
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med., 23 August 2023
Sec. Cardiovascular Imaging
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1245213
This article is part of the Research TopicCase Reports in Cardiovascular Imaging: 2023View all 13 articles
 Yi Yu1*†
Yi Yu1*† Ming Ding2,†
Ming Ding2,† Jin-Lan Chen1
Jin-Lan Chen1 Ting Wang1
Ting Wang1 Yu-Han Chen1
Yu-Han Chen1 Xiao-Min Yang1
Xiao-Min Yang1 Su-Yun Chen3
Su-Yun Chen3 Yue-Peng Wang1
Yue-Peng Wang1 Yi-Gang Li1*
Yi-Gang Li1*
Background: Lipomatous atrial septal hypertrophy (LASH) with atrial septal defect (ASD) is a rare congenital anomaly. Although LASH is a histologically benign cardiac lesion characterized by excessive fat deposition in the interatrial septum that spares the fossa ovale, it has been associated with supraventricular arrhythmias or sick sinus syndrome. Application of multimodal imaging is crucial for accurate diagnosis, appropriate treatment of LASH with ASD, and follow-up.
Case summary: A 68-year-old female patient presented with recurrent chest tightness and palpitation. Multimodal imaging revealed the characterizations of LASH and ASD. Two-dimensional transesophageal echocardiography showed a “dumbbell”-shaped involvement of the cephalad and caudal regions with sparing of a single secundum ASD. The septum with a brightness feature is an uncommon condition characterized by the deposition of unencapsulated fat cells in the atrial septum. Real-time four-dimensional transesophageal echocardiography reflected the lipomatous hypertrophy of the atrial septum and an oval-shaped ASD. Cardiac computer tomography angiography later confirmed this finding. The patient achieved a good clinical response with an ASD percutaneous occlusion guided by intracardiac echocardiography (ICE).
Conclusion: This case demonstrates a LASH combined with ASD. Multimodality imaging can provide an accurate diagnosis and may guide the procedure for precise occlusion.
Lipomatous atrial septal hypertrophy (LASH) with atrial septal defect (ASD) is a rare abnormality. LASH is a histologically benign cardiac lesion characterized by excessive fat deposition in the interatrial septum that spares the fossa ovale (1–3). The prevalence rate of LASH was reported at approximately 2.2% in patients referred for a multislice computed tomography (CT) scan and 8% in patients undergoing transesophageal echocardiography (TEE) (1). Only a few cases of LASH were found to be related to hemodynamic alterations (congestive heart failure, superior vena cava obstruction), and surgical intervention should only be reserved for patients who show marked superior vena cava or right atrium obstruction (4–8). Although LASH has been associated with supraventricular arrhythmias (9, 10) or sick sinus syndrome (11), there are few reports in the literature on patients with both LASH and ASD (9, 12). Advances in multimodal imaging techniques may aid in diagnosing, treating, and following up LASH with ASD (13).
A 68-year-old female patient with a 10-year history of hypertension was hospitalized in our department due to recurrent chest tightness and palpitation for half a month, accompanied by limb edema and dizziness. She had a history of multiple ground-glass nodules in both lungs, pulmonary bullae in the lower lobe of the right lung, esophageal papilloma, reflux esophagitis, gastric body submucosal eminence, and erosive gastritis. On presentation, she was afebrile, with a heart rate of 75 beats per minute, a blood pressure of 175/90 mmHg, and normal oxygen saturation.
An electrocardiogram documented that the patient had sinus arrhythmia and intermittent atrial premature beats, with short atrial tachycardia. The levels of serum N-terminal pro-B-type natriuretic peptide (NT-proBNP) (20.59 pg/ml) were elevated. The glycosylated hemoglobin level was 6.5%. Transthoracic echocardiography showed a secundum ASD with a size of 12 mm × 13.2 mm, and the interatrial septum was significantly thickened (19.4 mm). The right atrium and right ventricle were dilated, with a pulmonary-to-systemic flow ratio (Qp/Qs) of 2.0. There was mild tricuspid regurgitation, and the systolic pulmonary artery pressure was ≈49.8 mm Hg. The global left ventricle ejection fraction measured using the Simpson method was 63.4%, and grade I diastolic dysfunction was detected.
