsell Y, Gissler M, Lavebratt C. Association of preeclampsia and perinatal complications with offspring neurodevelopmental and psychiatric disorders. JAMA Netw Open. (2022) 5(1):e2145719. doi: 10.1001/jamanetworkopen.2021.45719
10. Hedermann G, Hedley PL, Thagaard IN, Krebs L, Ekelund CK, Sørensen TIA, et al. Maternal obesity and metabolic disorders associate with congenital heart defects in the offspring: a systematic review. PLoS One. (2021) 16(5):e0252343. doi: 10.1371/journal.pone.0252343
11. Xu Q, Dai L, Chen HQ, Xia W, Wang QL, Zhu CR, et al. Specific changes and clinical significance of plasma D-dimer during pregnancy and puerperium: a prospective study. BMC Pregnancy Childbirth. (2023) 23(1):248. doi: 10.1186/s12884-023-05561-1
12. Bhutani N, Jethani V, Jethani S, Ratwani K. Coagulation profile and platelet parameters in pregnancy induced hypertension cases and normotensive pregnancies: a cross-sectional study. Ann Med Surg. (2022) 80:104124. doi: 10.1016/j.amsu.2022.104124
13. Moraes D, Milioni C, Vieira CF, Parera EA, Silva BD, Baron MV, et al. Immature platelet fraction and thrombin generation: preeclampsia biomarkers. Fração de plaquetas imaturas e geração de trombina: biomarcadores da pré-eclâmpsia. Rev Bras Ginecol Obstet. (2022) 44(8):771–5. doi: 10.1055/s-0042-1743100
14. Bellos I, Fitrou G, Pergialiotis V, Papantoniou N, Daskalakis G. Mean platelet volume values in preeclampsia: a systematic review and meta-analysis. Pregnancy Hypertens. (2018) 13:174–80. doi: 10.1016/j.preghy.2018.06.016
15. Jakobsen C, Larsen JB, Fuglsang J, Hvas AM. Platelet function in preeclampsia—a systematic review and meta-analysis. Platelets. (2019) 30(5):549–62. doi: 10.1080/09537104.2019.1595561
16. Walle M, Gelaw Y, Getu F, Asrie F, Getaneh Z. Preeclampsia has an association with both platelet count and mean platelet volume: a systematic review and meta-analysis. PLoS One. (2022) 17(9):e0274398. doi: 10.1371/journal.pone.0274398
17. Machlus KR, Thon JN, Italiano JE Jr. Interpreting the developmental dance of the megakaryocyte: a review of the cellular and molecular processes mediating platelet formation. Br J Haematol. (2014) 165(2):227–36. doi: 10.1111/bjh.12758
18. Amsalem I, Asher E, Blaufeld I, Hitter R, Levi N, Taha L, et al. Mean platelet volume as a predictor of coronary artery disease severity and its association with coronary artery calcification. Clin Appl Thromb Hemost. (2023) 29:10760296231159113. doi: 10.1177/10760296231159113
19. Li J, Zhang J, Chen Y, Gao L, Yan X, Zhang M, et al. Mean platelet volume modifies the contribution of homocysteine to cardiovascular disease: a real-world study. Nutr Metab Cardiovasc Dis. (2023) 33(1):194–202. doi: 10.1016/j.numecd.2022.10.013
20. Vélez-Páez JL, Legua P, Vélez-Páez P, Irigoyen E, Andrade H, Jara A, et al. Mean platelet volume and mean platelet volume to platelet count ratio as predictors of severity and mortality in sepsis. PLoS One. (2022) 17(1):e0262356. doi: 10.1371/journal.pone.0262356
21. Colak E, Ozcimen EE, Ceran MU, Tohma YA, Kulaksızoglu S. Role of mean platelet volume in pregnancy to predict gestational diabetes mellitus in the first trimester. J Matern Fetal Neonatal Med. (2020) 33(21):3689–94. doi: 10.1080/14767058.2019.1583730
22. Yayla Abide Ç, Vural F, Kılıççı Ç, Bostancı Ergen E, Yenidede İ, Eser A, et al. Can we predict severity of intrahepatic cholestasis of pregnancy using inflammatory markers? Turk J Obstet Gynecol. (2017) 14(3):160–5. doi: 10.4274/tjod.67674
