- Department of Cardiothoracic Surgery, Shanghai Children’s Medical Center, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
Background: During cardiac surgery that involved cardiopulmonary bypass (CPB) procedure, gastrointestinal (GI) system was known to be vulnerable to complications such as GI bleeding. Our study aimed to determine the incidence and risk factors associated with GI bleeding in children who received CPB as part of cardiac surgery.
Methods: This retrospective study enrolled patients aged <18 years who underwent cardiac surgery with CPB from 2013 to 2019 at Shanghai Children's Medical Center. The primary outcome was the incidence of postoperative GI bleeding in children, and the associated risk factors with postoperative GI bleeding episodes were evaluated.
Results: A total of 21,893 children who underwent cardiac surgery with CPB from 2013 to 2019 were included in this study. For age distribution, 636 (2.9%) were neonates, 10,984 (50.2%) were infants, and 10,273 (46.9%) were children. Among the 410 (1.9%) patients with GI bleeding, 345 (84.2%) survived to hospital discharge. Incidence of GI bleeding in neonates, infants and children were 22.6% (144/636), 2.0% (217/10,984) and 0.5% (49/10,273), respectively. The neonates (22.6%) group was associated with highest risk of GI bleeding. Patients with GI bleeding showed longer length of hospital stays (25.8 ± 15.9 vs. 12.5 ± 8.9, P < 0.001) and higher mortality (15.9% vs. 1.8%, P < 0.001). Multivariate logistic regression analysis showed that age, weight, complicated surgery, operation time, use of extracorporeal membrane oxygenation (ECMO), low cardiac output syndrome (LCOS), hepatic injury, artery lactate level, and postoperative platelet counts were significantly associated with increased risk of GI bleeding in children with congenital heart disease (CHD) pediatric patients that underwent CPB procedure during cardiac surgery.
Conclusion: The study results suggest that young age, low weight, long operation time, complicated surgery, use of ECMO, LCOS, hepatic injury, high arterial lactate level, and low postoperative platelet counts are independently associated with GI bleeding after CPB in children.
1. Introduction
In data from previous studies, 6–9 of every 1,000 newborns were diagnosed with different forms of congenital heart disease (CHD), in which majority require surgical correction during childhood (1, 2). The gastrointestinal (GI) system is most vulnerable to complications (i.e., GI bleeding) after cardiac surgery due to low abdominal perfusion after prolonged ischemia (3, 4). Despite advanced improvements in CPB techniques, anesthesia procedure, and intensive care support, GI bleeding remains a serious complication after cardiopulmonary bypass (CPB), with incident rate ranging from 0.2% to 2%, with a significantly high mortality rate observed between 8.8% and 19.0% (3–13). Despite the apparent association in between postoperative GI bleeding with length of intensive care unit stays and increased morbidity, risk factors associated with GI bleeding after CPB in children remains unclear (14–17).
Our study aimed to determine risk factors of GI bleeding after CPB in children aged <18 years. We extensively collected potential preexisted factors and variables during whole course of hospitalization. By identifying potential risk factors, this might help surgeons to deploy preventive measures for high-risk patients and enhance early recognition of postoperative GI bleeding, thus improving prognostic outcomes in this cardiovascular surgical population.
2. Methods
2.1. Patient and study design
This retrospective study was carried out and approved by the Ethical Committee of Shanghai Children's Medical Center. Medical files of all patients who underwent cardiac surgery with CPB from January 2013 to December 2019 were collected from database and analyzed. Inclusion criteria refers to patients <18 years of age who underwent cardiac surgery with CPB. Condition such as cardiac surgery without CPB procedures, incomplete medical records or GI bleeding occurs within 30 days prior to cardiac surgery were excluded. Variables investigated in the study include the preexisted demographic data, laboratory test data, and surgical related variables. All these study data were obtained from electronic medical record system of Shanghai Children's Medical Center. This study was approved by the Ethical Committee of Shanghai Children's Medical Center.
2.2. Definition and variables
We retrospectively reviewed 21,893 consecutive cardiac surgery patients. Cardiac surgical procedure refers to any cardiac or intrathoracic great vessel procedure. GI bleeding refers to hemorrhage from the upper or lower GI tract. The clinical presentation of GI bleeding varies according to severity and localization of bleeding. Definition of GI bleeding refers to a positive occult blood test (OB) results in specimen including feces or gastric juice.
