
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 21 August 2023
Sec. Cardiovascular Epidemiology and Prevention
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1249401
This article is part of the Research TopicNutrition in the Prevention and Treatment of Cardiovascular DiseasesView all 11 articles
Background: In the United States, the relationship between visceral obesity and the risk of developing atherosclerosis cardiovascular disease (ASCVD) for the first time in 10 years is unclear.
Methods: Data for this cross-sectional study came from the National Health and Nutrition Examination Survey (NHANES) from 2011 to 2020. We collected variable information related to 10-year ASCVD risk and visceral obesity reliable indicators [Visceral obesity index (VAI) and Lipid accumulation product (LAP)]. And we used multiple logistic regression to analyze the correlation of visceral obesity indicators (VAI and LAP) with 10-year ASCVD risk. In addition, we assessed the linear relationship between VAI or LAP and 10-year ASCVD risk by smoothing curve fitting. Finally, we conducted subgroup analysis and sensitivity analysis after excluding participants with extreme VAI and LAP values to ensure that we obtained accurate and reliable results.
Results: Our study included a total of 1,547 participants (mean age: 56.5 ± 10.1, 60% of males). The results of the multiple logistic regression showed that compared with participants with the lowest VAI in the 1st Quartile (≤0.79), the adjusted OR values for VAI and elevated 10-year ASCVD risk in Q3 (1.30–2.14), and Q4 (≥2.15) were 2.58 (95% CI: 1.24–5.36, P = 0.011), 15.14 (95% CI: 6.93–33.05, P < 0.001), respectively. Compared with participants with the lowest LAP in the 1st Quartile (≤28.29), the adjusted OR values for VAI and elevated 10-year ASCVD risk in Q3 (46.52–77.00), and Q4 (≥77.01) were 4.63 (95% CI: 2.18–9.82, P < 0.001), 16.94 (95% CI: 6.74–42.57, P < 0.001), respectively. Stratified analysis showed that the association between VAI or LAP and the first ASCVD event was more pronounced in males.
Conclusion: Higher VAI or LAP scores are significantly associated with elevated 10-year ASCVD risk in adults aged 40 to 79 in the USA, which suggested that monitoring visceral obesity is crucial to reduce the risk of a first ASCVD event.
Despite encouraging achievements in the prevention, diagnosis, and treatment of ASCVD in recent years, ASCVD remains a leading cause of disability and premature death worldwide (1, 2). Due to the fact that most patients with early-onset ASCVD have modifiable risk factors before onset (3), exploring risk-related indicators for ASCVD and conducting early intervention is of paramount importance in reducing ASCVD mortality and alleviating the healthcare burden.
Obesity is a key risk factor for ASCVD (4–6), and the dramatic increase in obesity prevalence in recent years has undermined the gains made in controlling ASCVD risk factors and advancing medical technology (7). Body Mass Index (BMI) is a widely recognized standard for measuring obesity (8, 9). However, BMI can not distinguish between lean fat and whole fat, nor does it reflect the distribution of abdominal fat and body fat, and therefore it has some limitations in estimating the risk of ASCVD (10–12). Epidemiological findings suggested that visceral fat measured by imaging techniques such as CT or MRI is an independent risk factor for cardiovascular metabolic diseases and death (13). And there is evidence that ectopic fat deposition may be related to atherosclerosis and the increased risk of cardiometabolic (14). However, using techniques such as CT or MRI to measure ectopic fat deposition is expensive and limited by the detection instrument (15, 16). Therefore, several simple clinical tools have been developed to assess changes in visceral fat and ectopic fat deposition, of which VAI and LAP have been widely accepted and used clinically as two reliable indicators for assessing visceral obesity. VAI is a simple and reliable indicator of visceral adiposity dysfunction that reflects cardiometabolic risk, and is calculated by anthropometric parameters [waist circumference (WC) and BMI] and lipid measurement parameters [triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C)] (17–19). LAP is an index of lipid hyperaccumulation based on WC and TG, and is considered to be a good continuous indicator to describe visceral obesity (20–22).
To our knowledge, few studies have been conducted on the association between visceral obesity and 10-year ASCVD risk, and the relationship between the two remains controversial. Therefore, we analyzed the association of VAI and LAP with 10-year ASCVD risk through a cross-sectional study to provide a scientific basis for clinical application.
The National Health and Nutrition Examination Survey (NHANES) aims to assess and track the health and nutritional status of the non-institutionalized population in the United States through comprehensive health-related studies. A face-to-face interview is conducted at the individual's home to obtain information on demographics and medical history. Data from examinations, which include physiological, laboratory, and anthropometric data, were collected at the Mobile Examination Center (MEC). The NHANES protocol obtained approval from the National Center for Health Statistics ethics review committee and received written informed consent from all participants (23). For this cross-sectional study, we merged the NHANES data from 2011 to 2012, 2013–2014, 2015–2016, and 2017–2020. Participants included in this study had to meet the following criteria: (1) age between 40 and 79 years old, (2) no existing diagnosis of ASCVD, (3) HDL-C between 20 and 100 mg/dl, (4) total cholesterol (TC) between 130 and 320 mg/dl, and (5) systolic blood pressure (SBP) between 90 and 200 mmHg.
The VAI and LAP was used as exposure variable and was calculated using gender-specific equations, as detailed below. VAI: male [WC/39.68 + (1.88 × BMI)] × (TG/1.03) × (1.31/HDL-C); female [WC/36.58 + 1.89 × (BMI)] × (TG/0.81) × (1.52/HDL-C) (24). LAP: male [WC – 65] × TG; female [WC – 58] × TG (25). TG (mmol/L) was measured using the Wahlefeld method and HDL-C (mmol/L) was measured using the magnesium sulfate/glucan method. The calculation method for BMI is to divide weight (kilograms, kg) by height (meters, m) squared (kg/m2). WC (cm) was measured with an accuracy of millimeters using electronic sports measurements.
