- 1Department of Cardiology, University Hospital Marburg, Philipps University Marburg, Marburg, Germany
- 2Department of Cardiology, Cardiovascular Center Bad Neustadt/Saale, Bad Neustadt an der Saale, Germany
- 3Department of Cardiology, Cardiovascular Center Rotenburg/Fulda, Rotenburg an der Fulda, Germany
- 4Department of Cardiology, University Hospital Oldenburg, Carl von Ossietzky University Oldenburg, Oldenburg, Germany
- 5Department of Cardiology, University Hospital Gießen, Justus Liebig University Gießen, Gießen, Germany
- 6Institute for Bioinformatics and Biostatistics, Philipps University Marburg, Marburg, Germany
Background: Atrial fibrillation (AF) is the most common concomitant disease in patients undergoing transcatheter edge-to-edge repair (TEER) for mitral regurgitation (MR) and detrimentally affects their outcome. While there is increasing evidence for prognostic improvement and safety of catheter ablation (CA) of AF in the overall cohort of heart failure patients, corresponding data in TEER patients are lacking.
Objectives: To investigate the impact of treatment regimens for concomitant AF on survival of TEER patients.
Methods: In a multicenter observational cohort study consecutive patients successfully undergoing TEER were analyzed and survival of patients receiving CA of concomitant AF was compared with that of patients on pharmacological AF treatment and with that of patients without a history of AF, using propensity score matching (PSM).
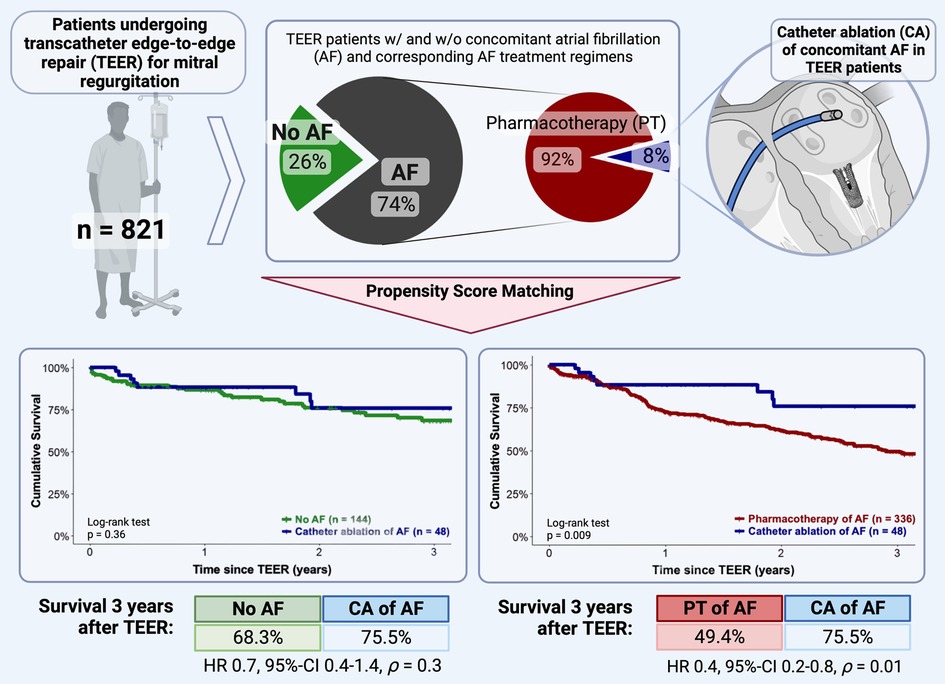
Results: A total of 821 patients were analyzed. Of these, 608 (74.1%) had concomitant AF, of whom 48 patients received CA. Patients with CA in AF showed significantly higher 3-year-survival after TEER compared to PSM-patients on pharmacological AF treatment (75.5% [36/48] vs. 49.4% [166/336], p = 0.009). The 3-year-survival after TEER of patients with concomitant AF treated with CA was not significantly different from PSM-patients without AF (75.5% [36/48] vs. 68.3% [98/144], p = 0.36).
Conclusions: CA of AF is superior to pharmacotherapy as it significantly improves the survival of TEER patients in a PSM analysis. CA even offsets the prognostic disadvantage of coexisting AF in TEER patients. Given the growing evidence of prognostic benefits in the overall cohort of HF patients, our data point out the importance of treating concomitant AF and support CA as an essential part of a holistic management of TEER patients.
Introduction
Mitral regurgitation (MR) represents one of the most common valvular heart diseases (VHD) in developed and industrialized countries. Especially in the elderly population over 75 years of age, it is the predominant form of VHD, with a prevalence of about 10%, and a major contributor to the development of heart failure (HF) (1). According to the projected demographic trends of aging societies, the number of these patients will increase dramatically in the decennia to come (2). However, already today as many as half of all patients with MR requiring therapy are not treated surgically due to advanced HF and multiple comorbidities associated with an unacceptably high perioperative risk (3, 4). For this particular high-risk cohort, transcatheter edge-to-edge repair (TEER) has proven to be a safe and beneficial treatment modality, alleviating HF symptoms (5) and even improving prognosis in selected MR etiologies (6, 7). With a prevalence of up to 75.4% (8), atrial fibrillation (AF) is the most common comorbidity in TEER collectives and, as demonstrated in a multitude of recent studies, dramatically worsens the medium- and long-term outcome of successfully treated TEER patients (9–13). In contrast to the collective of surgically treated MR patients, in whom there is convincing evidence of prognostically favorable concomitant rhythm control of AF (14), reflected in a class I-A recommendation in the relevant guideline (15), the evidence in TEER patients regarding the prognostic impact of AF treatment regimens is limited.
We recently demonstrated that the majority of TEER patients with coexistent AF were on rate control therapy, which was associated with a more favorable long-term outcome than a pharmacological rhythm control (13, 16). Data on the prognostic impact of state-of-the-art interventional rhythm control in this unique patient population, however, are lacking. Using the established statistical methods of propensity score matching (PSM) and multivariable Cox regression, we analyze the prognostic effect of catheter ablation (CA) of concomitant AF in comparison to pharmacological AF management in a well-characterized multicenter collective of TEER patients.
