
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 23 October 2023
Sec. Hypertension
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1247244
This article is part of the Research Topic Global Excellence in Cardiovascular Medicine: Africa View all 15 articles
Background: The available data on the association between parity and hypertension are inconclusive. This study was conducted to investigate the prevalence of hypertension and its association with parity among adult Sudanese women.
Methods: A multi-stage sampling survey was conducted in four villages in the River Nile State in Sudan between July and September 2022. The World Health Organization's three-level stepwise questionnaire was used to gather the participants' sociodemographic characteristics (age, sex, marital status, parity, educational level, occupation, obstetric history, family history of hypertension, weight and height). Regression analyses were performed.
Results: A total of 408 women were recruited. The median [measured in terms of interquartile range (IQR)] age was 45.0 years (33.0–55.7 years). A linear regression analysis revealed a significant association between parity and diastolic blood pressure (coefficient, 0.60; P = 0.011). The prevalence of hypertension (55.9%) increased with parity and ranged from 43.7% to 74.9%. In the multivariate analyses, increasing age (adjusted odds ratio [AOR], 1.03; 95% confidence interval [CI], 1.02–1.05), increasing parity (AOR, 1.09; 95% CI, 1.01–1.19), family history of hypertension (AOR, 1.79; 95% CI, 1.15–2.77), and increasing body mass index (AOR, 1.09; 95% CI, 1.05–1.13) were associated with hypertension. In women of ages ≥ 50 years, increasing parity was significantly associated with hypertension (AOR, 1.15; 95% CI, 1.2–1.29). Para > 5 (AOR, 2.73; 95% CI, 1.11–6.73) was associated with hypertension.
Conclusion: A high prevalence of hypertension was found among Sudanese women, and that parity at 5 or more is linked to hypertension.
Hypertension is one of the major non-communicable diseases (1). Around one-third (31.1%) of the global adult population (1.39 billion people) have hypertension (1, 2). Low- and middle-income countries (31.5%) and African countries (46.0%) (3) have higher prevalence rates of hypertension than high-income countries (28.5%) (1, 2). Sociodemographic, environmental, behavioural factors, high sodium intake, low potassium intake, obesity, alcohol consumption, smoking, lack of physical activity, and nutrition are the identified risk factors for developing hypertension (1, 2). Hypertension is the leading preventable risk factor of cardiovascular diseases and all-cause mortality across the globe (4). Several factors such as lack of awareness of health status, delayed diagnosis, poorly controlled hypertension, and a weak health system expose patients with hypertension in Africa to the highest risk of stroke and heart and renal diseases (3). Ethnicity and race can influence the management of hypertension and its related complications (5). In the global initiatives of the International Society of Hypertension for the screening and management of hypertension, early diagnosis and treatment of hypertension are recommended (6, 7).
The effect of parity on blood pressure levels or hypertension has been reported in several studies (8–13). This may be explained by increased blood volume, increased heart rate, altered myocardial contractility, and reduced afterload and preload, which lead to expanded cardiac output during pregnancy (14). While some studies have shown that parity is associated with an increased risk of developing hypertension (11, 12, 15), others have reported no such association (16). Most of these studies were conducted outside of sub-Saharan African countries. A higher prevalence of parity among women in sub-Saharan Africa was recently reported (17). In studies on the global epidemiology of hypertension, Sudan was identified as one of the countries with a hypertension prevalence rate >34% (1, 2). This is consistent with the findings of some recently published studies on the prevalence of hypertension among the general Sudanese population (35.2%‒41.0%) (18, 19). A higher prevalence of hypertension among females was reported in Eastern Sudan (41.0%) (19). Sudanese women have high parity, with most of them having five deliveries (grand multiparity) at a younger age, before 35 years old (20). Given the importance of the two clinical entities, their potential coexistence, and the meagre published clinical data on this issue in Sudan and in the region, the present study aimed to investigate the prevalence of hypertension among Sudanese women and the influence of parity, especially high parity, and other factors on the development of hypertension among Sudanese women.
