- 1Tehran Heart Center, Cardiovascular Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran
- 2Students’ Scientific Research Center (SSRC), Tehran University of Medical Sciences, Tehran, Iran
- 3Isfahan Cardiovascular Research Center, Cardiovascular Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran
- 4Cardiac Primary Prevention Research Center, Cardiovascular Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran
Background and objectives: Atrioventricular block (AVB) is a serious complication following coronary artery bypass grafting (CABG) surgery, and its high-grade form may necessitate the implantation of a permanent pacemaker (PPM). AVB is associated with increased morbidity and mortality rates. This study aims to estimate the incidence of AVB and subsequent PPM implantation after isolated CABG surgery.
Material and methods: We searched electronic databases of PubMed, Embase, and Scopus from inception to 18 November 2022. Clinical trials and observational studies reporting the incidence of post-CABG AVB or subsequent PPM implantation in adult patients were included. The total incidence for all included outcomes was calculated using the inverse variance method, and the I2 statistic was reported to evaluate the heterogeneity of studies.
Results: A total of 28 studies met the inclusion criteria. Four studies [3 cohorts, 1 randomized controlled trial (RCT)] reported AVB without specifying its type; one (cohort) reported different degrees of AVB, 20 (12 cohorts, 8 RCTs) reported complete heart block (CHB) (or AVB requiring temporary pacing), and nine (8 cohorts, 1 RCT) reported the number of PPM inserted due to AVB. The pooled incidence of AVB, CHB (or AVB requiring temporary pacing), and PPM due to AVB was 1.16%, 1.73%, and 0.58%, respectively. Meta-regression analysis revealed that age, gender, diabetes, hypertension, hyperlipidemia, or smoking were not significantly associated with AVB, CHB, or PPM implantation.
Conclusion: This study highlights the incidence of AVB and the need for PPM implantation following CABG surgery. The findings emphasize the importance of postoperative monitoring and surveillance to improve patient outcomes.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022377181, identifier PROSPERO CRD42022377181.
1. Introduction
Coronary artery bypass grafting (CABG) surgery is a well-known therapeutic strategy for treating coronary artery disease that uses autologous arteries or veins to bypass clogged arteries. It is recommended when a severe blockage in one of the major coronary arteries cannot be relieved by percutaneous coronary intervention (1, 2). Approximately 400,000 CABG procedures are performed annually in the United States, making it one of the most performed major surgeries (1). Early cardiac complications of CABG include myocardial infarction (MI), graft occlusion, low cardiac output, vasodilatory shock, pericarditis, and arrhythmias (3). Arrhythmias encompasses bradyarrhythmias, including sinus node dysfunction and atrioventricular block (AVB) (4, 5). Surgical trauma, local edema, inflammation, and ischemia are thought to be involved in the development of AVB (6, 7). Studies have reported various incidence rates for post-CABG AVB from 0% to 13% (8, 9). Several risk factors, such as age, number of bypassed vessels, aortic cross-clamp (ACC) time, preoperative arrhythmia, and left ventricular dysfunction (LVD), have been associated with the occurrence of AVB and the need for permanent pacemaker (PPM) implantation after CABG (10–12). Pacemaker implantation is considered to be the main treatment of high-grade AVB. In most cases, AVB recovers spontaneously in the postoperative period and does not require a PPM (7). However, if high-grade AVB persists for more than seven days postoperatively, implantation of a PPM should be considered (13, 14).
Patients with high-grade AVB have a higher risk for syncope, congestive heart failure, ventricular tachycardia, asystole, or sudden cardiac death (15–17). High-grade AVB after CABG prolongs hospital stays, and as a result, it may considerably increase healthcare costs. Moreover, high-grade AVB requiring temporary pacing (TP) can increase mortality after CABG by up to 10% compared with patients without AVB (10). Besides, pacemaker implantation has several complications, such as infection, hematoma, cardiac injury, pneumothorax, thrombosis, lead malfunction, and dislodgement (18).
Despite the importance of this issue, few studies have investigated the incidence of AVB after CABG, and to our knowledge, no systematic review or meta-analysis has been performed on this subject. The present systematic review and meta-analysis aims to estimate the incidence of AVB and subsequent PPM implantation following CABG and to review associated risk factors. It helps to better understand the significance of AVB as a complication of CABG and to build the foundation for preventive measures.
2. Methods
2.1. Search strategy
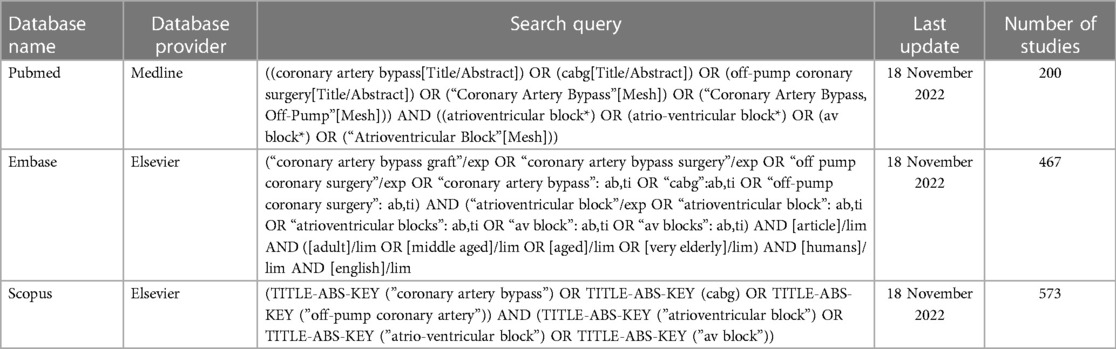
This systematic review was conducted according to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statements (19). The study protocol was registered on PROSPERO (registration number CRD42022377181). Three electronic databases, PubMed, Embase, and Scopus, were comprehensively searched from inception to 18 November 2022. The search was performed by combining the keywords and medical subject heading (MeSH) terms. Customized search queries for each database are presented in Table 1.
2.2. Study selection and data extraction
The inclusion and exclusion criteria were as follows: randomized clinical trials and observational studies reporting the incidence of AVB or PPM implantation due to AVB after isolated CABG surgery in adult patients (18 years or older) were included. Animal studies, studies not in English, conference abstracts, review articles, research letters, or studies whose full texts were not available were excluded. After the initial search and removing duplicates, two authors screened the titles and abstracts to identify relevant studies. The full texts of potentially relevant studies were independently reviewed following inclusion and exclusion criteria, and relevant data were extracted into a pre-defined Microsoft Excel spreadsheet (Microsoft Corporation, Redmond, WA, USA). The way of consensus resolved any conflict in this step. The following variables were extracted from each study: first author, publication year, study design, sample size and the major cardiovascular risk factors (including age, sex, diabetes, hypertension, hyperlipidemia, smoking, left ventricular ejection fraction), mean follow-up time, number of AVBs, complete heart blocks (CHB) (or AVBs requiring TP) and PPMs inserted due to AVB. We also extracted the list of risk factors reported to have a statistically significant relationship with the review outcomes from the included studies.
It is worth mentioning that some studies also reported pacemaker implantations for indications other than post-CABG AVB, such as sinus bradycardia, bundle branch block, atrial fibrillation, and cardiac arrest. To maintain the focus and integrity of our analysis, we only entered data related to pacemakers that were specifically indicated for post-CABG high-grade AVB. Furthermore, while some included studies did not exclude patients with pre-existing AVB, and some did not explain whether they did, all of the studies clearly stated that they were reporting on newly developed AVBs after CABG. Due to the limited availability of studies that exclude patients with pre-existing AVB, we deemed it appropriate to include all relevant studies that reported post-CABG AVB.
