- 1The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, China
- 2Qingdao Hiser Hospital Affiliated of Qingdao University (Qingdao Traditional Chinese Medicine Hospital), Qingdao, China
- 3Department of Cardiology, Zhejiang Hospital, Hangzhou, China
Background: Newly developed catheter ablation (CA) techniques, such as laser balloon ablation (LBA) and cryoballoon ablation (CBA), have been introduced in recent years and emerged as valuable alternatives to conventional radiofrequency CA strategies for paroxysmal atrial fibrillation (PAF) patients. However, evidence comparing LBA and CBA remain controversial. Thus, we conducted this meta-analysis to assess the efficacy and safety between these two techniques.
Methods: Scientific databases (PubMed, Embase) and relevant websites (the Cochrane Library, ClinicalTrials.gov) were systematically searched from inception to March 2023. The primary outcomes of interest were the AF recurrence and the procedure-related complications. Secondary outcomes included procedural time, fluoroscopy time, and left atrial (LA) dwell time.
Results: Seven clinical trials with a total of 637 patients were finally enrolled. No significant differences were found between LBA and CBA in terms of AF recurrence [16.3% vs. 22.7%, odds ratio (OR) = 0.66, 95% confidence interval (CI): 0.42–1.05, p = 0.078] or total procedural-related complications (8.4% vs. 6.4%, OR = 1.33, 95% CI: 0.71–2.51, p = 0.371). LBA had a significantly longer procedural time [weighted mean difference (WMD) = 38.03 min, 95% CI: 13.48–62.58 min, p = 0.002] and LA dwell time (WMD = 46.67 min, 95% CI: 14.63–78.72 min, p = 0.004) than CBA, but tended to have shorter fluoroscopy time.
Conclusions: LBA and CBA treatment have comparable efficacy and safety for PAF patients. LBA was associated with longer procedural and LA dwell times compared with CBA. Further large-scale studies are warranted to compare these two techniques with the newest generations.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=426513, identifier (CRD42023426513).
1. Introduction
Atrial fibrillation (AF) is a common cardiac arrhythmia, and patients with AF are known to be at increased risk of morbidity and mortality (1). Catheter ablation (CA) has been the most effective therapeutic approach in restoring and maintaining sinus rhythm for symptomatic AF patients, and pulmonary vein isolation (PVI) has been recognized as the cornerstone and fundamental therapeutic strategy of CA (2).
Several balloon-based catheter ablation techniques, including laser balloon ablation (LBA) and cryoballoon ablation (CBA), have been introduced in recent years and emerged as valuable alternatives to conventional radiofrequency CA strategies (3). Previous studies have demonstrated that balloon-based CA techniques not only have comparable efficacy and safety outcomes but also provide several superiorities, such as shorter procedural and fluoroscopy durations, especially for paroxysmal AF (PAF) patients (4, 5).
Several studies have compared the characteristics, efficacy, and safety between LBA and CBA as initial therapies for PAF patients; however, the results remain controversial (6, 7). Therefore, the aim of the present meta-analysis was to investigate the efficacy, safety, and procedural characteristics between LBA and CBA for PAF patients in light of the latest evidence.
2. Materials and methods
2.1. Search strategy and selection criteria
Scientific databases (PubMed, Embase) and relevant websites (the Cochrane Library, ClinicalTrials.gov) were systematically searched from inception to March 2023. The following keywords and the corresponding variants were used: “laser balloon,” “cryoballoon,” and “paroxysmal atrial fibrillation.” In addition, the reference lists of all eligible articles were manually checked for potentially relevant studies. Full-text articles in English that directly compared LBA and CBA in the treatment of PAF and reporting interested outcomes were included.
2.2. Data collection and quality assessment
Data extraction and quality assessment were performed by two authors (WY and GF) independently with divergences resolved with a third author (JL). The following data were extracted: author's name, publication year, sample size, participant characteristics, ablation protocol, duration of follow-up, and outcomes of interest. Two authors working independently assessed the risk of bias using the Cochrane Collaboration tool (8) for randomized controlled trials (RCTs) and the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool (9) for the non-randomized studies. The Cochrane Collaboration tool included six domains: random sequence generation, allocation concealment, blinding for outcome assessment, incomplete outcome data, selective reporting, and other bias. And the ROBINS-I tool included seven domains: confounding, selection of participants into the study, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of the reported result.
2.3. Primary and secondary outcomes
The primary outcomes of interest were the AF recurrence, defining as AF/atrial flutter/atrial tachycardia documented on the ECG or Holter continuing longer than 30 s during follow-up, and the procedure-related complications. Secondary outcomes included procedural time, fluoroscopy time and left atrial (LA) dwell time.
