- 1Department of Cardiovascular Medicine, Saga University, Saga, Japan
- 2Department of Medical and Health Information Management, National Cerebral and Cardiovascular Center, Suita, Osaka, Japan
- 3The Department of Cardiovascular Medicine, The University of Tokyo, Tokyo, Japan
- 4The Department of Advanced Cardiology, The University of Tokyo, Tokyo, Japan
- 5Department of Urology, Saga University, Saga, Japan
Introduction: Benign prostate hyperplasia (BPH) and prostate cancer (PCa) are major prostate diseases that potentially share cardiometabolic risk factors and an elevated risk for cardiovascular disease (CVD). However, the prevalence of prostate diseases among patients with established CVD remains unclear.
Materials and methods: This nationwide retrospective study assessed the prevalence and temporal trend of prostate diseases (i.e., BPH or PCa) among patients hospitalized for CVDs in Japan. We used a claims database (the Japanese Registry of All Cardiac and Vascular Diseases–Diagnosis Procedure Combination), which included data on 6,078,487 male patients recorded from 1,058 hospitals between April 2012 and March 2020. We conducted the Cochran–Armitage trend test and calculated the adjusted odds ratio (aOR) with 95% confidence intervals (CIs).
Results: The prevalence of prostate diseases over the entire study period was 5.7% (BPH, 4.4%; PCa, 1.6%). When dividing the overall cohort into age categories (<65, 65–74, and ≥75 years old), the prevalence was 1.1%, 4.7%, and 9.9%, respectively (P for trend <0.05). In addition, the annual prevalence showed a modest increasing trend over time. Patients admitted for heart failure (HF) were significantly associated with a higher incidence of coexisting prostate diseases than those admitted for non-HF causes [aOR 1.02 (95% CI, 1.01–1.03)] or acute coronary syndrome [aOR 1.19 (95% CI, 1.17–1.22)].
Conclusions: The nationwide real-world database revealed that the prevalence of prostate diseases is increasing among patients hospitalized for CVD, particularly HF. Attention to detailed causality and continued surveillance are needed to further clarify the clinical characteristics of prostate diseases among patients with CVD.
Introduction
Benign prostate hyperplasia (BPH) and prostate cancer (PCa) are major prostate diseases among older men, and the global burden of these diseases continues to increase in the current aging and long-lived society (1–5). BPH is the most common cause of male lower urinary tract symptoms (LUTS) (6), adversely affecting the quality of life and cardiovascular outcomes (7). Patients with PCa also have an increased risk of developing cardiovascular diseases (CVDs) (8), which are a major cause of noncancer death among PCa survivors (9, 10).
The incidence of major CVDs, such as coronary heart disease and heart failure (HF), generally increases as age, dysregulated cardiometabolic risk factors, and inflammation increase (11–13). Furthermore, metabolic syndrome and coexisting proinflammatory status play a pivotal role in the pathogenesis of major prostate diseases, including BPH and PCa, and disrupted cardiometabolic conditions are strongly associated with an increased prevalence of these prostate diseases (14–18). Hence, prostate diseases share foundational risk factors with CVD and can represent an aspect of cardiometabolic syndrome (19). Therefore, a biological rationale for close interplay exists between such prostate diseases and CVD entities.
Numerous epidemiological and observational studies have shown an increased risk for CVD among patients with major prostate diseases (7, 8). This finding is helpful, especially for urologists (rather than cardiologists), allowing them to recognize that male patients with prostate diseases have a substantial risk for CVD and require a careful cardiovascular risk assessment in the urological care setting (20, 21). However, real-world clinical reports on the prevalence of prostate diseases among patients with entire or specific CVD are limited. Although prostate diseases are closely linked to CVD, knowledge and evidence concerning the burden of prostate diseases in the cardiovascular care setting are still insufficient.
The present study therefore assessed the burden of major prostate diseases and recent associated temporal trends among hospitalized patients with established CVD using a nationwide real-world database collected throughout Japan.
Materials and methods
Ethics
This study was approved by the Japanese Circulation Society (JCS) (No. 2020-08) and the Ethics Committee of Saga University Hospital (No. 2021-08-01 on November 01, 2021) and conducted according to the principles of the Declaration of Helsinki. The database used in this study deidentified personal information; therefore, informed consent from our study participants was not needed.
Data sources and availability
This retrospective study obtained data from the Japanese Registry of All Cardiac and Vascular Diseases–Diagnosis Procedure Combination (JROAD-DPC), a nationwide claims database developed by the JCS. Details in the database were previously described elsewhere (22–25). In brief, the JROAD-DPC database, which contains data from cardiovascular training facilities certified by the JCS, includes inpatients' clinical information, such as their age, sex, diagnosis, comorbidity, hospitalization duration, and discharge status. Patients' diagnoses and comorbidities were coded using the International Classification of Disease and Related Health Problems 10th version (ICD-10) codes.
Given that the JROAD-DPC dataset is owned by the JCS for the purpose of research, the data that support our findings will not be shared without JCS' permission. The dataset will be available upon reasonable request from researchers and after approval by the JCS. Inquiries are to be addressed to the JCS (contact viaaXRkYXRhYmFzZUBqLWNpcmMub3IuanA=).
Diagnoses, measurements, and definitions
In the JROAD-DPC database, each diagnosis is coded for the main diagnosis, admission-precipitating diagnosis, most resource-consuming diagnosis, and second-most resource-consuming diagnosis. A maximum of 10 diagnoses are coded for comorbidities encountered during admission and conditions that arise after admission.