2.3. Quality assessment
Two reviewers independently evaluated the quality of each study, and disagreements were resolved with the help of a third reviewer. The quality of observational studies was assessed following the Newcastle-Ottawa Quality Assessment Scale (20). The scale evaluates three main areas: (a) study selection, (b) comparability, (c) outcome, and the maximum score for cohort and case-control studies is 9. Study quality was considered high if the Newcastle-Ottawa Scale score was at least 7 points and categorized as good (3 or 4 points in the selection domain, 2 or 3 points in the exposure and outcome domain, and 1 or 2 points in the comparability domain). The quality of the study was otherwise considered low. We used Cochrane's Risk of Bias (RoB 2) tool (21) to assess randomized trials, which evaluates five main domains: randomization processes, deviations from intended interventions, missing outcome data, outcome measurement, and selection of reported results. We classified each domain individually as high risk, low risk, or some concerns. The overall risk of bias was considered low risk if all domains were recognized as low risk (Supplementary File S1).
2.4. Outcomes
The primary outcomes were the incidence of AVB, CHB (or AVB requiring TP), and PPM implantation. The secondary outcome was to determine if major cardiovascular risk factors are associated with the occurrence of primary outcomes.
AVB is a significant complication after CABG surgery that may range from transient and self-resolving to persistent and requiring medical intervention such as pacemaker implantation. High-grade AVB or AVB requiring TP increase morbidity and mortality (10, 22). Understanding the incidence of different grades of AVB is crucial in evaluating its overall burden and impact on patient outcomes. Furthermore, the need for PPM implantation is an important clinical endpoint as it reflects the long-term consequences of post-CABG AVB. In addition, we aimed to explore the association between major cardiovascular risk factors and the occurrence of AVB, high-grade AVB, and PPM implantation as our secondary outcome. This analysis seeks to identify potential risk factors that may contribute to the development of AVB and assist in risk stratification and postoperative surveillance strategies for high-risk patients.
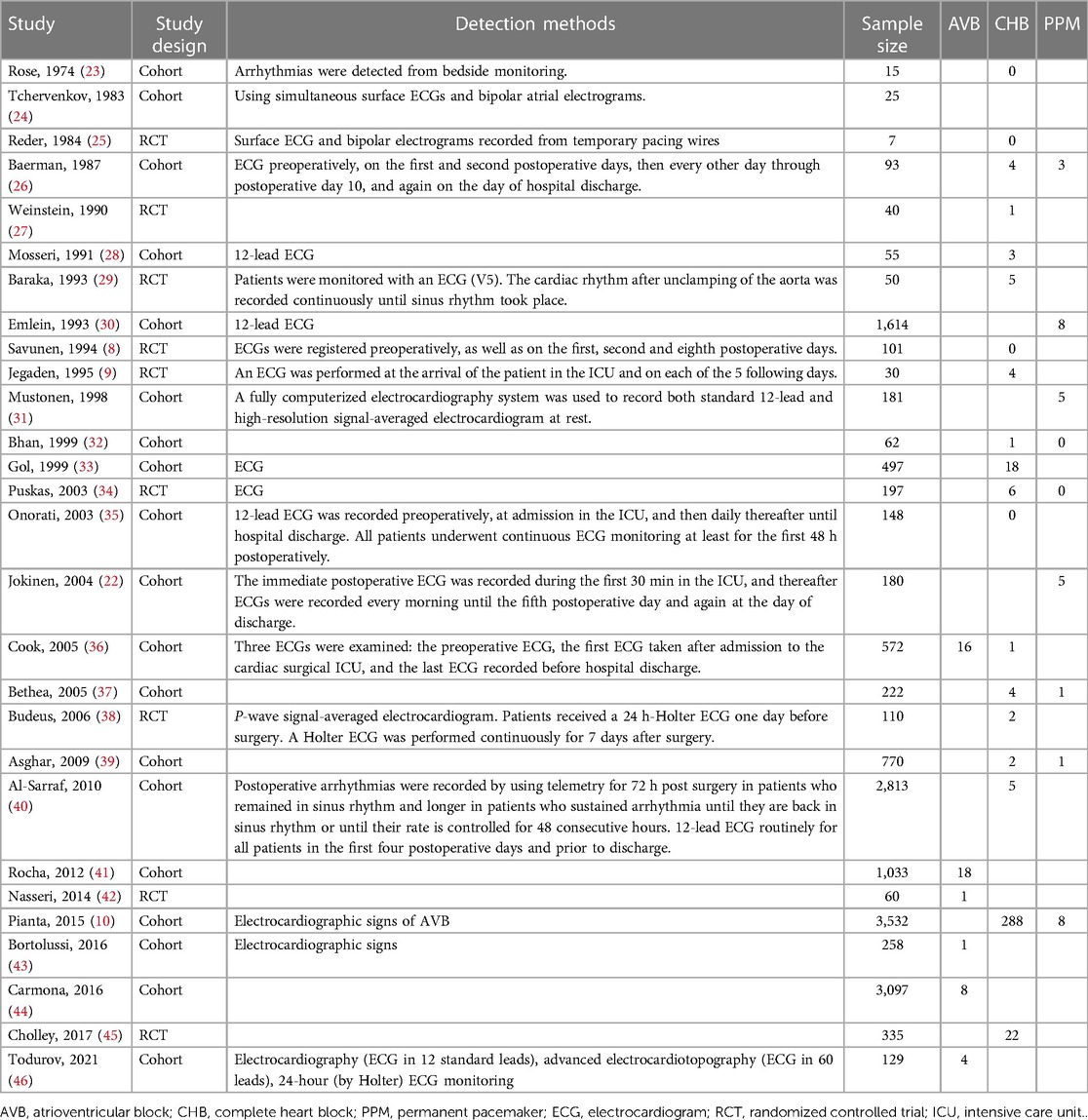
The frequency of desired outcomes and methods used to identify them in each study are shown in Table 2.
2.5. Data synthesis and statistical analysis
Baseline patient characteristics were reported in mean ± standard deviation (SD) format for continuous variables and percentage (number) for categorical variables. The total incidence for all included outcomes was calculated using data from the longest follow-up of each study and through Freeman-Tukey double arcsine transformation and back transformation in the inverse variance method (47). Between-study variance (τ2) was calculated through the restricted maximum-likelihood estimator (REML) (48). We also reported the I2 statistic to evaluate the heterogeneity of studies.
Furthermore, a meta-regression analysis was conducted to assess the relationship between desired outcomes and risk factors such as age, gender, diabetes, hypertension, hyperlipidemia, and smoking. We assessed publication bias using funnel plots and Egger's test. However, there is no universally accepted definition of what constitutes a positive result in a proportional meta-analysis. The presumption that positive results are more frequently published is not always valid for proportional studies (49, 50). R Programming language (R for Windows, version 4.2.1, Vienna, Austria), R Studio version 1.1.463 (Posit PBC, Boston, MA, United States), packages “meta” (version 5.5.0) and “metafor” (version 3.4.0), and STATA software version 16.0 (StataCorp LLC, College Station, TX, USA, version 16.0) were used for all statistical analyses.