The Pooled Cohort Equations (PCE) were implemented in 2013 by the American College of Cardiology/American Heart Association (ACC/AHA) as a tool for estimating the likelihood of developing ASCVD over ten years. This risk prediction model specifically caters to individuals aged 40–79 who are non-Hispanic white. This risk assessment equation includes characteristics such as age, gender, race, SBP, diastolic blood pressure (DBP), TC, HDL-C, low-density lipoprotein cholesterol (LDL-C), smoking status, hypertension treatment, statin use, and aspirin therapy. The 10-year risk of a first hard ASCVD event can be estimated by https://tools.acc.org/ASCVD-Risk-Estimator-Plus/#!/calculate/estimate/. Participants who scored ≥7.5% were classified as having an elevated 10-year ASCVD risk, whereas those who scored <7.5% were identified as low-risk individuals (26).
Continuous variables with normal distribution were expressed as mean ± standard deviation (SD), while those with skewed distribution were expressed as the median [interquartile range, (IQR)]. Categorical variables were presented as frequencies (%). The baseline characteristics of different 10-year ASCVD risk groups were compared using One-Way ANOVA when the data were normally distributed, Kruskal-Wallis H when the distribution was skewed, and the chi-square test for categorical variables analysis. We used logistic regression to investigate the association between VAI and LAP with 10-year ASCVD risk (odds ratios [OR] and 95% confidence interval [CI]). Both non-adjusted and multivariate adjusted models were utilized in this study. Model 1 included adjustments for age, gender, and race. Model 2 was adjusted for sociodemographic characteristics such as age, gender, race, education level, marital status, PIR, smoking status, and BMI. Model 3 encompassed full adjustments, including sociodemographic characteristics, blood pressure measurements (SBP and DBP), TC, LDL-C, diabetes, statin use, and aspirin therapy.
Furthermore, we employed a smoothed curve fitting approach to evaluate the linear association between VAI or LAP and 10-year ASCVD risk. To ensure the accuracy of the findings from this study, multivariate logistic regression models were used for subgroup analysis. Possible variations in the relationship between VAI or LAP and 10-year ASCVD risk were examined, including gender, race, diabetes, statin use, and aspirin therapy. The interaction between subgroups was assessed using the likelihood ratio test. Moreover, participants with extreme VAI and LAP outside the mean ± 3 SD were excluded, for sensitivity analyses. All statistical analyses were conducted utilizing R version 3.3.2 (The R Foundation, http://www.R-project.org) and Free Statistics software version 1.7). A two-sided P value <0.05 was regarded as having statistical significance.
This study included 45,462 prospective participants from NHANES (2011–2020), of which 3,468 adults (40–79 years) who met the inclusion criteria completed interviews and were subjected to MEC screening. Participants with missing data for age, gender, race, SBP, DBP, TC, HDL-C, LDL-C, diabetes, smoking status, hypertension treatment, statin, and aspirin therapy were excluded (n = 1,147). After excluding participants with incomplete covariate data (n = 774), a total of 1,547 participants were enrolled in this cross-sectional study. The flowchart of population screening is shown in Figure 1.
The mean participants' age was 56.5 ± 10.1 years, and 928 (60.0%) were men. The mean baseline VAI and LAP were 1.73 ± 1.3 and 58.5 ± 42.3. There were 803 (51.9%) participants with elevated 10-year ASCVD risk. Table 1 presents the baseline characteristics of study participants based on their 10-year ASCVD risk profile. There were obvious differences in age, gender, race, educational level, PIR, smoking status, SBP, DBP, diabetes status, statin use, and aspirin therapy between the two groups (P < 0.05). Marital status, BMI, TC, and LDL-C were comparable between the two groups (P > 0.05).
The univariate analysis demonstrated that age, gender, race, education level, marital status, PIR, smoking status, SBP, DBP, diabetes status, statin use, and aspirin therapy were associated with elevated 10-year ASCVD risk (Supplementary Table S1).
The results of multifactor logistic regression analysis showed that after adjustment in multivariable analyses, VAI and LAP were significantly associated with elevated 10-year ASCVD risk. When VAI was assessed as a continuous variable, the adjusted OR was 3.46 (95% CI: 2.65–4.52) for elevated 10-year ASCVD risk in the full variables adjusted model (model 3). There was a significant positive correlation between VAI and elevated 10-year ASCVD risk after adjusting for all variables, when VAI was analyzed using quartiles. In model 3, compared with participants with the lowest VAI in the 1st Quartile (≤0.79), the adjusted OR values for VAI and elevated 10-year ASCVD risk in Q2 (0.79–1.29), Q3 (1.30–2.14), and Q4 (≥2.15) were 1.50 (95% CI: 0.75–3.00, P = 0.254), 2.58 (95% CI: 1.24–5.36, P = 0.011), 15.14 (95% CI: 6.93–33.05, P < 0.001), respectively (Table 2). When LAP was assessed as a continuous variable, the adjusted OR was 1.04 (95% CI: 1.03–1.05) for elevated 10-year ASCVD risk in model 3. When LAP was analyzed using quartiles, compared with participants with the lowest LAP in the 1st Quartile (≤28.29), the adjusted OR values for VAI and elevated 10-year ASCVD risk in Q2 (28.31–46.44), Q3 (46.52–77.00), and Q4 (≥77.01) were 3.00 (95% CI: 1.49–6.00, P = 0.254), 4.63 (95% CI: 2.18–9.82, P < 0.001), 16.94 (95% CI: 6.74–42.57, P < 0.001), respectively, in model 3 (Table 2). All of the models were statistically significant (Table 2, P for trend <0.05).