2.4. Statistical analysis
Statistical analysis was performed using the STATA software package (version 14.1 for macOS; STATA Corporation, College Station, TX, USA). Categorical variables were described as n (%) and continuous variables were described as median and standard deviation (SD). Odds ratio (OR) and weighted mean difference (WMD) with the 95% confidence interval (CI) were calculated to demonstrate the summary statistics for comparisons between LBA and CBA. The random-effects model was applied. The between-study heterogeneity was assessed using the inconsistency index (I2) statistic (I2 < 25% = low, I2: 25%–50% = moderate, and I2 > 50% high heterogeneity). When significant heterogeneity was present, possible causes were investigated. The likelihood of publication bias was analyzed by funnel plots graphically and by Egger's and Begg's tests statistically. The protocol for this systematic review and meta-analysis was registered on PROSPERO (doi: 10.15124/CRD42023426513).
3. Results
3.1. Eligible studies and characteristics
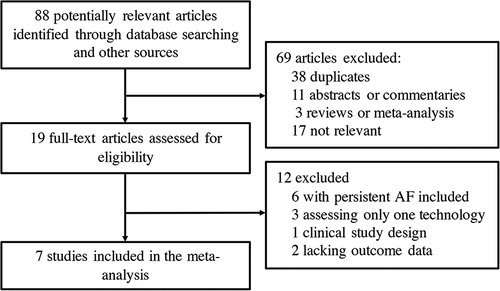
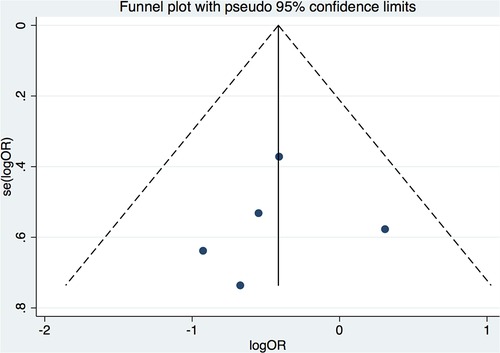
Seven clinical trials (6, 7, 10–14) from 88 potentially relevant studies were finally included in the meta-analysis (Figure 1). A total of 637 patients receiving initial ablation for PAF (LBA, n = 311 vs. CBA, n = 326) were studied. The main characteristics of the studies and the participants included are reported in Table 1. Briefly, across the trials, two studies (10, 12) were RCTs while the rest studies were non-randomized prospective clinical trials. There were 311 patients in the LBA group and 326 patients in the CBA group. LBA with the first-generation laser balloon (LB1) was performed in all studies. CBA with the first-generation CB (CB1) was applied in one study (6), while CBA with the second-generation CB (CB2) were applied in three studies (11, 13, 14). The mean age of the patients ranged from 57.6 to 73 years. The mean left ventricular ejection fraction (LVEF) ranged from 61.8% to 70% and the mean left atrium dimeter (LAd) ranged from 36 to 43 mm. Median follow-up length was 12.4 months. All the included studies had good qualities according to the Cochrane Collaboration tool (8) and ROBINS-I tool (9) (Supplementary Tables S1, S2). No significant publication bias was found by funnel plot or Egger's and Begg's tests based on the primary outcomes (Egger's: p = 0.789; Begg's: p = 0.462) (Figure 2).
3.2. Primary end points
Of the included trials, five studies (6, 10, 11, 13, 14) provided information on AF recurrence after LBA and CBA treatments. Results demonstrated that there was no significant difference between LBA and CBA regarding AF recurrence (16.3% vs. 22.7%, OR = 0.66, 95% CI: 0.42–1.05, p = 0.078). No significant heterogeneity was detected (I2 = 0%) (Figure 3).
Four studies (6, 10, 11, 13) additionally provided data regarding the needs of touch-up ablation (TUA) during LBA and CBA procedures and showed that LBA and CBA had comparable TUA rates (8.4% vs. 10.7%, OR = 1.00, 95% CI: 0.34–2.94, p = 1.00). No significant heterogeneity was detected (I2 = 39.1%) (Figure 3) Additional meta-regression analyses did not show significant associations between AF recurrence and the study and patient characteristics, such as year of publication, ablation protocols, participant number, mean LAd, mean LVEF, monitoring protocols, and follow-up lengths (p > 0.05 for all), whereas the leave-one-out analysis was further performed and showed that, when the study by Yano et al. (14) was removed, LBA treatment was associated with significantly lower AF recurrence rate compared with that of CBA (OR = 0.57, 95% CI: 0.35–0.95, p = 0.03).