The following data were recorded for each cardiac surgical procedure: patient demographics [age, gender, premature birth (birth <37 weeks)], operative procedure, CPB time (minutes), temperature of CPB, perfusion mode of CPB, and postoperative factors [(length of hospital stay, length of mechanical ventilation, use of renal replacement therapy, and use of ECMO, postoperative ejection fraction, postoperative dialysis, postoperative lactate, postoperative hepatic function (ALT and AST), postoperative platelet, and survival to hospital discharge (%)]. Patients' age was classified into three categories: neonates (<28 days), infants (29 days to 1 year), and children (1–18 years). Operative procedure risk stratification was categorized using the Risk Adjusted Classification for Congenital Heart Surgery (RACHS-1), and RACHS-1 scoring 3–6 was defined as complicated surgery. In our study, hepatic injury was defined as detection of ALT ≥ 80 U/L and/or AST ≥ 80 U/L in 30 days after surgery. Low cardiac output syndrome (LCOS) refers to a decrease in cardiac output that is due to myocardial dysfunction. Diagnostic criteria for LCOS: Cardiac output index (CI) <2.5 L·min−1·m−2.
2.3. Statistical analysis
All statistical analyses were performed using SPSS 22.0. The measurement data were expressed by mean ± standard deviation , the mean between the two groups was compared by Mann-Whitney U-test, the count data were expressed by the number of cases (constituent ratio), and the comparison between groups was expressed by the χ2 test. Logistic regression analysis was applied to assess the association between potential risk factors and GI bleeding after cardiopulmonary bypass. A P value of 0.05 was considered statistical significant. Based on the risk factors obtained from the multivariate logistic analysis, the Receiver Operating Characteristic Curve (ROC) were plotted for each risk factor to predict GI bleeding after CPB in Children. Risk prediction models were developed, column line plots were generated using R software package. The discriminative ability of nomograms was evaluated by ROC curve and Area under the Curve (AUC).
3. Results
3.1. Patient characteristics
A total of 21,893 children who underwent cardiac surgery with cardiac pulmonary bypass from January 1 2013 to December 31 2019 were included in this study. At the time of operation, 636 (2.9%) were neonates, 10,984 (50.2%) were infants, and 10,273 (46.9%) were children. Of 21,893 children, 410 (1.9%) developed GI bleeding as a post-operative complication. Regarding outcomes of patients with GI bleeding, 65 patients (15.9%) died during their hospital stay, while 345 patients (84.1%) survived to hospital discharge. The incidence of GI bleeding in neonates, infants and children were 22.6% (144/636), 2.0% (217/10,984) and 0.5% (49/10,273), respectively. As shown in Table 1, in the non-GI bleeding group, the top 5 congenital heart diseases were ventricular septal defect (44%), atrial septal defect (10.5%), tetralogy of Fallot (8.3%), double outlet of right ventricle (3.6%) and atrioventricular septal defect (3.3%). While in the GI bleeding group, the top 5 congenital heart diseases were coarctation of aorta (25.4%), transposition of great arteries (14.9%), ventricular septal defect (10.0%), anomalous pulmonary venous connection (8.3%) and pulmonary atresia (6.1%). There are statistical differences in length of hospital stay and discharge status between different age groups with GI bleeding or no GI bleeding (Table 2). Patients with GI bleeding after CPB was associated with higher mortality (15.9% vs. 1.8%) compared to non-GI bleeding patients. The overall 30-day mortality rate was 2.0% (443/21,893).