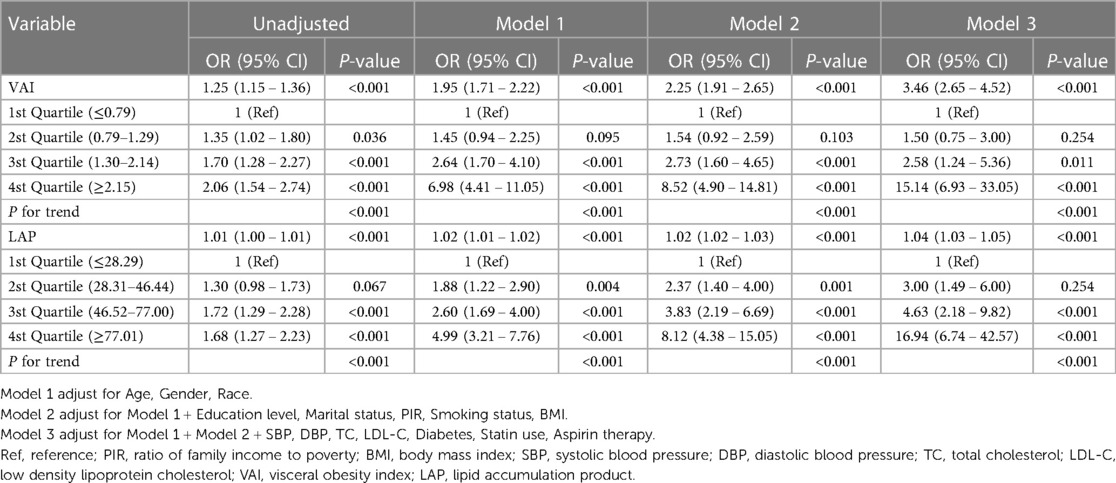
Table 2. Multivariable-adjust ORs and 95% CI of the VAI and LAP quartiles associated with elevated 10-year ASCVD risk.
In addition, we used generalized additive models and smoothed curve fittings to assess the links between VAI or LAP and elevated 10-year ASCVD risk (Figure 2). There was a linear relationship of elevated 10-year ASCVD risk with VAI and LAP (P for non-linearity >0.05), which indicated that 10-year ASCVD risk increased with VAI and LAP.
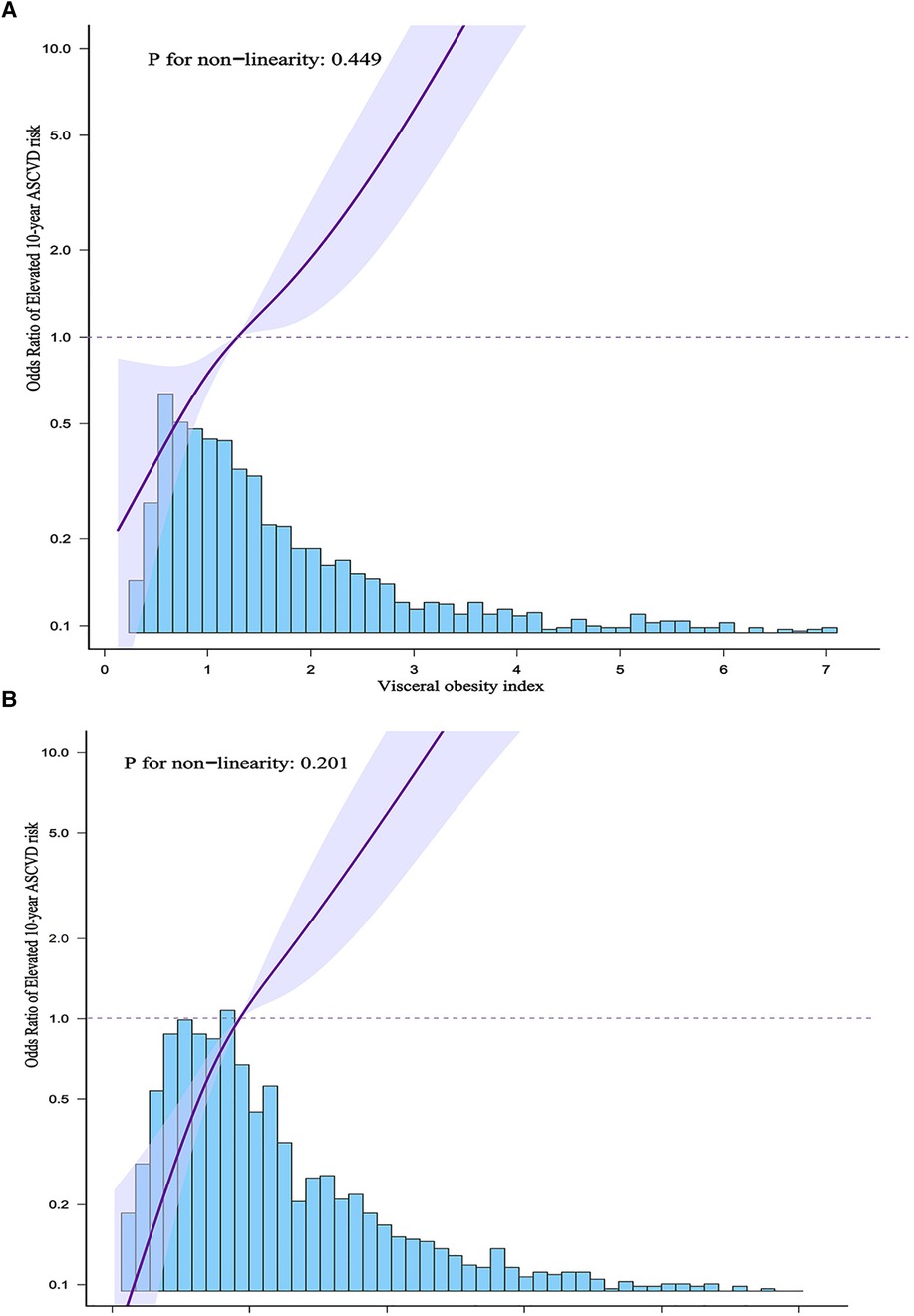
Figure 2. (A) Shows the association between VAI and elevated 10-year ASCVD risk. (B) Shows the association between LAP and elevated 10-year ASCVD risk. The solid purple line indicates the estimated or predicted value, the shaded area around the solid purple line indicates the 95% confidence interval, and the blue bar provides information on the sample size.
Stratified analyses were conducted in various subgroups to examine the potential modification effect of VAI and LAP on the relationship with elevated 10-year ASCVD risk (Figure 3). No significant interactions were found in any of the subgroups after stratification by race, diabetes status, statin use, and aspirin therapy (P for interaction >0.05). After stratifying by gender, significant interactions were observed in both VAI and LAP groups (P for interaction <0.05).
