- 1Department of Cardio-Thoracic-Vascular Diseases, Foundation IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
- 2Dyspnea Lab, Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy
- 3Cardioncology Unit, European Institute of Oncology, IRCCS, Milan, Italy
- 4Haematology Unit, Foundation IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
Purpose: To describe the efficacy and safety of sodium-glucose cotransporter 2 inhibitors as a specific treatment for anthracycline-related cardiac dysfunction in a small real-world population.
Methods: Seven patients with anthracycline-related cardiac dysfunction were clinically and echocardiographically evaluated before and after the introduction of sodium-glucose cotransporter 2 inhibitors.
Results: After a median period of 24 weeks with uninterrupted sodium-glucose cotransporter 2 inhibitors treatment, a significant clinical improvement was observed with at least one New York Heart Association Functional Class (NHYA FC) improvement in all patients (median NYHA FC: I vs. III, p < 0.010). A noteworthy left ventricular reserve remodeling (median left ventricular end diastolic volume indexed: 53 vs. 82.5 ml/m2, p = 0.018; median left ventricular ejection fraction: 50% vs. 40%, p = 0.17) was also observed. Sodium-glucose cotransporter 2 inhibitors therapy was well tolerated by every patients; no cases of discontinuation or relevant side effects were observed.
Conclusion: Sodium-glucose cotransporter 2 inhibitors induce a significant clinical improvement and left ventricular reserve remodeling in patients affected by anthracycline-related cardiac dysfunction.
Highlights
• Sodium-glucose cotransporter 2 inhibitors induce a significant clinical improvement in patients affected by anthracycline-related cardiac dysfunction.
• Sodium-glucose cotransporter 2 inhibitors induce a significant increase in left ventricle ejection fraction and reduction in indexed left ventricle end-diastolic volumes.
Introduction
Cardiovascular disease and cancer are the main leading causes of death in developed countries. While advancements in oncological treatment have led to improved survival rates, they have also resulted in an increase in cardiac side effects. Anthracyclines are effective and widely used oncological treatments, although they are associated with the risk of severe myocardial damage which can often necessitate the discontinuation of chemotherapy. The European Society of Cardiology (ESC) has recently published the first edition of guidelines on cardio-oncology, defining anthracycline-related cardiac dysfunction (ARCD) as a form of heart failure (HF) characterized by left ventricle (LV) remodeling and mild-to-severe impairment of ejection fraction (1). Currently, no specific treatment is available, and all efforts are directed towards preventing damage and its progression. The ESC Guidelines on cardio-oncology recommend treating ARCD following the indications of the ESC Guidelines on HF. To highlight the potential role of sodium-glucose cotransporter 2 inhibitors (SGLT2-i) in preventing ARCD, our research team recently conducted a meta-analysis showing promising results on mouse models (2). Furthermore, two retrospective analysis of patients with diabetes and cancer provided preliminary evidence of the protective role of SGLT2-i in humans (3, 4). Given the potential cardioprotective effects of SGLT2-i, we investigated their efficacy in treating ARCD, as no evidence on this issue had been published. Here, we present a case series of consecutive patients diagnosed with ARCD who were given SGLT2-i in addition to guideline-directed medical treatment (GDMT).
Case description
We collected seven cases of patients with ARCD and symptomatic HF with reduced ejection fraction (HFrEF). They had all been exposed to a high dose of anthracycline for treating different types of cancer. No other cause of HF was found, and coronary angiography was negative in all cases. All the patients had clinical and echocardiographic assessments in three different time periods: before chemotherapy (T0), after chemotherapy when ARCD was diagnosed (T1), and after SGLT2-i therapy (T2). New York Heart Association functional class (NYHA FC), two-dimension LV ejection fraction (LVEF), LV end-diastolic and volume indexed (LVEDVi), were considered to describe the clinical status and LV remodeling. We compared T0 vs. T1, T1 vs. T2, and T2 vs. T0.
The non-parametric statistical Wilcoxon Signed Rank Test was adopted. The significance level (α) was considered at a two-tailed probability level of significance of 95% (p < 0.05). The Chi2 test through Person's analysis was used to test NYHA functional class changes. The statistical analysis was carried out using SPSS version 28.0.1.0.