Materials and methods
Data collection and definitions
Data on all consecutive patients scheduled for TEER after a consensus decision by a multidisciplinary heart team at four tertiary cardiac centers in Germany between October 2011 and December 2022 were recorded in registries of the participating center and subsequently pooled for the present analysis. Eligibility for TEER was defined according to relevant guidelines (17): generally, patients with severe symptomatic primary MR, and patients with secondary MR who remain highly symptomatic despite guideline-guided treatment of heart failure, who are at high or prohibitive risk for surgery, and who meet echocardiographic eligibility criteria for TEER. Here, the multidisciplinary heart teams mainly oriented on the echocardiographic criteria defined in the Endovascular Valve Edge-to-Edge Repair Study (EVEREST) II for primary MR and on those defined in the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT) trial for secondary MR (5, 6). Regarding procedural outcome, successful TEER was defined as MR reduction to less than or equal to moderate severity and a pressure gradient across the mitral valve of 5 mmHg or less after clip implantation. The definition of AF types and therapies and procedural aspects were as defined in recent publications (13, 16), which also addressed how patient selection was carried out. In brief, the definition of AF types was made according to the recent guidelines of the European Society of Cardiology (18). Correspondingly, paroxysmal AF was defined as lasting seven days or less. All AF episodes exceeding seven days were defined as persistent AF, presuming that a rhythm control strategy was still being pursued. Permanent atrial fibrillation was defined as when rhythm-controlling measures were no longer performed by mutual agreement between the patient and the treating physician.
All patients with non-permanent AF who underwent catheter ablation (CA) of AF within 24 months before or after the TEER procedure were identified and assigned to the interventional rhythm control group. The ablation procedure always included antral isolation of the pulmonary vein ostia. The creation of additional lesions was left to the discretion of the individual physician. CA-associated major complications were defined as access site bleeding or hematoma requiring blood transfusion or surgical intervention, pericardial effusion requiring pericardiocentesis, systemic and/or cerebral embolization, phrenic nerve palsy, pulmonary vein stenosis, esophageal ulceration and/or fistula or periprocedural mortality. All remaining AF patients were assigned to the pharmacological AF treatment group. Furthermore, all patients with non-permanent AF treated with antiarrhythmic drugs (AAD) of Vaughan-Williams classes I-IV, or a combination of these were defined to be on pharmacological rhythm control therapy. The indication for the use of AAD, especially that for amiodarone (class III AAD), was carefully considered in each case to exclude indications other than rhythm control in AF (e.g., ventricular arrhythmias) from further analysis. In accordance with the determinant decision to forgo further rhythm-maintaining therapies, all patients with permanent AF were defined to be on rate control therapy. Major adverse cardiac and cerebrovascular events (MACCE) were defined as the occurrence of a cerebral and/or systemic thromboembolic event, a hemorrhage requiring intervention and/or transfusion or in-hospital morality from a cardiovascular cause. The local ethics committee approved the study (reference number 120/18, Philipps University Marburg, Germany).
Statistical analysis
All statistical analyses were performed by using R Studio V3.6.1 (R Foundation for Statistical Computing, Vienna, Austria), including the “survival”-, “MatchIt”-, “survminer”-, “stddiff”-, “My.Stepwise”- and “dplyr”-Packages as well as GraphPad Prism 6.0 (Dotmatics, Boston, MA, USA). Continuous variables are presented with mean and standard deviation for normally distributed variables and with median and interquartile ranges (IQR: 25th–75th percentile) for non-normally distributed variables. Categorical variables are presented as frequencies and percentages (%). Differences between two groups were compared for categorical variables with the chi-square test when the expected cell size was ≥20 and with Fisher's exact test when the expected cell size in one or more cells was <20. For continuous variables, Student's t test was used for normally distributed variables and Wilcoxon's test was used for non-normally distributed variables. The normal distribution of continuous variables was validated with the Shapiro-Wilk test. A two-tailed p-value of <0.05 was considered statistically significant. To address differences in baseline characteristics and to achieve the most unbiased comparison possible between outcomes of patients with interventional and pharmacological rhythm control, with rate control, and of patients without a history for AF, propensity score matching (PSM) analysis was performed using the nearest neighbor matching with a caliper width set at 0.2 standardized difference of the logit of the estimated propensity scores. In order to include as many subjects as possible from the total cohort in the analyses, the groups to be studied were matched at different ratios. The matching ratio was based on the group with the smallest number of patients, the interventional rhythm control group, and on ensuring a sufficient balance of baseline characteristics. With regard to appropriate matching parameters, we used statistically significantly different (p-value cut off 0.05) parameters, in the corresponding baseline characteristics and previously published and generally accepted mortality predictors and mortality predictors revealed or confirmed by univariable and multivariable Cox regression analyses in the present collective. The selected matching parameters were age, coronary artery disease, pre-existing cardiac resynchronization therapy, chronic obstructive pulmonary disease, New York Heart Association (NYHA) functional class IV, angiotensin receptor-neprilysin inhibitor usage, STS risk score as well as concomitant severe tricuspid regurgitation. Following matching, time-to-event analysis for the different AF treatment strategies was conducted according to the Kaplan-Meier method; differences between groups were compared using the log-rank test. Both univariable and multivariable Cox regression were performed to determine independent predictors of mortality. Variables with p < 0.1 in the univariate analysis were included in the multivariable Cox regression model. The primary end point in the survival analyses was death from any cause. Figure 1 provides a flowchart of the study design.