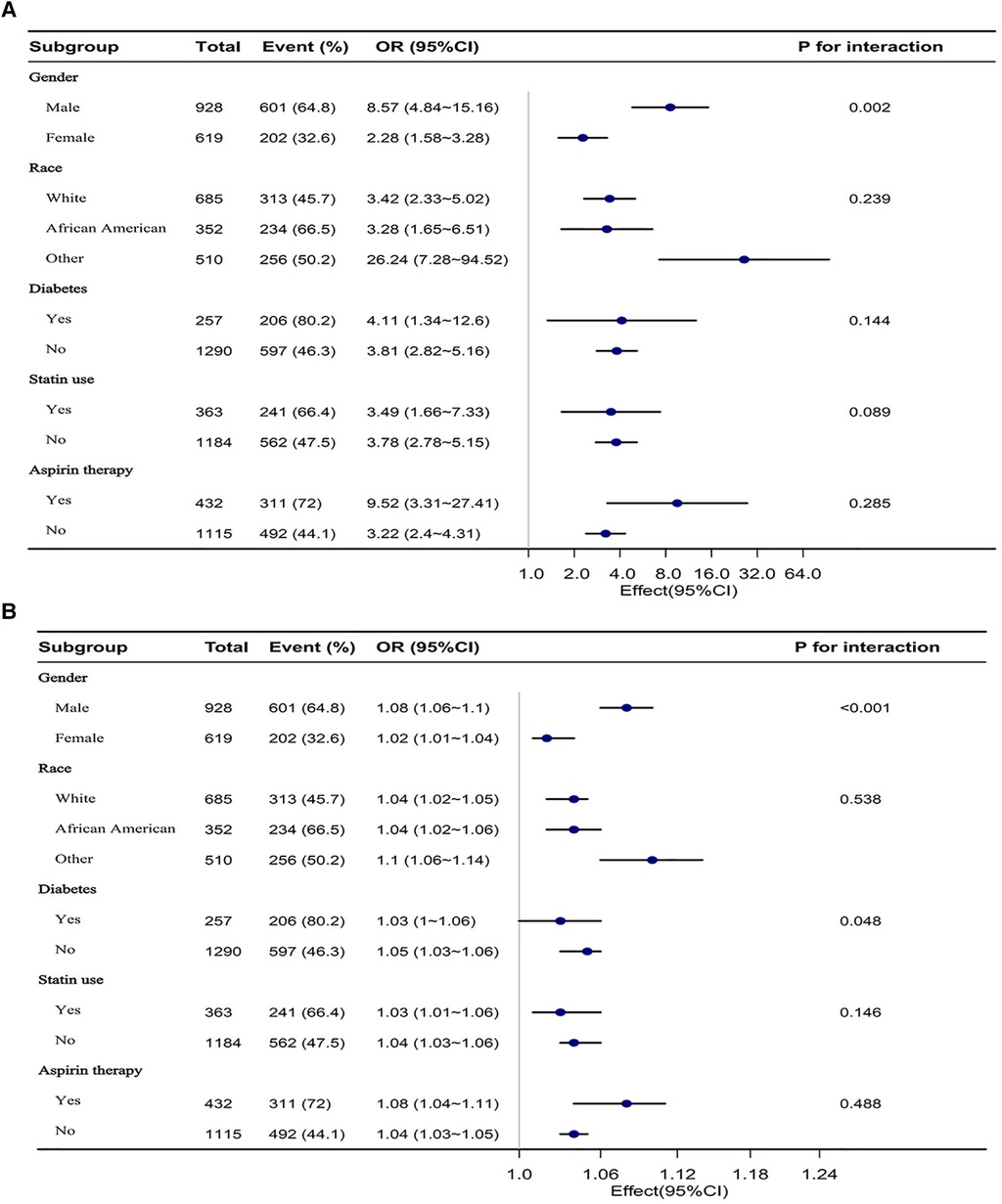
Figure 3. (A) Is a forest plot of the subgroup analysis of VAI and elevated 10-year ASCVD risk. (B) Is a forest plot of the subgroup analysis of LAP and elevated 10-year ASCVD risk. Each subgroup was adjusted for all other variables except the grouping factor itself.
After excluding participants with extreme VAI and LAP, 1,490 and 1,487 participants were remaining, respectively, and the association between elevated 10-year ASCVD risk with VAI and LAP remained stable. When VAI or LAP was assessed as a continuous variable, in the fully adjusted models, the adjusted OR for a 10-year ASCVD risk increase was 3.04 (95% CI: 2.24–4.12, P < 0.001) and 1.03 (95% CI: 1.02–1.04, P < 0.001), respectively (Supplementary Table S2).
This is a large cross-sectional study of American adults aged 40–79 years using NHANES data from 2011 to 2020. And the results of the study showed that VAI or LAP, whether as a continuous or categorical variable, was positively and linearly associated with elevated 10-year ASCVD risk when adjusted for potential confounding factors. The relationship between 10-year ASCVD risk with VAI and LAP remained robust after stratified and sensitivity analyses were performed. Interestingly, the stratified analysis also showed that this relationship was more pronounced among men.