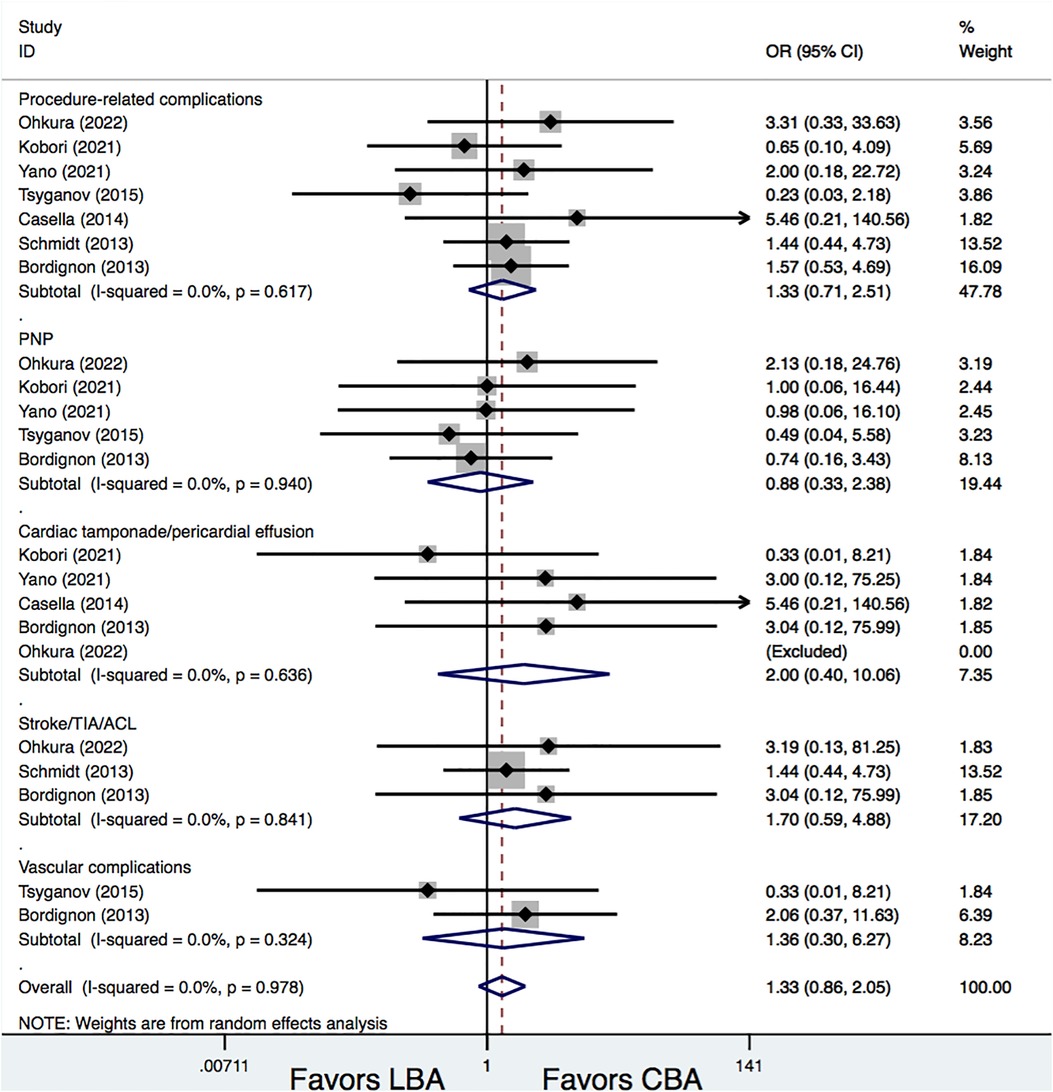
All the studies included provided information on procedure-related complications. Results demonstrated that the total procedure-related complications rates were similar between the LBA and CBA treatments (8.4% vs. 6.4%, OR = 1.33, 95% CI: 0.71–2.51, p = 0.371). Additional subgroup analyses were conducted according to different complication types, and the results showed that, there were no significances between LBA and CBA regarding phrenic nerve palsy (PNP) (2.6% vs. 2.8%, OR = 0.88, 95% CI: 0.33–2.38, p = 0.807), cardiac tamponade/pericardial effusion (1.0% vs. 0.3%, OR = 2.00, 95% CI: 0.40–10.06, p = 0.399), stroke/transient ischemic attacks (TIA)/asymptomatic cerebral lesions (ACL) (3.2% vs. 1.8%, OR = 1.70, 95% CI: 0.59–4.88, p = 0.325), and vascular complications (1.3% vs. 0.9%, OR = 1.36, 95% CI: 0.30–6.27, p = 0.689). No significant heterogeneities were detected for all the comparisons (I2 = 0%) (Figure 4).