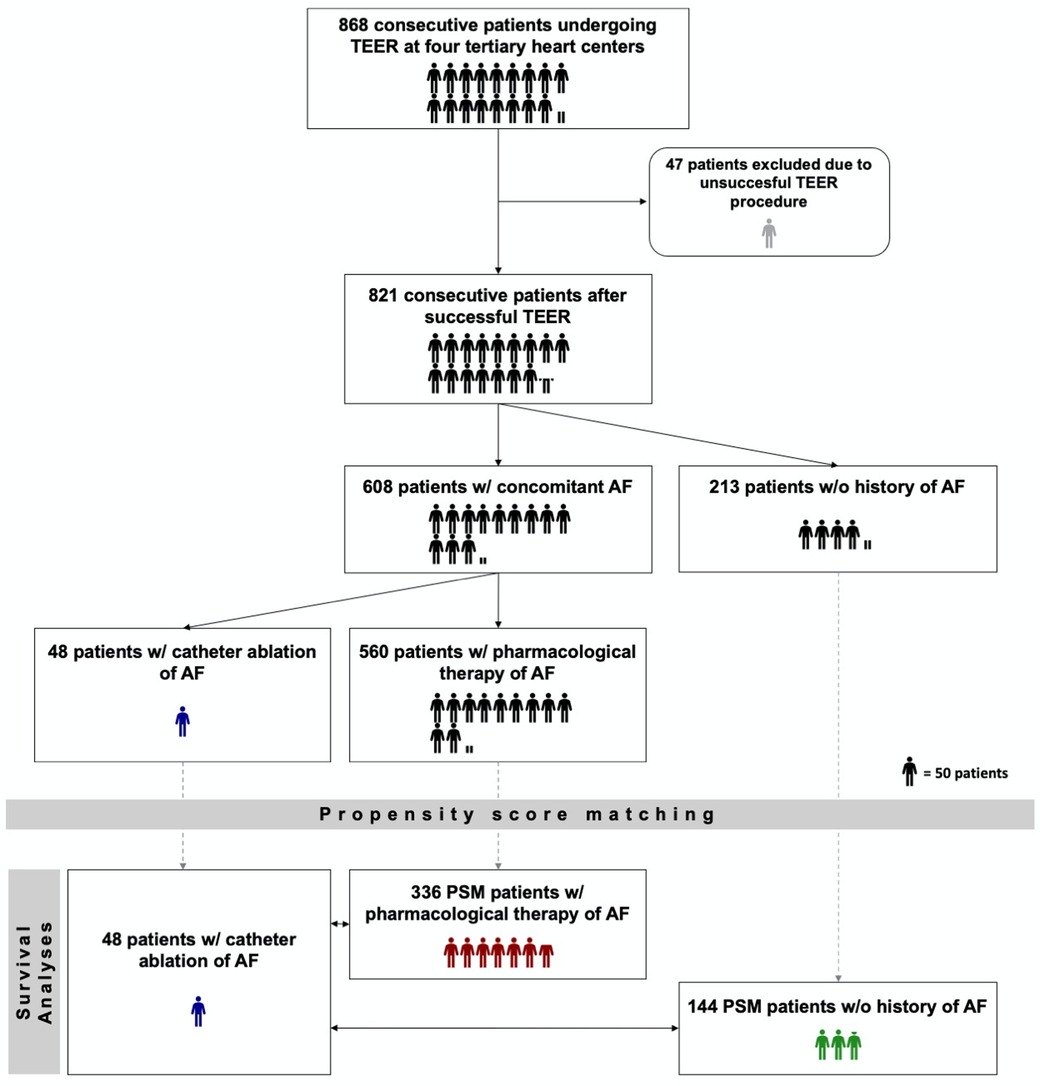
Figure 1. Study design flow chart. TEER, transcatheter edge-to-edge mitral valve repair; AF, atrial fibrillation; PSM, propensity score matching. w/ - with. w/o – without. One humanoid icon corresponds to 50 patients. If the number of patients could not be divided by 50, a correspondingly proportional humanoid icon was used (e.g. one half humanoid icon corresponds to 25 patients.).
Missing data
In cases where follow-up data were insufficient, they were supplemented by a survival query to the registry office for patients lost to follow-up. In spite of all efforts, 55 patients (6.7%) could not be followed-up on during the indicated study period because of an unreported change of residence. There was no evidence of informative missingness and no significant impact of “lost to follow-up” patients on the results presented. Supplementary Table S1 provides baseline characteristics of patients lost to follow-up compared with the entire cohort studied.
Results
During the study period, 868 patients undergoing TEER for severe MR were identified at the four participating centers. For the TEER procedure, the MitraClip® device (Abbott Vascular, Santa Clara, CA, USA) was used in 650 (79.2%) and the PASCAL™ system (Edwards Lifescience, Irvine, CA, USA) in 171 (20.8%) of cases. Forty-seven patients (5.4%) underwent conservative treatment or surgical intervention due to insufficient reduction in MR severity and were therefore excluded from further analysis. The type of TEER device used had no significant influence on the success or safety of the procedure (Odds ratio [OR] 0.9, 95%-confidence interval [CI] 0.5–1.4, p = 0.6 and OR 1.1, 95%-CI 0.5–2.2, p = 0.8, respectively). Concomitant AF or underlying AF treatment regimen also did not affect the risk of unsuccessful TEER intervention, as reported previously (13, 16). The well-established association of concomitant AF with adverse outcomes after successful TEER was also confirmed in the studied collective [Hazard ratio (HR) 1.3, 95%-CI 1.03–1.7, p = 0.046].
Clinical and procedural characteristics of the entire cohort are presented in Table 1 (first column). The median follow-up time was 397 days (IQR 890 days).
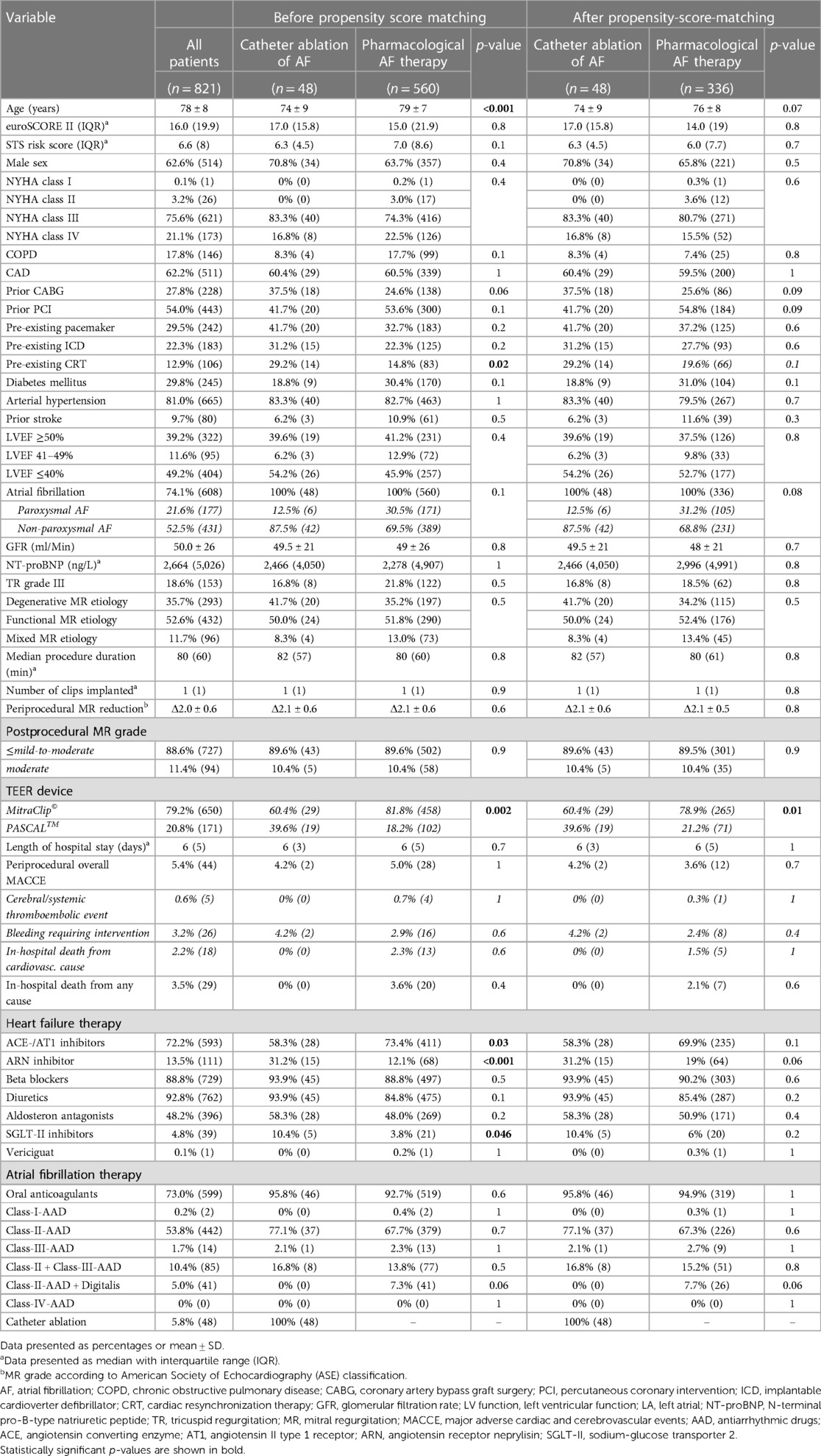
Table 1. Clinical and procedural characteristics of the overall cohort studied and of patients with catheter ablation of concomitant AF and of patients with pharmacological AF therapy before and after propensity score matching at the time of the TEER procedure.