We defined prostate diseases as BPH and PCa, coded as N40 and C61 in the ICD-10 codes, respectively, and identified them in any of the following data categories: the main diagnosis, admission-precipitating diagnosis, most resource-consuming diagnosis, second-most resource-consuming diagnosis, comorbidities during admission, and conditions arising after admission. Complications (hypertension, diabetes, dyslipidemia, and atrial fibrillation/flutter) were identified using the Charlson comorbidity index. Furthermore, two cardiovascular causes [acute coronary syndrome (ACS) and HF] of the index hospitalization were extracted from data categories, including the main diagnosis, admission-precipitating diagnosis, and most resource-consuming diagnosis, using the ICD-10 codes I200 (unstable angina pectoris) and I21 (acute myocardial infarction) for ACS and I50 for HF. We also extracted data on the age, sex, height, weight, body mass index (BMI), and smoking status.
Study population
Of the 9,825,635 patients hospitalized for any CVDs recorded from 1,058 JCS-certified hospitals between April 2012 and March 2020 (Figure 1), 6,078,487 men without missing clinical data were included in the main analysis (main cohort). Next, we extracted six specific subgroups according to comorbidities (hypertension, diabetes, dyslipidemia, and atrial fibrillation/flutter) (subcohort 1) and cardiovascular causes of admission (ACS and HF) (subcohort 2). In addition, the cohorts were subdivided into 3 age groups: <65, 65–74, and ≥75 years old.
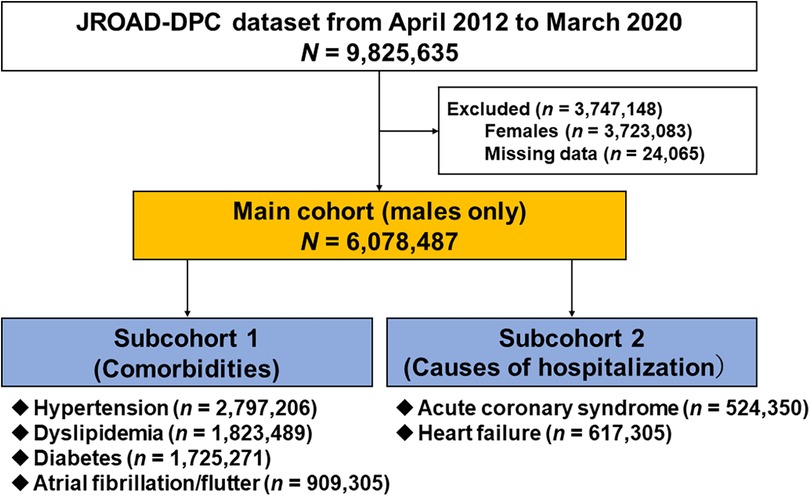
Figure 1. Study flowchart. JROAD-DPC, Japanese registry of all cardiac and vascular diseases–diagnosis procedure combination.
Statistical analyses
We presented categorical data as numbers (percentages) and continuous data as medians [interquartile ranges (IQRs)]. Annual frequencies of prostate diseases were calculated according to the data obtained from April of the corresponding year to March of the following year. Using the Cochran–Armitage trend test, we assessed the prevalence of prostate diseases by age group (<65, 65–74, and ≥75 years old). To determine the odds ratios and 95% confidence intervals, we constructed multilevel mixed-effects logistic regression with institution as a random intercept and adjusting for confounding factors, such as age, smoking status, hypertension, dyslipidemia, diabetes, and atrial fibrillation/flutter. In the subcohort analyses, patients with both ACS and HF were excluded in order to analyze the associations between each cardiovascular cause of admission (ACS and HF) and prostate disease individually.
All statistical data were analyzed using the STATA16 software program (College Station, TX, USA).
Results
Clinical characteristics
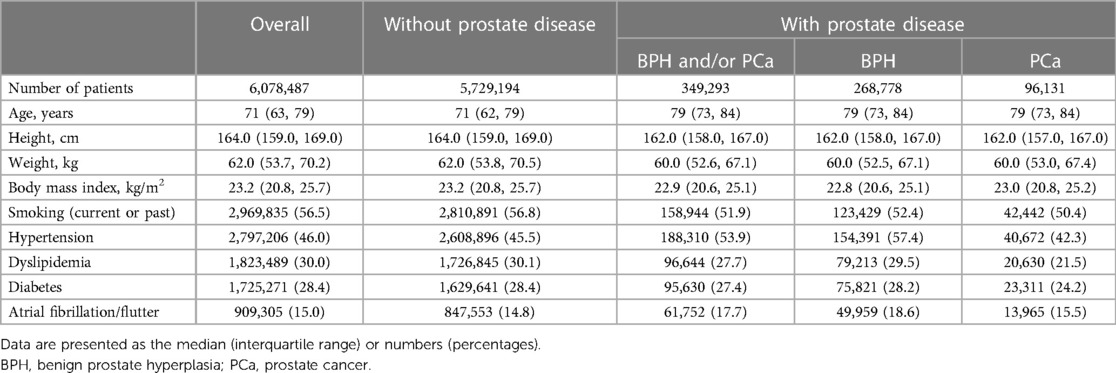
Of the 9,825,635 patients registered in the JROAD-DPC database between April 2012 and March 2020, 2,723,083 women and 24,065 cases with missing data were excluded (Figure 1). Ultimately, 6,078,487 male patients were analyzed as the main cohort. Overall, the median age was 71 (IQR: 63, 79) years old, and the median BMI was 23.2 (IQR: 20.8, 25.7) kg/m2. The prevalences of current or past smoking and coexisting hypertension, dyslipidemia, diabetes, and atrial fibrillation/flutter were 56.5%, 46.0%, 30.0%, 28.4%, and 15.0%, respectively (Table 1).