Two-dimensional transesophageal echocardiography revealed a “dumbbell”-shaped involvement of the cephalad and caudal regions with sparing of a single secundum ASD with a size of 9 mm × 13.2 mm. The maximal thickness of the interatrial septum was 20.7 mm, and the thinnest atrial septum was 1.6 mm from the view at 0°–180°. The atrial septum presented with a brightness feature, a rare condition characterized by the deposition of unencapsulated fat cells in this area (Figures 1A,B). There was no obstruction in the superior and inferior vena cava. A two-dimensional color Doppler ultrasound evidenced a left-to-right shunt through ASD (Figures 1C,D). The diagnosis of LASH with ASD was established based on the above findings. Real-time four-dimensional transesophageal echocardiography (RT4D-TEE) confirmed the lipomatous hypertrophy of the atrial septum and an oval-shaped ASD (Figure 1E and Supplementary Movie I). In addition, a color Doppler ultrasound of RT4D-TEE demonstrated the defect appeared foraminal in a location with a significant left-to-right shunt, suggesting a “foraminal” or “fossa” ASD (Figure 1F and Supplementary Movie II).
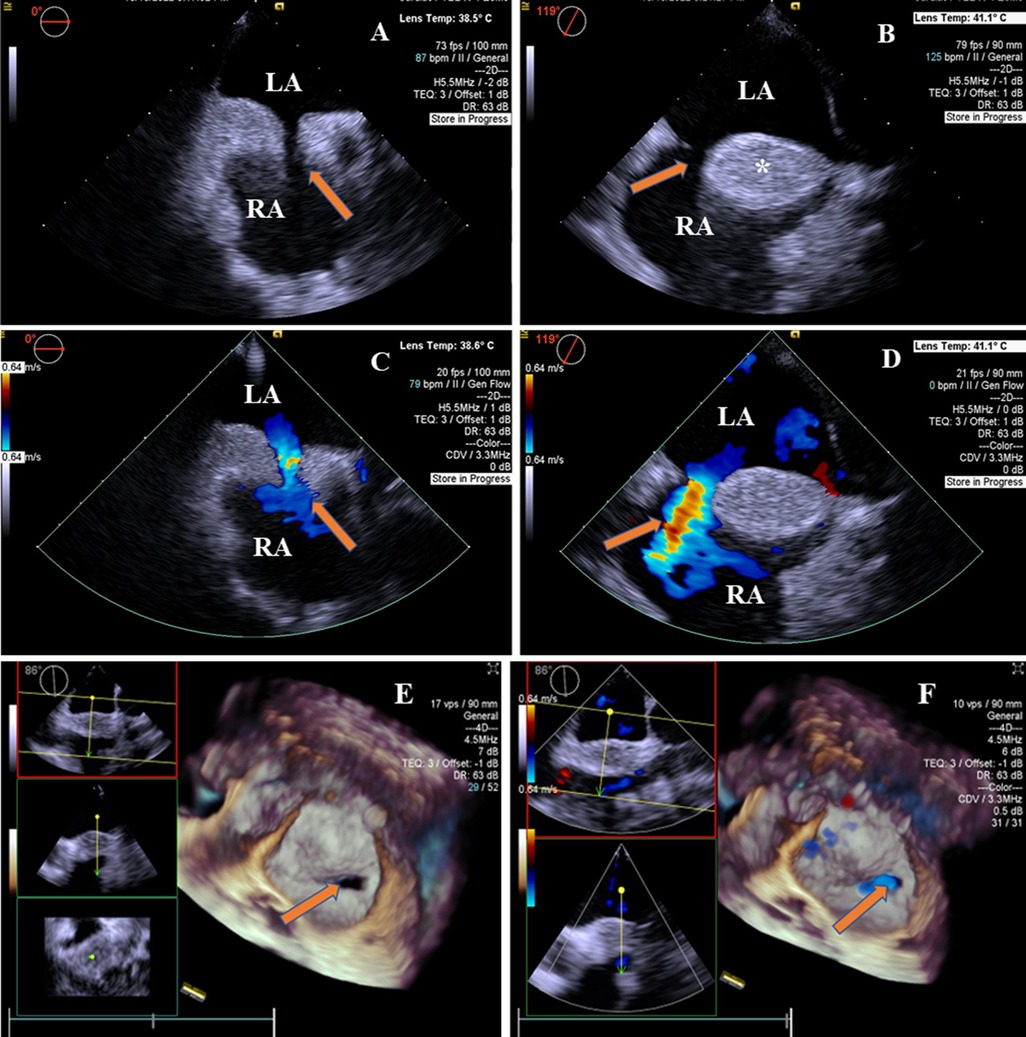
Figure 1. 2D-TEE and 4D-TEE images revealed lipomatous hypertrophy of the atrial septum with atrial septal defect. (A) On 2D-TEE imaging, a single secundum ASD (arrow) and interatrial septum thickened to 20.7 mm in diameter were observed from 0° view. The septum with brightness features is characterized by the deposition of unencapsulated fat cells in the atrial septum. (B) 2D-TEE further documented a “dumbbell”-shaped involvement of the cephalad and caudal regions with sparing of the fossa ovalis (arrow) from 119° view. The