Comparison of long-term outcomes of catheter ablation vs. pharmacological therapy of concomitant atrial fibrillation in TEER patients
Within the overall collective, 48 patients were identified who received interventional rhythm control by CA of AF. CA was performed in 50% (24/48) of patients within a mean period of 487 days (±243 days) before and in the remaining half of patients (24/48) within a mean period of 246 days (±174 days) after TEER intervention. With the exception of one case (1/48), which was treated with cryoablation, CA was performed with radiofrequency (RF) energy (47/48). The mean procedure time for CA amounted 110 min (±39 min), and acute procedural success defined as achieving bidirectional block of all pulmonary veins was reached in all patients. No CA-related periprocedural major complications or interferences with the TEER device were observed. The timing of CA, whether performed before or after TEER, did not affect the long-term prognosis (HR 1.0, 95%-CI 0.97–1.002, p = 0.75). However, to address a potential immortal time bias (19), the follow-up period was started after completion of both the TEER and CA procedures.
Propensity score matching (PSM) was used to identify patients with pharmacological treatment (PT) of AF from the total cohort and to assign them to the CA group. To achieve a full balance of the baseline characteristics and to include as many subjects as possible in the subsequent survival analysis, a matching ratio of 1:7 was selected. Except for the type of TEER device used, a balance of all relevant baseline characteristics was thus achieved, as shown in Table 1. Despite PSM, the PASCAL device was utilized statistically significantly more often in the CA group compared to the PT group (39.6% vs. 20.3%, p = 0.01).
A statistically significant reduced estimated cumulative survival of pharmacological AF treatment compared to interventional rhythm control patients was observed after 3 years (166/336 (49.4%) vs. 36/48 (75.5%), p = 0.009). The corresponding Kaplan-Meier diagram is plotted in Figure 2. Multivariable Cox regression revealed severe tricuspid regurgitation (TR) (HR 1.5, 95%-CI 1.3–2.2, p < 0.001), chronic obstructive pulmonary disease (HR 1.3, 95%-CI 1.03–1.9, p = 0.03) and New York Heart Association (NYHA) functional class IV (HR 1.4, 95%-CI 1.02–1.8, p = 0.04) to be statistically significant negative predictors of long-term survival. Whereas a statistically significant positive association was found between CA of AF and long-term outcome (HR 0.4, 95%-CI 0.2–0.8, p = 0.01).
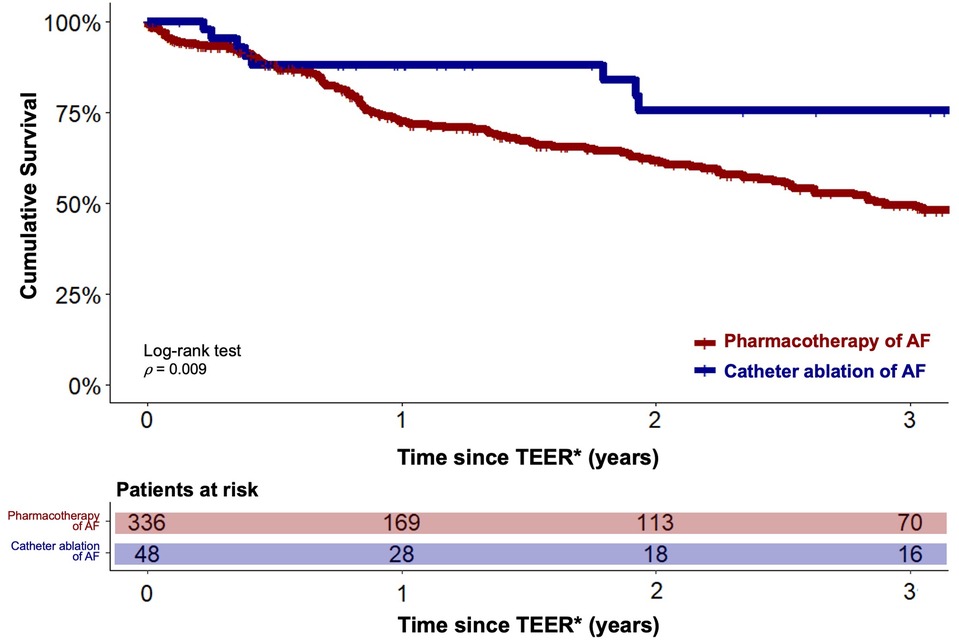
Figure 2. Estimated cumulative survival of TEER patients with catheter ablation and pharmacotherapy of concomitant AF. Kaplan-Meier plots showing cumulative survival of patients with catheter ablation of AF (blue graph) and of patients under pharmacotherapy of AF (red graph). Graphs indicating means and 95% confidence intervals. * - For the catheter ablation group, the follow-up period was initiated after completion of both the TEER and CA procedures.