Prevalence of prostate diseases
In the main cohort, 349,293 (5.7%) patients had prostate diseases (BPH: 268,778 [4.4%]; PCa: 96,131 [1.6%]), and they tended to be older and smoked less than those without such diseases. In addition, hypertension and atrial fibrillation/flutter were more common in those with prostate diseases than in those without such diseases, especially those with BPH (Table 1).
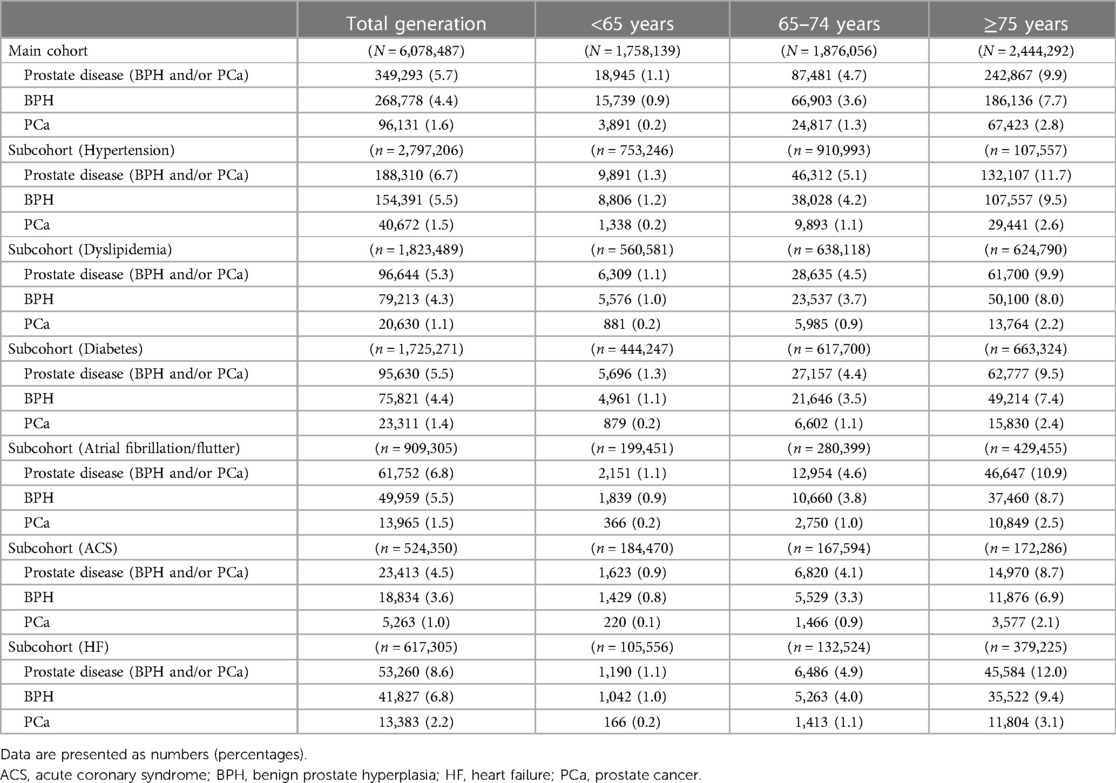
Regarding age, the prevalence of prostate diseases and individual components (BPH or PCa) significantly increased as the age increased (all P-values for trend <0.05) (Table 2). Figure 2 shows the annual prevalence of prostate diseases and individual components in the overall cohort and each age category from 2012 to 2019. The overall prevalence modestly increased over time in all generations, especially from 2015 to 2016 and among those who were ≥65 years old.
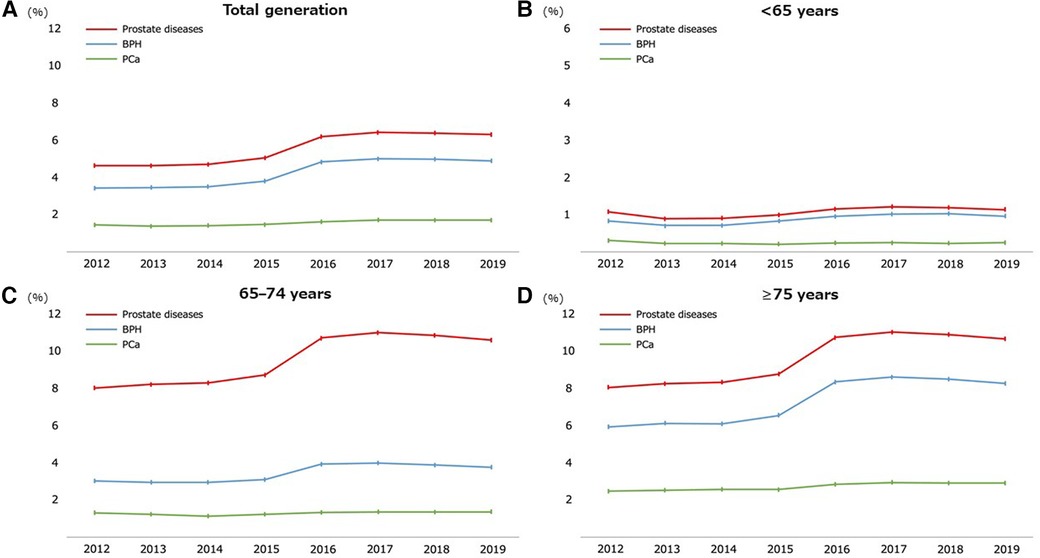
Figure 2. Temporal trend of the prevalence of prostate diseases from 2012 to 2019. Participants throughout the total generation (A) and stratified by age groups of <65 (B), 65–74 (C), and ≥75 years old (D). BPH, benign prostate hyperplasia; PCa, prostate cancer.