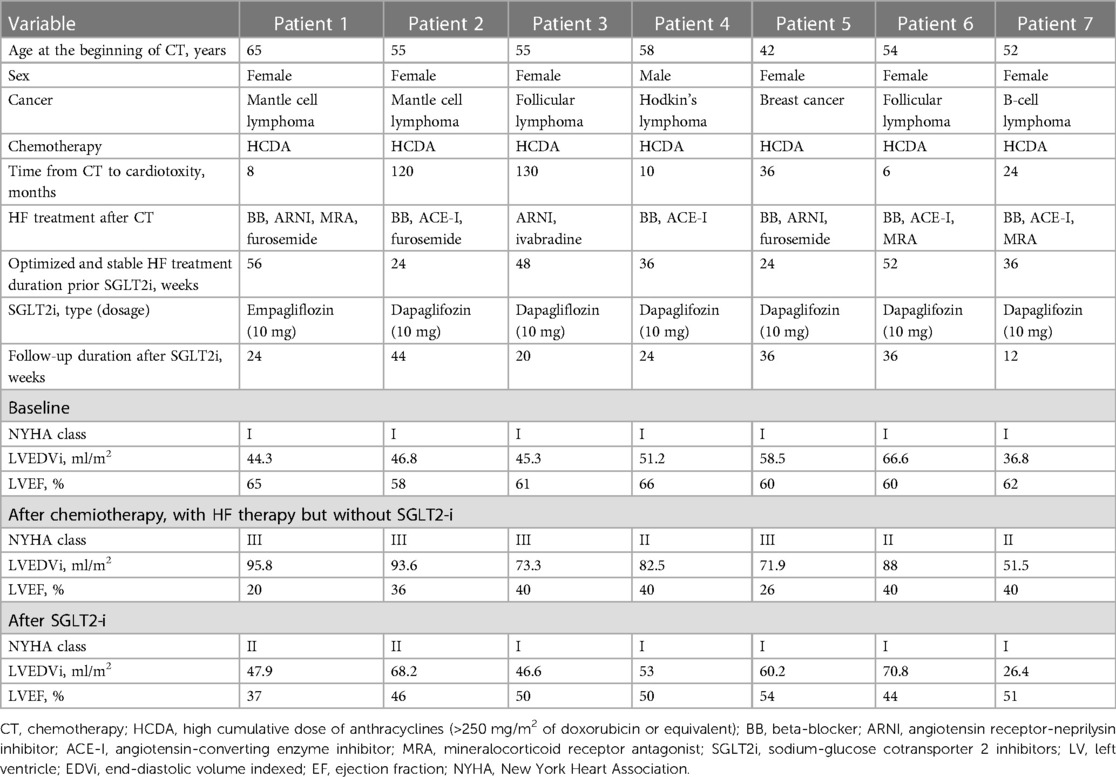
The demographic, clinical, and echocardiographic characteristics are reported in Table 1. Among the seven patients, six were female, and the median age was 55 years. All patients underwent chemotherapy with a high cumulative anthracycline dose (>250 mg/m2 of doxorubicin or equivalent). All the patients had a NYHA FC I before completing the chemotherapy cycles, and none had previous cardiovascular disease or diabetes. All patients had normal echocardiographic morpho-functional parameters before chemotherapy, and none had more than mild valvular disease.
Since ARCD was diagnosed, all parameters showed a significant statistical difference from T0 to T1: patients presented with remarkable LV remodeling (median LVEDVi 46.8 vs. 82.5 ml/m2, p = 0.018), significant reduction in LVEF (median LVEF 61% vs. 40%, p = 0.018), and a relevant degradation of symptoms (median NYHA FC I vs. III, p < 0.001). At T1, all patients had been treated with at least two HF drugs for at least 6 months, the most common combination being a beta-blocker and angiotensin-converting enzyme inhibitor (ACE-I) or angiotensin receptor-neprilysin inhibitor (ARNI). Symptomatic hypotension was the main limitation to the simultaneous administration of GDMT for HFrEF. If indicated, cardiac resynchronization therapy (CRT) was implanted, and SGLT2-i initiation occurred at least 6 months after the implantation.
Of the seven patients, six were given dapagliflozin 10 mg and one empagliflozin 10 mg daily. After a median period of 24 weeks with uninterrupted SGLT2-i treatment, significant clinical improvement was observed at T2 compared to T1 with at least one NYHA FC improvement in all patients (median NYHA FC I vs. III, p < 0.010). We also observed noteworthy LV reserve remodeling (median LVEDVi 53 vs. 82.5 ml/m2, p = 0.018; median LVEF 50% vs. 40%, p = 0.17).
No significant difference in clinical parameters was found when comparing T2 vs. T0, except for LVESV (median value 27.7 vs. 17.7 ml/m2, p = 0.028) (Figure 1).