3.3. Secondary end points
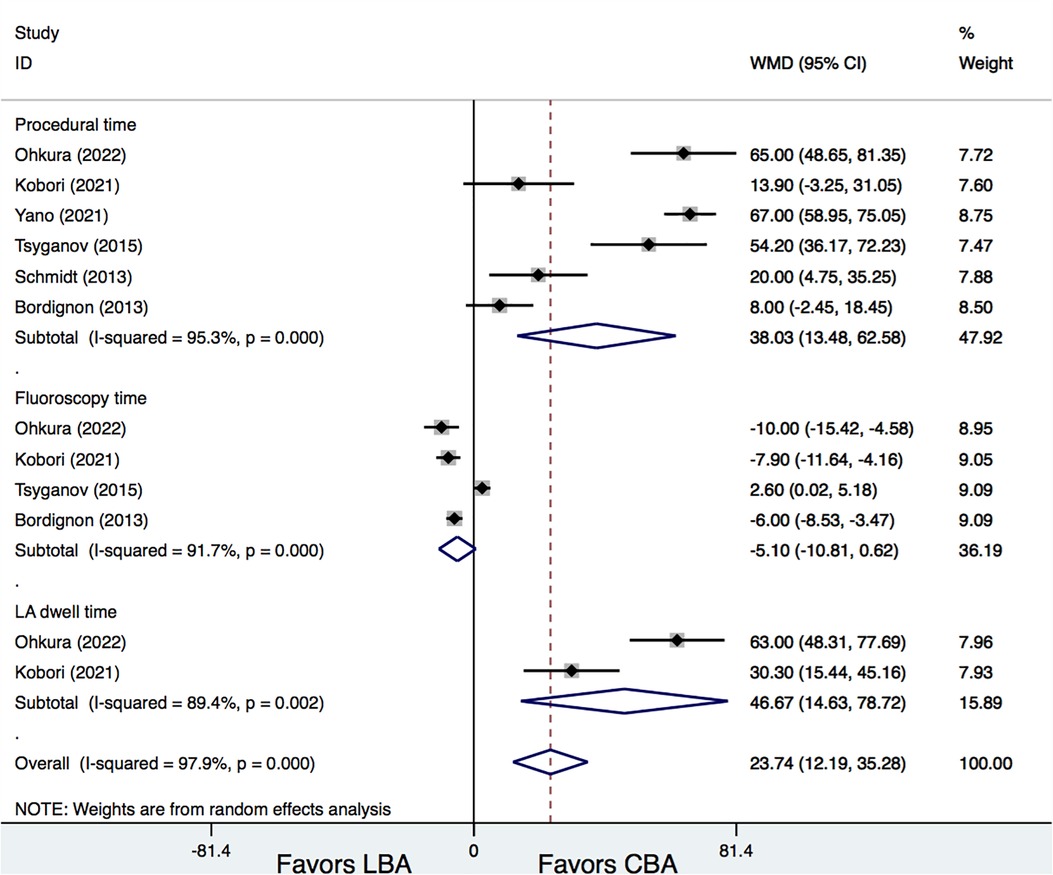
Six studies (6, 7, 11–14) provided information on procedural time of LBA and CBA. Results demonstrated that, LBA had a significantly longer procedural time than CBA (WMD = 38.03 min, 95% CI: 13.48–62.58 min, p = 0.002). In addition, LBA also needed a longer LA dwell time than CBA (WMD = 46.67 min, 95% CI: 14.63–78.72 min, p = 0.004) (Figure 5). No significant difference was found regarding fluoroscopy time between LBA and CBA therapy (WMD = −5.10 min, 95% CI: −10.81 to 0.62 min, p = 0.081). However, significant heterogeneities were detected for comparisons (I2 = 95.3%, 89.4%, and 91.7%, respectively). Meta-regression analysis was further conducted, whereas no significantly associations between procedural time and the study and patient characteristics were detected (p > 0.05 for all). Additional subgroup and leave-one-out analysis were also performed and showed that, when the study by Tsyganov et al. (13) was removed, LBA treatment was associated with significantly shorter fluoroscopy time compared with that of CBA (WMD = −7.04 min, 95% CI: −9.00 to −5.08 min, p = 0.00) (Figure 5).
4. Discussion
This meta-analysis included seven studies with a total of 637 patients. The major findings were as follows: (1) LBA had a non-significant lower AF recurrence rate compared with CBA (16.3% vs. 22.7%); (2) The needs of TUA during procedure were comparable between the two technologies; (3) LBA and CBA showed comparable safety profile; (4) LBA had significantly longer procedural and LA dwell time than CBA, but tends to have shorter fluoroscopy time.