For further comparison of interventional vs. pharmacological rhythm control of AF and interventional rhythm control vs. rate control of AF, multivariable Cox regression analysis was performed in the respective patient cohorts defined according to the criteria specified in the Methods section. Again, treatment of concomitant AF by CA was statistically significantly associated with better outcome in TEER patients compared with pharmacological rhythm control (HR 0.6, 95%-CI 0.3–0.9, p = 0.03) and rate control (HR 0.45, 95% CI 0.3–0.7, p = 0.006).
Comparison of long-term outcomes of TEER patients with concomitant AF treated with catheter ablation vs. TEER patients without a history of AF
Analogous to the previous analysis, the long-term outcomes of TEER patients under interventional rhythm control were compared with those of TEER patients without concomitant AF using PSM in a 1:3 ratio. Balanced characteristics are provided in Table 2. There were no statistically significant differences in the estimated cumulative survival of TEER patients with interventional rhythm-controlled AF and TEER patients without concomitant AF at 3 years after TEER procedure (98/144 (68.3%) vs. 36/48 (75.5%), p = 0.36). The corresponding Kaplan-Meier diagram is shown in Figure 3. Multivariable Cox regression identified NYHA functional class IV (HR 1.9, 95%-CI 1.1–3.3, p = 0.01) and concomitant chronic obstructive pulmonary disease (HR 2.0, 95%-CI 1.2–3.3, p = 0.002) to be statistically significant negative predictors of long-term survival in this comparison. CA of AF was not statistically significant associated with the long-term outcome (HR 0.8, 95%-CI 0.3–1.4, p = 0.3).
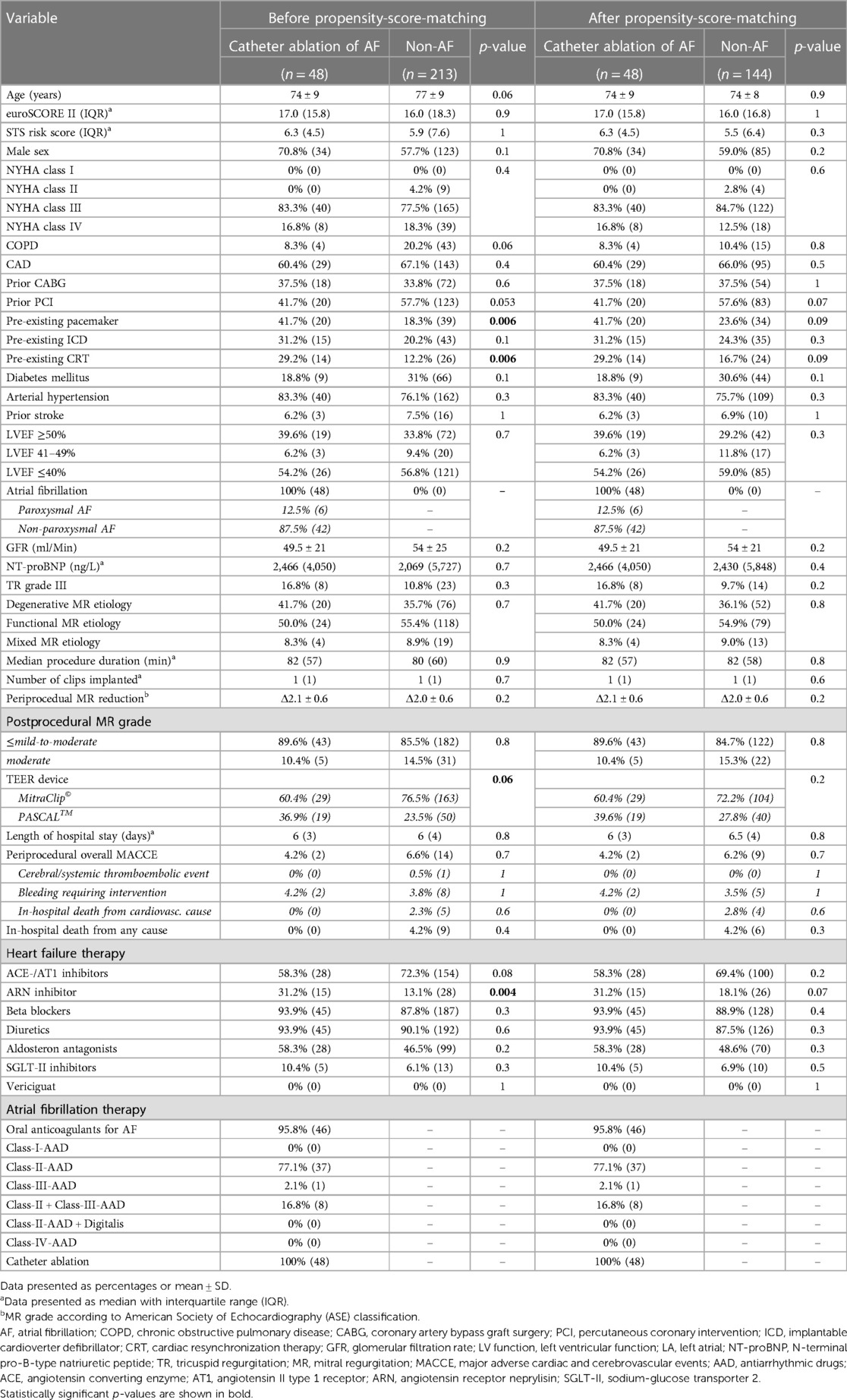
Table 2. Clinical and procedural characteristics of patients with catheter ablation of concomitant AF and of patients without a history of AF before and after propensity score matching.