This study was conducted in accordance with the principles stipulated in the Declaration of Helsinki. Ethical approval was obtained from the health authority of Almatamah, Sudan (reference No. 03/2021). Signed written informed consent forms were collected from all participants. A multi-stage sampling study was conducted in the River Nile State, northern Sudan, between July and September 2022. River Nile State is one of the 18 states of Sudan and has a total population of 1,120,441 (21). Almatamah is one of the seven localities (the smallest administrative unit in Sudan) in River Nile State and was initially selected by simple random sampling. Adult women in the households of four villages (Hajer Alteer, Athawra Kabota, Alkoumer, and Wadi Alshohda) were selected randomly from the Almatamah locality on the basis of the population size of all sectors. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) standard checklists were followed (22). Only Sudanese women (>18 years of age) from the selected households who agreed to participate in the study were selected. Two trained medical officers interviewed the participants during the study period.
After signing an informed consent form, the participants completed a questionnaire that collected their sociodemographic information, clinical and physical measurements, blood pressures, and weights and heights. Pregnant women; those with known causes of secondary hypertension, renal diseases, medication use (steroid therapy), substance abuse, mental illness, disabilities, or congenital deformities; and those who refused to participate in the study were excluded. The World Health Organization's (WHO) three-level stepwise approach questionnaire was used to collect data (23) on the participants' sociodemographic characteristics, including age; marital status, categorised as married, widow, or divorced; educational level (≤secondary level or >secondary level); and past medical history of hypertension and drug history (steroid therapy). Moreover, a detailed history was obtained regarding the women's menopausal status, history of miscarriage, and live birth/parity. According to the Sudanese tradition, smoking and alcohol consumption are not female habits; hence, we did not include these in the questionnaire to avoid a loss of cooperation among the participants.
An OMRON 3 (with an appropriate cuff size) automated blood measuring device was used to obtain two blood pressure readings after the participant had rested for at least 10 min. The measurement was performed with the participant's arm placed at the level of the heart. The mean of two blood pressure readings (at an interval of 1–2 min) was computed and registered. When the difference between the two readings was significant, that is, >5 mmHg, new measurements were taken until a stable reading was obtained. The method adopted for measuring blood pressure was based on recent recommendations and requirements (24). Women were considered hypertensive on the basis of a reading of ≥140 mmHg for systolic blood pressure and ≥90 mmHg for diastolic blood pressure or both of them. Both criteria were recorded in repeated measurements or reported as having previous hypertension and receiving anti-hypertensive medications (1). The body mass index (BMI) was calculated from the patient's weight and height and grouped according to the WHO's classification for females as follows: underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), or obese (≥30.0 kg/m2) (25).
Parity is defined as the number of times a woman had given birth to a foetus with a gestational age of ≥24 weeks, irrespective of whether the baby was born alive or stillborn (26). Nulliparity is considered as para 0, or no previous delivery, and multipara is defined as a woman who has given birth 2 or more times (26).
A sample size of 408 women was calculated using the Open Epi Menu (27), with an assumption of a type I error of 5% and an adequate power of 80% (β = 0.2). The estimated sample size (n = 408) was calculated on the basis of the assumed hypertension prevalence rate (41.0%) among women. This assumption was based on our previous observations in eastern Sudan (19). Thus, the ratio of women with hypertension to women without hypertension was expected to be 2:3. We assumed that 27.0% of women with hypertension and 15.0% of women without hypertension would have a para ≥5. This assumption was based on our recent work on reproductive health in Sudan (28).
The Statistical Package for the Social Sciences (SPSS) for Windows (IBM SPSS v.25) was used to analyse the data. The chi-square test was used to compare the proportions between the women with and those without hypertension. Continuous data were assessed for normality using the Shapiro-Wilk test. A t-test and the Mann-Whitney test were used to compare the normally distributed and non-normally distributed data, respectively, between the two groups of women (hypertensive and non-hypertensive). Spearman correlations were performed between continuous variables (age, parity and BMI). Multiple linear regression analysis was conducted for parity with systolic and diastolic blood pressures to assess the risk factors. Logistic regression analyses were performed by entering the dependent (hypertension) and independent variables (age, BMI, educational level, occupation, past medical history of hypertension, and live birth/parity number). Variance inflation factor (<4) and the presence of high correlations (r = 0.9) were used to assess the presence of multi-collinearity and there was no multi-collinearity between the independent variables including age, parity and BMI. The independent variables with a univariate P value < 0.20 were entered into the model. The adjusted odds ratio (AORs) and 95% confidence intervals (Cis) were calculated, with P values < 0.05 considered statistically significant. Backward likelihood ratio adjustments were then performed in the different models.