Subcohort analyses
Table 2 also lists the prevalence of prostate diseases by each subcohort. Similar to that in the main cohort, the prevalence increased significantly with age in each subcohort (all P-values for trend <0.05), and the time trend for the prevalence also showed a modest increase over time (Supplementary Figures S1–S6).
In the subcohort analysis, the lowest and highest prevalence rates of prostate diseases were observed in the subcohorts with ACS (4.5%) and HF (8.6%) (Table 2). The closer association of prostate diseases with HF was validated through univariate and multivariate logistic regression analyses (Table 3 and Supplementary Table S1). Overall, the subcohort admitted for HF had significantly higher incidences of prostate diseases, dominated by BPH, than the subcohorts admitted for non-HF causes or ACS (Table 3). When stratifying these subcohorts into three age categories, the association of subcohorts admitted for HF with the coexistence of prostate diseases was more pronounced in the older group (≥65 years old) (Supplementary Table S1).
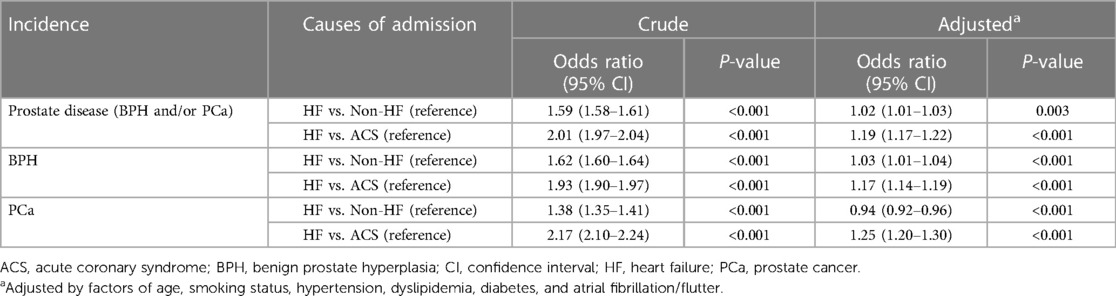
Table 3. Univariate and multivariate logistic regression analyses of prostate disease incidences in subcohorts according to the cause of admission.
Discussion
This study used a nationwide claims database recorded from JCS-certified hospitals between April 2012 and March 2020. To our knowledge, this is the first study to reveal the real-world prevalence of prostate diseases among patients hospitalized for CVDs. Prostate diseases were more prevalent in older patients than younger ones and showed a modest temporal increasing trend in the past eight years (2012–2019). Furthermore, the coexistence of prostate diseases was more common in patients hospitalized for HF than in those hospitalized for other cardiovascular causes, including ACS. Our findings highlight the potential clinical importance for cardiologists and even general physicians to recognize the potential association between CVD and the risk for prostate disease.
Metabolic syndrome and relevant cardiometabolic disorders, which are fully established as key risk factors for most CVDs, have been recently reported to play an important role in the pathogenesis of major prostate diseases (BPH and PCa) (17, 19, 26–29), highlighting an increased burden of prostate diseases coexisting with CVD in actual clinical settings. Epidemiological studies have reported that CVD is prevalent among patients with LUTS (primarily caused by BPH) or PCa (7, 30–32). Furthermore, some PCa medications, including ADT (androgen deprivation therapy), have a potency that adversely affects the cardiometabolic status and increases the risk for cardiovascular complications (33–36). Chan et al. (37) recently demonstrated temporal increasing trends of recipients of ADT therapy with cardiovascular risk factors, frequently used cardiovascular and metabolic medications, and developed cardiovascular events. CVD is the leading cause of mortality in PCa survivors (9, 10). These studies have contributed to emphasizing the clinical need for cardiovascular risk assessments and appropriate risk management in this population (20, 38, 39).
Currently, studies assessing the real-world burden of prostate diseases among patients with established CVD remain limited. In Poland, Semczuk-Kaczmarek et al. (40) reported that 62 (37.3%) out of 166 patients hospitalized with CVD had moderate-to-severe LUTS according to the International Prostate Symptoms Score. In a nationwide survey using the National Inpatient Sample in the United States between 2004 and 2014 (41), PCa was the most prevalent in a specific population with cancer who underwent percutaneous coronary intervention. Relative to these studies, the strength of the present study was being the first to attempt to survey the burden of two major prostate diseases, namely BPH and PCa, in patients hospitalized with CVD in a real-world nationwide dataset. In addition, we found a modestly increasing temporal trend of prevalent prostate diseases in the cohort examined, consistent with the global trend of increased burden of prostate diseases in general populations (1–5). This increase may reflect aging and indicate the need to promote public and clinical awareness of prostate diseases (3, 42) and PCa screening with prostate-specific antigen tests (43–45). Collectively, continued surveillance is needed to further clarify the clinical characteristics of prostate diseases among patients with CVD. Details concerning casualties should also be further examined.