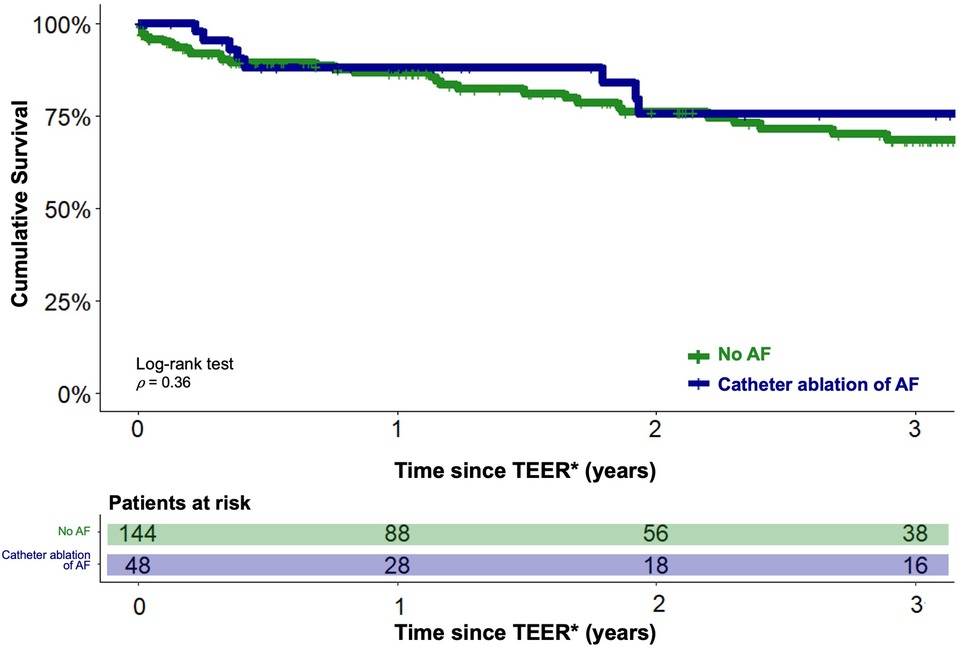
Figure 3. Estimated cumulative survival of TEER patients with concomitant AF treated with catheter ablation and of TEER patients without a history of AF. Kaplan-Meier plots showing cumulative survival of TEER patients with catheter ablation of concomitant AF (blue graph) and of TEER patients without a history of AF (green graph). Graphs indicating means and 95% confidence intervals. * - For the catheter ablation group, the follow-up period was initiated after completion of both the TEER and CA procedures.
Discussion
Reflecting the complex and intimate pathophysiological interactions, AF represents the most common concomitant disease in severe MR and has been shown to dramatically worsen the prognosis of patients undergoing TEER. This underscores the urgent need for additional strategies to improve the outcomes in this unique HF patient population. Addressing this highly relevant but still unmet clinical need, we demonstrate in a large, well-characterized multicenter collective that an interventional strategy for rhythm control of AF, as opposed to pharmacological rhythm control or rate control, is associated with a more favorable long-term outcome in TEER patients. Here, interventional rhythm control even offsets the prognostic disadvantage of coexisting AF, as the outcome is not significantly different from that of TEER patients without a history of AF. The present key findings parallel evidence derived from patients undergoing surgery for MR, which also demonstrate a beneficial impact of surgical ablation (SA) of concomitant AF as part of the valve procedure. Thus, multiple studies show that SA can be safely added to the intended surgical intervention and that it improves the long-term survival considerably compared with conservatively managed or untreated preoperative AF, which is subsequently not even significantly different form that of patients without AF (20–23). This body of evidence led to a class I recommendation (level of evidence A) for SA of AF with concomitant mitral valve surgery in the 2017 STS issued “Clinical Practice Guidelines in Surgical Treatment of Atrial Fibrillation” (15). Of course, it must also be recognized that due to the nature of TEER, these patients differ significantly from surgical collectives in terms of HF severity and degree of multimorbidity. In further agreement with the present results, substantial evidence of a prognostically favorable effect of CA of AF has also emerged in recent years for the overall collective of HF patients. Thus, the “Ablation Versus Amiodarone for Treatment of Persistent Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted Device” (AATAC) randomized controlled trial (RCT), the “Catheter Ablation vs. Standard Conventional Therapy in Patients with Left Ventricular Dysfunction and Atrial Fibrillation” (CASTLE-AF) RCT, subgroup analyses of the “Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation” (CABANA) RCT and recent pooled analyses of randomized data and meta-analyses demonstrated a reduced all-cause mortality in heart failure patients undergoing CA for AF compared to the medical AF treatment (24–29). However, these collectives were on average about 10 years younger, involved fewer patients in NYHA functional class III/IV than the present study, and, obviously, excluded patients with high-grade MR. In addition to prognostic efficacy, the safety of the procedure is also a critical consideration. In the present study no major complications or periprocedural interferences with the TEER device associated with CA were observed. Also, a case series limited to 14 patients with CA of AF after TEER reported no major complications except for a transient ischemic attack which proved to be completely reversible in a patient on vitamin-k antagonists two days postprocedurally (30). The complication rate reported in the landmark studies cited above is also encouragingly low, at approximately 2%, with the vast majority being access related bleeding complications and percutaneously manageable pericardial effusions (24–26). Here, however, elderly patients such as those in the present collective are not well represented. “Real-world” data derived from observational studies and meta-analyses suggest that patients over 75 years of age experience similar to slightly higher complication rates, again mainly due to non-fatal bleeding at the access site and pericardium rather than systemic or cerebral emboli (31–34). Considering this low rate of expectable major complications associated with CA, the present sample size is insufficient to reliably assess the safety of interventional rhythm control in this collective. Overall, however, it can be stated that CA for AF performed in an experienced and high-volume center can be considered safe even in elderly and multimorbid patients, and most likely applies to the TEER collective as well.
Limitations
Given the nature of an observational cohort study, the results cannot demonstrate a causal relationship.
Despite careful adjustment for baseline differences by the two established and independent statistical procedures, PSM and multivariable Cox regression, the possibility of residual bias remains. Thus, despite PSM, it was not possible to fully balance the type of TEER device used between the CA and PT groups, as evidenced by the more frequent use of the PASCAL device in the CA group compared with the PT group. An impact of this imbalance on the primary endpoint all-cause mortality however, is unlikely, as both devices do not differ in their basic mode of operation and as no relevant differences in clinical efficacy or safety were observed between PASCAL and the MitraClip device in the randomized controlled Edwards PASCAL Transcatheter Valve Repair System Pivotal Clinical (CLASP-IID) Trial, which was also confirmed by our data (35).
Due to the design of the present study, however, it is theoretically possible that in spite of careful adjustment for the comprehensively recorded clinical and procedural characteristics, there is a bias in the selection (selection bias) of the individual cohorts analyzed.
Compared with clinical trials, the proportion of missing data, although small, and the use of a registry may limit precision, which may limit internal validity. In addition, we cannot evaluate the efficacy of AF therapies in terms of sinus rhythm stability or individual AF burden, respectively, and no detailed information on incidence of rehospitalizations or on individual causes of death during the follow-up period because the relevant data were not fully available. Nevertheless, clinically highly relevant hard end points were addressed.
Conclusion
Due to intimately shared pathophysiological mechanisms, MR, HF, and AF constitute a highly prevalent and yet equally fatal trio that continues to dramatically darken a patient's prognosis even after successful TEER. In the present study, we demonstrate with PSM and Cox regression that CA of concomitant AF is associated with a significantly better long-term outcome compared to pharmacological therapy and may even reverse the evident prognostic disadvantage of AF in the TEER collective.