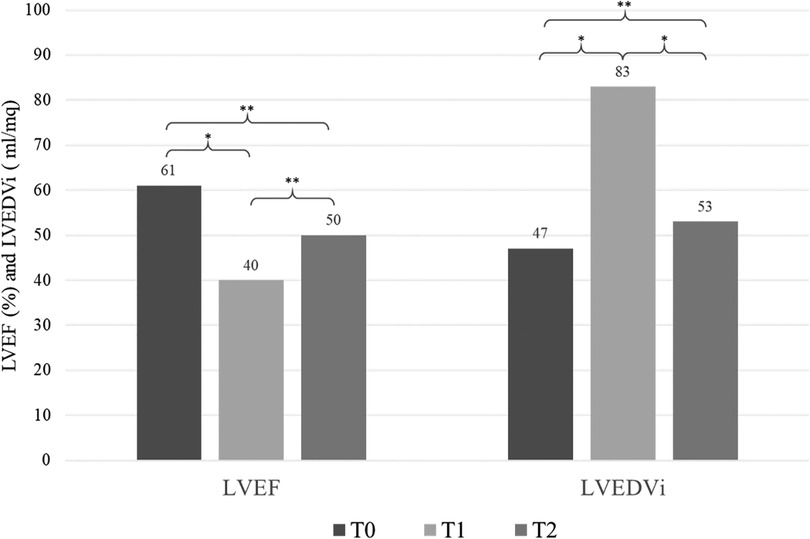
Figure 1. Echocardiographic variations over time (T0, T1, T2). T0 = pre-chemoteraphy, T1 = after chemotherapy when ARCD was diagnosed, T2 = after SGLT2-i therapy. LVEF, left ventricular ejection fraction; LVEDVi, left ventricular end diastolic volume indexed. *, p < 0.05; **, not significant.
SGLT2-i therapy was well tolerated by every patients; no cases of discontinuation or relevant side effects were observed.
We report below the two most significant cases:
Case 1
Patient 1 was diagnosed with follicular lymphoma and treated with a cumulative doxorubicin equivalent dose >250 mg/m2. The echocardiographic follow-up showed normal LV volume and preserved LVEF, and the patient remained asymptomatic for 10 years following the end of chemotherapy. However, the patient showed severe dyspnea and LV systolic dysfunction. Cardiac magnetic resonance (CMR) revealed an initial dilatation of the LV (LVEDVi 106 ml/m2), a significant reduction in LVEF (34%), and a small LGE stria in the interventricular septum. Coronary angiography yielded negative results. Due to persistent symptoms and severe reduction in LVEF under GDMT, a CRT-D was implanted. One year after the implantation, clinical picture was unchanged. At this point, SGLT2-i was added to previous therapy [ARNI, mineralocorticoid receptor antagonist (MRA), and ivabradine]. After 6 months, LVEDVi and LVEF normalized (respectively 46.6 ml/m2 and 50%) and NYHA FC improved to I.
Case 2
Patient 2 was diagnosed with Hodgkin's lymphoma and underwent chemotherapy with a cumulative doxorubicin equivalent dose >250 mg/m2. The echocardiography at baseline showed normal LV volumes and LVEF >60%. However, 1 year after the end of chemotherapy, the LV was mildly dilated (LVEDVi 82 ml/m2), and LVEF had decreased to 45%. The patient was started on treatment with bisoprolol and ramipril. Three months later, a further decrease in LVEF to 40% was observed through echocardiography. Therefore, CMR was performed, which revealed LV dilatation (105 ml/m2), diffuse hypokinesis, and a significant reduction in LVEF (37%). The patient also reported dyspnea during moderate physical activity (NYHA FC II). Coronary angiography was negative. At this time, SGLT2-i was added. At the follow-up 6 months later, the patient was asymptomatic (NYHA class I), and a recovery in LV volumes and contractility was observed (LVEDVi 46 ml/m2 and LVEF 50% at echocardiography).
Discussion
To our knowledge, this is the first case series that demonstrates the benefits of SGLT2-i in ARCD. All patients showed a significant improvement in symptoms and echocardiography assessment after adding SGLT2-i to their HF GDMT. Additionally, we observed that SGLT2-i was well-tolerated and safe, with no cases of discontinuation or recorded side effects.
Despite none of our patients having a diabetes diagnosis, our findings are consistent with a recent retrospective study by Gongora et al. In their study, SGLT2-i were found to be effective in preserving LV function and reducing the incidence of cardiac events among patients with diabetes who were treated with anthracyclines. It is worth noting that no cases of ARCD were observed in the group treated with SGLT2-i (3). Our data suggest that SGLT2-i not only has a preventive role in patients at risk for ARCD but can also induce reverse remodeling. Although we cannot exclude the possibility of synergistic mechanisms with other concomitant drugs, we can speculate that SGLT2-i has pharmacodynamic characteristics that target the pathophysiology of ARCD.