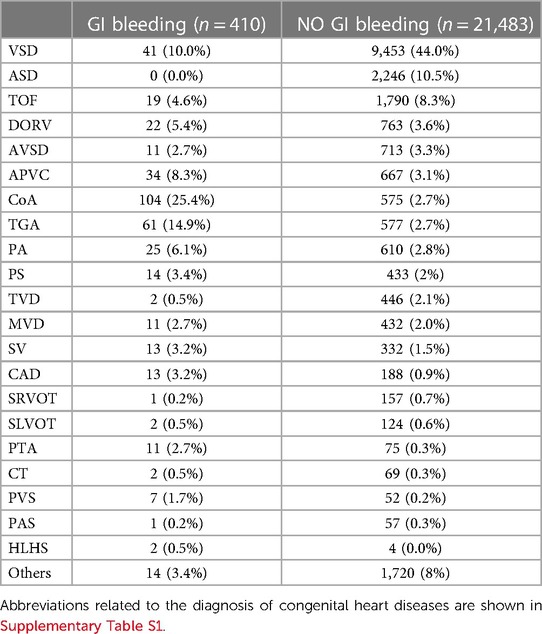
Table 1. Preoperative diagnosis of congenital heart diseases in patients with GI bleeding or without GI bleeding.
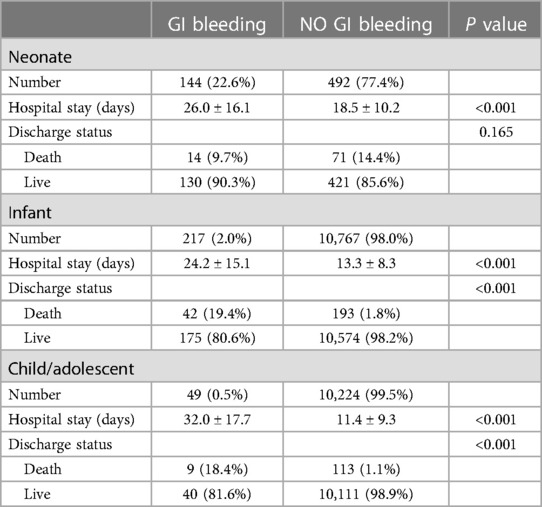
Table 2. Hospital stay and discharge status comparison between GI bleeding or without GI bleeding in different age group.
3.2. Univariate analysis for GI bleeding
To analysis independent variables and features associated with GI bleeding after CPB, all enrolled patients were categorized into GI bleeding group (n = 410) and non-GI bleeding group (n = 21,483) based on the post-operative occult blood test results. In consideration of pathophysiology of GI bleeding, types of surgical procedures and findings interpreted from preexisted studies, a series of preoperative, intraoperative and postoperative variables were compared between the two groups.
Results of univariate analysis for GI bleeding after CPB were shown in Table 3. In this CPB cohort (21,893 children), 410 (1.9%) had postoperative GI bleeding. In regards of demographic and clinical variables, patients with GI bleeding were of younger age (278.6 ± 712.1 vs. 720.0 ± 931.6 days, P < 0.001) and lower weight. Incidence of preterm birth was more prevalent in the GI-bleeding group, nearly five times (5.4% vs. 1.1% P < 0.001) higher than non-GI-bleeding group. Pre-operative assessment such as surgical options (radical or palliative) and RACHS-1 category that largely depends on CHD diagnosis, were significantly different between the two groups. During operation, patients with GI bleeding has longer CPB time (131.8 ± 87.2 vs. 68.8 ± 46.4, P < 0.001), aortic cross-clamp time (73.9 ± 40.3 vs. 39.5 ± 38.6 P < 0.001) and operation time (252.9 ± 110.9 vs. 151.8 ± 65.0 P < 0.001).