3. Results
3.1. Search results
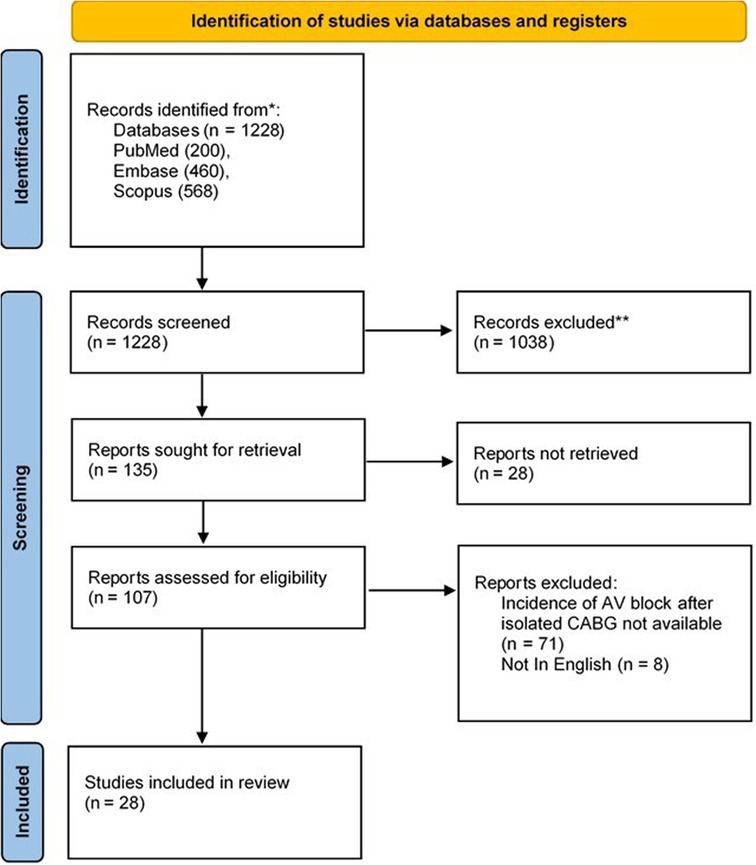
The initial search of electronic databases returned 1,228 documents. After removing duplicates and initial screening, the full text of 135 articles was evaluated for inclusion and exclusion criteria. Finally, reviewers extracted the data of the thirty included articles into pre-defined spreadsheets. The PRISMA flowchart of the study is presented in Figure 1.
3.2. Study characteristics
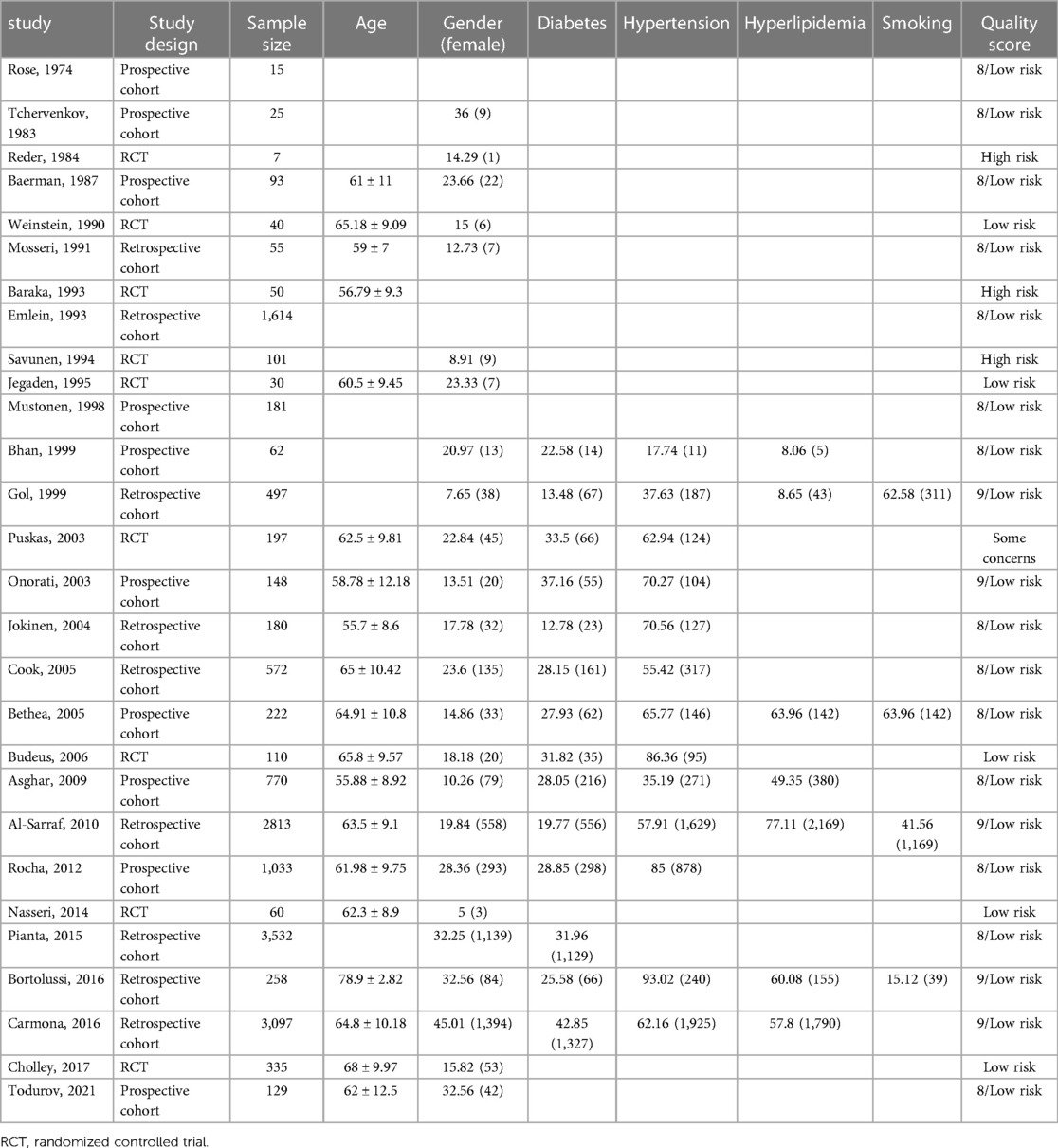
The baseline demographic features and comorbidities of patients have been presented in Table 3. All observational studies that entered the review had a low risk of bias, and from nine randomized controlled trials (RCTs), three were assessed to have a high risk of bias (Table 3). The included studies reported the following postoperative outcomes: four studies reported AVB without specifying its type, one study reported different types of AVB, 20 studies reported CHB (or AVB requiring TP), and nine studies reported the number of PPM inserted due to AVB. Separate meta-analyses were performed on each of the mentioned outcomes to enhance the accuracy of the results.
3.3. Atrioventricular block
Five studies (4 cohorts, 1 RCT) reported AVBs following CABG. The pooled incidence of AVB was 1.16% [95% CI (0.00; 3.60), τ2 = 0.003, I2 = 88.7%; Figure 2] calculated with random effects model with a prediction interval ranging from 0% to 10.01%. Todurov et al. reported the highest incidence of post-CABG AVB (3.10%), and Carmona et al. reported the lowest incidence (0.26%) (44, 46). Furthermore, the meta-regression results indicated no significant relationship between the incidence of post-CABG AVB, age, gender, the prevalence of diabetes, and hypertension in studied populations (p-values of 0.425, 0.273, 0.727, and 0.611, respectively).
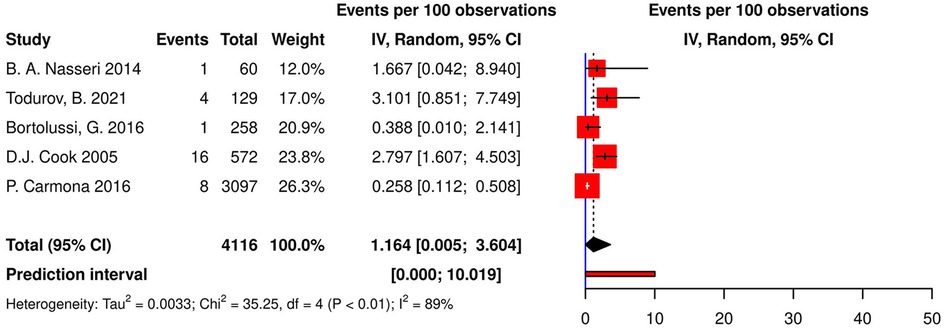
Figure 2. Forest plot demonstrating the incidence of AVB in patients following CABG. AVB, atrioventricular block; CABG, coronary artery bypass grafting; IV, individual value.