In the present study, prostate diseases, dominated by BPH, were more prevalent in inpatients with HF than in those with non-HF causes or ACS, highlighting a possible association between HF and prostate diseases. Although the reason for the difference is still uncertain, it may be at least caused by the epidemiological fact that the incidence of corresponding diseases reflects demographics with aging. Lusty et al. (46) recently revealed that the most common medications for BPH (5-alpha reductase inhibitor, α-blocker, and combination therapy) were associated with an increased risk for incident cardiac failure in older (median: 73 years old) patients with BPH. More recently, HF was proven to be the leading cause of CVD admission and an increasing cause of mortality among patients across cancer types, including PCa (47, 48). Thus, considering the increasing global burden of HF (49), clinicians need to recognize that HF is an emerging and prognostic complication in patients with prostate diseases.
Although not limited to prostate diseases, estimation of the risk and early screening of the coexistence of non-CVD in patients with established CVD may lead to better overall patient outcomes. With recent improvements in diagnostics and therapeutics in various fields, including cardiology and urology, the resultant increase in the number of survivors from individual diseases and aging will further augment the subsequent risk of developing cardiovascular and noncardiovascular complications. In particular, patients with cancer generally have an increased risk for CVD (50–52), and the development of a better healthcare system is urgently needed in order to provide multidisciplinary clinical management and appropriate cardiovascular care (“onco-cardiology”) to patients with cancer.
In the present study, we showed real-world evidence concerning the burden and temporal increasing trend of major prostate diseases (BPH and PCa) among patients hospitalized for CVD. Given the close epidemiological relationship based on shared risk factors between prostate diseases and CVD entities, the prevalence of prostate diseases identified in inpatients may be merely part of a larger and more complex issue and is also common in patients with chronic CVD and even in those at risk for CVD (14–18). However, conducting clinical interviews regarding the presence of LUTS and screening tests for prostate diseases in cardiovascular care settings is currently uncommon. Our findings may be clinically useful to motivate cardiologists and even general physicians to screen patients with CVD for the presence of prostate diseases and to share clinical information with urologists. Furthermore, our study may promote clinical and research collaboration among specialists (especially urologists and cardiologists), leading to the development of a novel academic field of “uro-cardiology” in the near future.
Limitations
Our study has several limitations that need to be considered, and they were mainly based on the use of data sources obtained from medical claims. First, the present analysis was based only on the DPC data, and these data might contain certain inevitable errors, leading to the over- or underestimation of the clinical diagnosis accuracy. In particular, the JROAD-DPC data were obtained from the cardiovascular unit of several JCS-certified training facilities, and cardiologists might not be knowledgeable enough in the clinical diagnosis of prostate diseases. In addition, data were only collected from patients hospitalized for CVDs in JCS-certified hospitals; thus, selection bias is possible. We also did not compare the inpatients with CVD with healthy individuals or inpatients without CVD. Second, the DPC data system cannot share individual information among hospitals. If patients were admitted to another hospital, the previous DPC data could not be carried over, and the individuals could not be identified; thus, duplicates in the dataset are likely. Third, the DPC dataset did not include clinical information on the etiology, laboratory and imaging data, medications, or disease severity and staging of CVD and prostate diseases. Fourth, we had no information about the subsequent clinical course, rehospitalization, or mortality. Therefore, further research is needed to assess long-term outcomes in patients suffering from prostate diseases with established CVD. Finally, given that CVD and prostate diseases often vary in their prevalence by ethnicity and region (1, 3, 4), whether or not our findings from this Japanese patient population can be applied to different populations remains uncertain.
Conclusions
The nationwide real-world dataset used in this study revealed the increasing prevalence of prostate diseases among patients hospitalized for CVDs, particularly HF. Cardiologists and even general physicians must recognize that prostate diseases and CVDs often share underlying risk factors and that the frequency of their coexistence is increasing. This insight may facilitate the early screening and diagnosis of prostate diseases and help improve healthcare management.
Data availability statement
Given that the JROAD-DPC dataset is owned by the JCS for the purpose of research, the data that support our findings will not be shared without JCS' permission. The dataset will be available upon reasonable request from researchers and after approval by the JCS. Inquiries are to be addressed to the JCS (contact viaaXRkYXRhYmFzZUBqLWNpcmMub3IuanA=).
Ethics statement
This study was approved by the JCS (No. 2020-08) and the Ethics Committee of Saga University Hospital (No. 2021-08-01 on November 01, 2021) and conducted according to the principles of the Declaration of Helsinki. The database used in this study deidentified personal information; therefore, informed consent from our study participants was not needed.
Author contributions
KK, AT, and KN designed the research (project conception, development of overall research plan, and study oversight) and wrote the manuscript; KK, AT, MN, YS, and KN conducted the research (hands-on conduct of the experiments and data collection); KK, AT, MN, and YS analyzed the data or performed statistical analyses; HK, MN, and KN revised the manuscript; AT and KN had primary responsibility for the final content. All authors contributed to the article and approved the submitted version.
Funding
This work was partly supported by the Bayer Academic Support (No. BASJ20210416007) and research funding from Bristol Myers Squibb (No. 74241135).
Acknowledgments
The authors are grateful to Mikiko Kagiyama (Saga University) and Shizuka Okada (Saga University) for their dedicated assistance with the study.