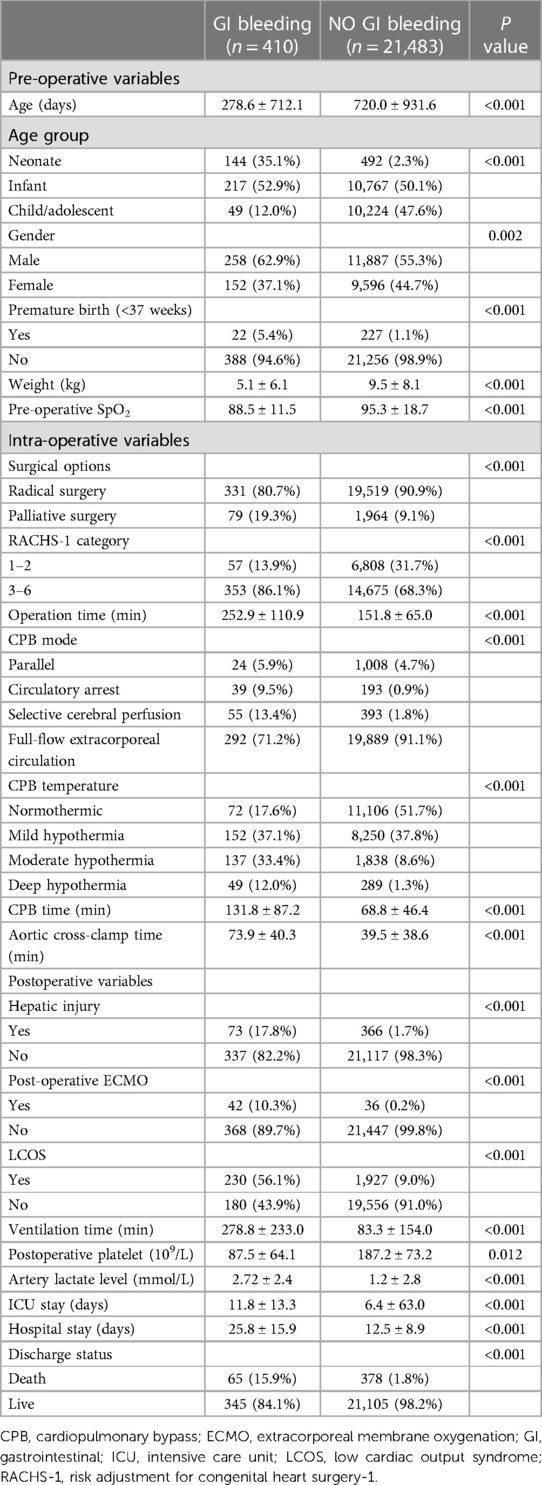
Table 3. Univariate analysis for gastrointestinal bleeding after cardiopulmonary bypass in children.
As to postoperative platelet counts, patients with GI bleeding had lower level of platelet counts (87.5 ± 64.1 vs. 187.2 ± 73.2, P = 0.012). Postoperative artery lactate level was higher in patients with GI bleeding compared with those who did not develop GI bleeding (2.72 ± 2.4 vs. 1.2 ± 2.8, P < 0.001).
Overall, patients with GI bleeding had longer hospital stays (25.8 ± 15.9 vs. 12.5 ± 8.9, P < 0.001) and intensive care unit (ICU) stays (11.8 ± 13.3 vs. 6.4 ± 63.0, P < 0.001). Mortality rate was significantly higher among patients with GI bleeding compared with those who did not develop GI bleeding (15.9% vs. 1.8%, P < 0.001).
3.3. Multivariate logistic regression analysis of risk factors
The results of multivariate analysis of risk factors for GI bleeding after CPB in children were shown in Table 4 Multivariate analysis showed that the operation time [95% confidence interval (CI): 1–1.004; P = 0.01], age (95% CI: 0.999–1.000; P < 0.001), complicated surgery (95% CI: 2.304–5.211; P < 0.001), weight (95% CI: 0.912–0.988; P = 0.011), low cardiac output syndrome (LCOS) (95% CI: 2.203–3.945 P < 0.001), use of ECMO (95% CI: 2.736–9.508; P < 0.001), hepatic injury (95% CI: 1.05–2.257 P = 0.027), artery lactate level (95% CI: 1.194–1.426; P < 0.001), and postoperative platelet counts (95% CI: 0.989–0.994; P < 0.001) were associated with GI bleeding in CHD children with CPB.
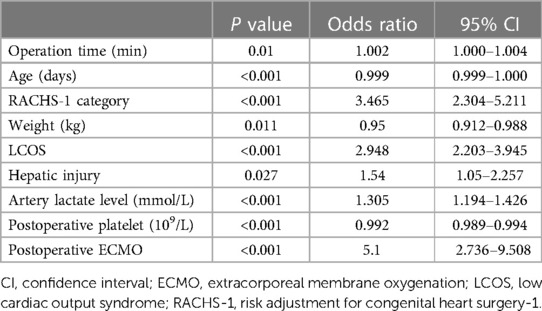
Table 4. Multivariate logistic regression analysis of risk factors for gastrointestinal bleeding after cardiopulmonary bypass in children.