interatrial septal fatty infiltration was demonstrated (*). (C) Color Doppler ultrasound of 2D-TEE showing a blood shunt from the left atrium to the right atrium (arrow) from 0° view. (D) Color Doppler ultrasound of 2D-TEE showing left-to-right shunt through ASD (arrow) from 119° view. (E) RT4D-TEE imaging reflected the whole ASD was surrounded by lipomatous hypertrophy of atrial septum (arrow) from 86° view. (F) Color Doppler ultrasound of RT4D-TEE showing blood flow of the ASD from the left to the right atrium (arrow). LA, left atrium; RA, right atrium.
A cardiac computer tomography angiography (CCTA) scan was performed to further characterize the atrial septum and evaluate the status of the coronary artery. The CCTA images demonstrated a 9 mm × 13 mm defect on the atrial septum (Figures 2A,B). The volume rendering image of the CCTA scan showed a non-enhancing, smooth, well-marginated mass with an appearance similar to subcutaneous fat (Figures 2C,D). In addition, the CCTA scan revealed a myocardial bridge in the middle of the left anterior descending artery.
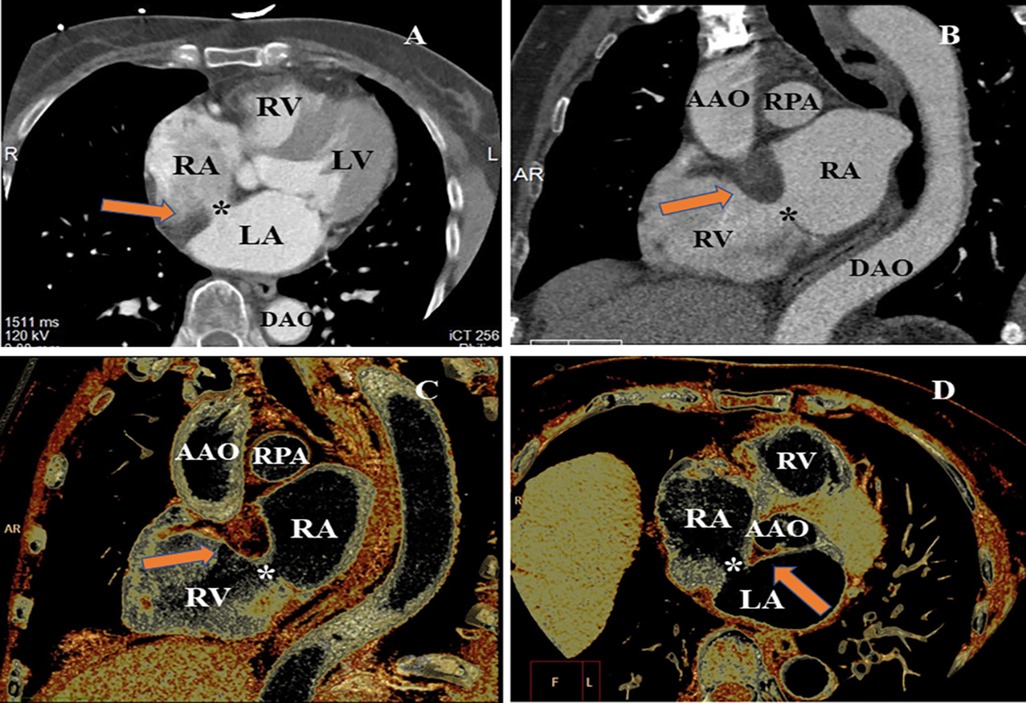
Figure 2. CCTA evaluating the LASH with ASD from different perspectives. (A) MPR image of CCTA found the loss of echogenicity between the left atrium and the right atrium (*). (B) MPR showed significant hypertrophy of the atrial septum (arrow), producing a dumbbell shape. (C) VR image indicated the thickened interatrial septum (arrow). (D) VR image of CCTA demonstrating the ASD and significant hypertrophy of atrial septum (arrow and *). MPR, multiplanar reconstruction; VR, volume rendering; LV, left ventricle; RV, right ventricle; RPA, right pulmonary artery; AAO, ascending aorta; DAO, descending aorta.