Four hundred and eight women were enrolled in this study. Their median [interquartile range (IQR)] age was 45.0 years (33.0‒55.7 years). Their parity ranged from 0 to 10, with a median of 2. A total of 158 women (38.7%) were nulliparous, whereas 95 (23.3%), 73 (17.9%), and 82 (20.1%) had para 1 to 3, 4 or 5, and more than 5, respectively. Of the 408 women, 276 (67.6%) had an educational level ≥ secondary education, and 223 (54.7%) were housewives. Age increased with parity, and women who had para >5 had the highest median (IQR) age [53.0 years (42.0‒60.0), years]. Of 408 enrolled women, 131(32.1%), 39 (9.6%), 123(30.1%) and 115 (28.2%) were normal weight, underweight, overweight and obese, respectively. The women who had para 4 or 5 had the highest BMI [27.6 kg/m2 (23.7‒32.9 kg/m2)]. There was a borderline correlation between age (r = 0.294), parity (r = 0.139) and BMI. No significant difference in educational level was found between the women in the different parity groups. A significantly higher number of women with para >5 were housewives (see Table 1). While no significant difference in median (IQR) systolic blood pressure was found, diastolic blood pressure was significantly higher in the women with para 4 or 5 [85.0 mmHg (80.0‒95.0 mmHg); see Table 1].
In the multiple linear regression analysis, parity was not associated with systolic blood pressure, however there was a significant association between parity and diastolic blood pressure (coefficient, 0.60; P = 0.011; see Table 2).
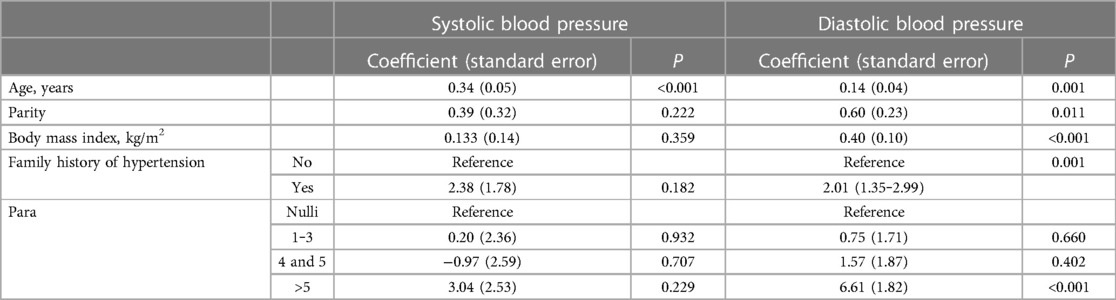
Table 2. Multiple linear regression analysis of the adjusted factors associated with systolic and diastolic blood pressure among women in Sudan, 2022.
A total of 228 women (55.9%) had hypertension, 71 women (17.4%) had known hypertension, and 157 women (38.5%) had newly discovered hypertension. The prevalence of hypertension increased with parity and ranged from 43.7% to 74.9%. Women who had para >5 had the highest prevalence of hypertension (74.9%; see Table 1 and Figure 1). In the univariate analysis, increasing age, increasing parity, family history of hypertension, and increasing BMI were associated with hypertension. Educational level and occupation were not associated with hypertension (see Table 3). When these variables were adjusted in the multivariate analysis, increasing age (AOR, 1.03; 95% CI, 1.02‒1.05), increasing parity (AOR, 1.09; 95% CI, 1.01‒1.19), family history of hypertension (AOR, 1.79; 95% CI, 1.15‒2.77), and increasing BMI (AOR, 1.09; 95% CI, 1.05‒1.13) were associated with hypertension. Next, we removed parity as a continuous variable and entered the parity groups into the model. In this case, compared with the nulliparity (reference), para 1 to 3 (AOR, 1.35; 95% CI, 0.77‒2.38), para 4 or 5 (AOR, 1.37; 95% CI, 0.73‒2.55) was not associated with hypertension. Women with para >5 (AOR, 2.40; 95% CI, 1.26‒4.58) were at higher risk of hypertension (see Table 4).