Current guidelines recommend PVI by means of CA as treatment for drug-refractory PAF (4). Point-by-point radiofrequency catheter ablation (RFCA) has been a standard of care for PVI; however, it still has shortcomings, such as technical complexity, long procedure time, high rates of complications, and long learning curve (15). The balloon-based CA technologies including LBA and CBA have been introduced in recent years to overcome the complexity of the conventional point-by-point RFCA procedure, which also have shown simplicity, reproducibility, and similar effectiveness compared with RFCA, especially for PAF patients (4, 16). For persistent AF patients, RFCA has shown advantages, when additional ablations of atrial myocardium beyond pure PVI are needed, though the STAR-AF2 trial proved that pure PVI was non-inferior to more extensive atrial ablation in patients with persistent AF (17). However, persistent AF has a more complex pathophysiologic basis than PAF, and pure PVI is sufficient in most cases for PAF patients. Thus, the balloon-based CA techniques have advantages over RFCA for PAF patients, with comparable efficacy but shorter procedure duration.
In the present study, LBA and CBA were directly compared for PAF patients and demonstrated similar AF recurrence at a median of 12.4 months. However, it should be noted that AF recurrence seemed to be lower after LBA compared with CBA during further sensitivity analysis. Possible reasons may be that, first, though low heterogeneity was detected (I2 = 0%) for this outcome, the definitions of AF recurrence and rhythm monitoring strategies were non-uniform across the studies. The rates of freedom from AF recurrences in the present study were 83.7% in the LBA group and 77.3% in the CBA group, which were higher than that in the studies using continuous rhythm monitoring strategies by Rovaris et al. (18) and Andrade et al. (19). They reported that the 1-year freedom from recurrences was 66.9%, 81.0%, and 86.8% considering any, 5.5-h, and 24-h cut-off duration after LBA (18), and was 52.2% and 51.7% after 4-min CBA and 2-min CBA, respectively (19). Continuous rhythm monitoring is thought to be essential and the most accurate approach to assess the post-procedure AF burden and the true value of certain AF ablation techniques (20). However, all the included trials in this study used only Holter ECGs as monitoring tools. This intermittent monitoring tool is thought to be a very limited technique to truly assess AF recurrences, which may inevitably cause bias (18). Second, except the study by Yano et al. (14), non-significant lower AF recurrences were seen after LBA in the remaining studies. It was reported that the PVI lesions created by LBA had a durability of 86% at 3 months during repeat mapping (21). In addition, in patients with clinical recurrence and repeat procedures, the chronic isolation rates were reported to be only 32% after CBA, compared with that of 59% after LBA (6). The relatively high durability of electrical PVI may indicate better arrhythmic outcomes of LBA compared with CBA.
Though the lesion size created by CBA was reported to be larger than that by LBA, it was not seen to be correlated with clinical outcome after a single procedure during 1-year follow-up (22). In a multicenter, randomized trial of LBA that compared with RFCA, 94.1% of patients were able to achieve electrical PVI with the LBA alone (16), whereas in the STOP AF Trial, only 83% PVI was achieved with the CBA alone, and additional ablation was required (23). However, in the present study, data were limited to compare the acute PVI rates, but the incidence of additional TUA was found to be comparable between these two techniques, which were consistent with the previously work by Seki et al., comparing hot balloon, LBA, and CBA for both PAF and persistent AF patients (24).
As for the safety profile, LBA and CBA had comparable overall complication rates, with no significant differences in complication types. The total procedure-related complication rate in the present study (7.4%) was a little higher than that reported in the study by Chun et al. (5.4%), which investigated the complications in 3,000 AF patients after CBA and LBA procedures (25). However, the total complications rates of both LBA and CBA in the present study were still lower than that reported in their initial randomized comparisons to standard RFCA (11.8% for LBA in the HeartLight Study and 12.8% for CBA in the FIRE and ICE trial) (4, 16). Possible reasons may be the different definitions of procedural complications across these trials, as ACL was recognized as a complication in the present study, while dyspnea, gastrointestinal complication, and contrast media reaction were defined as complications in other trials (4, 16).
PNP was a common periprocedural complication of balloon-based procedures for AF (26). Though it was reported that PNP recovery time was significantly longer during LBA than that during CBA, due to their greatly different lesion formations (27), the majority of PNP were usually transient, most of which could recover during follow-up.