It is well known that atherosclerosis is strongly associated with the risk of cardiovascular mortality worldwide (27, 28). Visceral obesity is strongly associated with increased atherosclerotic burden and is an emerging risk factor for CVD (13). And there is research showing that visceral obesity is significantly associated with the risk of recurrent ASCVD after myocardial infarction, residual cardiovascular risk, and CVD mortality (29). What's more a study on the South American population found that visceral obesity accounts for 15.4% of the 12 modifiable risk factors for CVD, ranking second. And its contribution to CVD mortality is 9.7% (30). ASCVD, recurrent cardiovascular events, and residual CVD risks impose a heavy burden on human health and the development of the economy and society. Therefore, paying attention to visceral obesity is crucial for reducing the burden of atherosclerosis. CT, MRI and other imaging methods are the gold standard for detecting visceral obesity, providing a visual display of the thickness and area of visceral fat. However, due to the high cost, time-consuming nature, and the need for professional operators, these imaging examinations are not suitable for large-scale surveys of the general population in clinical settings (31–33). VAI and LAP are considered sensitive and reliable indicators for assessing visceral obesity, especially VAI has been proven to be highly correlated with visceral fat measured through gold standard methods (17, 22). Its advantages of high safety, easy operation, and low cost make it replace complex imaging methods and become an alternative indicator for evaluating visceral obesity. Amato et al. showed for the first time in a retrospective study of Alkam metabolic syndrome (AlkaMeSy) that an increase in VAI was independently associated with increased cardiovascular and cerebrovascular events (18). Subsequent studies suggested that VAI was independently associated with coronary atherosclerosis and could assess cardiometabolic risk, ASCVD risk, and CVD mortality (34–38). However, the relationship between VAI and 10-year ASCVD risk remains controversial. In a prospective cohort study conducted in Europe, Koulii et al. found that VAI was independently associated with a 10-year risk of CVD, especially in males, and its relevance was not affected by potential confounding factors such as lifestyle factors (39). On the contrary, Aysegul et al. conducted a prospective cohort study on 55 postmenopausal women and found that there was no significant association between VAI and 10-year CVD risk (40). Our research on American adults aged 40–79 shows that there is a significant correlation between AVI and the 10-year ASCVD risk, and this relationship is more pronounced in males, which is similar to the findings of Koulii et al. This gender difference may be due to the fact that men and women differ greatly in body fat distribution, with men being more prone to visceral fat accumulation than women (41, 42). And a study showed that the measurement of visceral fat tissue in men using CT scans is twice as high as that in premenopausal women, and postmenopausal women also have lower accumulation of visceral fat tissue. As a result, women have lower risk of cardiovascular metabolic disorders (43, 44). In addition, hormones have a great impact on fat distribution patterns. Research has shown that androgens can promote the accumulation of visceral fat, while estrogens have less impact on the accumulation of visceral fat (43). Therefore, the VAI in males may be relatively higher than in females, with a greater increase in 10-year ASCVD risk. However, whether there are gender differences in the association between VAI and 10-year ASCVD risk still requires further validation through large-scale clinical studies. In 2005, Henry Kahn et al. based on the cross-sectional study of the NHANES III first proposed the LAP index and pointed out that compared with BMI, LAP had a better correlation with key risk factors for CVD (such as heart rate and blood lipids, as well as uric acid circulation levels), and may better predict the incidence of CVD (22). Subsequent studies have shown that LAP was associated with atherosclerosis in elderly and menopausal women, and can independently predict the risk of cardiovascular events in women with polycystic ovary syndrome (PCOS) as well as in participants with normal BMI (35, 44–46). Ioachimescu et al. found that LAP, rather than BMI, can predict the mortality of non-diabetes patients with high CVD risk, which suggested that LAP may be a useful tool for risk stratification of obesity-related adverse consequences in clinical practice (47). Li et al.'s cross-sectional study conducted in China showed that the alternative indicators of visceral obesity, VAI and LAP, may be related to the risk of intracranial Atherosclerosis stenosis (ICAS) in women ≥40 years (48). Kyrou et al. found that LAP was independently related to the long-term incidence of CVD in a prospective study of the Greek population (49), which was similar to our research findings. However, unlike the findings of Huang et al. in 3,143 Taiwanese adults, our study did not find any differences in the association strength between VAI and LAP with 10-year ASCVD risk, which may be due to ethnic differences in the study population (50).
The mechanism by which central obesity indicators (VAI and LAP) are associated with 10-year ASCVD risk is still unclear. There are several possible explanations for the research results. Firstly, the study indicates that abnormal distribution and accumulation of adipose tissue are fundamental causes of atherosclerosis, and VAI and LAP are representative indices for assessing adipose distribution and accumulation (35). Secondly, the characteristic of visceral obesity is an increased deposition of visceral and ectopic fat, which is associated with insulin resistance (51), elevated blood pressure (52), dyslipidemia (53), and inflammation (54), all of which are closely related to ASCVD risk. Visceral adipose tissue can increase basal fat breakdown, release free fatty acids (FFA), and specific cytokines secreted by visceral adipocytes, such as leptin and adiponectin, which can increase insulin resistance (55–57). In addition, inflammatory cytokines (tumor necrosis factor-alpha and interleukin-6) released by macrophages accumulated in visceral adipose tissue can weaken insulin sensitivity and thus promote insulin resistance (58). In insulin resistance, co-causative factors including glucotoxicity, lipotoxicity, and inflammation selectively impair PI3K-dependent insulin signaling pathways, thereby inducing the atherogenic process and leading to the occurrence of ASCVD (59). Hypertension is a recognized risk factor for ASCVD. Visceral adiposity patients have increased insulin and leptin which promotes sympathetic nervous system (SNS) activity (60–62). The SNS stimulates renin release and the production of angiotensin II, which increases the activity of the Renin-Angiotensin-Aldosterone System (RAAS) and therefore raises blood pressure (63, 64). In addition, visceral obesity causes the kidneys to reabsorb sodium through the SNS, hormones (aldosterone and insulin), and renal vasculature (angiotensin II). The increase in sodium also contributes to higher blood pressure to maintain sodium balance and volume homeostasis (65, 66). Dyslipidemia is highly associated with ASCVD risk. Abnormal lipid metabolism causes the blood to be in a highly cohesive state, the blood viscosity increases, and promotes the formation of atherosclerotic plaque (67). Insulin resistance, abnormal metabolism of fat factors [pro-inflammatory adipokines (leptin, resistin, TNF-α), anti-inflammatory adipokine (adiponectin), specific adipokine Sfrp5], and vitamin D deficiency are all possible causes of abnormal blood lipids in visceral obese individuals (53). In addition, excessive production of very low-density lipoprotein (VLDL) by the liver and reduced breakdown of triglycerides (TG) during lipid metabolism circulation, damaged peripheral FFA uptake, increased FFA from adipocytes to the liver and other tissues, and the formation of small dense LDL as well as damage to the ASP/C3adesArg pathway are also possible mechanisms of obesity-induced abnormal blood lipids (68). Inflammation is a key link in the occurrence and development of ASCVD. When there is excessive visceral fat, subcutaneous enlarged adipocytes secrete pro-inflammatory cytokines such as IL-6, reducing the secretion of possible anti-inflammatory and insulin sensitized cytokines adiponectin, and prone to cell apoptosis, leading to macrophage invasion (69–71). Macrophages infiltrate into enlarged adipocytes, further leading to an increase in the production of inflammatory cytokines such as tumor necrosis factor-alpha and interleukin-6, a decrease in the production of protective adipokine adiponectin, and harmful cross-talk between macrophages and enlarged adipocytes resulting in the production of detrimental secreted products (13, 72).