3.4. ROC curve of independent risk factor predicting GI bleeding
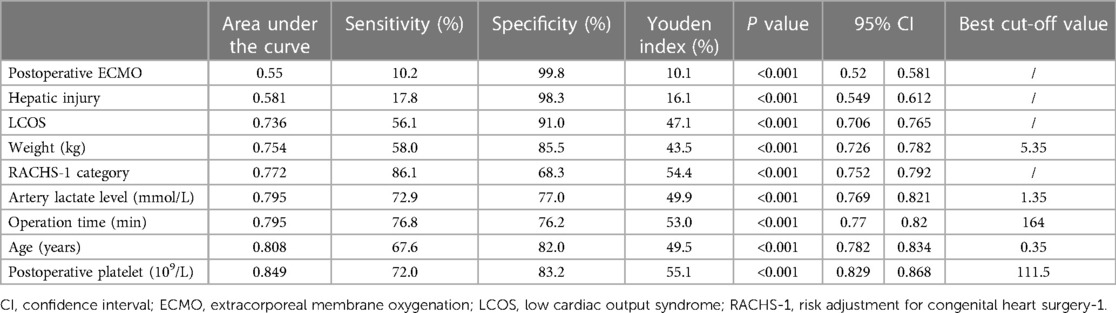
The independent risk factors obtained from the multivariate logistic analysis are further visually presented through ROC curves (Figure 1). Incidence prediction of gastrointestinal bleeding based on ROC analysis of age, operation time, RACHS-1 category, weight, LCOS, hepatic injury, artery lactate level, postoperative platelet and postoperative ECMO. The area under the curve of ROC predicting GI bleeding risk after cardiopulmonary bypass in children. Table 5 displays the specific details of each ROC curve.
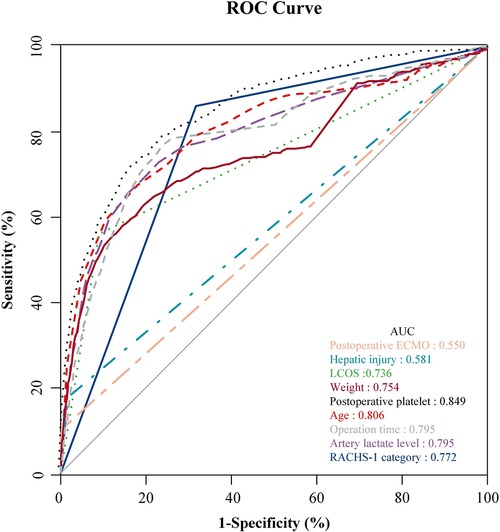
Figure 1. Incidence prediction of GI bleeding based on ROC analysis of age, operation time, RACHS-1 category, weight, LCOS, hepatic injury, artery lactate level, postoperative platelet and postoperative ECMO. The area under the curve of ROC predicting GI bleeding risk after CPB in children.