23. Lailler G, Grave C, Gabet A, Regnault N, Deneux-Tharaux C, Kretz S, et al. Aspirin for the prevention of early and severe pre-eclampsia recurrence: a real-world population-based study. Drugs. (2023) 83(5):429–37. doi: 10.1007/s40265-023-01842-3
24. Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. (2018) 218(3):287–93.e1. doi: 10.1016/j.ajog.2017.11.561
25. Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. (2017) 216(2):110–20.e6. doi: 10.1016/j.ajog.2016.09.076
26. Horgan R, Hage Diab Y, Waller J, Abuhamad A, Saade G. Low-dose aspirin therapy for the prevention of preeclampsia: time to reconsider our recommendations? Am J Obstet Gynecol. (2023) 229(4):410–8. doi: 10.1016/j.ajog.2023.04.031
27. Kim MA, Han GH, Kwon JY, Kim YH. Clinical significance of platelet-to-lymphocyte ratio in women with preeclampsia. Am J Reprod Immunol. (2018) 80(1):e12973. doi: 10.1111/aji.12973
28. Örgül G, Aydın Haklı D, Özten G, Fadiloğlu E, Tanacan A, Beksaç MS. First trimester complete blood cell indices in early and late onset preeclampsia. Turk J Obstet Gynecol. (2019) 16(2):112–7. doi: 10.4274/tjod.galenos.2019.93708
29. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. (2021) 10(1):89. doi: 10.1186/s13643-021-01626-4
30. Hypertensive Disorders in Pregnancy Subgroup, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association. [Diagnosis and treatment of hypertension and pre-eclampsia in pregnancy: a clinical practice guideline in China (2020)]. Zhonghua Fu Chan Ke Za Zhi. (2020) 55(4):227–38. doi: 10.3760/cma.j.cn112141-20200114-00039
31. ACOG Committee on Practice Bulletins–Obstetrics. ACOG Practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. (2002) 99(1):159–67. doi: 10.1016/s0029-7844(01)01747-1
32. Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy. (2001) 20(1):IX–XIV. doi: 10.1081/PRG-100104165
34. Jiaolan L, Qiong L, Zhongjun L, Xiao Zhengqin, Shen Guxiu, Rao Yi, et al. Clinical significance of placental growth factor, platelet parameters and uterine artery blood flow parameters in predicting preeclampsia. Curr Med Res Pract. (2022) 7(04):41–4. doi: 10.19347/j.cnki.2096-1413.202204011
35. Walle M, Asrie F, Gelaw Y, Getaneh Z. The role of platelet parameters for the diagnosis of preeclampsia among pregnant women attending at the University of Gondar Comprehensive Specialized Hospital Antenatal Care Unit, Gondar, Ethiopia. J Clin Lab Anal. (2022) 36(4):e24305. doi: 10.1002/jcla.24305
36. Oğlak SC, Tunç Ş, Ölmez F. First trimester mean platelet volume, neutrophil to lymphocyte ratio, and platelet to lymphocyte ratio values are useful markers for predicting preeclampsia. Ochsner J. (2021) 21(4):364–70. doi: 10.31486/toj.21.0026
37. Li Y, Sun L, Zheng X, Liu J, Zheng R, Lv Y. The clinical value of platelet parameters combined with sFlt-1/PlGF in predicting preeclampsia. Ann Palliat Med. (2021) 10(7):7619–26. doi: 10.21037/apm-21-1244
38. Wei Y, Yingying C, Ni L, Kuangyi S, Suzhen L, Fanfan L, et al. Predictive value of platelet parameters and coagulation indexes for preeclampsia. Jiangsu Med J. (2021) 47(01):36–9. doi: 10.19460/j.cnki.0253-3685.2021.01.010
39. Pingping W. Correlation between coagulation index and platelet parameters and progression of preeclampsia in late pregnancy. Laboratory Med Clinic. (2021) 18(15):2232–4. doi: 10.3969/j.issn.1672-9455.2021.15.02