3.4. CHB, or AVB requiring TP
Twenty-one studies (13 cohorts, 8 RCTs) reported the incidence of post-CABG CHB or AVB requiring TP, resulting in an aggregate incidence of 1.73% [95% CI (0.59; 3.26), τ2 = 0.007, I2 = 96.0%; Figure 3] on random effects model. The prediction interval ranged from 0% to 10.78%. Additionally, a meta-regression of the relationship between the incidence of post-CABG CHB, or AVB requiring TP and age, gender, the prevalence of diabetes, hypertension, hyperlipidemia, and smoking was performed, which found no significant relationships (p-values of 0.972, 0.110, 0.366, 0.929, 0.569 and 0.619, respectively).
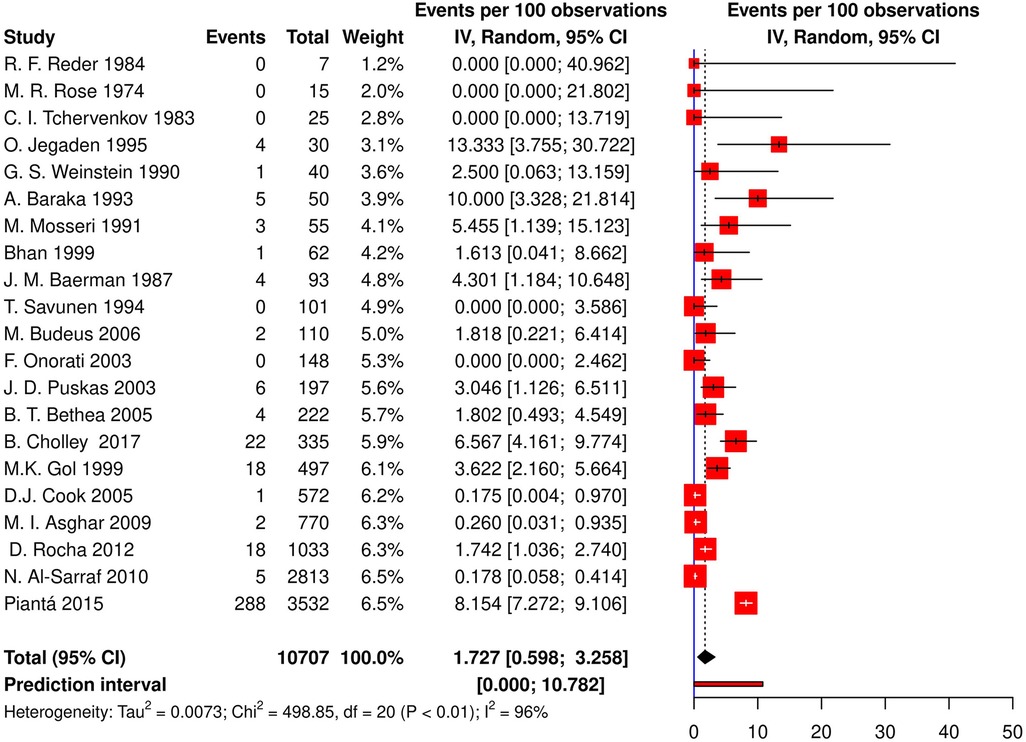
Figure 3. Forest plot demonstrating incidence of CHB/AVB requiring TP in patients following CABG. CHB, complete heart block; AVB, atrioventricular block; TP, temporary pacing; CABG, coronary artery bypass grafting; IV, individual value.
3.5. Postoperative permanent pacing due to atrioventricular block
The pooled estimate for post-CABG pacing was calculated using data from nine studies (8 cohorts, 1 RCT) and equaled 0.58% [95% CI (0.07; 1.42), τ2 = 0.002, I2 = 73%; Figure 4] on the random effects model. The prediction interval for this analysis ranged from 0% to 4.35%. The highest reported percentage for permanent pacing after CABG was 3.23% (26); in two studies, no permanent pacing was required (32, 34). Moreover, in the meta-regression analysis, no significant correlation was observed between post-CABG pacing, age, gender, the prevalence of diabetes, hypertension, and hyperlipidemia (p-values of 0.997, 0.947, 0.784, 0.858, and 0.978, respectively).
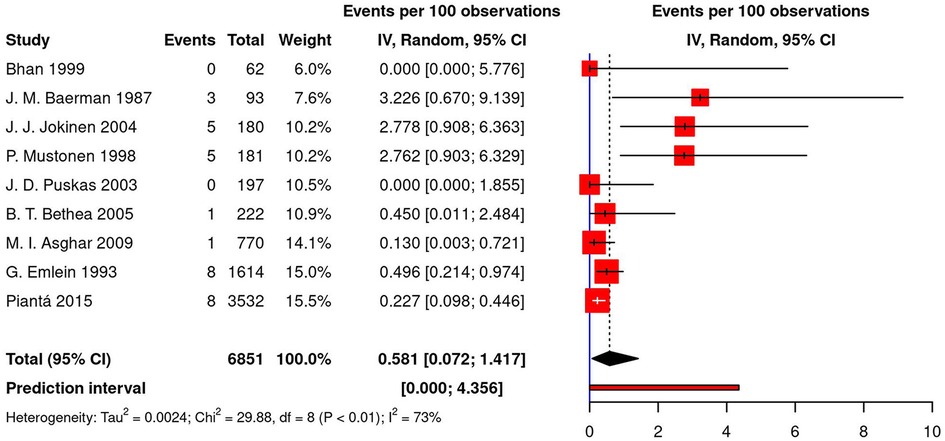
Figure 4. Forest plot demonstrating percentage of permanent pacing in patients following CABG. CABG, coronary artery bypass grafting; IV, individual value.
3.6. Publication bias
Funnel plots were used to evaluate publication bias for all three outcomes (Figure 5). The plots for post-CABG AVB and CHB (or AVB requiring TP) were symmetrical, yet relative asymmetry was observed regarding post-CABG permanent pacing. However, Egger's test for plot asymmetry did not suggest any asymmetry for any of the outcomes. The p-values of Egger's test for AVB, CHB, and permanent pacing were 0.1821, 0.8425, and 0.0623, respectively.