Conflict of interest
KK received an academic support from Bayer. AT has received honoraria from Boehringer Ingelheim and research funding from GlaxoSmithKline, Takeda, Bristol Myers Squibb, and Novo Nordisk. HK has received research funding and scholarship funds from Medtronic Japan Co., LTD, Abbott Medical Japan Co., LTD, Boston Scientific Japan Co., LTD, and Fukuda Denshi, Central Tokyo Co., Ltd. MN has received a research funding from Pfizer. KN has received honoraria from MSD, Astellas, AstraZeneca, Novartis, Ono, Daiichi Sankyo, Mitsubishi Tanabe, Eli Lilly, Boehringer Ingelheim, and Takeda; research grants from Asahi Kasei, Astellas, Mitsubishi Tanabe, Teijin, Terumo, Boehringer Ingelheim, Eli Lilly and Company, Mochida, and Fuji; and scholarships from Daiichi Sankyo Healthcare, Teijin, Medtronic, and Bayer.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1236144/full#supplementary-material
References
1. The global, regional, and national burden of benign prostatic hyperplasia in 204 countries and territories from 2000 to 2019: a systematic analysis for the global burden of disease study 2019. Lancet Healthy Longev. (2022) 3:e754–76. doi: 10.1016/S2666-7568(22)00213-6
2. Egan KB. The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urol Clin North Am. (2016) 43:289–97. doi: 10.1016/j.ucl.2016.04.001
3. Xu XF, Liu GX, Guo YS, Zhu HY, He DL, Qiao XM, et al. Global, regional, and national incidence and year lived with disability for benign prostatic hyperplasia from 1990 to 2019. Am J Mens Health. (2021) 15:15579883211036786. doi: 10.1177/15579883211036786
4. Cao Y, Zhang W, Li Y, Fu J, Li H, Li X, et al. Rates and trends in stage-specific prostate cancer incidence by age and race/ethnicity, 2000–2017. Prostate. (2021) 81:1071–7. doi: 10.1002/pros.24204
5. Zhang H, Huang D, Zhang Y, Wang X, Wu J, Hong D. Global burden of prostate cancer attributable to smoking among males in 204 countries and territories, 1990–2019. BMC Cancer. (2023) 23:92. doi: 10.1186/s12885-023-10552-8
6. Gratzke C, Bachmann A, Descazeaud A, Drake MJ, Madersbacher S, Mamoulakis C, et al. EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. (2015) 67:1099–109. doi: 10.1016/j.eururo.2014.12.038
7. Gacci M, Corona G, Sebastianelli A, Serni S, De Nunzio C, Maggi M, et al. Male lower urinary tract symptoms and cardiovascular events: a systematic review and meta-analysis. Eur Urol. (2016) 70:788–96. doi: 10.1016/j.eururo.2016.07.007
8. Schoormans D, Vissers PAJ, van Herk-Sukel MPP, Denollet J, Pedersen SS, Dalton SO, et al. Incidence of cardiovascular disease up to 13 year after cancer diagnosis: a matched cohort study among 32,757 cancer survivors. Cancer Med. (2018) 7:4952–63. doi: 10.1002/cam4.1754
9. Shikanov S, Kocherginsky M, Shalhav AL, Eggener SE. Cause-specific mortality following radical prostatectomy. Prostate Cancer Prostatic Dis. (2012) 15:106–10. doi: 10.1038/pcan.2011.55
10. Ng HS, Koczwara B, Roder D, Vitry A. Development of comorbidities in men with prostate cancer treated with androgen deprivation therapy: an Australian population-based cohort study. Prostate Cancer Prostatic Dis. (2018) 21:403–10. doi: 10.1038/s41391-018-0036-y
11. Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. (2004) 109:II2–10. doi: 10.1161/01.CIR.0000110642.73995.BF
12. Raggi P, Genest J, Giles JT, Rayner KJ, Dwivedi G, Beanlands RS, et al. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis. (2018) 276:98–108. doi: 10.1016/j.atherosclerosis.2018.07.014
13. Jaiswal S, Libby P. Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat Rev Cardiol. (2020) 17:137–44. doi: 10.1038/s41569-019-0247-5
14. Russo GI, Castelli T, Privitera S, Fragalà E, Favilla V, Reale G, et al. Increase of framingham cardiovascular disease risk score is associated with severity of lower urinary tract symptoms. BJU Int. (2015) 116:791–6. doi: 10.1111/bju.13053
15. Yoo S, Oh S, Park J, Cho SY, Cho MC, Jeong H, et al. The impacts of metabolic syndrome and lifestyle on the prevalence of benign prostatic hyperplasia requiring treatment: historical cohort study of 130,454 men. BJU Int. (2019) 123:140–8. doi: 10.1111/bju.14528
16. Gacci M, Russo GI, De Nunzio C, Sebastianelli A, Salvi M, Vignozzi L, et al. Meta-analysis of metabolic syndrome and prostate cancer. Prostate Cancer Prostatic Dis. (2017) 20:146–55. doi: 10.1038/pcan.2017.1
17. Hammarsten J, Damber JE, Haghsheno MA, Mellström D, Peeker R. A stage-dependent link between metabolic syndrome components and incident prostate cancer. Nat Rev Urol. (2018) 15:321–33. doi: 10.1038/nrurol.2018.8