3.5. Nomograms predicting GI bleeding risk after cardiopulmonary bypass in children
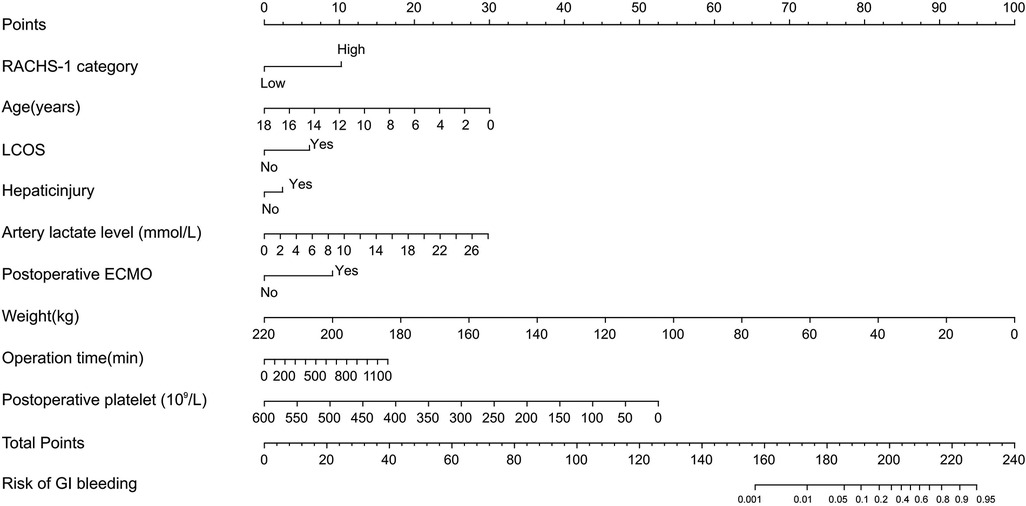
Probability of GI bleeding after CPB in children can be estimated with the nomograms (Figure 2). In order to predict GI bleeding risk after CPB in children with congenital heart disease, each parameter has a corresponding score on the point axis, and the sum of the scores is plotted on the “total point” axis. The probability of GI bleeding risk after cardiopulmonary bypass in children is the value at a vertical line from corresponding total points. As shown in Figure 3, the area under the ROC curve of the nomograms prediction model for GI bleeding was 0.898 (95% CI: 0.884–0.914, P < 0.001), with a sensitivity of 80.9% and specificity of 84.2%.
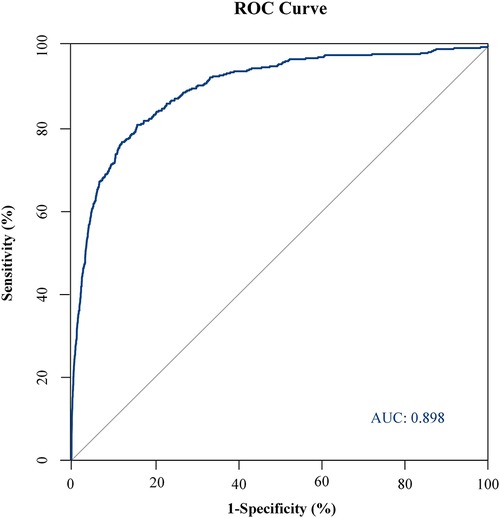
Figure 3. ROC curve of GI bleeding risk prediction model. The area under the ROC curve of the nomogram prediction model for GI bleeding was 0.898 (95% CI: 0.884–0.914, P < 0.001), with a sensitivity of 80.9% and specificity of 84.2%.
4. Discussion
CHD has gradually turned into the most common congenital defect, in which majority situation require cardiac surgery correction with cardiopulmonary bypass. The ultimate goal of CPB is to maintain sufficient tissue perfusion of all organs and tissues, but it is difficult to fully simulate normal blood circulation and pulsation processes. During this period, due to the redistribution of blood perfusion, blood flow is redistributed and its pathophysiological changes are similar to those observed in shock patients (18–21). Cardiopulmonary bypass give priority to guarantee effective blood supply to the brain and other important organs, which in turn leading to progressive reduction in gastrointestinal mucosa blood flow. GI bleeding after CPB is an unusual fatal event. GI bleeding was found to occur after 0.2%–2% of cardiac surgery cases with CPB and was associated with high mortality rates (8.8%–19.0%) (3–13). In our study, the observed GI bleeding incidence rate was 1.9% (410/21,893), which was similar to that reported in preexisted literature. The mortality rate in this series, 15.9%, well agreed with those previously reported (3–13). We observed a nine-fold increase in mortality risk (15.9% vs. 1.8%, P < 0.001) and a significantly longer hospital stay (26.0 ± 15.9 vs. 12.5 ± 8.9, P < 0.001) in patients with postoperative GI bleeding. Generally, children undergoing congenital heart surgery were confronted with increased risk of GI complications than adults (22, 23). Frequent monitoring for symptoms and related clinical indicators in these high-risk patients are necessary. Due to the absence of typical clinical signs or masking by signs of other more common complications, GI bleeding demands early clinical recognition so to secure favorable outcomes.