To our knowledge, this is the first exploration of the relationship between VAI and LAP with 10-year risk of first ASCVD events in US adults. However, there also are some limitations in our study. Firstly, although regression models, subgroup analysis, and sensitivity analysis are used, residual confounding effects of unmeasured or unknown factors cannot be completely excluded. Secondly, the current research results are based on a survey of adults aged 40–79 in the United States, and further research is still needed to determine whether the results of this study are applicable to other populations. In addition, although one of the indicators of visceral obesity, VAI, is a composite calculated from BMI, WC, TG and HDL-C. However, it has a similar parameter to the ASCVD, and some validation bias may exist even though the primary results did not change after adjusting for the similar parameter. Finally, the cross-sectional study can only explore the correlation, and can not further draw causal inferences, thus future longitudinal studies or randomized controlled trials are needed for further validation.
In conclusion, in American adults, especially males, VAI or LAP score is positively correlated with 10-year risk of first ASCVD events. Our research indicates that doctors should assess the degree of visceral obesity to identify individuals at high risk for ASCVD.
Publicly available datasets were analyzed in this study. This data can be found here: www.cdc.gov/nchs/nhanes/.
The studies involving humans were approved by the National Center for Health Statistics Ethics review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MX, LZ, and AS designed the study and extracted the relevant data. SH and RQ collated and cleaned the data. RW and XG examined the cleaned data. LZ and AS analyzed the data and wrote the original manuscript. MX reviewed and revised the manuscript. LZ and AS contributed equally to the study. All authors contributed to the article and approved the submitted version.
This study was supported by Major research project of scientific and technological innovation project of Chinese Academy of Chinese Medicine Sciences (no. CI2021A00913), National Natural Science Foundation of China (no. 81973686), and National Key Programme for Research and Development from the Ministry of Science and Technology, China (no. 2019YFC0840608).
We are grateful to the NHANES database for providing public data and to the participants for their considerable effort in collecting and collating data for the NHANES project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1249401/full#supplementary-material
1. Safiri S, Karamzad N, Singh K, Carson-Chahhoud K, Adams C, Nejadghaderi SA, et al. Burden of ischemic heart disease and its attributable risk factors in 204 countries and territories, 1990–2019. Eur J Prev Cardiol. (2022) 29(2):420–31. doi: 10.1093/eurjpc/zwab213
2. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010
3. Stone NJ, Smith SC Jr., Orringer CE, Rigotti NA, Navar AM, Khan SS, et al. Managing atherosclerotic cardiovascular risk in young adults: JACC state-of-the-art review. J Am Coll Cardiol. (2022) 79(8):819–36. doi: 10.1016/j.jacc.2021.12.016
4. Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. (2009) 6(6):399–409. doi: 10.1038/nrcardio.2009.55
5. Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res. (2016) 118(11):1723–35. doi: 10.1161/circresaha.115.306825
6. Csige I, Ujvárosy D, Szabó Z, Lőrincz I, Paragh G, Harangi M, et al. The impact of obesity on the cardiovascular system. J Diabetes Res. (2018) 2018:3407306. doi: 10.1155/2018/3407306
7. Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. (2005) 352(11):1138–45. doi: 10.1056/NEJMsr043743
8. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults–the evidence report. National institutes of health. Obes Res. (1998) 6(Suppl 2):51s–209s. doi: 10.1002/j.1550-8528.1998.tb00690.x
9. Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. Circulation. (2014) 129(25 Suppl 2):S102–38. doi: 10.1161/01.cir.0000437739.71477.ee
10. Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. (2005) 366(9497):1640–9. doi: 10.1016/s0140-6736(05)67663-5
11. Zeller M, Steg PG, Ravisy J, Lorgis L, Laurent Y, Sicard P, et al. Relation between body mass index, waist circumference, and death after acute myocardial infarction. Circulation. (2008) 118(5):482–90. doi: 10.1161/circulationaha.107.753483
12. Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America’s health: association for weight management and obesity prevention; NAASO, the obesity society; the American society for nutrition; and the American diabetes association. Am J Clin Nutr. (2007) 85(5):1197–202. doi: 10.1093/ajcn/85.5.1197
13. Neeland IJ, Ross R, Després JP, Matsuzawa Y, Yamashita S, Shai I, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. (2019) 7(9):715–25. doi: 10.1016/s2213-8587(19)30084-1
14. Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. (2012) 126(10):1301–13. doi: 10.1161/circulationaha.111.067264
15. Kaul S, Rothney MP, Peters DM, Wacker WK, Davis CE, Shapiro MD, et al. Dual-energy x-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring). (2012) 20(6):1313–8. doi: 10.1038/oby.2011.393
16. Neeland IJ, Grundy SM, Li X, Adams-Huet B, Vega GL. Comparison of visceral fat mass measurement by dual-x-ray absorptiometry and magnetic resonance imaging in a multiethnic cohort: the Dallas heart study. Nutr Diabetes. (2016) 6(7):e221. doi: 10.1038/nutd.2016.28
17. Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, et al. Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. (2010) 33(4):920–2. doi: 10.2337/dc09-1825
18. Amato MC, Giordano C, Pitrone M, Galluzzo A. Cut-off points of the visceral adiposity index (VAI) identifying a visceral adipose dysfunction associated with cardiometabolic risk in a Caucasian sicilian population. Lipids Health Dis. (2011) 10:183. doi: 10.1186/1476-511x-10-183