Indeed, our research group recently reviewed basic science data supporting the potential role of SGLT2-i in preventing ARCD. Through a meta-analysis of 8 studies conducted on mouse models we showed that LVEF after chemotherapy with anthracyclines was significantly higher in rats preventively treated with SGLT2i (2). Additionally, SGLT2i have been shown to decrease plasma levels of cardiac troponin, brain natriuretic peptide, tumor necrosis factor-alfa, and fibroblast growing factor-2 in mouse models treated with anthracyclines. Myocardial fibrosis, intracellular radical oxygen species (ROS) formation, and lipid peroxidation were also lower in rats treated with SGLT2-inhibitors and anthracycline than in rats treated with only anthracycline (5, 6). Taken together, this evidence supports the hypothesis that SGLT2i may play a key role in the prevention and management of ARCD.
The pathophysiological mechanisms of ARCD are complex and include oxidative stress, intracellular ROS formation, lipid peroxidation, mitochondrial dysfunction, intracellular calcium dysregulation and microenvironmental cardiac inflammation (7). Therefore, the effect of SGLT2-i on cardiac metabolism, mitochondrial function, intracellular calcium homeostasis and inflammation may account for a specific and targeted cardioprotective effect. Indeed, SGLT2-i induces a shift of cardiac metabolism toward oxidation of fatty acids and consumption of ketone bodies, while reducing glucose utilization. Ketone bodies exert an anti-inflammatory effect and their use have been shown to improve cardiac function and remodeling in a canine model of cardiomyopathy and in nondiabetic subjects with HF. This substrate shift is also associated with an increase in myocardial adenosine triphosphate (ATP) content (8). Moreover, SGLT2-i are supposed to improve mitochondrial function and autophagy, thus reducing ROS formation. Gliflozins also directly inhibits Na+/Hydrogen exchanger 1, consequently reducing cytoplasmatic sodium and calcium concentrations, and increasing mitochondrial calcium concentration. This effect on calcium homeostasis is likely to improve myocardial contractility and reduce oxidative stress. Finally, SGLT2-i reduce inflammation, which plays a crucial role in the pathogenesis of ARCD. This effect is mediated by an increased activation of 5' adenosine monophosphate-activated protein kinase and a reduction in pro-inflammatory cytokines (9).
Patient perspective and conclusions
Currently, evidence regarding the management of ARCD is scarce, and no trial on the use of SGLT2-i in this specific context has been published. Indeed, in previous large studies demonstrating the benefit of SGLT2-i, patients with active cancer were systematically excluded. Some promising data are expected from an ongoing randomized clinical trial (EMPACT trial, NCT05271162), which is investigating the efficacy of SGLT2-i in preventing ARCD.
At present, the ESC guidelines on cardio-oncology recommend treating patients with symptomatic ARCD and asymptomatic moderate or severe ARCD as indicated by HF guidelines. Therefore, the concomitant use and up-titration at the maximal tolerated dose of a beta-blocker, an ACE-I/angiotensin II receptor blocker (ARB) or ARNI, a MRA and a SGLT2-I is suggested (1). However, in clinical practice, it may be challenging to manage the treatment with all recommended HF drugs in patients with ARCD because of clinical frailty. Indeed, as shown in this case series, only one patient of seven was treated with four pharmacological classes (patient 1 in Table 1), and no specific indications are available on how to start and up-titrate treatments. The early diagnosis and initiation of targeted treatment are crucial to obtain a recovery of LV function in HF as well as in ARCD and beta-blockers and ACE-Is have been effective in improving prognosis (10). Our observation suggests that SGLT2-i should be considered as front-line treatment in ARCD together with the rest of the validated strategies. SGLT2-i showed a crucial role to induce reverse remodeling and restore cardiac function in ARCD. Despite obtaining a before-after evaluation someway allows each patient to serve as their own control, the main limitation of our study is precisely the lack of a control group. Prospective study and controlled clinical trials are needed to address properly this issue and obtain definitive results.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study received the proper ethical oversight; Protocol n.0043050 (15/09/2022). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Conception and design or analyses and interpretation of data, or both: all the authors; drafting of the manuscript or revising it critically for important intellectual content: all the authors; All authors contributed to the article and approved the submitted version.