Different from PNP, cardiac tamponade and pericardial effusion are serious perioperative complications. It was reported that, the incidence of cardiac tamponade was approximately 1.3% in all the CA techniques for AF (28). The incidence of cardiac tamponade for LBA and CBA procedure was reported to be 0.1% in the study with 3,000 AF patients in a high-volume center (25). The incidences of cardiac tamponade and pericardial effusion in the present study were inconsistent with the published data. However, it should be noted that, the majority of events of cardiac tamponade/pericardial effusion occurred in the LBA group (3/4) in the present study. In a large RCT trial that compared LBA and RFCA, the incidence of cardiac tamponade/pericardial effusion was reported to be 1.2% (16), whereas in the FIRE and ICE trial, this incidence was only 0.3% for CBA (4).
These results indicated that LBA may be more vulnerable to cardiac tamponade/pericardial effusion than CBA. Possible reasons may be that the CBA procedure was navigated through a guidewire but LBA was not; in addition, unlike the single-shot CBA technique, more catheter manipulations were required for LBA in the LA with segment-by-segment ablation (27). Operator experiences and the generations of the techniques used may also be important potential reasons. As it was reported, adverse event endpoints were improved with increased operator experience over 15 LBA cases, demonstrating the learning curve effect with the newly introduced technologies such as LBA (16, 29).
These new balloon-based CA techniques were designed to reduce the complexity of the conventional RFCA procedure, which could greatly reduce the total procedural time (20). In the present study, LBA was shown to have a significantly longer procedural time and LA dwell time than CBA. As mentioned above, the non-compliant CBA system represents as a single-shot PVI tool, in contrast to the segment-by-segment ablation procedure by LBA (27). LBA could offer direct PV visualization and provide a more precise regional energy titration, at the cost of more procedural times. In addition, LBA lacks the technique for real-time PV potentials recording as CBA, contributing to increased procedural time when PVI validation was needed, especially in the case of failed first-round PVI (27).
On the other hand, owing to the inherent visually guiding characteristic of LBA during procedure, less fluoroscopy time was supposed to be required (6). In our study, LBA was found to be associated with a non-significantly shorter fluoroscopy time compared with CBA, whereas during further sensitivity analysis, LBA showed a significant reduction in fluoroscopy time. This is reasonable as CBA requires a serial angiogram to achieve optimal PV occlusion, while LBA offers visually guided balloon control and deployment. It should still be noted that the first-generation laser balloon was applied in all the studies included. It was reported that the more compliant second- and third-generation laser balloon has been applied, offering an automated continuous lesion deployment, which contributes to the improvements in PV occlusion, energy delivery, and shorter procedural time (30, 31). Thus, future larger prospective multicenter randomized studies are warranted to reveal the efficacy, safety profiles, and procedure characteristics between LBA and CBA, especially for these techniques with the newest generations.
The current study has a number of limitations. First, the number of trials and the total sample size of the present analysis were relatively small, and large-scale clinical trials, especially RCTs, comparing LBA and CBA, were rare. Second, AF recurrence definitions and monitoring strategies across trials were non-uniform, and all the included studies used Holter ECG monitoring rather than continuous rhythm monitoring, which is limited in assessing AF recurrences and may cause possible bias. However, we performed an additional meta-regression analysis to further investigate the potential moderator effect of the study and patient features on AF recurrence. Third, LB1 was applied in all the studies and CB2 was exclusively used in only three studies. As LBA with the second- and third-generation and CBA with the third-generation have been introduced and widely applied in recent years, and the improvement of the LBA between the first and the second/third generation was enormous, the results need to be further updated to provide up-to-date evidence. Finally, considerable heterogeneities were detected in the analysis of the secondary outcomes; though meta-regression and sensitive analyses have been performed, the interpretation should still be taken with caution.