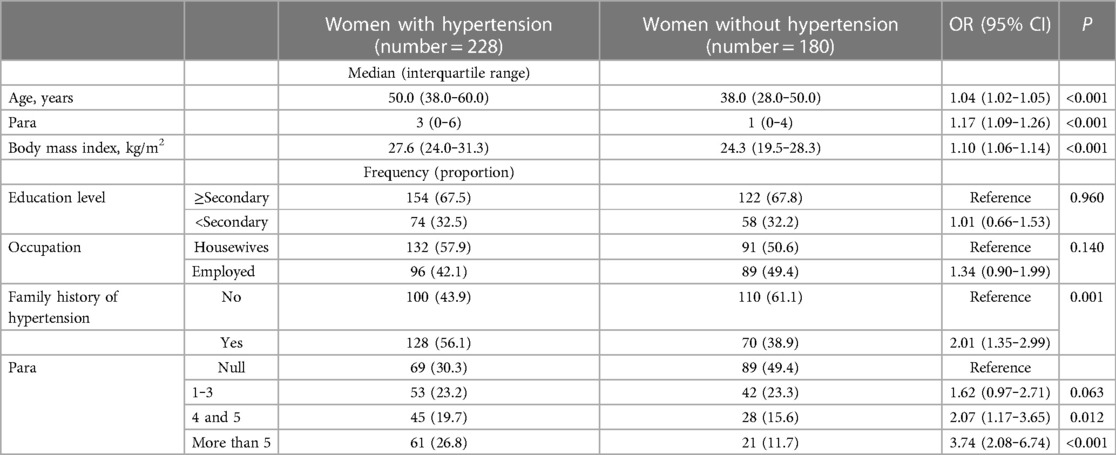
Table 3. Univariate analysis of the factors (unadjusted) associated with hypertension among women in Sudan, 2022.
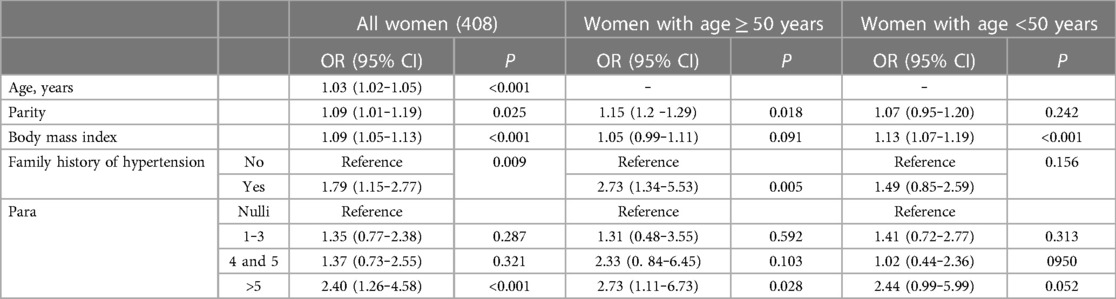
Table 4. Multivariate analysis of the adjusted factors associated with hypertension among women in Sudan, 2022.
We then divided the women into two age groups (≥50 and <50 years). In the women aged ≥50 years, increasing parity was associated with hypertension (AOR, 1.15; 95% CI, 1.2‒1.29). Compared with the nulliparous women (reference), para 1 to 3 (AOR, 1.31; 95% CI, 0.48‒3.55) and para 4 or 5 (AOR, 2.33; 95% CI, 0.84‒6.45) were not associated with hypertension. Para >5 (AOR, 2.73; 95% CI, 1.11‒6.73) was associated with hypertension (see Table 4). In the women aged <50 years, parity and parity groups were not associated with hypertension (see Table 4).
The main findings of this study were a higher hypertension prevalence rate, and after adjusting for age and BMI, parity and increasing parity became significant risk factors for developing hypertension among Sudanese women. The hypertension prevalence rate among the Sudanese women (55.9%) in our study was comparatively higher than that obtained in eastern Sudan (40.8%) (19) and in some African countries such as Ethiopia (19.1%) (29) and Ghana (16%) (11). The differences in hypertension prevalence rate could be explained by the differences in sodium intake, potassium intake, alcohol consumption, obesity, nutrition, and physical activity across the regions (1, 2). The main findings of this study indicate that after adjusting for age and BMI, increasing parity was associated with hypertension (in terms of diastolic blood pressure) and women aged ≥50 years. Parity was not associated with systolic blood pressure or hypertension in the women aged <50 years.