Myocardial perfusion imaging was performed to evaluate metabolic activity. Results showed no hypermetabolic lesions or abnormal myocardial blood perfusion in the heart. The left ventricular systolic and diastolic functions were normal.
The patient underwent an ASD percutaneous occlusion under the guidance of intracardiac echocardiography (ICE) (Supplementary Movie III). During the operation, the LASH with ASD could be visualized by ICE from different angles. After balloon sizing, an 18 mm septal occluder device (Pushi, Shanghai) was successfully deployed (Figures 3A,B and Supplementary Movie IV). The occluder embraced the thick lipomatous cephalad rim and the thin “normal” caudal rim of the fossa ovalis. The left-to-right shunt disappeared, and no procedural complications, such as erosion or embolization, were observed. The patient was asymptomatic postoperation and followed up in an outpatient clinic (Supplementary Figures 1A–C). The atrial septum no longer increased in thickness. The systolic pulmonary artery pressure was ≈25.8 mmHg after ASD closure.
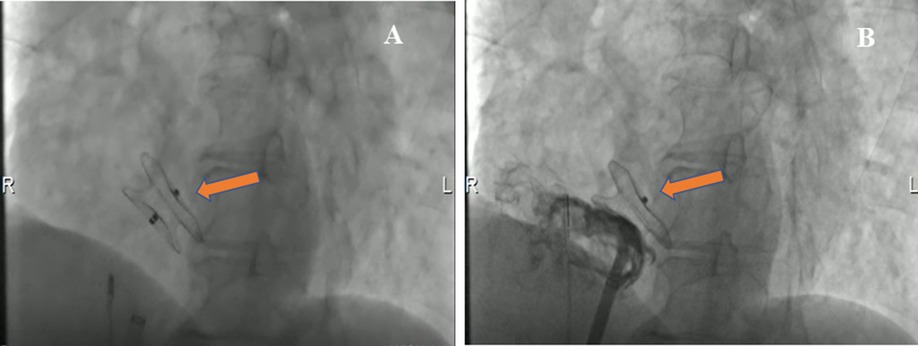
Figure 3. Cardioangiography (CAG) images demonstrating the closure with a Pushi septal occluder device. (A) CAG image visualized the position of a Pushi septal occluder device during the operation (arrow). (B) CAG image revealed the position of the septal occluder device was good, and there was no shunt between the left atrium and the right atrium after occlusion (arrow).
The patient was examined using multimodality imaging, including two-dimensional TEE (2D-TEE), RT4D-TEE, and CCTA. The diagnostic features of LASH with ASD include the following: (1) initial 2D-TEE imaging showed a “dumbbell”-shaped involvement of the cephalad and caudal regions with sparing of a single secundum ASD, (2) RT4D-TEE demonstrated lipomatous hypertrophy of the atrial septum and an oval-shaped ASD with a significant left-to-right shunt, and (3) CCTA images demonstrated the defect structure of the atrial septum and showed a non-enhancing, smooth, well-marginated mass with an appearance similar to subcutaneous fat.
The pathognomonic “dumbbell” shape is due to hypertrophy of the septum primum and secundum with sparing of the fossa ovalis (12). Echocardiographic features of LASH include a diffuse, echo-dense globular thickening anteroinferior or posterosuperior, and the magnitude of fat accumulation is >15 mm in thickness (14). As we all know, most cases of LASH are benign; nevertheless, patients with significant hypertrophy may develop obstruction of right atrial filling, dyspnea, or symptoms similar to congestive heart failure (15). A massive LASH larger than 20 mm may alter the nearby atrial musculature, leading to disturbed atrial conduction, resulting in arrhythmias (16–18). Heyer et al. (19) and Breuer et al. (6) reported that the obstruction of the right atrium by massive septal hypertrophy potentially requires surgical resection. In addition, older age and obesity are contributors to the pathogenesis of LASH; both are risk factors for the development of atrial fibrillation. However, there are few reports in the literature on patients with both LASH and ASD (9). Moir et al. (20) reported a case of successful percutaneous transcatheter closure using an FSO device for combined LASH and ASD with rim deficiency. In our case, 2D-TEE imaging indicated that LASH was associated with ASD, as evidenced by a left-to-right shunt flow signal on TEE. Finally, using 4D imaging, we confirmed the diagnosis of LASH combined with ASD, an uncommon condition illustrated as the deposition of unencapsulated fat cells in the atrial septum and oval hole.