18. Hernández-Pérez JG, Torres-Sánchez L, Hernández-Alcaráz C, López-Carrillo L, Rodríguez-Covarrubias F, Vázquez-Salas RA, et al. Metabolic syndrome and prostate cancer risk: a population case-control study. Arch Med Res. (2022) 53:594–602. doi: 10.1016/j.arcmed.2022.07.003
19. Hammarsten J, Peeker R. Urological aspects of the metabolic syndrome. Nat Rev Urol. (2011) 8:483–94. doi: 10.1038/nrurol.2011.112
20. Tanaka A, Node K. The emerging and promising role of care for cardiometabolic syndrome in prostate cancer. JACC CardioOncol. (2019) 1:307–9. doi: 10.1016/j.jaccao.2019.09.005
21. Merseburger A, Bro Falkenberg A, Kornilova OJ. New study suggests patients with advanced prostate cancer on androgen deprivation therapy need more dialogue with health care provider, especially around cardiovascular risk. World J Urol. (2019) 37:1085–93. doi: 10.1007/s00345-018-2495-0
22. Yasuda S, Nakao K, Nishimura K, Miyamoto Y, Sumita Y, Shishido T, et al. The current status of cardiovascular medicine in Japan—analysis of a large number of health records from a nationwide claim-based database, JROAD-DPC. Circ J. (2016) 80:2327–35. doi: 10.1253/circj.CJ-16-0196
23. Yasuda S, Miyamoto Y, Ogawa H. Current status of cardiovascular medicine in the aging society of Japan. Circulation. (2018) 138:965–7. doi: 10.1161/CIRCULATIONAHA.118.035858
24. Nakai M, Iwanaga Y, Sumita Y, Wada S, Hiramatsu H, Iihara K, et al. Associations among cardiovascular and cerebrovascular diseases: analysis of the nationwide claims-based JROAD-DPC dataset. PLoS One. (2022) 17:e0264390. doi: 10.1371/journal.pone.0264390
25. Ogawa M, Yoshida N, Nakai M, Kanaoka K, Sumita Y, Kanejima Y, et al. Hospital-associated disability and hospitalization costs for acute heart failure stratified by body mass index- insight from the JROAD/JROAD-DPC database. Int J Cardiol. (2022) 367:38–44. doi: 10.1016/j.ijcard.2022.08.044
26. Russo GI, Cimino S, Castelli T, Favilla V, Gacci M, Carini M, et al. Benign prostatic hyperplasia, metabolic syndrome and non-alcoholic fatty liver disease: is metaflammation the link? Prostate. (2016) 76:1528–35. doi: 10.1002/pros.23237
27. Di Francesco S, Robuffo I, Caruso M, Giambuzzi G, Ferri D, Militello A, et al. Metabolic alterations, aggressive hormone-naïve prostate cancer and cardiovascular disease: a Complex relationship. Medicina (Kaunas). (2019) 55:62. doi: 10.3390/medicina55030062
28. Geng JH, Plym A, Penney KL, Pomerantz M, Mucci LA, Kibel AS. Metabolic syndrome and its pharmacologic treatment are associated with the time to castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. (2022) 25:320–6. doi: 10.1038/s41391-022-00494-w
29. Bhindi B, Locke J, Alibhai SMH, Kulkarni GS, Margel DS, Hamilton RJ, et al. Dissecting the association between metabolic syndrome and prostate cancer risk: analysis of a large clinical cohort. Eur Urol. (2015) 67:64–70. doi: 10.1016/j.eururo.2014.01.040
30. Wollersheim BM, Boekhout AH, van der Poel HG, van de Poll-Franse LV, Schoormans D. The risk of developing cardiovascular disease is increased for patients with prostate cancer who are pharmaceutically treated for depression. BJU Int. (2020) 125:433–41. doi: 10.1111/bju.14961
31. Tanaka Y, Matsuyama S, Tada H, Hayashi K, Takamura M, Kawashiri MA, et al. Association of lower urinary tract symptoms based on the international prostate symptom score and cardiovascular disease. Circ J. (2021) 85:2092–9. doi: 10.1253/circj.CJ-21-0278
32. Wang X, Su Y, Yang C, Hu Y, Dong JY. Benign prostatic hyperplasia and cardiovascular risk: a prospective study among Chinese men. World J Urol. (2022) 40:177–83. doi: 10.1007/s00345-021-03817-z
33. Liu JM, Lin CC, Chen MF, Liu KL, Lin CF, Chen TH, et al. Risk of major adverse cardiovascular events among second-line hormonal therapy for metastatic castration-resistant prostate cancer: a real-world evidence study. Prostate. (2021) 81:194–201. doi: 10.1002/pros.24096
34. Zhang KW, Reimers MA, Calaway AC, Fradley MG, Ponsky L, Garcia JA, et al. Cardiovascular events in men with prostate cancer receiving hormone therapy: an analysis of the FDA adverse event reporting system (FAERS). J Urol. (2021) 206:613–22. doi: 10.1097/JU.0000000000001785
35. Lin E, Garmo H, Van Hemelrijck M, Zethelius B, Stattin P, Hagström E, et al. Association of gonadotropin-releasing hormone agonists for prostate cancer with cardiovascular disease risk and hypertension in men with diabetes. JAMA Netw Open. (2022) 5:e2225600. doi: 10.1001/jamanetworkopen.2022.25600
36. Schmidt C. Does prostate-cancer treatment place a strain on the heart? Nature. (2022) 609:S46–s47. doi: 10.1038/d41586-022-02863-w