Funding
This work was supported partially by Italian Ministry of Health, Ricerca Corrente Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European hematology association (EHA), the European society for therapeutic radiology and oncology (ESTRO) and the international cardio-oncology society (IC-OS). Eur Heart J. (2022) 43(41):4229–361. doi: 10.1093/eurheartj/ehac244
2. Faggiano A, Gherbesi E, Cardinale D, Vicenzi M, Carugo S. SGLT2-i prevent left ventricular dysfunction induced by anthracycline in mouse model: a systematic-review and meta-analysis. Vascul Pharmacol. (2023) 13:107171. doi: 10.1016/j.vph.2023.107171
3. Gongora CA, Drobni ZD, Quinaglia Araujo Costa Silva T, Zafar A, Gong J, Zlotoff DA, et al. Sodium-glucose co-transporter-2 inhibitors and cardiac outcomes among patients treated with anthracyclines. JACC Heart Fail. (2022) 10(8):559–67. doi: 10.1016/j.jchf.2022.03.006
4. Chiang CH, Chiang CH, Chiang CH, Ma KS, Peng CY, Hsia YP, et al. Impact of sodium-glucose cotransporter-2 inhibitors on heart failure and mortality in patients with cancer. Heart. (2023) 109:470–7. doi: 10.1136/heartjnl-2022-321545
5. Belen E, Canbolat IP, Yigittürk G, Cetinarslan Ö, Akdeniz CS, Karaca M, et al. Cardio-protective effect of dapagliflozin against doxorubicin induced cardiomyopathy in rats. Eur Rev Med Pharmacol Sci. (2022) 26(12):4403–8. doi: 10.26355/eurrev_202206_29079
6. Quagliariello V, De Laurentiis M, Rea D, Barbieri A, Monti MG, Carbone A, et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc Diabetol. (2021) 20(1):150. doi: 10.1186/s12933-021-01346-y
7. Rawat PS, Jaiswal A, Khurana A, Bhatti JS, Navik U. Doxorubicin-induced cardiotoxicity: an update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed Pharmacother. (2021) 139:111708. doi: 10.1016/j.biopha.2021.111708
8. Honka H, Solis-Herrera C, Triplitt C, Norton L, Butler J, DeFronzo RA. Therapeutic manipulation of myocardial metabolism: JACC state-of-the-art review. J Am Coll Cardiol. (2021) 77(16):2022–39. doi: 10.1016/j.jacc.2021.02.057
9. Zelniker TA, Braunwald E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 75(4):422–34. doi: 10.1016/j.jacc.2019.11.031
Keywords: sodium-glucose cotransporter 2 inhibitors, anthracycline-related cardiac dysfunction, left ventricular remodeling, cardiotoxicity, heart faillure
Citation: Giangiacomi F, Faggiano A, Cardinale D, Rossi FG, Pollina A, Gherbesi E, Gnan E, Carugo S and Vicenzi M (2023) Case report: Sodium-glucose cotransporter 2 inhibitors induce left ventricular reverse remodeling in anthracycline-related cardiac dysfunction—a case series. Front. Cardiovasc. Med. 10:1250185. doi: 10.3389/fcvm.2023.1250185
Received: 29 June 2023; Accepted: 8 August 2023;
Published: 22 August 2023.
Edited by:
Reto Asmis, Wake Forest University, United StatesReviewed by:
Diana Mihalcea, Carol Davila University of Medicine and Pharmacy, RomaniaHarald Becher, University of Alberta Hospital, Canada
© 2023 Giangiacomi, Faggiano, Cardinale, Rossi, Pollina, Gherbesi, Gnan, Carugo and Vicenzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Faggiano YW5kcmVhZmFnZ2lhbm85NUBnbWFpbC5jb20= Stefano Carugo c3RlZmFuby5jYXJ1Z29AdW5pbWkuaXQ=
Abbreviations ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARCD, anthracycline-related cardiac dysfunction; ARNI, angiotensin receptor-neprilysin inhibitor; ATP, adenosine triphosphate; CMR, cardiac magnetic resonance; CPET, cardiopulmonary exercise test; CRT, cardiac resynchronization therapy; ESC, European Society of Cardiology; GDMT, guideline-directed medical treatment; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; LV, left ventricle; LVEDVi, left ventricle end-diastolic volume indexed; LVEF, left ventricle end-diastolic and end-systolic volume indexed ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA FC, New York Heart Association functional class; ROS, radical oxygen species; SGLT2-I, sodium-glucose cotransporter 2 inhibitors.
 Francesco Giangiacomi1,2
Francesco Giangiacomi1,2 Andrea Faggiano
Andrea Faggiano Daniela Cardinale
Daniela Cardinale Elisa Gherbesi
Elisa Gherbesi Eleonora Gnan
Eleonora Gnan Stefano Carugo
Stefano Carugo Marco Vicenzi
Marco Vicenzi