In addition, the images could be misinterpreted as a tumor or other structural abnormalities (21–23). Masses in and near the interatrial septum may be either benign or malignant tumors. Kleiman et al. (22) reported a left atrial myxoma attached to the interatrial septum, increasing its thickness, a condition known as LASH. They then made an accurate diagnosis through TEE imaging. The interatrial septum was well visualized by echocardiography, although the image quality with TTE imaging is suboptimal compared with that of TEE imaging. If diagnosing a mass in or near the atrial septum is difficult, other available imaging modalities include cardiac magnetic resonance (CMR) imaging, myocardial perfusion imaging, and PET-CT. CMR imaging is useful in elaborating on imaging features, such as the location, shape, and signal intensity of LASH. It can reveal the presence of fatty tissues in the interatrial septum with the characteristic “dumbbell” shape and confirm the diagnosis. CMR can evaluate potential obstructions in the inflow of the right atrium and outflow of the right ventricle and accurately diagnose benign and malignant tumors (3, 24).
In this case, TEE played an important role in diagnosing LASH with ASD. Multiple sectional views at 0°–180° could be presented during TEE examinations (25), which help visualize LASH with ASD and exclude cardiac tumors. However, in patients with significant hypertrophy of the interatrial septum, diagnosing LASH with ASD by 2D-TEE may be technically challenging. Thus, it is crucial to further evaluate LASH with ASD in one cardiac cycle using RT4D-TEE imaging, which could provide some additional valuable information. By imaging in >1 RT4D-TEE planes, LASH can be seen and confirmed with an oval hole in the center of the atrial septum. In addition, RT4D-TEE offers a thorough evaluation of the interatrial septum. Thus, it can visualize the entire significant hypertrophy of the interatrial septum and ASD in the center of the septum. Meanwhile, the diagnosis of LASH should exclude atrial septal tumors. LASH has identified a uniform internal echo of the atrial septal tissue, distinct from the appearance of the neoplasm. Next, the high spatial resolution of CCTA has the advantage of visualizing significant hypertrophy of the atrial septum. ICE may provide additional information to aid a successful operation. It used to believe that catheter-based closure of ASD was contraindicated in patients with LASH (14). Lin et al. (12) reported a successful closure of ASDs in two patients with LASH using the Amplatzer muscular ventricular septal defect closure device. This device fitted well to the atrial septum and had no residual shunts at the 1-month follow-up. In our case, intraoperative ICE guidance combined with preoperative imaging examination results is safer, and the ASD occluder can be selected more accurately. To the best of our knowledge, this study is the first report of a successful percutaneous transcatheter closure using a Pushi septal occluder device for ASD combined with LASH.
In conclusion, in this case, the multimodality imaging techniques, especially for RT4D-TEE imaging, are pivotal for diagnosing a rare LASH with ASD and for deciding to perform the occlusion of ASD guided by ICE.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine approved the studies involving humans. The studies were conducted in accordance with local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent for the publication of any potentially identifiable images or data included in this article was obtained from the individual(s).
YY participated in conception and design. Y-GL and Y-PW provided administrative support. S-YC and J-LC provided materials or patients. MD and TW performed collection and assembly of data. X-MY and Y-HC performed data analysis and interpretation. All authors contributed to the article and approved the submitted version.
Funding for this study was provided in part by grant number 17411954800 from Shanghai Science and Technology Committee Clinical Field Project Fund (YY), grant number 202240110 from the Shanghai Health and Family Planning Commission (YY), and grant number XHKC2021-07 from Xinhua Hospital Affiliated with the School of Medicine (YY).