In this large cohort study, we aimed to identify potential risk factor of GI bleeding after CPB by multivariate logistic regression model. Clinical, laboratory and surgical variables during perioperative period were considered. Increased surgical complexity (OR = 3.465, P < 0.001) was one of the independent risk factor for GI bleeding. We also observed significant longer CPB time and aortic cross-clamp time in the GI-bleeding group, however multivariate analysis failed to prove its association with GI complication (not shown in table). In earlier studies, CPB time and aortic cross-clamp durations were widely suggested as influential factors of abdominal perfusion. Some evidences pointed out that splanchnic perfusion during CPB procedure might led to inadequate metabolic supply that further enhance GI complications. As reported by Andersson et al. (24), CPB procedure exceeding 150 min was one of the independent factors for predicting GI complications after cardiac surgery. In another prospective study, patients who received CPB longer than 100 min showed significant increase in gut permeability and gut impairment (25). Others conclude the associated risk of cardiopulmonary bypass time on intestinal ischemia damage by measuring biomarkers (26). Though ample evidences mentioned longer CPB time as risk factor of GI complication. However, most of these evidences focus on outcomes such as intestinal ischemic damages but not solely gastrointestinal bleeding. In other words, we cannot fully agree the independent role of extracorporeal circulation variables (such as CPB and aortic cross-clamp duration) in GI bleeding.
In our circulation system, GI system account to 20%–25% of the body's cardiac output and provide 20% of the oxygen supply in adult. Pathogenesis of GI complication could be multifactorial, while major contributing mechanisms during cardiac surgery is reduced systemic blood flow, which leads to insufficient oxygen delivery and energy deficit. Our study findings suggest that a LCOS (95% CI: 2.203–3.945, P < 0.001) after surgery is associated with GI bleeding. LCOS was a common postoperative complication, occurring in 9.9% (2,157/21,893) of the patients in this study in the first 24 h after surgery and we observed increased mortality of 6.4% (139/2,157) in this particular subgroup (not shown in table).
In our results, there was a statistical difference in pre-operative SpO2 between the bleeding and non-bleeding groups (88.5 ± 11.5 vs. 95.3 ± 18.7, P < 0.001), but SpO2 was not a risk factor after multivariate analysis. In our analysis of preoperative diagnosis (Table 1), the proportion of TOF in the bleeding group and the non-pulmonary bleeding group was (4.6% vs. 8.3%), respectively. Most congenital heart conditions in cyanotic CHD are complex congenital heart conditions. There are few studies of hypoxia tolerance between cyanotic and acyanotic CHD, and we need to further explore the relationship between them (27, 28). Recently, it has been suggested that cyanotic patients with CHD are characterized by increased arterial stiffness, expressed as a worse vasodilator response (29).
Among all organs, the gut is extremely vulnerable to ischemic injuries during postoperative LCOS because the splanchnic circulation is susceptible to endogenous and exogenous catecholamines. Immobilization, administration of high doses of opioid drugs, and delayed or absent enteral alimentation can aggravate the adverse effects of intraoperative mucosal damage caused by on pump surgery (30, 31). More importantly, prolonged and severe hypoperfusion might result in insufficient splanchnic blood flow. Pathophysiological events include uneven blood flow distribution, oxygen supply abnormalities, oxygen demand imbalance, and systemic inflammation (25, 32). Consequently, impairment of intestinal barrier integrity gradually results in translocation of bacteria and toxins, systemic inflammatory response syndrome and finally remote organ injury. We showed that the GI bleeding following cardiac surgery was common in neonates but rare in children. This may cause by neonates likelier to have LCOS and the sensitivity of neonatal gastrointestinal (33). In our study, we found patients with GI bleeding were younger than non-GI bleeding cases (278.6 ± 712.1 vs. 712.0 ± 931.6). Multivariate logistic regression analysis suggested that a younger age is closely associated with a higher incidence of GI bleeding. Our present series showed an incidence of postoperative GI bleeding of 22.6% (144/636) in neonates, 2.0% (217/10,984) in infants, and 0.5% (49/10,273) in children, which are similar to results reported in earlier literature. As in neonates, low body weight possess increased risk in development of GI bleeding, particularly necrotizing enterocolitis (NEC) (33, 34). Low body weight usually indicates preterm with organ immaturity. Poor visceral regulation to hypoperfusion changes might be the underlined mechanism.