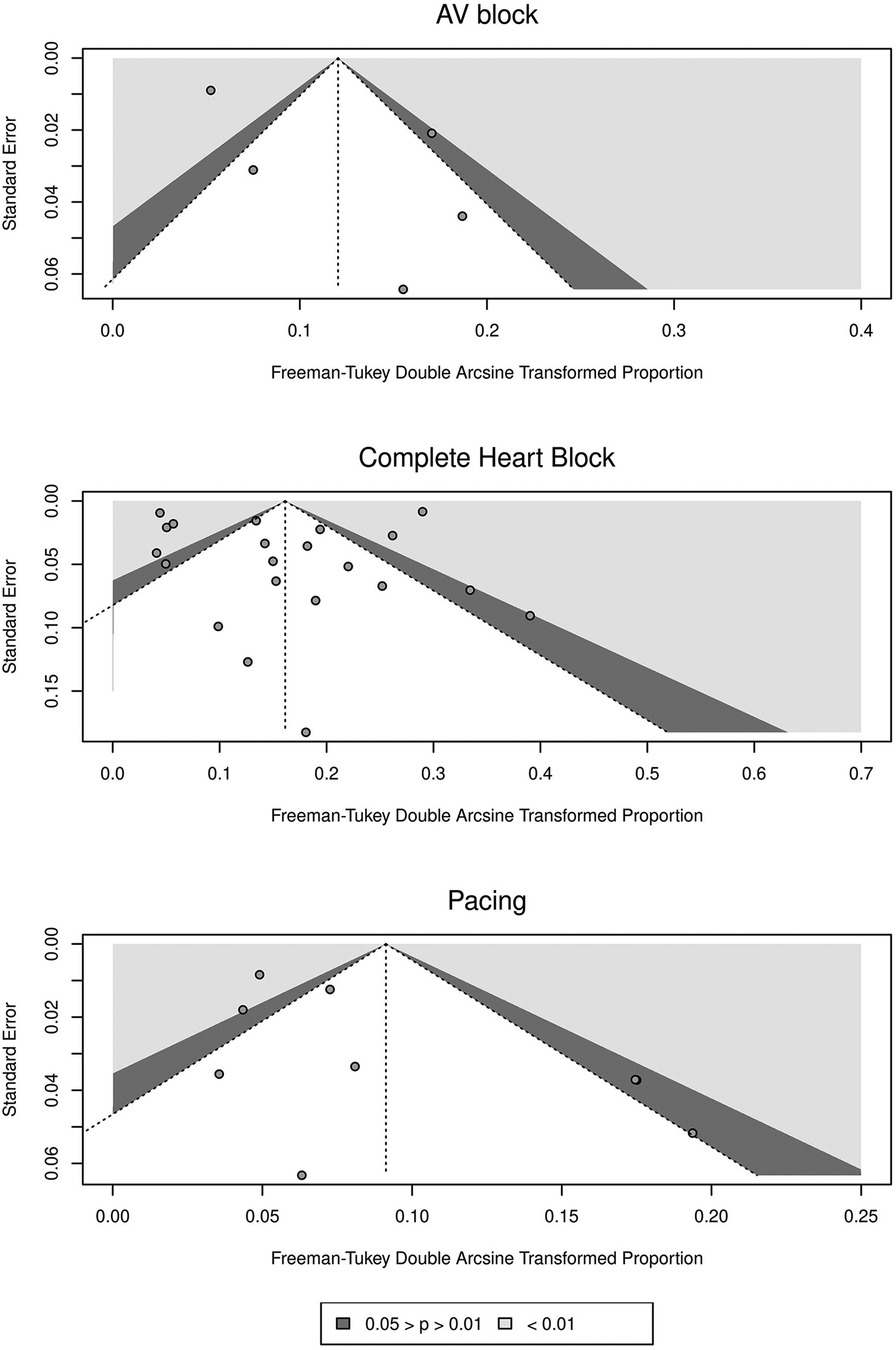
Figure 5. Funnel plots of standard error against freeman-tukey double arcsine transformed proportions for included studies for different outcomes.
4. Discussion
To our knowledge, this is the first systematic review and meta-analysis of the incidence of postoperative AVB and subsequent pacing requirements in patients undergoing isolated CABG surgery. The current meta-analysis found that after isolated CABG, the incidence of AVB in 4,116 patients was 1.16%, the incidence of CHB or AVB requiring pacemaker implantation in 10,707 patients was 1.73%, and the percentage of PPM implantation due to advanced AVB in 6,851 patients was 0.58%. Based on these results, 0.33 or one-third of patients with CHB or AVB requiring TP will need PPM implantation. In the meta-regression analysis, age, gender, diabetes, hypertension, hyperlipidemia, or smoking had no significant association with AVB, CHB, or PPM implantation. However, these variables were reported for study populations, and we were not able to access the individual data required for a more robust analysis. In this review, we considered studies that reported AVB without further explanation as high-grade AVB due to the similarity between their incidence rates and those that reported high-grade AVB requiring TP.
The results of this study show that the incidence of high-grade AVB and the subsequent need for PPM implantation is a serious complication after CABG surgery. Therefore, it is crucial to take preventive measures, especially in high-risk patients. For this purpose, the risk factors should be further investigated, and then clinical guidelines should be designed to identify high-risk patients.
4.1. Heterogeneity of studies
We observed a high variability between studies, especially in those reporting CHB. The overall heterogeneity between 21 studies that reported CHB or AVB requiring TP was high, with an I2 of 96.0%. The overall heterogeneity for AVB and PPM implantation was lower (I2 88.7% and 73.2%, respectively). Since the diagnosis method was serial electrocardiograms in most studies, it does not seem to be the source of variability. Instead, two outlier studies in the CHB group (9, 29) and three outlier studies in the PPM group (22, 26, 31) with relatively small sample sizes and wide confidence intervals could be considered the primary source of heterogeneity.
In a study by Baraka et al., it was found that of 19 patients (38%) who needed pacing after releasing the ACC, only five (10%) required it upon discharge from the operating room (29). Accordingly, there is a significant difference between the number of intraoperative and postoperative AVB. In other words, many intraoperative AVBs resolve before discharge from the operating room. In confirmation of this assertion, three excluded studies reported the incidence of CHB after releasing the ACC, 16% (51), 24% (11), and 30% (12). These numbers are much higher than those reported in our included studies. In one of these studies, CHB after the release of ACC was referred to as postoperative CHB (51). Thus, there is a possibility that the studies we included also misreported postoperative AVB. In this case, this may account for some of the observed heterogeneity.
4.2. Risk factors
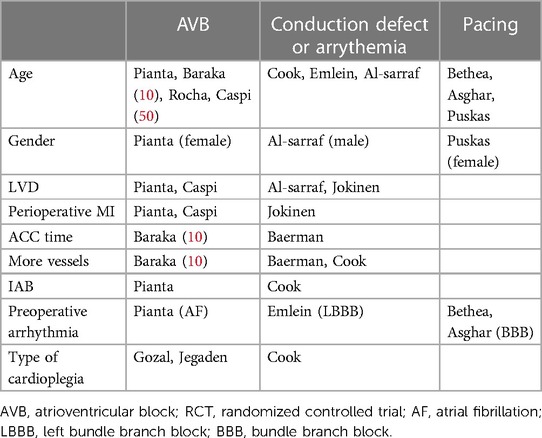
Literature has reported many risk factors associated with post-CABG AVB and the subsequent need for pacing. Here we review the risk factors in the studies we included in our meta-analysis. These consisted of old age, gender, LVD, perioperative MI, number of vessels bypassed, longer ACC time, perioperative use of an intra-aortic balloon, preoperative arrhythmia, and the type of cardioplegia (Table 4).
One of the most frequently mentioned risk factors was age. In four studies, old age was associated with a higher incidence of AVB after CABG or after releasing the ACC. Four studies had a significant association between LVD and post-CABG AVB or pacing. In a large cohort study, Pianta et al. found that perioperative MI increased post-CABG AVB requiring TP, but the same was not true for patients with previous MI. In the same study, post-CABG AVB was more frequent in patients with preoperative atrial fibrillation (10). In other studies by Emlein et al., Bethea et al., and Asghar et al. preoperative arrhythmia, especially bundle branch block, was associated with an increased risk of post-CABG bradyarrhythmias or pacing (30, 37, 39). In a study by Baraka et al., longer ACC time and more grafted vessels were associated with the incidence of AVB after releasing the ACC (11). In one study, using an intra-aortic balloon was related to the incidence of post-CABG AVB (10). Few included studies suggested a relationship between gender and AVB or post-CABG pacing. Pianta et al. suggested that post-CABG high-grade AVB appeared more frequently in females (10). Also, females were more likely to experience post-CABG pacing in a study by Puskas et al. (34).