Clinical perspectives
In light of the growing evidence of prognostic benefit and safety of CA of AF in the overall cohort of HF patients, our data, albeit not randomized, provide strong evidence for interventional rhythm control of AF as an essential part of a holistic management of TEER patients. We point out the importance of treating concomitant AF, as this is a very promising approach to improve the prognosis of this unique and continually growing HF population. Prospective randomized confirmation of the present results and further investigation of procedural aspects of CA in a larger TEER collective are strongly encouraged.
Data availability statement
The raw data supporting the conclusion of this article are available upon request.
Ethics statement
The studies involving humans were approved by the Ethics committee of the Medical Faculty of the Philipps University of Marburg. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because of the nature of the observational cohort study.
Author contributions
Conceptualization, CW, FA, and UL; Methodology, UL, FA, HM, CW; Software, FA; Validation, UL, HM, CW; Formal Analysis FA, HM, CW; Funding acquisition, CW; Investigation, FA, UL, CW; Resources, JM, SL, GC; Data curation FA, UL, HM, SB, DF, and CW; Writing—original draft preparation CW; Writing—review & editing FA, UL, BS, PH, SB, DF, HM, JM, SK; Visualization, FA, CW; Supervision UL, CW and BS; Project administration CW, FA, UL, BS, DD, SK, TD, HN, and PH; All authors contributed to the article and approved the submitted version.
Funding
The present work required no dedicated founding. CW was supported by the clinician scientist program (SUCCESS) of the Medical Faculty of the Philipps University Marburg, Germany. Open Access funding was provided by the Open Acess Publishing Fund of Philipps-Universität Marburg with support of the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation).
Acknowledgment
We would like to thank Erik Wade and Eberhard Weihe for helpful comments on the manuscript. The graphical abstract was created with BioRender.com.
Conflict of interest
SB received speaker's honoraria from Abbott Vascular.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1229651/full#supplementary-material
References
1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG. Enriquez-Sarano, M. Burden of valvular heart diseases: a population-based study. Lancet. (2006) 368:1005–11. doi: 10.1016/s0140-6736(06)69208-8
2. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. (2020) 22:1342–56. doi: 10.1002/ejhf.1858
3. Wu S, Chai A, Arimie S, Mehra A, Clavijo L, Matthews RV, et al. Incidence and treatment of severe primary mitral regurgitation in contemporary clinical practice. Cardiovasc Revasc Med. (2018) 19:960–3. doi: 10.1016/j.carrev.2018.07.021
4. Mirabel M, Iung B, Baron G, Messika-Zeitoun D, Détaint D, Vanoverschelde J-L, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J. (2007) 28:1358–65. doi: 10.1093/eurheartj/ehm001
5. Feldman T, Foster E, Glower DD, Glower DG, Kar S, Rinaldi MJ, et al. Percutaneous repair or surgery for mitral regurgitation. New Engl J Med. (2011) 364:1395–406. doi: 10.1056/nejmoa1009355
6. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, et al. Transcatheter mitral-valve repair in patients with heart failure. New Engl J Med. (2018) 379:2307–18. doi: 10.1056/nejmoa1806640
7. Stone GW, Abraham WT, Lindenfeld J, Kar S, Grayburn PA, Lim DS, et al. Five-year follow-up after transcatheter repair of secondary mitral regurgitation. New Engl J Med. (2023) 388:2037–48. doi: 10.1056/nejmoa2300213
8. Waechter C, Ausbuettel F, Chatzis G, Cheko J, Fischer D, Nef H, et al. Antithrombotic treatment and its association with outcome in a multicenter cohort of transcatheter edge-to-edge mitral valve repair patients. J Cardiovasc Dev Dis. (2022) 9:366. doi: 10.3390/jcdd9110366
9. Velu JF, Kortlandt FA, Hendriks T, Schurer RAJ, van Boven AJ, Koch KT, et al. Comparison of outcome after percutaneous mitral valve repair with the MitraClip in patients with versus without atrial fibrillation. Am J Cardiol. (2017) 120:2035–40. doi: 10.1016/j.amjcard.2017.08.022
10. Keßler M, Pott A, Mammadova E, Seeger J, Wöhrle J, Rottbauer W, et al. Atrial fibrillation predicts long-term outcome after transcatheter edge-to-edge mitral valve repair by MitraClip implantation. Biomol. (2018) 8:152. doi: 10.3390/biom8040152
11. Arora S, Vemulapalli S, Stebbins A, Ramm CJ, Kosinski AS, Sorajja P, et al. The prevalence and impact of atrial fibrillation on 1-year outcomes in patients undergoing transcatheter mitral valve repair results from the society of thoracic surgeons/American college of cardiology transcatheter valve therapy registry. Jacc Cardiovasc Interv. (2019) 12:569–78. doi: 10.1016/j.jcin.2018.12.012
12. Saad AM, Kassis N, Gad MM, Abdelfattah O, Ahuja KR, Farwati M, et al. Impact of atrial fibrillation on outcomes following MitraClip: a contemporary population-based analysis. Catheter Cardio Interv. (2021) 97:1252–6. doi: 10.1002/ccd.29310
13. Waechter C, Ausbuettel F, Chatzis G, Cheko J, Fischer D, Nef H, et al. Impact of rhythm vs. rate control in atrial fibrillation on the long-term outcome of patients undergoing transcatheter edge-to-edge mitral valve repair. J Clin Med. (2021) 10:5044. doi: 10.3390/jcm10215044
14. Halas M, Kruse J, McCarthy PM. Concomitant treatment of atrial fibrillation during mitral valve surgery. J Cardiovasc Electr. (2021) 32:2873–8. doi: 10.1111/jce.15019
15. Badhwar V, Rankin JS, Damiano RJ, Gillinov AM, Bakaeen FG, Edgerton JR, et al. The society of thoracic surgeons 2017 clinical practice guidelines for the surgical treatment of atrial fibrillation. Ann Thorac Surg. (2017) 103:329–41. doi: 10.1016/j.athoracsur.2016.10.076