19. Mohammadreza B, Farzad H, Davoud K, Fereidoun Prof AF. Prognostic significance of the complex “visceral adiposity index” vs. Simple anthropometric measures: tehran lipid and glucose study. Cardiovasc Diabetol. (2012) 11:20. doi: 10.1186/1475-2840-11-20
20. Taverna MJ, Martínez-Larrad MT, Frechtel GD, Serrano-Ríos M. Lipid accumulation product: a powerful marker of metabolic syndrome in healthy population. Eur J Endocrinol. (2011) 164(4):559–67. doi: 10.1530/eje-10-1039
21. Kahn HS, Valdez R. Metabolic risks identified by the combination of enlarged waist and elevated triacylglycerol concentration. Am J Clin Nutr. (2003) 78(5):928–34. doi: 10.1093/ajcn/78.5.928
22. Kahn HS. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord. (2005) 5:26. doi: 10.1186/1471-2261-5-26
23. Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999−2010. National Center for Health Statistics. Vital Health Stat 1. (2013) (56):1–37.25078429
24. Amato MC, Giordano C. Visceral adiposity index: an indicator of adipose tissue dysfunction. Int J Endocrinol. (2014) 2014:730827. doi: 10.1155/2014/730827
25. Liu PJ, Lou HP, Zhu YN. Screening for metabolic syndrome using an integrated continuous Index consisting of waist circumference and triglyceride: a preliminary cross-sectional study. Diabetes Metab Syndr Obes. (2020) 13:2899–907. doi: 10.2147/dmso.S259770
26. Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. (2014) 129(25 Suppl 2):S49–73. doi: 10.1161/01.cir.0000437741.48606.98
27. Basili S, Loffredo L, Pastori D, Proietti M, Farcomeni A, Vestri AR, et al. Carotid plaque detection improves the predictive value of CHA(2)DS(2)-VASc score in patients with non-valvular atrial fibrillation: the ARAPACIS study. Int J Cardiol. (2017) 231:143–9. doi: 10.1016/j.ijcard.2017.01.001
28. Libby P. The changing landscape of atherosclerosis. Nature. (2021) 592(7855):524–33. doi: 10.1038/s41586-021-03392-8
29. Moura L, Pagotto V, Camargo Pereira C, de Oliveira C, Silveira EA. Does abdominal obesity increase all-cause, cardiovascular disease, and cancer mortality risks in older adults? A 10-year follow-up analysis. Nutrients. (2022) 14(20):4315. doi: 10.3390/nu14204315
30. Lopez-Jaramillo P, Joseph P, Lopez-Lopez JP, Lanas F, Avezum A, Diaz R, et al. Risk factors, cardiovascular disease, and mortality in South America: a PURE substudy. Eur Heart J. (2022) 43(30):2841–51. doi: 10.1093/eurheartj/ehac113
31. Wu FZ, Huang YL, Wu CC, Wang YC, Pan HJ, Huang CK, et al. Differential effects of bariatric surgery versus exercise on excessive visceral fat deposits. Medicine (Baltimore). (2016) 95(5):e2616. doi: 10.1097/md.0000000000002616
32. Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. American association of clinical endocrinologists and American college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. (2016) 22(Suppl 3):1–203. doi: 10.4158/ep161365.Gl
33. Flegal KM, Shepherd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. (2009) 89(2):500–8. doi: 10.3945/ajcn.2008.26847
34. Yang F, Wang G, Wang Z, Sun M, Cao M, Zhu Z, et al. Visceral adiposity index may be a surrogate marker for the assessment of the effects of obesity on arterial stiffness. PLoS One. (2014) 9(8):e104365. doi: 10.1371/journal.pone.0104365
35. Sun J, Meng X, Huang H, Jing J, Pan Y, Mei L, et al. Higher visceral adiposity index and lipid accumulation product in relation to increased risk of atherosclerotic burden in community-dwelling older adults. Exp Gerontol. (2023) 174:112115. doi: 10.1016/j.exger.2023.112115
36. Oh SK, Cho AR, Kwon YJ, Lee HS, Lee JW. Derivation and validation of a new visceral adiposity index for predicting visceral obesity and cardiometabolic risk in a Korean population. PLoS One. (2018) 13(9):e0203787. doi: 10.1371/journal.pone.0203787
37. Xie X, Li Q, Zhang L, Ren W. Lipid accumulation product, visceral adiposity index, and Chinese visceral adiposity Index as markers of cardiometabolic risk in adult growth hormone deficiency patients: a cross-sectional study. Endocr Pract. (2018) 24(1):33–9. doi: 10.4158/ep-2017-0007
38. Tamosiunas A, Luksiene D, Kranciukaite-Butylkiniene D, Radisauskas R, Sopagiene D, Bobak M. Predictive importance of the visceral adiposity index and atherogenic index of plasma of all-cause and cardiovascular disease mortality in middle-aged and elderly Lithuanian population. Front Public Health. (2023) 11:1150563. doi: 10.3389/fpubh.2023.1150563
39. Kouli GM, Panagiotakos DB, Kyrou I, Georgousopoulou EN, Chrysohoou C, Tsigos C, et al. Visceral adiposity index and 10-year cardiovascular disease incidence: the ATTICA study. Nutr Metab Cardiovasc Dis. (2017) 27(10):881–9. doi: 10.1016/j.numecd.2017.06.015
40. Gulbahar A, Caglar GS, Arslanca T. Evaluation of visceral adiposity index with cardiovascular risk factors, biomarkers in postmenopausal women to predict cardiovascular disease: a 10 year study. Exp Gerontol. (2022) 170:111986. doi: 10.1016/j.exger.2022.111986
41. Pond CM. An evolutionary and functional view of mammalian adipose tissue. Proc Nutr Soc. (1992) 51(3):367–77. doi: 10.1079/pns19920050
42. Kuk JL, Lee S, Heymsfield SB, Ross R. Waist circumference and abdominal adipose tissue distribution: influence of age and sex. Am J Clin Nutr. (2005) 81(6):1330–4. doi: 10.1093/ajcn/81.6.1330
43. Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. (2013) 93(1):359–404. doi: 10.1152/physrev.00033.2011
44. Tongdee P, Nimkuntod P. Novel mathematic indexes to identify subclinical atherosclerosis in different obesity phenotypes of perimenopausal/menopausal women. J Med Assoc Thai. (2016) 99(Suppl 7):S62–8.29901951
45. Velija-Asimi Z, Burekovic A, Dujic T, Dizdarevic-Bostandzic A, Semiz S. Incidence of prediabetes and risk of developing cardiovascular disease in women with polycystic ovary syndrome. Bosn J Basic Med Sci. (2016) 16(4):298–306. doi: 10.17305/bjbms.2016.1428