The type of cardioplegia can be a risk factor since different types of cardioplegia methods have been related to increased postoperative conduction disorders. A study by Cook et al. suggested that crystalloid cardioplegia increased post-CABG conduction defects compared with blood cardioplegia (36). Gozal et al. indicated that hypothermic blood cardioplegia was associated with more CHB than normothermic cardioplegia (12). In another study by Jegaden et al., antegrade cardioplegia caused more AVB requiring pacing than combined antegrade-retrograde cardioplegia (9). The other factor that has been proposed is the type of CABG surgery. Carmona et al. showed that the incidence of AVB following on-pump and off-pump CABG was not significantly different (44).
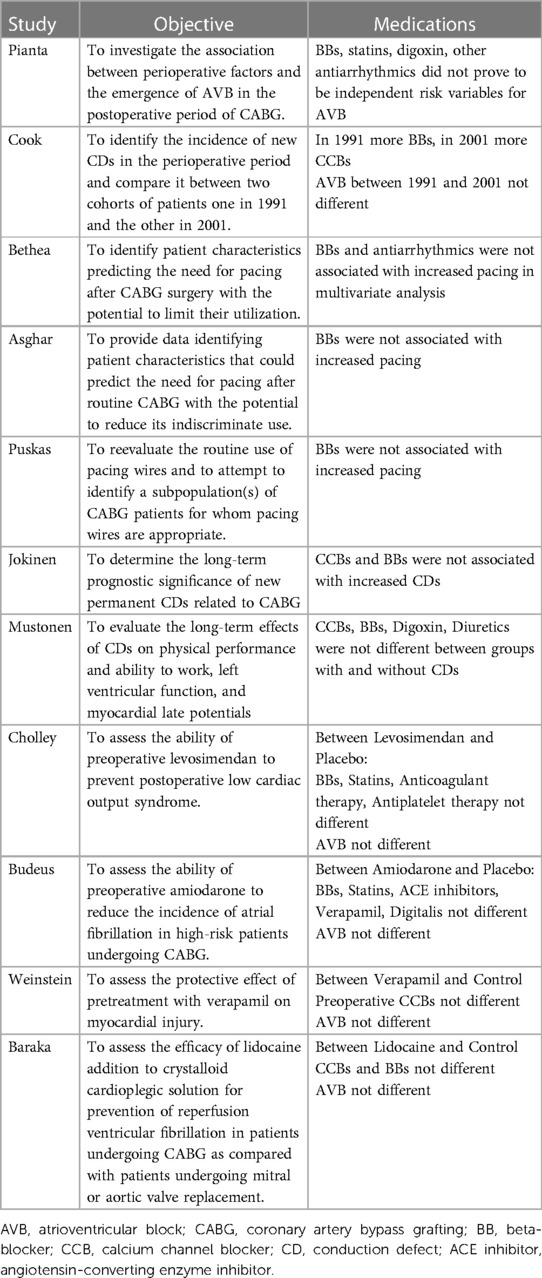
The other factor that should be further investigated is the effect of medications. Pianta et al. found that the incidence of high-grade AVB after CABG was similar between patients using beta-blockers and those without (10). In a cohort study on the prognosis of permanent conduction defects related to CABG by Jokinen et al., calcium channel blockers and beta-blockers were not associated with increased conduction defects (22). In a single-center retrospective study, Cook et al. compared two cohorts of patients who underwent CABG surgery, one in 1991 and the other in 2001, regarding conduction disorders. In 2001, compared to 1991, more patients were using beta-blockers; the opposite was true for calcium channel blockers. In the 2001 cohort, the incidence of post-CABG conduction defects decreased, but the incidence of different degrees of AVB did not differ (36). In other studies, Puskas et al., Bethea et al., and Asghar et al. found that preoperative beta-blocker use did not increase the risk of pacemaker implantation after CABG (34, 37, 39).
Because only one study (10) specifically investigated the potential association of medications with the incidence of post-CABG AVB or pacemaker implantation due to AVB, we could not analyze this association. Table 5 includes an outline of the studies that provided information about the medications used by patients.
4.3. Prognosis
Preventing post-CABG AVB and its related complications is crucial to avoid adverse consequences. Several studies suggest the incidence of AVB after CABG surgery is associated with increased mortality. Pianta et al. indicated AVB requiring TP prolonged hospital stay and increased mortality after CABG (10). In a study by Jokinen et al. that investigated the long-term prognosis of permanent conduction defects after CABG, CHB showed a significant association with increased mortality (22). In another study by Caspi et al., AVB after releasing the ACC was associated with postoperative low cardiac output and higher mortality rates (51).
4.4. Limitations
There are several restrictions on how broadly applicable our findings can be. First, we only searched three online databases and included full-text English journal articles, which may have resulted in selection bias. Second, five studies reported post-CABG AVB without further explanation of its type, which limited analysis of the incidence of different types of AVB. However, we analyzed them separately to prevent the pooling of heterogeneous data. Third, studies did not separately report characteristics in patients with and without AVB, except in one (10). Consequently, we could not accurately analyze their potential associations with post-CABG AVB. Fourth, since almost all studies did not provide a precise definition of postoperative AVB, intraoperative AVB may also have been reported.
4.5. Clinical implications and recommendations
Our study provides important insights into the incidence of post-CABG AVB. The pooled prevalence rates calculated for the incidence of CHB (or AVB requiring TP) and PPM implantation can serve as reference points for clinicians. These incidence rates highlight the need for increased postoperative surveillance and monitoring to reduce the risk of high-grade AVB sequelae. The identification of high-risk patients should be prioritized. Risk factors such as age, preoperative arrhythmia, LVD, perioperative MI, and the use of certain medications should be considered when assessing the patients.
As for future research, we recommend conducting large-scale prospective cohort studies investigating the incidence of post-CABG AVB, its risk factors, and its long-term prognosis. These studies would provide a more comprehensive understanding of the multiple factors influencing post-CABG AVB and facilitate the development of risk prediction models. Moreover, including other patient-centered outcomes, such as quality of life measures and healthcare resource utilization, would contribute to a more holistic assessment of the impact of post-CABG AVB. The STROBE guidelines should be used for complete and transparent reporting.
One of the most significant limitations of this review was the nonuniform definitions and reporting across different studies. It underscores the importance of a uniform definition and diagnostic criteria for post-CABG AVB, which should be concise, clear, and clinically relevant. This consistency will allow for more accurate comparisons and assessment of the incidence and outcomes of post-CABG AVB across different studies. The following are our recommendations in this regard:
1. Determine whether the study focuses on developing new-onset AVB or worsening pre-existing AVB.
2. Identify the type of AVB of interest (e.g., first-degree, second-degree, third-degree).
3. Determine the time frame for defining post-CABG AVB (e.g., starting upon discharge from the operating room until one month after surgery).
4. Define the criteria for determining the presence of AVB. It could include identifying new electrocardiographic findings consistent with the type of AVB of interest.
5. Provide clear exclusion criteria. It could include:
• Pre-existing AVB.
• pre-existing pacemakers or implantable cardioverter-defibrillators.
• Previous cardiac surgeries.
• Severe comorbidities that may affect the study outcomes, such as mortality.
• Use of medication that can exacerbate AVB.
• Inadequate medical records.