The present study indicates that compared with nulliparity (reference), para 1 to 3 and para 4 or 5 are not at higher risk of hypertension. Para > 5 are 2.40 times more likely to have hypertension. This is in concordance with the results obtained in Mali, in which women with para ≥5 had significantly higher blood pressures (in terms of increased systolic blood pressure) than those with para 1 to 3 (30). In Ghana, women (ages 45–49 years) with para 2 or 3 had a higher likelihood of being hypertensive than younger and nulliparous women (11). A similar finding was observed in our study among Chinese post-menopausal women with para ≥5 had higher blood pressures than women with para 0 or 1; however, parity was not associated with hypertension in pre-menopausal women (15). Similarly, Turkish women with para ≥4 were at a higher risk of having hypertension than those who had para less than 4 (31). Likewise, in rural Bangladesh, high parity was positively associated with a risk of hypertension among women with obesity who had ≥4 pregnancies compared with those aged 15–75 years who only had one pregnancy (32). A previous study showed that in Iran, compared with para 2, para ≥3 was significantly associated with hypertension; however, these findings were mainly among younger women, that is, <50 years of age (12).
By comparison, Khalid (2006) reported no association between parity and hypertension among 441 women aged between 15 and 60 years in Abha, Saudi Arabia (16). A Swiss study recruited 2,837 women aged 30–73 years and demonstrated that parity had a significant adverse effect on the development of hypertension in women at 60 years; however, parity had a protective effect against hypertension in women aged <40 years (9). Giubertoni et al. reported that in Italy, while parity is associated with early hypertension during the transition to menopause, parity is not associated with hypertension in the post-menopausal period (13). In Bangladesh, women with para 1 as the reference, diastolic blood pressure was higher in nulliparous women and in para ≥3. The association between increased diastolic blood pressure and nulliparity was mainly observed in women aged >45 years, and the same association was observed among women in Bangladesh (8). Likewise, a global epidemiological study in sub-Saharan Africa demonstrated higher diastolic blood pressures (6). Our study showed that a family history of hypertension was not associated with hypertension among women with increased parity, which may reflect the heterogeneity of essential hypertension.
The results of these studies must be compared with caution. The discrepancies and modelling differences in the methods used in these studies were obvious: some studies compared women with high parity (≥5 children) with those with low-to-moderate parity, and some studies did not include nulliparous women in their analyses. Differences in lifestyle, cultural factors, genetic influence, and hypertension prevalence rate might have contributed to the differences in the results of these studies and hypertension prevalence rates among different populations (33, 34).
Parity exposes women to the risk of clinical placental syndrome (pregnancy loss, foetal growth restriction, and pre-eclampsia) as a result of altered uterine and intervillous blood flow, which is linked to inflammatory processes that lead to maternal vascular endothelial dysfunction and permanent vascular damage, thereby accelerating the development of atherosclerosis, hypertension, and cardiovascular diseases (35). Higher parity has been associated with increases in some inflammatory markers (fibrinogen, D-dimer, GlycA, high-sensitivity C-reactive protein, and interleukin-6 levels), which reflect increased risks of cardiovascular diseases and metabolic syndrome (36). In addition, the loss of the protective effect of oestrogen in postmenopausal women might lead to endothelial dysfunction and increased BMI, which are the main negative indicators of hypertension, particularly among women aged >50 years (37). The renin-angiotensin-aldosterone system in females is influenced significantly (38). Our study and several previous studies have documented significant associations between parity, BMI, and hypertension (4, 24, 28, 32). The prevalence 28.2% of obesity in the current study was slightly lower than the prevalence (33.5%) of obesity reported in eastern Sudan (19).