We appreciate the doctors in the Department of Cardiology, Xinhua Hospital of Jiaotong University, for treating these patients.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1245213/full#supplementary-material
1. Laura DM, Donnino R, Kim EE, Benenstein R, Freedberg RS, Saric M. Lipomatous atrial septal hypertrophy: a review of its anatomy, pathophysiology, multimodality imaging, and relevance to percutaneous interventions. J Am Soc Echocardiogr. (2016) 29:717–23. doi: 10.1016/j.echo.2016.04.014
2. Xanthos T, Giannakopoulos N, Papadimitriou L. Lipomatous hypertrophy of the interatrial septum: a pathological and clinical approach. Int J Cardiol. (2007) 121:4–8. doi: 10.1016/j.ijcard.2006.11.150
3. Xanthopoulos A, Giamouzis G, Alexopoulos N, Kitai T, Triposkiadis F, Skoularigis J. Lipomatous hypertrophy of the interatrial septum: a case report and review of the literature. CASE. (2017) 1:182–9. doi: 10.1016/j.case.2017.06.005
4. Takayama S, Sukekawa H, Arimoto T, Ikeno E, Komatu T, Rikimaru H, et al. Lipomatous hypertrophy of the interatrial septum with cutaneous lipomatosis. Circ J. (2007) 71:986–9. doi: 10.1253/circj.71.986
5. Bielicki G, Lukaszewski M, Kosiorowska K, Jakubaszko J, Nowicki R, Jasinski M. Lipomatous hypertrophy of the atrial septum—a benign heart anomaly causing unexpected surgical problems: a case report. BMC Cardiovasc Disord. (2018) 18:152. doi: 10.1186/s12872-018-0892-3
6. Breuer M, Wippermann J, Franke U, Wahlers T. Lipomatous hypertrophy of the interatrial septum and upper right atrial inflow obstruction. Eur J Cardiothorac Surg. (2002) 22:1023–5. doi: 10.1016/S1010-7940(02)00619-X
7. Tugcu A, Yildirimturk O, Rizaoglu E, Sagbas E, Akpinar B, Aytekin S. Lipomatous hypertrophy of the interatrial septum presenting as an obstructive right atrial mass in a patient with exertional dyspnea. J Am Soc Echocardiogr. (2007) 20:1319.e3–5. doi: 10.1016/j.echo.2007.04.012
8. Søholm H, Iversen K, Olsen PS, Andersen CB, Hassager C. Superior vena cava syndrome as a rare complication to lipomatous atrial septal hypertrophy (LASH). Eur Heart J Cardiovasc Imaging. (2013) 14:717. doi: 10.1093/ehjci/jes324
9. Takafuji H, Obunai K, Kato N, Honda M, Watanabe H. Lipomatous atrial septal hypertrophy and atrial septal defect with rim deficiency. JACC Cardiovasc Interv. (2022) 15:e31–3. doi: 10.1016/j.jcin.2021.09.040
10. Brienesse SC, Sugito S, Mejia R, Leitch J, Wilsmore B. An electrophysiological and anatomical space-occupying lesion: lipomatous hypertrophy of the interatrial septum in a patient presenting with atrial tachycardia. Heart Rhythm Case Reports. (2021) 7:542–5. doi: 10.1016/j.hrcr.2021.05.003
11. Sato Y, Matsuo S, Kusama J, Kunimasa T, Yoda S, Matsumoto N, et al. Lipomatous hypertrophy of the interatrial septum presenting as sick sinus syndrome. Int J Cardiol. (2007) 119:280–1. doi: 10.1016/j.ijcard.2006.07.161
12. Lin CH, Balzer DT, Lasala JM. Defect closure in the lipomatous hypertrophied atrial septum with the Amplatzer muscular ventricular septal defect closure device: a case series. Catheter Cardiovasc Interv. (2011) 78:102–7. doi: 10.1002/ccd.22858
13. Czekajska-Chehab E, Tomaszewska M, Olchowik G, Tomaszewski M, Adamczyk P, Drop A. Lipomatous hypertrophy of the interatrial septum in ECG-gated multislice computed tomography of the heart. Med Sci Monit. (2012) 18:MT54–9. doi: 10.12659/MSM.883197
14. Zanchetta M, Rigatelli G, Pedon L, Zennaro M, Maiolino P, Onorato E. Role of intracardiac echocardiography in atrial septal abnormalities. J Intervent Cardiol. (2003) 16:63–77. doi: 10.1046/j.1540-8183.2003.08004.x