16. Waechter C, Ausbuettel F, Chatzis G, Fischer D, Nef H, Barth S, et al. Analysis of atrial fibrillation treatment regimes in a multicenter cohort of transcatheter edge-to-edge mitral valve repair patients. J Interv Cardiol. (2020) 2020:1–7. doi: 10.1155/2020/6542028
17. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart DiseaseDeveloped by the task force for the management of valvular heart disease of the European society of cardiology (ESC) and the European association for cardio-thoracic surgery (EACTS). Eur Hear J. (2021) 43:ehab395. doi: 10.1093/eurheartj/ehab395
18. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association of cardio-thoracic surgery (EACTS)The task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2020) 42:ehaa612. doi: 10.1093/eurheartj/ehaa612
19. Yadav K, Lewis RJ. Immortal time bias in observational studies. JAMA. (2021) 325:686–7. doi: 10.1001/jama.2020.9151
20. Iribarne A, DiScipio AW, McCullough JN, Quinn R, Leavitt BJ, Westbrook BM, et al. Surgical atrial fibrillation ablation improves long-term survival: a multicenter analysis. Ann Thorac Surg. (2019) 107:135–42. doi: 10.1016/j.athoracsur.2018.08.022
21. Musharbash FN, Schill MR, Sinn LA, Schuessler RB, Maniar HS, Moon MR, et al. Performance of the cox-maze IV procedure is associated with improved long-term survival in patients with atrial fibrillation undergoing cardiac surgery. J Thorac Cardiovasc Surg. (2018) 155:159–70. doi: 10.1016/j.jtcvs.2017.09.095
22. McCarthy PM, Manjunath A, Kruse J, Andrei A-C, Li Z, McGee EC, et al. Should paroxysmal atrial fibrillation be treated during cardiac surgery? J Thorac Cardiovasc Surg. (2013) 146:810–23. doi: 10.1016/j.jtcvs.2013.05.015
23. Lee R, McCarthy PM, Wang EC, Vaduganathan M, Kruse J, Malaisrie SC, et al. Midterm survival in patients treated for atrial fibrillation: a propensity-matched comparison to patients without a history of atrial fibrillation. J Thorac Cardiovasc Surg. (2012) 143:1341–51. doi: 10.1016/j.jtcvs.2012.02.006
24. Biase LD, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device. Circulation. (2016) 133:1637–44. doi: 10.1161/circulationaha.115.019406
25. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. Catheter ablation for atrial fibrillation with heart failure. New Engl J Med. (2018) 378:417–27. doi: 10.1056/nejmoa1707855
26. Packer DL, Piccini JP, Monahan KH, Al-Khalidi HR, Silverstein AP, Noseworthy PA, et al. Ablation versus drug therapy for atrial fibrillation in heart failure. Circulation. (2021) 143:1377–90. doi: 10.1161/circulationaha.120.050991
27. Saglietto A, Ponti RD, Biase LD, Matta M, Gaita F, Romero J, et al. Impact of atrial fibrillation catheter ablation on mortality, stroke, and heart failure hospitalizations: a meta-analysis. J Cardiovasc Electr. (2020) 31:1040–7. doi: 10.1111/jce.14429
28. Chen S, Pürerfellner H, Meyer C, Acou W-J, Schratter A, Ling Z, et al. Rhythm control for patients with atrial fibrillation complicated with heart failure in the contemporary era of catheter ablation: a stratified pooled analysis of randomized data. Eur Heart J. (2020) 41:2863–73. doi: 10.1093/eurheartj/ehz443
29. Magnocavallo M, Parlavecchio A, Vetta G, Gianni C, Polselli M, Vuono FD, et al. Catheter ablation versus medical therapy of atrial fibrillation in patients with heart failure: an updated systematic review and meta-analysis of randomized controlled trials. J Clin Med. (2022) 11:5530. doi: 10.3390/jcm11195530
30. Rottner L, Lemes C, Dotz I, Wohlmuth P, Schmidt T, Mathew S, et al. The clip and the tip: lessons learned from ablation of atrial fibrillation in patients postpercutaneous mitral valve repair. J Cardiovasc Electr. (2019) 30:1207–14. doi: 10.1111/jce.13964
31. Kautzner J, Peichl P, Sramko M, Cihak R, Aldhoon B, Wichterle D. Catheter ablation of atrial fibrillation in elderly population. J Geriatric Cardiol Jgc. (2017) 14:563–8. doi: 10.11909/j.issn.1671-5411.2017.09.008
32. Prasitlumkum N, Tokavanich N, Trongtorsak A, Cheungpasitporn W, Kewcharoen J, Chokesuwattanaskul R, et al. Catheter ablation for atrial fibrillation in the elderly >75 years old: systematic review and meta-analysis. J Cardiovasc Electr. (2022) 33:1435–49. doi: 10.1111/jce.15549
33. Lee W-C, Wu P-J, Chen H-C, Fang H-Y, Liu P-Y, Chen M-C. Efficacy and safety of ablation for symptomatic atrial fibrillation in elderly patients: a meta-analysis. Front Cardiovasc Med. (2021) 8:734204. doi: 10.3389/fcvm.2021.734204
34. Müller J, Nentwich K, Berkovitz A, Ene E, Sonne K, Chakarov I, et al. Efficacy and safety of high-power short duration atrial fibrillation ablation in elderly patients. J Cardiovasc Electr. (2022) 33:1425–34. doi: 10.1111/jce.15504
Keywords: pulmonary vein isolation, atrial fibrillation, mortality, heart failure, mitraclip, PASCAL, mitral regurgitation, catheter ablation
Citation: Ausbuettel F, Barth S, Chatzis G, Fischer D, Kerber S, Mueller J, List S, Halbfass P, Deneke T, Nef H, Mueller H-H, Divchev D, Schieffer B, Luesebrink U and Waechter C (2023) Catheter ablation of concomitant atrial fibrillation improves survival of patients undergoing transcatheter edge-to-edge mitral valve repair. Front. Cardiovasc. Med. 10:1229651. doi: 10.3389/fcvm.2023.1229651
Received: 26 May 2023; Accepted: 28 July 2023;
Published: 14 August 2023.
Edited by:
Hoda Hatoum, Michigan Technological University, United StatesReviewed by:
Shelley Gooden, Georgia Institute of Technology, United StatesBrototo Deb, Georgetown University, United States
Ahmed El Shaer, University of Wisconsin-Madison, United States
© 2023 Ausbuettel, Barth, Chatzis, Fischer, Kerber, Mueller, List, Halbfass, Deneke, Nef, Mueller, Divchev, Schieffer, Luesebrink and Waechter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian Waechter Y2hyaXN0aWFuLndhZWNodGVyQHVuaS1tYXJidXJnLmRl
 Felix Ausbuettel1
Felix Ausbuettel1 Julian Mueller
Julian Mueller Bernhard Schieffer
Bernhard Schieffer Christian Waechter
Christian Waechter