Several studies have reported a significant association between increasing parity and metabolic syndrome (obesity, diabetes mellitus, and dyslipidaemia), which is associated with oxidative stress and inflammation that induces endothelial dysfunction, vascular stiffening, atherosclerosis, and hypertension (39, 40). Furthermore, the physiologic cardiometabolic changes associated with pregnancy, such as insulin resistance, increased plasma glucose, weight gain, dyslipidaemia, and cardiovascular complications, increase the potential risk for developing hypertension (12, 41, 42). However, the previous studies have found positive correlations in women with a much lower number of children than what the current study is reporting. Perhaps, some other possible contributors to hypertension such as geographic location and high levels of stress among these women who raised 5 or more children and were homemakers.
The hypertension prevalence rate in the Sudanese women in this study was significantly high, and that parity at 5 or more is linked to hypertension.
This study has certain limitations that should be considered. The study was a questionnaire-based survey conducted over a 3-month period. The participants' reproductive histories were self-reported, which might have increased the possibility of misclassification of parity and gravidity, particularly among the older women. Similarly, the self-reporting of menopausal status might have resulted in some misclassification. In addition, other risk factors such as history of gestational hypertension or preeclampsia, salt intake, physical exercise, oral contraceptive use, smoking, alcohol consumption, lipid profile, and blood sugar status were not assessed. Moreover, all the data obtained (apart from the blood pressure measurements) were declarative, so descriptive elements regarding the causes of secondary hypertension and other factors were lacking.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Ethical approval was obtained from the health authority of Almatamah, Sudan (reference No. 03/2021). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
IRM and OEO conceived the study; OEO and IA supervised the work, guided the analysis and critically reviewed the manuscript; IRM and IA prepared the analysis plan, performed the data analysis and wrote the first draft of the paper; IRM and OEO supervised data collection All authors reviewed and approved the final manuscript. All authors contributed to the article and approved the submitted version.
The authors would like to thank all the participants who participated in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. (2016) 134(6):441–50. doi: 10.1161/CIRCULATIONAHA.115.018912
2. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. (2020) 16(4):223–37. doi: 10.1038/s41581-019-0244-2
3. Dzudie A, Rayner B, Ojji D, Schutte AE, Twagirumukiza M, Damasceno A, et al. Roadmap to achieve 25% hypertension control in Africa by 2025. Glob Heart. (2018) 13(1):45–59. doi: 10.1016/j.gheart.2017.06.001
4. Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Abate KH, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease stu. Lancet. (2018) 392(10159):1923–94. doi: 10.1016/S0140-6736(18)32225-6
5. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International society of hypertension global hypertension practice guidelines. Hypertens (Dallas, Tex 1979). (2020) 75(6):1334–57. doi: 10.1161/HYPERTENSIONAHA.120.15026
6. Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. (2021) 18(11):785–802. doi: 10.1038/s41569-021-00559-8
7. Ogah O, Arije A, Xin X, Beaney T, Adebiyi A, Sani MU. May measurement month 2017: screening for hypertension in Nigeria—sub-saharan Africa. Eur Heart J Suppl. (2019) 21(Suppl D):D86–8. doi: 10.1093/eurheartj/suz064
8. Shih YH, Scannell Bryan M, Parvez F, Uesugi KH, Shahriar M, Ahmed A, et al. Gravidity, parity, blood pressure and mortality among women in Bangladesh from the HEALS cohort. BMJ Open. (2020) 10(8):e037244. doi: 10.1136/bmjopen-2020-037244