40. Bhamri SS, Singh U, Mehrotra S, Solanki V. Association of mean platelet volume in the late first trimester of pregnancy and development of preeclampsia. J South Asian Fed Obstetrics Gynaecology. (2018) 11(3):172–4. doi: 10.5005/jp-journals-10006-1672
41. Tesfay F, Negash M, Alemu J, Yahya M, Teklu G, Yibrah M, et al. Role of platelet parameters in early detection and prediction of severity of preeclampsia: a comparative cross-sectional study at Ayder Comprehensive Specialized and Mekelle General Hospitals, Mekelle, Tigray, Ethiopia. PLoS One. (2019) 14(11):e0225536. doi: 10.1371/journal.pone.0225536
42. Lu W, Rui L, Xinming H, Songtao Z. Correlation analysis between blood cell parameters and preeclampsia. J Bengbu Med Coll. (2019) 44(07):943–5. doi: 10.13898/j.cnki.Issn.1000-2200.2019.07.027
43. Rezk M, Gaber W, Shaheen A, Nofal A, Emara M, Gamal A, et al. First versus second trimester mean platelet volume and uric acid for prediction of preeclampsia in women at moderate and low risk. Hypertens Pregnancy. (2018) 37(3):111–7. doi: 10.1080/10641955.2018.1483508
44. Chen Y, Lin L. Potential value of coagulation parameters for suggesting preeclampsia during the third trimester of pregnancy. Am J Med Sci. (2017) 354(1):39–43. doi: 10.1016/j.amjms.2017.03.012
45. Mannaerts D, Heyvaert S, De Cordt C, Macken C, Loos C, Jacquemyn Y. Are neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and/or mean platelet volume (MPV) clinically useful as predictive parameters for preeclampsia? J Matern Fetal Neonatal Med. (2019) 32(9):1412–9. doi: 10.1080/14767058.2017.1410701
46. Gezer C, Atalay E, Solmaz U, Ozeren M, Taner CE, Gokhan Tosun G The value of red cell distribution width for predicting subsequent preeclampsia. Cukurova Med J. (2016) 41(2):224–8. doi: 10.17826/cutf.199198
47. Kurtoglu E, Kokcu A, Celik H, Bildircin FD, Tosun M, Alper T, et al. Validity of platelet indices in predicting the risk of developing preeclampsia. Clin Res. (2016) 33(2):57–61. doi: 10.5835/jecm.omu.33.02.002
48. Nooh AM, Abdeldayem HM. Changes in platelet indices during pregnancy as potential markers for prediction of preeclampsia development. Open J Obstet Gynecol. (2015) 5(12):703. doi: 10.4236/ojog.2015.512099
49. Han L, Liu X, Li H, Zou J, Yang Z, Han J, et al. Blood coagulation parameters and platelet indices: changes in normal and preeclamptic pregnancies and predictive values for preeclampsia. PLoS One. (2014) 9(12):e114488. doi: 10.1371/journal.pone.0114488
50. Kanat-Pektas M, Yesildager U, Tuncer N, Arioz DT, Nadirgil-Koken G, Yilmazer M. Could mean platelet volume in late first trimester of pregnancy predict intrauterine growth restriction and pre-eclampsia? J Obstet Gynaecol Res. (2014) 40(7):1840–5. doi: 10.1111/jog.12433
51. Kashanian M, Hajjaran M, Khatami E, Sheikhansari N. Evaluation of the value of the first and third trimester maternal mean platelet volume (MPV) for prediction of pre-eclampsia. Pregnancy Hypertens. (2013) 3(4):222–6. doi: 10.1016/j.preghy.2013.06.001
52. Dundar O, Yoruk P, Tutuncu L, Erikci AA, Muhcu M, Ergur AR, et al. Longitudinal study of platelet size changes in gestation and predictive power of elevated MPV in development of pre-eclampsia. Prenat Diagn. (2008) 28(11):1052–6. doi: 10.1002/pd.2126
53. Yamazaki T, Cerdeira AS, Agrawal S, Koh I, Sugimoto J, Vatish M, et al. Predictive accuracy of soluble FMS-like tyrosine kinase-1/placental growth factor ratio for preeclampsia in Japan: a systematic review. Hypertens Res Pregnancy. (2021) 9(1):1–7. doi: 10.14390/jsshp.HRP2020-012
54. Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. (2010) 376(9741):631–44. doi: 10.1016/S0140-6736(10)60279-6
55. Mayer-Pickel K, Stern C, Eberhard K, Lang U, Obermayer-Pietsch B, Cervar-Zivkovic M. Comparison of mean platelet volume (MPV) and sFlt-1/PlGF ratio as predictive markers for preeclampsia. J Matern Fetal Neonatal Med. (2021) 34(9):1407–14. doi: 10.1080/14767058.2019.1638356
56. Bhutani N, Jethani V, Jethani S, Ratwani K. Coagulation profile and platelet parameters in pregnancy induced hypertension cases and normotensive pregnancies: a cross-sectional study. Ann Med Surg (Lond). (2022) 80:104124. doi: 10.1016/j.amsu.2022.104124
57. Wu J, Zhang J, Yang J, Zheng TQ, Chen YM. Association between platelet indices and risk of preeclampsia in pregnant women. J Obstet Gynaecol. (2022) 42(7):2764–70. doi: 10.1080/01443615.2022.2109136
58. Elbasuony WA, Hodeib HAem, Eljejawy AE, Shaheen KAef. The role of platelet indices in prediction of pre-eclampsia. J Adv Med Med Res. (2021) 33(2):69–77. doi: 10.9734/JAMMR/2021/v33i230808
59. Freitas LG, Alpoim PN, Komatsuzaki F, Carvalho MdG, Dusse LMS. Preeclampsia: are platelet count and indices useful for its prognostic? Hematology (Amsterdam, Netherlands). (2013) 18(6):360–4. doi: 10.1179/1607845413Y.0000000098
60. Kamel Ammar WAE, Hei MAEHA, Ibrahim Mohamed MAGM. Evaluation of platelet indices and their significance in preeclampsia. Nat Sci. (2014) 12(3):147–53. doi: 10.7537/marsnsj120314.21
61. Gandhi S, Sun D, Park AL, Hladunewich M, Silversides CK, Ray JG. The Pulmonary Edema Preeclampsia Evaluation (PEPE) study. J Obstet Gynaecol Can. (2014) 36(12):1065–70. doi: 10.1016/S1701-2163(15)30383-2
62. Adam I, Mutabingwa TK, Malik EM. Red cell distribution width and preeclampsia: a systematic review and meta-analysis. Clin Hypertens. (2019) 25:15. doi: 10.1186/s40885-019-0119-7
63. Sachan R, Patel ML, Sachan P, Shyam R. Role of platelet count and mean platelet volume and red cell distribution width in the prediction of preeclampsia in early pregnancy. J Family Med Prim Care. (2021) 10(2):838–43. doi: 10.4103/jfmpc.jfmpc_1528_20
64. Kang Q, Li W, Yu N, Fan L, Zhang Y, Sha M, et al. Predictive role of neutrophil-to-lymphocyte ratio in preeclampsia: a meta-analysis including 3982 patients. Pregnancy Hypertens. (2020) 20:111–8. doi: 10.1016/j.preghy.2020.03.009
65. Zheng WF, Zhan J, Chen A, Ma H, Yang H, Maharjan R. Diagnostic value of neutrophil-lymphocyte ratio in preeclampsia: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). (2019) 98(51):e18496. doi: 10.1097/MD.0000000000018496
Keywords: mean platelet volume, prevention, diagnosis, pre-eclampsia, meta-analysis
Citation: Ye D, Li S, Ding Y, Ma Z and He R (2023) Clinical value of mean platelet volume in predicting and diagnosing pre-eclampsia: a systematic review and meta-analysis. Front. Cardiovasc. Med. 10:1251304. doi: 10.3389/fcvm.2023.1251304
Received: 1 July 2023; Accepted: 20 September 2023;
Published: 6 October 2023.
Edited by:
Redhwan Ahmed Al-Naggar, National University of Malaysia, MalaysiaReviewed by:
Ioana Pavaleanu, Grigore T. Popa University of Medicine and Pharmacy, RomaniaRamu Adela, National Institute of Pharmaceutical Education and Research, India
© 2023 Ye, Li, Ding, Ma and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongxia He bmluaTYxNThAMTYzLmNvbQ==
 Dan Ye
Dan Ye Shuwen Li2
Shuwen Li2