5. Conclusion
The current study was designed to determine the overall incidence of AVB, CHB, and subsequent PPM implantation following the CABG procedure and identify its associated risk factors. We calculated a pooled prevalence of 1.16% for postoperative AVB incidence, 1.73% for CHB or AVB requiring TP, and 0.58% for PPM implantation. These findings underscore the significance of AVB as a serious complication of CABG, emphasizing the need for postoperative monitoring and surveillance to ensure satisfactory patient outcomes. As for further research, we recommend conducting large-scale prospective cohorts with primary outcomes focusing on the incidence, risk factors, and prognosis of post-CABG AVB to yield more precise and comprehensive results. Using a uniform definition and diagnostic criteria for post-CABG AVB and adhering to the reporting standards will allow for more accurate comparisons and assessment of the incidence and outcomes of post-CABG AVB across different studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
RY: designed the search strategy and contributed to the screening, data extraction, analysis, and manuscript drafting. RH: contributed to the analysis and manuscript drafting. HS: contributed to the manuscript drafting and revising the final draft. SS, MM, PM, and GA: contributed to the screening, data extraction, and manuscript drafting. KH: contributed to the development of the idea and search strategy, supervised the data extraction and analysis, revised the final manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1225833/full#supplementary-material
References
1. Alexander JH, Smith PK. Coronary-artery bypass grafting. N Engl J Med. (2016) 374:1954–64. doi: 10.1056/NEJMra1406944
2. Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation. (2011) 124:e652–735. doi: 10.1161/CIR.0b013e31823c074e
3. Aranki S, Cutlip D. Early cardiac complications of coronary artery bypass graft surgery. UpToDate in Waltham, MA (2022). Available at: https://www.uptodate.com/contents/early-cardiac-complications-of-coronary-artery-bypass-graft-surgery (Accessed December 18, 2022).
4. Hosseini Dolama R, Eghbal AH, Rezaee M, Farahani AV, Jalali A, Hosseini K. Sinus node dysfunction and related permanent pacemaker implantation after major cardiac surgeries, systematic review, and meta-analysis. Front Cardiovasc Med. (2023) 10:1091312. doi: 10.3389/fcvm.2023.1091312
5. Pires LA, Wagshal AB, Lancey R, Huang SK. Arrhythmias and conduction disturbances after coronary artery bypass graft surgery: epidemiology, management, and prognosis. Am Heart J. (1995) 129:799–808. doi: 10.1016/0002-8703(95)90332-1
6. Knotzer H, Dünser MW, Mayr AJ, Hasibeder WR. Postbypass arrhythmias: pathophysiology, prevention, and therapy. Curr Opin Crit Care. (2004) 10:330–5. doi: 10.1097/01.ccx.0000135512.18753.bc
7. Clay-Weinfeld K, Callans M. Common postcardiothoracic surgery arrhythmias. Crit Care Nurs Clin North Am. (2019) 31:367–88. doi: 10.1016/j.cnc.2019.05.006
8. Savunen T, Kuttila K, Rajalin A, Inberg M, Niinikoski J, Jalonen J, et al. Combined cardioplegia delivery offers no advantage over antegrade cardioplegia administration in coronary surgical patients with a preserved left ventricular function. Eur J Cardiothorac Surg. (1994) 8:640–4. doi: 10.1016/s1010-7940(05)80102-2
9. Jegaden O, Eker A, Montagna P, Ossette J, Vial C, Guidollet J, et al. Antegrade/retrograde cardioplegia in arterial bypass grafting: metabolic randomized clinical trial. Ann Thorac Surg. (1995) 59:456–61. doi: 10.1016/0003-4975(94)00863-3
10. Piantá RM, Ferrari AD, Heck AA, Ferreira DK, Piccoli Jda C, Albuquerque LC, et al. Atrioventricular block in coronary artery bypass surgery: perioperative predictors and impact on mortality. Rev Bras Cir Cardiovasc. (2015) 30:164–72. doi: 10.5935/1678-9741.20140086
11. Baraka AS, Taha SK, Yazbeck VK, Rizkallah PA, Zughbi JP, Aouad MJ, et al. Transient atrioventricular block after release of aortic cross-clamp. Anesth Analg. (1995) 80:54–7. doi: 10.1097/00000539-199501000-00009
12. Gozal Y, Glantz L, Luria MH, Milgalter E, Shimon D, Drenger B. Normothermic continuous blood cardioplegia improves electrophysiologic recovery after open heart surgery. Anesthesiology. (1996) 84:1298–306. doi: 10.1097/00000542-199606000-00004
13. Raza SS, Li JM, John R, Chen LY, Tholakanahalli VN, Mbai M, et al. Long-term mortality and pacing outcomes of patients with permanent pacemaker implantation after cardiac surgery. Pacing Clin Electrophysiol. (2011) 34:331–8. doi: 10.1111/j.1540-8159.2010.02972.x
14. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American college of cardiology/American heart association task force on practice guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices) developed in collaboration with the American association for thoracic surgery and society of thoracic surgeons. J Am Coll Cardiol. (2008) 51:e1–62. doi: 10.1016/j.jacc.2008.02.032
15. Aste M, Brignole M. Syncope and paroxysmal atrioventricular block. J Arrhythm. (2017) 33:562–7. doi: 10.1016/j.joa.2017.03.008
16. Gizurarson S, Lorentzon M, Råmunddal T, Waagstein F, Bergfeldt L, Omerovic E. Effects of complete heart block on myocardial function, morphology, and energy metabolism in the rat. Europace. (2007) 9:411–6. doi: 10.1093/europace/eum065
17. Hamm W, Rizas KD, Stülpnagel LV, Vdovin N, Massberg S, Kääb S, et al. Implantable cardiac monitors in high-risk post-infarction patients with cardiac autonomic dysfunction and moderately reduced left ventricular ejection fraction: design and rationale of the SMART-MI trial. Am Heart J. (2017) 190:34–9. doi: 10.1016/j.ahj.2017.05.006
18. Clémenty N, Fernandes J, Carion PL, de Léotoing L, Lamarsalle L, Wilquin-Bequet F, et al. Pacemaker complications and costs: a nationwide economic study. J Med Econ. (2019) 22:1171–8. doi: 10.1080/13696998.2019.1652186
19. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
20. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. Ott Ott Hosp Res Inst. Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed December 10, 2022).
21. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
22. Jokinen JJ, Mustonen PK, Hippeläinen MJ, Rehnberg LS, Hartikainen JE. Effects of coronary artery bypass related conduction defects: a 10-year follow-up study. Scand Cardiovasc J. (2004) 38:235–9. doi: 10.1080/14017430410016323
23. Rose MR, Glassman E, Spencer FC. Arrhythmias following cardiac surgery: relation to serum digoxin levels. Am Heart J. (1975) 89:288–94. doi: 10.1016/0002-8703(75)90077-0
24. Tchervenkov CI, Wynands JE, Symes JF, Malcolm ID, Dobell AR, Morin JE. Electrical behavior of the heart following high-potassium cardioplegia. Ann Thorac Surg. (1983) 36:314–9. doi: 10.1016/s0003-4975(10)60134-8
25. Reder RF, Mindich B, Halperin J, Litwak RS, Kupersmith J. Acute effects of oral labetalol on myocardial conduction after coronary artery bypass grafting. Clin Pharmacol Ther. (1984) 35:454–60. doi: 10.1038/clpt.1984.59
26. Baerman JM, Kirsh MM, de Buitleir M, Hyatt L, Juni JE, Pitt B, et al. Natural history and determinants of conduction defects following coronary artery bypass surgery. Ann Thorac Surg. (1987) 44:150–3. doi: 10.1016/s0003-4975(10)62027-9
27. Weinstein GS, Rao PS, Tyras DH. Reduction of myocardial injury with verapamil before aortic cross-clamping. Ann Thorac Surg. (1990) 49:419–23. doi: 10.1016/0003-4975(90)90247-4
28. Mosseri M, Meir G, Lotan C, Hasin Y, Applebaum A, Rosenheck S, et al. Coronary pathology predicts conduction disturbances after coronary artery bypass grafting. Ann Thorac Surg. (1991) 51:248–52. doi: 10.1016/0003-4975(91)90796-s
29. Baraka A, Hirt N, Dabbous A, Taha S, Rouhana C, El-Khoury N, et al. Lidocaine cardioplegia for prevention of reperfusion ventricular fibrillation. Ann Thorac Surg. (1993) 55:1529–33. doi: 10.1016/0003-4975(93)91104-u
30. Emlein G, Huang SK, Pires LA, Rofino K, Okike ON, Vander Salm TJ. Prolonged bradyarrhythmias after isolated coronary artery bypass graft surgery. Am Heart J. (1993) 126:1084–90. doi: 10.1016/0002-8703(93)90658-v
31. Mustonen P, Hippeläinen M, Vanninen E, Rehnberg S, Tenhunen-Eskelinen M, Hartikainen J. Significance of coronary artery bypass grafting-associated conduction defects. Am J Cardiol. (1998) 81:558–63. doi: 10.1016/s0002-9149(97)00981-8
32. Bhan A, Gupta V, Choudhary SK, Sharma R, Singh B, Aggarwal R, et al. Radial artery in CABG: could the early results be comparable to internal mammary artery graft? Ann Thorac Surg. (1999) 67:1631–6. doi: 10.1016/s0003-4975(99)00223-4
33. Göl MK, Yilmazkaya B, Göksel S, Sener E, Mavitaş B, Taşdemir O, et al. Results of right coronary artery endarterectomy with or without patchplasty. J Card Surg. (1999) 14:75–81. doi: 10.1111/j.1540-8191.1999.tb00954.x
34. Puskas JD, Sharoni E, Williams WH, Petersen R, Duke P, Guyton RA. Is routine use of temporary epicardial pacing wires necessary after either OPCAB or conventional CABG/CPB? Heart Surg Forum. (2003) 6:E103–6. doi: 10.1532/hsf.1061
35. Onorati F, Renzulli A, De Feo M, Santarpino G, Gregorio R, Biondi A, et al. Does antegrade blood cardioplegia alone provide adequate myocardial protection in patients with left main stem disease? J Thorac Cardiovasc Surg. (2003) 126:1345–51. doi: 10.1016/s0022-5223(03)00736-0
36. Cook DJ, Bailon JM, Douglas TT, Henke KD, Westberg JR, Shirk-Marienau ME, et al. Changing incidence, type, and natural history of conduction defects after coronary artery bypass grafting. Ann Thorac Surg. (2005) 80:1732–7. doi: 10.1016/j.athoracsur.2005.04.029
37. Bethea BT, Salazar JD, Grega MA, Doty JR, Fitton TP, Alejo DE, et al. Determining the utility of temporary pacing wires after coronary artery bypass surgery. Ann Thorac Surg. (2005) 79:104–7. doi: 10.1016/j.athoracsur.2004.06.087
38. Budeus M, Hennersdorf M, Perings S, Röhlen S, Schnitzler S, Felix O, et al. Amiodarone prophylaxis for atrial fibrillation of high-risk patients after coronary bypass grafting: a prospective, double-blinded, placebo-controlled, randomized study. Eur Heart J. (2006) 27:1584–91. doi: 10.1093/eurheartj/ehl082
39. Asghar MI, Khan AA, Iqbal A, Arshad A, Afridi I. Placing epicardial pacing wires in isolated coronary artery bypass graft surgery–a procedure routinely done but rarely beneficial. J Ayub Med Coll Abbottabad. (2009) 21:86–90.20364750
40. Al-Sarraf N, Thalib L, Hughes A, Houlihan M, Tolan M, Young V, et al. The risk of arrhythmias following coronary artery bypass surgery: do smokers have a paradox effect? Interact Cardiovasc Thorac Surg. (2010) 11:550–5. doi: 10.1510/icvts.2010.242586
41. Rocha AS, Pittella FJ, Lorenzo AR, Barzan V, Colafranceschi AS, Brito JO, et al. Age influences outcomes in 70-year or older patients undergoing isolated coronary artery bypass graft surgery. Rev Bras Cir Cardiovasc. (2012) 27:45–51. doi: 10.5935/1678-9741.20120008
42. Nasseri BA, Ebell W, Dandel M, Kukucka M, Gebker R, Doltra A, et al. Autologous CD133 + bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: the cardio133 trial. Eur Heart J. (2014) 35:1263–74. doi: 10.1093/eurheartj/ehu007
43. Bortolussi G, Bejko J, Gallo M, Comisso M, Carrozzini M, Guglielmi C, et al. Coronary artery bypass grafting in elderly patients: insights from a comparative analysis of total arterial and conventional revascularization. J Cardiovasc Transl Res. (2016) 9:223–9. doi: 10.1007/s12265-016-9688-y
44. Carmona P, Paredes F, Mateo E, Mena-Durán AV, Hornero F, Martínez-León J. Is off-pump technique a safer procedure for coronary revascularization? A propensity score analysis of 20 years of experience. Interact Cardiovasc Thorac Surg. (2016) 22:612–8. doi: 10.1093/icvts/ivw005
45. Cholley B, Caruba T, Grosjean S, Amour J, Ouattara A, Villacorta J, et al. Effect of levosimendan on low cardiac output syndrome in patients with low ejection fraction undergoing coronary artery bypass grafting with cardiopulmonary bypass: the LICORN randomized clinical trial. Jama. (2017) 318:548–56. doi: 10.1001/jama.2017.9973
46. Todurov B, Bitsadze A, Shorikova D. Early postoperative complications in patients with acute myocardial infarction during emergency coronary bypassing. Int J Health Sci. (2021) 5:550–64. doi: 10.53730/ijhs.v5n3.2381
47. Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. (1950) 21:607–11. doi: 10.1214/aoms/1177729756
48. Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behav Stat. (2005) 30:261–93. doi: 10.3102/10769986030003261
49. Barker TH, Migliavaca CB, Stein C, Colpani V, Falavigna M, Aromataris E, et al. Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med Res Methodol. (2021) 21:189. doi: 10.1186/s12874-021-01381-z
50. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
Keywords: coronary artery bypass grafting (CABG), complete heart block (CHB), atrioventricular (AV) block, temporary pacing, temporary pacemaker, permanent pacemaker (PPM), conduction defect (CD)
Citation: Yaghoobian R, Hosseini Dolama R, Soleimani H, Saeidi S, Mashayekhi M, Mirzayi P, Alavi Tabatabaei G and Hosseini K (2023) Incidence of atrioventricular block after isolated coronary artery bypass grafting: a systematic review and pooled-analysis. Front. Cardiovasc. Med. 10:1225833. doi: 10.3389/fcvm.2023.1225833
Received: 19 May 2023; Accepted: 19 July 2023;
Published: 1 August 2023.
Edited by:
Massimo Bonacchi, University of Florence, ItalyReviewed by:
Arian Arjomandi Rad, University of Oxford, United KingdomGiorgia Bonalumi, Monzino Cardiology Center (IRCCS), Italy
© 2023 Yaghoobian, Hosseini Dolama, Soleimani, Saeidi, Mashayekhi, Mirzayi, Alavi Tabatabaei and Hosseini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaveh Hosseini a2F2ZWhfaG9zc2VpbmkxMzBAeWFob28uY29t
 Ramin Yaghoobian
Ramin Yaghoobian Reza Hosseini Dolama
Reza Hosseini Dolama Hamidreza Soleimani1
Hamidreza Soleimani1 Sahar Saeidi
Sahar Saeidi Mahtab Mashayekhi
Mahtab Mashayekhi Parsa Mirzayi
Parsa Mirzayi Ghazaal Alavi Tabatabaei
Ghazaal Alavi Tabatabaei Kaveh Hosseini
Kaveh Hosseini