9. Dratva J, Schneider C, Schindler C, Stolz D, Gerbase M, Pons M, et al. Is there a differential impact of parity on blood pressure by age? J Hypertens. (2014) 32(11):2146–51. doi: 10.1097/HJH.0000000000000325
10. Wang J, Sun W, Wells GA, Li Z, Li T, Wu J, et al. Differences in prevalence of hypertension and associated risk factors in urban and rural residents of the northeastern region of the people’s republic of China: a cross-sectional study. PLoS One. (2018) 13(4):e0195340. doi: 10.1371/journal.pone.0195340
11. Dorgbetor CI, Dickson KS, Ameyaw EK, Adde KS. Prevalence and associated factors of hypertension among women in southern Ghana: evidence from 2014 GDHS. Int J Hypertens. (2022) 2022:9700160. doi: 10.1155/2022/9700160
12. Moazzeni SS, Asgari S, Azizi F, Hadaegh F. Live birth/parity number and the risk of incident hypertension among parous women during over 13 years of follow-up. J Clin Hypertens. (2021) 23(11):2000. doi: 10.1111/jch.14369
13. Giubertoni E, Bertelli L, Bartolacelli Y, Origliani G, Modena MG. Parity as predictor of early hypertension during menopausal transition. J Hypertens. (2013) 31(3):501–7. doi: 10.1097/HJH.0b013e32835c1742
14. Liu LX, Arany Z. Maternal cardiac metabolism in pregnancy. Cardiovasc Res. (2014) 101(4):545–53. doi: 10.1093/cvr/cvu009
15. Liu D, Zhang M, Liu Y, Sun X, Yin Z, Li H, et al. Association of hypertension with parity and with the interaction between parity and body mass index in rural Chinese women. J Am Soc Hypertens. (2018) 12(11):789–97. doi: 10.1016/j.jash.2018.09.005
16. Khalid ME. TThe effect of age, obesity and parity on blood pressure and hypertension in non-pregnant married women. J Family Community Med. (2006) 13(3):103. doi: 10.4103/2230-8229.97536
17. Adu C, Frimpong JB, Mohammed A, Tetteh JK, Budu E, Ahinkorah BO, et al. Safer sex negotiation and parity among women in sub-saharan Africa. J Biosoc Sci. (2023) 55(1):74–86. doi: 10.1017/S0021932021000651
18. Shakil SS, Ojji D, Longenecker CT, Roth GA. Early stage and established hypertension in sub-saharan Africa: results from population health surveys in 17 countries, 2010-2017. Circ Cardiovasc Qual Outcomes. (2022) 15(12):E009046. doi: 10.1161/CIRCOUTCOMES.122.009046
19. Omar SM, Musa IR, Osman OE, Adam I. Prevalence and associated factors of hypertension among adults in Gadarif in eastern Sudan : a community-based study. BMC Public Health. (2020) 20(1):291. doi: 10.1186/s12889-020-8386-5
20. Alsammani MA, Jafer AM, Khieri SA, Ali AO, Shaaeldin MA. Effect of grand multiparity on pregnancy outcomes in women under 35 years of age: a comparative study. Med Arch. (2019) 73(2):92–6. doi: 10.5455/medarh.2019.73.92-96
21. 5th Sudan Population and Housing Census - 2008. 2009; (April 2009). Available at: Available at: https://microdata.worldbank.org/index.php/catalog/1014 (Acessed 9/25/2023).
22. Cuschieri S. The STROBE guidelines. Saudi J Anaesth. (2019) 13(Suppl 1):S31. doi: 10.4103/sja.SJA_543_18
23. Riley L, Guthold R, Cowan M, Savin S, Bhatti L, Armstrong T, et al. The world health organization STEPwise approach to noncommunicable disease risk-factor surveillance: methods, challenges, and opportunities. Am J Public Health. (2016) 106(1):74–8. doi: 10.2105/AJPH.2015.302962
24. Meng L, Zhao D, Pan Y, Ding W, Wei Q, Li H, et al. Validation of Omron HBP-1300 professional blood pressure monitor based on auscultation in children and adults. BMC Cardiovasc Disord. (2016) 16(1):9. doi: 10.1186/s12872-015-0177-z
25. Obesity : preventing and managing the global epidemic : report of a WHO consultation. Available at: https://apps.who.int/iris/handle/10665/42330
26. Prenatal Care. In: Cunningham F, Leveno KJ, Dashe JS, Hoffman BL, Spong CY, Casey BM, editors. Williams Obstetrics, 26e. McGraw Hill (2022). Available at: https://accessmedicine.mhmedical.com/content.aspx?bookid=2977§ionid=249763458 (Accessed October 17, 2023).