37. Chan JSK, Satti DI, Lee YHA, Hui JMH, Dee EC, Ng K, et al. Temporal trends in cardiovascular burden among patients with prostate cancer receiving androgen deprivation therapy: a population-based cohort study. Br J Cancer. (2023) 128:2253–60. doi: 10.1038/s41416-023-02271-5
38. Sun L, Parikh RB, Hubbard RA, Cashy J, Takvorian SU, Vaughn DJ, et al. Assessment and management of cardiovascular risk factors among US veterans with prostate cancer. JAMA Netw Open. (2021) 4:e210070. doi: 10.1001/jamanetworkopen.2021.0070
39. Katz AJ, Chen RC, Usinger DS, Danus SM, Zullig LL. Cardiovascular disease prevention and management of pre-existent cardiovascular disease in a cohort of prostate cancer survivors. J Cancer Surviv. (2023) 17:351–9. doi: 10.1007/s11764-022-01229-5
40. Semczuk-Kaczmarek K, Rys-Czaporowska A, Platek AE, Szymanski FM. Prevalence of lower urinary tract symptoms in patients with cardiovascular disease. Cent European J Urol. (2021) 74:190–5. doi: 10.5173/ceju.2021.0370.R1
41. Potts JE, Iliescu CA, Lopez Mattei JC, Martinez SC, Holmvang L, Ludman P, et al. Percutaneous coronary intervention in cancer patients: a report of the prevalence and outcomes in the United States. Eur Heart J. (2019) 40:1790–800. doi: 10.1093/eurheartj/ehy769
42. Ito K. Prostate cancer in Asian men. Nat Rev Urol. (2014) 11:197–212. doi: 10.1038/nrurol.2014.42
43. Okinaka Y, Kageyama S, Nishizawa K, Yoshida T, Ishitoya S, Shichiri Y, et al. Clinical, pathological, and therapeutic features of newly diagnosed prostate cancer predominantly detected by opportunistic PSA screening: a survey of shiga prefecture, Japan. Prostate. (2021) 81:1172–8. doi: 10.1002/pros.24212
44. Inoue T, Yoshimura K, Terada N, Tsukino H, Murota T, Kinoshita H, et al. Prostate-specific antigen density during dutasteride treatment for 1 year predicts the presence of prostate cancer in benign prostatic hyperplasia after the first negative biopsy (PREDICT study). Int J Urol. (2021) 28:849–54. doi: 10.1111/iju.14590
45. Lai SM, Keighley J, Garimella S, Enko M, Parker WP. Variations in age-adjusted prostate cancer incidence rates by race and ethnicity after changes in prostate-specific antigen screening recommendation. JAMA Netw Open. (2022) 5:e2240657. doi: 10.1001/jamanetworkopen.2022.40657
46. Lusty A, Siemens DR, Tohidi M, Whitehead M, Tranmer J, Nickel JC. Cardiac failure associated with medical therapy of benign prostatic hyperplasia: a population based study. J Urol. (2021) 205:1430–7. doi: 10.1097/JU.0000000000001561
47. Kobo O, Raisi-Estabragh Z, Gevaert S, Rana JS, Van Spall HGC, Roguin A, et al. Impact of cancer diagnosis on distribution and trends of cardiovascular hospitalizations in the USA between 2004 and 2017. Eur Heart J Qual Care Clin Outcomes. (2022) 8:787–97. doi: 10.1093/ehjqcco/qcac045
48. Raisi-Estabragh Z, Kobo O, Freeman P, Petersen SE, Kolman L, Miller RJH, et al. Temporal trends in disease-specific causes of cardiovascular mortality amongst patients with cancer in the USA between 1999 and 2019. Eur Heart J Qual Care Clin Outcomes. (2022) 9:54–63. doi: 10.1093/ehjqcco/qcac016
49. Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. (2023) 118:3272–87. doi: 10.1093/cvr/cvac013
50. Kobo O, Khattak S, Lopez-Mattei J, Van Spall HGC, Graham M, Cheng RK, et al. Trends in cardiovascular mortality of cancer patients in the US over two decades 1999–2019. Int J Clin Pract. (2021) 75:e14841. doi: 10.1111/ijcp.14841
51. Battisti NML, Welch CA, Sweeting M, de Belder M, Deanfield J, Weston C, et al. Prevalence of cardiovascular disease in patients with potentially curable malignancies: a national registry dataset analysis. JACC Cardiol Oncol. (2022) 4:238–53. doi: 10.1016/j.jaccao.2022.03.004
Keywords: prostate disease, benign prostate hyperplasia, prostate cancer, cardiovascular disease, acute coronary syndrome, heart failure, epidemiology, temporal trend
Citation: Kaneta K, Tanaka A, Nakai M, Sumita Y, Kaneko H, Noguchi M and Node K (2023) Prevalence and temporal trends of prostate diseases among inpatients with cardiovascular disease: a nationwide real-world database survey in Japan. Front. Cardiovasc. Med. 10:1236144. doi: 10.3389/fcvm.2023.1236144
Received: 7 June 2023; Accepted: 9 October 2023;
Published: 19 October 2023.
Edited by:
Zhonghua Sun, Curtin University, AustraliaReviewed by:
Hiroshi Asanuma, Meiji University of Integrative Medicine, JapanJeffrey Shi Kai Chan, Cardiovascular Analytics Group, Hong Kong SAR, China
© 2023 Kaneta, Tanaka, Nakai, Sumita, Kaneko, Noguchi and Node. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Atsushi Tanaka dGFuYWthYTJAY2Muc2FnYS11LmFjLmpw Koichi Node bm9kZUBjYy5zYWdhLXUuYWMuanA=
 Kohei Kaneta
Kohei Kaneta Atsushi Tanaka
Atsushi Tanaka Michikazu Nakai2
Michikazu Nakai2 Koichi Node
Koichi Node