46. Hosseinpanah F, Barzin M, Mirbolouk M, Abtahi H, Cheraghi L, Azizi F. Lipid accumulation product and incident cardiovascular events in a normal weight population: tehran lipid and glucose study. Eur J Prev Cardiol. (2016) 23(2):187–93. doi: 10.1177/2047487314558771
47. Ioachimescu AG, Brennan DM, Hoar BM, Hoogwerf BJ. The lipid accumulation product and all-cause mortality in patients at high cardiovascular risk: a PreCIS database study. Obesity (Silver Spring). (2010) 18(9):1836–44. doi: 10.1038/oby.2009.453
48. Li R, Li Q, Cui M, Ying Z, Li L, Zhong T, et al. Visceral adiposity index, lipid accumulation product and intracranial atherosclerotic stenosis in middle-aged and elderly Chinese. Sci Rep. (2017) 7(1):7951. doi: 10.1038/s41598-017-07811-7
49. Kyrou I, Panagiotakos DB, Kouli GM, Georgousopoulou E, Chrysohoou C, Tsigos C, et al. Lipid accumulation product in relation to 10-year cardiovascular disease incidence in Caucasian adults: the ATTICA study. Atherosclerosis. (2018) 279:10–6. doi: 10.1016/j.atherosclerosis.2018.10.015
50. Huang YC, Huang JC, Lin CI, Chien HH, Lin YY, Wang CL, et al. Comparison of innovative and traditional cardiometabolic indices in estimating atherosclerotic cardiovascular disease risk in adults. Diagnostics (Basel). (2021) 11(4):603. doi: 10.3390/diagnostics11040603
51. Hayashi T, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes. (2008) 57(5):1269–75. doi: 10.2337/db07-1378
52. Hayashi T, Boyko EJ, Leonetti DL, McNeely MJ, Newell-Morris L, Kahn SE, et al. Visceral adiposity is an independent predictor of incident hypertension in Japanese Americans. Ann Intern Med. (2004) 140(12):992–1000. doi: 10.7326/0003-4819-140-12-200406150-00008
53. Vekic J, Zeljkovic A, Stefanovic A, Jelic-Ivanovic Z, Spasojevic-Kalimanovska V. Obesity and dyslipidemia. Metab Clin Exp. (2019) 92:71–81. doi: 10.1016/j.metabol.2018.11.005
54. Després JP. Abdominal obesity and cardiovascular disease: is inflammation the missing link? Can J Cardiol. (2012) 28(6):642–52. doi: 10.1016/j.cjca.2012.06.004
55. Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. (2004) 89(6):2595–600. doi: 10.1210/jc.2004-0372
56. Rytka JM, Wueest S, Schoenle EJ, Konrad D. The portal theory supported by venous drainage-selective fat transplantation. Diabetes. (2011) 60(1):56–63. doi: 10.2337/db10-0697
57. Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. (2013) 2013:139239. doi: 10.1155/2013/139239
58. Hardy OT, Czech MP, Corvera S. What causes the insulin resistance underlying obesity? Curr Opin Endocrinol Diabetes Obes. (2012) 19(2):81–7. doi: 10.1097/MED.0b013e3283514e13
59. Muniyappa R, Iantorno M, Quon MJ. An integrated view of insulin resistance and endothelial dysfunction. Endocrinol Metab Clin North Am. (2008) 37(3):685–711, ix–x. doi: 10.1016/j.ecl.2008.06.001
60. Landsberg L. Insulin-mediated sympathetic stimulation: role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why). J Hypertens. (2001) 19(3 Pt 2):523–8. doi: 10.1097/00004872-200103001-00001
61. Kennedy A, Gettys TW, Watson P, Wallace P, Ganaway E, Pan Q, et al. The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J Clin Endocrinol Metab. (1997) 82(4):1293–300. doi: 10.1210/jcem.82.4.3859
62. Kazumi T, Kawaguchi A, Katoh J, Iwahashi M, Yoshino G. Fasting insulin and leptin serum levels are associated with systolic blood pressure independent of percentage body fat and body mass index. J Hypertens. (1999) 17(10):1451–5. doi: 10.1097/00004872-199917100-00013
63. Sarzani R, Salvi F, Dessì-Fulgheri P, Rappelli A. Renin-angiotensin system, natriuretic peptides, obesity, metabolic syndrome, and hypertension: an integrated view in humans. J Hypertens. (2008) 26(5):831–43. doi: 10.1097/HJH.0b013e3282f624a0
64. Bomback AS, Klemmer PJ. Interaction of aldosterone and extracellular volume in the pathogenesis of obesity-associated kidney disease: a narrative review. Am J Nephrol. (2009) 30(2):140–6. doi: 10.1159/000209744
65. Ahmed SB, Fisher ND, Stevanovic R, Hollenberg NK. Body mass index and angiotensin-dependent control of the renal circulation in healthy humans. Hypertension. (2005) 46(6):1316–20. doi: 10.1161/01.HYP.0000190819.07663.da
66. Rocchini AP, Key J, Bondie D, Chico R, Moorehead C, Katch V, et al. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med. (1989) 321(9):580–5. doi: 10.1056/nejm198908313210905
67. Neves JS, Newman C, Bostrom JA, Buysschaert M, Newman JD, Medina JL, et al. Management of dyslipidemia and atherosclerotic cardiovascular risk in prediabetes. Diabetes Res Clin Pract. (2022) 190:109980. doi: 10.1016/j.diabres.2022.109980
68. Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. (2013) 5(4):1218–40. doi: 10.3390/nu5041218
69. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. (1995) 95(5):2409–15. doi: 10.1172/jci117936
70. Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. (2003) 112(12):1785–8. doi: 10.1172/jci20514
71. Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. (2007) 117(9):2621–37. doi: 10.1172/jci31021
Keywords: atherosclerotic cardiovascular disease, visceral obesity, visceral obesity index, lipid accumulation product, NHANES
Citation: Zheng L, Sun A, Han S, Qi R, Wang R, Gong X and Xue M (2023) Association between visceral obesity and 10-year risk of first atherosclerotic cardiovascular diseases events among American adults: National Health and Nutrition Examination Survey. Front. Cardiovasc. Med. 10:1249401. doi: 10.3389/fcvm.2023.1249401
Received: 28 June 2023; Accepted: 7 August 2023;
Published: 21 August 2023.
Edited by:
Zhendong Liu, Shandong First Medical University, ChinaReviewed by:
Fu-Zong Wu, Kaohsiung Veterans General Hospital, Taiwan© 2023 Zheng, Sun, Han, Qi, Wang, Gong and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mei Xue bWVpYXJAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.