27. OpenEpi Menu. Available at: http://wwww.openepi.com/Menu/OE_Menu.htm
28. Elmugabil A, Alhabrdi NM, Rayis DA, Al-Wutayd O, Adam I. Evaluation of the association between haemoglobin levels and preterm birth at Khartoum, Sudan: a hospital-based study. Front Nutr. (2022) 9:933557. doi: 10.3389/fnut.2022.933557
29. Gedamu DK, Sisay W. Prevalence of hypertension and associated factors among public servants in north wollo zone, amhara region, Ethiopia, 2020. Vasc Health Risk Manag. (2021) 17:363–70. doi: 10.2147/VHRM.S298138
30. Taylor JY, Sampson DA, Anderson CM, Caldwell D, Taylor AD. Effects of parity on blood pressure among west African dogon women. Ethn Dis. (2012) 22(3):360. PMID: 22870582.22870582
31. Erem C, Hacihasanoglu A, Kocak M, Deger O, Topbas M. Prevalence of prehypertension and hypertension and associated risk factors among turkish adults: trabzon hypertension study. J Public Health (Oxf). (2009) 31(1):47–58. doi: 10.1093/pubmed/fdn078
32. Akter S, Jesmin S, Rahman MM, Islam MM, Khatun MT, Yamaguchi N, et al. Higher gravidity and parity are associated with increased prevalence of metabolic syndrome among rural Bangladeshi women. PLoS One. (2013) 8(8):e68319. doi: 10.1371/journal.pone.0068319
33. Connelly PJ, Azizi Z, Alipour P, Delles C, Pilote L, Raparelli V. The importance of gender to understand sex differences in cardiovascular disease. Can J Cardiol. (2021) 37(5):699–710. doi: 10.1016/j.cjca.2021.02.005
34. Ford ND, Robbins CL, Hayes DK, Ko JY, Loustalot F. Prevalence, treatment, and control of hypertension among US women of reproductive age by race/hispanic origin. Am J Hypertens. (2022) 35(8):723–30. doi: 10.1093/ajh/hpac053
35. Fraser A, Catov JM. Placental syndromes and long-term risk of hypertension. J Hum Hypertens. (2023) 37(8):671–4. doi: 10.1038/s41371-023-00802-4
36. Ezeigwe A, Ogunmoroti O, Minhas AS, Rodriguez CP, Kazzi B, Fashanu OE, et al. Association between parity and markers of inflammation: the multi-ethnic study of atherosclerosis. Front Cardiovasc Med. (2022) 9:922367. doi: 10.3389/fcvm.2022.922367
37. Modena MG. Hypertension in postmenopausal women: how to approach hypertension in menopause. High Blood Press Cardiovasc Prev. (2014) 21(3):201–4. doi: 10.1007/s40292-014-0057-0
38. Lu KT, Keen HL, Weatherford ET, Sequeira-Lopez MLS, Gomez RA, Sigmund CD. Estrogen receptor α is required for maintaining baseline renin expression. Hypertens (Dallas, Tex 1979). (2016) 67(5):992–9. doi: 10.1161/HYPERTENSIONAHA.115.07082
39. Stamatelopoulos K, Apostolakis M, Augoulea A, Paschou SA, Armeni E, Panoulis K, et al. Predictors of incident hypertension in healthy non-diabetic postmenopausal women with normal renal function. Gynecol Endocrinol. (2019) 35(12):1063–6. doi: 10.1080/0951359020191630607
40. Brodowski L, Rochow N, Yousuf EI, Kohls F, Von Kaisenberg CS, Schild RL, et al. The cumulative impact of parity on the body mass index (BMI) in a non-selected lower saxony population. J Perinat Med. (2020) 49(4):460–7. doi: 10.1515/jpm-2020-0261
41. Li W, Ruan W, Lu Z, Wang D. Parity and risk of maternal cardiovascular disease: a dose-response meta-analysis of cohort studies. Eur J Prev Cardiol. (2019) 26(6):592–602. doi: 10.1177/2047487318818265
Keywords: parity, age, hypertension, associated factor, Sudan, body mass index
Citation: Musa IR, Osman OE and Adam I (2023) The association between parity and hypertension: a cross-sectional, community-based study. Front. Cardiovasc. Med. 10:1247244. doi: 10.3389/fcvm.2023.1247244
Received: 25 June 2023; Accepted: 13 October 2023;
Published: 23 October 2023.
Edited by:
Elise Peery Gomez-Sanchez, University of Mississippi Medical Center, United StatesReviewed by:
Jessica Faulkner, Augusta University, United States© 2023 Musa, Osman and Adam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ishag Adam aXNoYWdhZGFtQGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.