5. Conclusions
LBA and CBA treatments have comparable efficacy and safety in terms of AF recurrence and procedure-related complications as initial therapies for PAF patients. CBA therapy is associated with a shorter procedural time and LA dwell time compared with that of LBA, while LBA therapy tends to have shorter fluoroscopy time. Further large-scale RCTs with the newest generations of LBA and CBA are needed to provide up-to-date evidence.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
WY, QC, and GF designed the research, performed the statistical analysis, and wrote the manuscript text. XZ and XW performed the literature search and data collation. WM and JL have jointly supervised the work and critically revised the article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Research Project of Zhejiang Chinese Medical University (grant number 2021JKZDZC03).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1229223/full#supplementary-material
References
1. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham heart study: a cohort study. Lancet. (2015) 386:154–62. doi: 10.1016/s0140-6736(14)61774-8
2. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42:373–498. doi: 10.1093/eurheartj/ehaa612
3. Boersma L. New energy sources and technologies for atrial fibrillation catheter ablation. Europace. (2022) 24:ii44–51. doi: 10.1093/europace/euab258
4. Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KR, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. (2016) 374:2235–45. doi: 10.1056/NEJMoa1602014
5. Zhou X, Dai J, Xu X, Lian M, Lou Y, Lv Z, et al. Comparative efficacy and safety of catheter ablation interventions for atrial fibrillation: comprehensive network meta-analysis of randomized controlled trials. J Interv Card Electrophysiol. (2021) 62:199–211. doi: 10.1007/s10840-020-00878-9
6. Bordignon S, Chun KR, Gunawardene M, Fuernkranz A, Urban V, Schulte-Hahn B, et al. Comparison of balloon catheter ablation technologies for pulmonary vein isolation: the laser versus cryo study. J Cardiovasc Electrophysiol. (2013) 24:987–94. doi: 10.1111/jce.12192
7. Ohkura T, Yamasaki T, Kakita K, Hattori T, Nishimura T, Iwakoshi H, et al. Comparison of maximum-sized visually guided laser balloon and cryoballoon ablation. Heart Vessels. (2022) 38(5):691–8. doi: 10.1007/s00380-022-02208-7
8. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. Br Med J. (2011) 343:d5928. doi: 10.1136/bmj.d5928
9. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. (2016) 355:i4919. doi: 10.1136/bmj.i4919
10. Casella M, Dello Russo A, Russo E, Al-Mohani G, Santangeli P, Riva S, et al. Biomarkers of myocardial injury with different energy sources for atrial fibrillation catheter ablation. Cardiol J. (2014) 21:516–23. doi: 10.5603/CJ.a2013.0153
11. Kobori A, Sasaki Y, Pak M, Okada T, Toyota T, Kim K, et al. Early experiences with three types of balloon-based ablation catheters in patients with paroxysmal atrial fibrillation. Heart Rhythm O2. (2021) 2:223–30. doi: 10.1016/j.hroo.2021.03.009
12. Schmidt B, Gunawardene M, Krieg D, Bordignon S, Fürnkranz A, Kulikoglu M, et al. A prospective randomized single-center study on the risk of asymptomatic cerebral lesions comparing irrigated radiofrequency current ablation with the cryoballoon and the laser balloon. J Cardiovasc Electrophysiol. (2013) 24:869–74. doi: 10.1111/jce.12151
13. Tsyganov A, Petru J, Skoda J, Sediva L, Hala P, Weichet J, et al. Anatomical predictors for successful pulmonary vein isolation using balloon-based technologies in atrial fibrillation. J Interv Card Electrophysiol. (2015) 44:265–71. doi: 10.1007/s10840-015-0068-3
14. Yano M, Egami Y, Ukita K, Kawamura A, Nakamura H, Matsuhiro Y, et al. Impact of myocardial injury and inflammation due to ablation on the short-term and mid-term outcomes: cryoballoon versus laser balloon ablation. Int J Cardiol. (2021) 338:102–8. doi: 10.1016/j.ijcard.2021.06.016
15. Wu C, Li X, Lv Z, Chen Q, Lou Y, Mao W, et al. Second-generation cryoballoon versus contact force radiofrequency ablation for atrial fibrillation: an updated meta-analysis of evidence from randomized controlled trials. Sci Rep. (2021) 11:17907. doi: 10.1038/s41598-021-96820-8
16. Dukkipati SR, Woollett I, Mc EH, Böhmer MC, Doshi SK, Gerstenfeld EP, et al. Pulmonary vein isolation using the visually guided laser balloon: results of the U.S. feasibility study. J Cardiovasc Electrophysiol. (2015) 26:944–9. doi: 10.1111/jce.12727