15. Laura DM, Donnino R, Kim EE, Benenstein R, Freedberg RS, Saric M. Lipomatous atrial septal hypertrophy: a review of its anatomy, pathophysiology, multimodality imaging, and relevance to percutaneous interventions. J Am Soc Echocardiogr. (2016) 8:717–23. doi: 10.1016/j.echo.2016.04.014
16. Augoustides JG, Weiss SJ, Ochroch AE, Weiner J, Mancini J, Savino JS, et al. Analysis of the interatrial septum by transesophageal echocardiography in adult cardiac surgical patients: anatomic variants and correlation with patent foramen ovale. J Cardiothorac Vasc Anesth. (2005) 19:146–9. doi: 10.1053/j.jvca.2005.01.021
17. López-Candales A. Is the presence of interatrial septal hypertrophy a marker for atrial fibrillation in the elderly? Am J Geriatr Cardiol. (2002) 11:399–403. doi: 10.1111/j.1076-7460.2002.01629.x
18. Abboud H, Brochet E, Amarenco P. Lipomatous hypertrophy of the inter-atrial septum and stroke. Cerebrovasc Dis. (2004) 18:178. doi: 10.1159/000079740
19. Heyer CM, Kagel T, Lemburg SP, Bauer TT, Nicolas V. Lipomatous hypertrophy of the interatrial septum. Chest. (2003) 124:2068–73. doi: 10.1378/chest.124.6.2068
20. Moir WS, McGaw DJ, Harper RW, Gelman J. Atrial septal defect device closure in a patient with lipomatous hypertrophy of the atrial septum. Circulation. (2003) 107:e217. doi: 10.1161/01.CIR.0000069908.63932.0A
21. Agmon Y, Meissner I, Tajik AJ, Seward JB, Petterson TM, Christianson TJH, et al. Clinical, laboratory, and transesophageal echocardiographic correlates of interatrial septal thickness: a population-based transesophageal echocardiographic study. J Am Soc Echocardiogr. (2005) 18:175–82. doi: 10.1016/j.echo.2004.09.002
22. Kleiman AM, Harding LM, Bechtel AJ. Concomitant lipomatous hypertrophy and left atrial mass: distinguishing benign from malignant. Echocardiography. (2018) 35:534–6. doi: 10.1111/echo.13834
23. Bassareo PP, Tumbarello R, Mercuro G. Cor triatriatum and lipomatous hypertrophy of the interatrial septum in the elderly: a case report. Cardiovasc Ultrasound. (2010) 8:4. doi: 10.1186/1476-7120-8-4
24. Hudzik B, Filipiak K, Zembala M, Szkodzinski J, Miszalski-Jamka K, Niklewski T, et al. Lipomatous hypertrophy of the interatrial septum: a rare cause of right ventricular impairment. J Card Surg. (2010) 25:171–4. doi: 10.1111/j.1540-8191.2009.00961.x
25. Silvestry FE, Cohen MS, Armsby LB, Burkule NJ, Fleishman CE, Hijazi ZM, et al. Guidelines for the echocardiographic assessment of atrial septal defect and patent foramen ovale: from the American Society of Echocardiography and Society for Cardiac Angiography and Interventions. J Am Soc Echocardiogr. (2015) 28:910–58. doi: 10.1016/j.echo.2015.05.015
Keywords: lipomatous atrial septal hypertrophy, atrial septal defect, multimodality imaging, transesophageal echocardiography, case report
Citation: Yu Y, Ding M, Chen J-L, Wang T, Chen Y-H, Yang X-M, Chen S-Y, Wang Y-P and Li Y-G (2023) Multimodality imaging in diagnosing lipomatous atrial septal hypertrophy with atrial septal defect: a case report. Front. Cardiovasc. Med. 10:1245213. doi: 10.3389/fcvm.2023.1245213
Received: 23 June 2023; Accepted: 7 August 2023;
Published: 23 August 2023.
Edited by:
Riccardo Liga, Pisana University Hospital, ItalyReviewed by:
Amitoj Singh, University of Arizona, United States© 2023 Yu, Ding, Chen, Wang, Chen, Yang, Chen, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Yu eXV5aTAxQHhpbmh1YW1lZC5jb20uY24= Yi-Gang Li bGl5aWdhbmdAeGluaHVhbWVkLmNvbS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.