17. Verma A, Sanders P, Macle L, Deisenhofer I, Morillo CA, Chen J, et al. Substrate and trigger ablation for reduction of atrial fibrillation trial-part II (STAR AF II): design and rationale. Am Heart J. (2012) 164:1–6.e6. doi: 10.1016/j.ahj.2012.04.002
18. Rovaris G, Ciconte G, Schiavone M, Mitacchione G, Gasperetti A, Piazzi E, et al. Second-generation laser balloon ablation for the treatment of atrial fibrillation assessed by continuous rhythm monitoring: the LIGHT-AF study. Europace. (2021) 23:1380–90. doi: 10.1093/europace/euab085
19. Andrade JG, Champagne J, Dubuc M, Deyell MW, Verma A, Macle L, et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. (2019) 140:1779–88. doi: 10.1161/circulationaha.119.042622
20. Schiavone M, Gasperetti A, Montemerlo E, Pozzi M, Sabato F, Piazzi E, et al. Long-term comparisons of atrial fibrillation ablation outcomes with a cryoballoon or laser-balloon: a propensity-matched analysis based on continuous rhythm monitoring. Hellenic J Cardiol. (2022) 65:1–7. doi: 10.1016/j.hjc.2022.03.006
21. Dukkipati SR, Neuzil P, Kautzner J, Petru J, Wichterle D, Skoda J, et al. The durability of pulmonary vein isolation using the visually guided laser balloon catheter: multicenter results of pulmonary vein remapping studies. Heart Rhythm. (2012) 9:919–25. doi: 10.1016/j.hrthm.2012.01.019
22. Perrotta L, Konstantinou A, Bordignon S, Fuernkranz A, Dugo D, Chun KJ, et al. What is the acute antral lesion size after pulmonary vein isolation using different balloon ablation technologies? Circ J. (2017) 81:172–9. doi: 10.1253/circj.CJ-16-0345
23. Andrade JG, Khairy P, Macle L, Packer DL, Lehmann JW, Holcomb RG, et al. Incidence and significance of early recurrences of atrial fibrillation after cryoballoon ablation: insights from the multicenter sustained treatment of paroxysmal atrial fibrillation (STOP AF) trial. Circ Arrhythm Electrophysiol. (2014) 7:69–75. doi: 10.1161/circep.113.000586
24. Seki R, Nagase T, Asano S, Fukunaga H, Inoue K, Sekiguchi Y, et al. Radiofrequency current versus balloon-based ablation for atrial fibrillation. Am J Cardiol. (2022) 178:52–9. doi: 10.1016/j.amjcard.2022.05.029
25. Chun KRJ, Perrotta L, Bordignon S, Khalil J, Dugo D, Konstantinou A, et al. Complications in catheter ablation of atrial fibrillation in 3,000 consecutive procedures: balloon versus radiofrequency current ablation. JACC Clin Electrophysiol. (2017) 3:154–61. doi: 10.1016/j.jacep.2016.07.002
26. Okumura Y, Henz BD, Bunch TJ, Dalegrave C, Johnson SB, Packer DL. Distortion of right superior pulmonary vein anatomy by balloon catheters as a contributor to phrenic nerve injury. J Cardiovasc Electrophysiol. (2009) 20:1151–7. doi: 10.1111/j.1540-8167.2009.01495.x
27. Chun JKR, Bordignon S, Last J, Mayer L, Tohoku S, Zanchi S, et al. Cryoballoon versus laserballoon: insights from the first prospective randomized balloon trial in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. (2021) 14:e009294. doi: 10.1161/circep.120.009294
28. Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol. (2009) 53:1798–803. doi: 10.1016/j.jacc.2009.02.022
29. Perrotta L, Bordignon S, Dugo D, Fürnkranz A, Chun KJ, Schmidt B. How to learn pulmonary vein isolation with a novel ablation device: learning curve effects using the endoscopic ablation system. J Cardiovasc Electrophysiol. (2014) 25:1293–8. doi: 10.1111/jce.12491
30. Heeger CH, Tiemeyer CM, Phan HL, Meyer-Saraei R, Fink T, Sciacca V, et al. Rapid pulmonary vein isolation utilizing the third-generation laserballoon—the PhoeniX registry. Int J Cardiol Heart Vasc. (2020) 29:100576. doi: 10.1016/j.ijcha.2020.100576
Keywords: paroxysmal atrial fibrillation, catheter ablation, laser balloon ablation, cryoballoon ablation, meta-analysis
Citation: Ye W, Chen Q, Fan G, Zhou X, Wang X, Mao W and Li J (2023) Efficacy and safety of visually guided laser balloon versus cryoballoon ablation for paroxysmal atrial fibrillation: a systematic review and meta-analysis. Front. Cardiovasc. Med. 10:1229223. doi: 10.3389/fcvm.2023.1229223
Received: 31 May 2023; Accepted: 9 August 2023;
Published: 22 August 2023.
Edited by:
Christian Hendrik Heeger, University Heart Center Luebeck, GermanyReviewed by:
Giuseppe Ciconte, IRCCS San Donato Polyclinic, ItalyAlessio Gasperetti, Johns Hopkins Medicine, United States
© 2023 Ye, Chen, Fan, Zhou, Wang, Mao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Mao bWFvd2VpbHdAMTYzLmNvbQ== JuanJuan Li anVhbmp1YW5fbGkyMDIzQDE2My5jb20=
†These authors have contributed equally to this work
 Wenyi Ye1,†
Wenyi Ye1,† Xinbin Zhou
Xinbin Zhou