The splanchnic circulation plays an important role during hypovolemia and possess several regulation mechanisms. For instance, splanchnic vasoconstriction stimulated by catecholamine and renin-angiotensin help compensate systemic circulation by increasing total systemic vascular resistance and auto-transfusion (35). Splanchnic hypoperfusion also ensure vital organ perfusion. In this situation, splanchnic flow respond slow to reperfusion even when systemic flow was restored. In more vulnerable patients, persistent hypoperfusion might result to splanchnic ischemia and visceral organ injuries such as GI bleeding. Physically, intestinal villous structure is highly sensitive to ischemia. During perfusion, arterial inflow enters from the base of intestinal villous which result in lower oxygen partial pressure at the tip. However, the high metabolic rate and oxygen demand observed at the tip make it incompatible to ischemia (35, 36).
Another important mechanism is the negative consequences of nonpulsatile blood flow during ischemia (32, 37). Earlier study pointed out that nonpulsatile blood flow as a determinant for renin release, activation of the renin–angiotensin–aldosterone axis and secretion of angiotensin II (38). Hypothermia is also associated with vasoconstriction and altered regional blood flow and distribution. Routine post-operative vasoactive drugs such as noradrenaline and vasopressin are also associated with splanchnic hypoperfusion.
Spotnitz et al. (39) first mentioned the importance of prolonged mechanical ventilation as an indeterminate risk factor for GI complications following cardiac surgery. D'Ancona et al. (7) reemphasized the significance of prolonged postoperative mechanical ventilation on GI bleeding and confirmed as an independent predictor based on multivariate analysis. Other studies have also observed prolonged ventilation as a risk factor during CPB surgery (8, 9, 13, 40, 41). Patients receiving mechanical ventilation often present signs of sympathetic nervous system activation and decreased cardiac output (42), which further contribute to splanchnic hypoperfusion and the injury of gastrointestinal mucosa. Elizalde et al. (43) have clarified the relationship of gastric mucosal ischemia and mechanically ventilated patients in the ICU. Nevertheless, it is reasonable to speculate that mechanical ventilation may enhance the adverse effects of critical illness on GI pathology. Similarly, our study showed a statistical difference in the length of mechanical ventilation between the GI-bleeding group and the non-GI bleeding group (278.8 ± 233.0 vs. 83.3 ± 154.0). However, multivariate analysis verified that long-term mechanical ventilation is not an independent risk factor for GI bleeding (P = 0.438).
The application of ECMO has saved the lives of many patients with cardiopulmonary insufficiency, but ECMO-related complications have also become thorny problems in clinical practice. The most common complications are bleeding and thrombus events. To a large extent, the occurrence of such complications is related to anticoagulation management during ECMO. At present, heparin is widely used in clinic, which mediates anticoagulation through its interaction with anti-thrombin (44–46). The results of our study suggest that post-operative use of ECMO (P < 0.001) may be a risk factor GI bleeding after CPB in children. This reminds us to pay attention to the effects of heparin during ECMO use and closely monitor relevant indicators such as APTT (activated partial thromboplastin time). At present, there is a shortage of specific anticoagulant preparations for children. Heparin has many disadvantages, such as binding to other plasma proteins and endothelial cells in addition to anti-thrombin, causing unpredictable reactions, difficulties in monitoring, and the risk of heparin-induced thrombocytopenia (HIT). HIT is a life-threatening complication of heparin exposure. HIT is a life-threatening complication of heparin exposure. It is initiated by immunoglobulin G (IgG) against the PF4–heparin complex (47, 48). Currently, a few studies confirm that direct thrombin inhibitors (DTIs), such as bivalirudin, may be a good option for anticoagulation (47–52). High-quality randomized controlled studies are needed to confirm the superiority of bivalirudin to provide a better option for future ECMO anticoagulation.
In order to minimize GI complications, high-risk patients must be identified to ensure optimal splanchnic perfusion. Intraoperative monitoring of gastrointestinal mucosal blood perfusion is essential to avoid gastrointestinal complications. However, splanchnic perfusion is currently difficult to monitor and optimal splanchnic perfusion was not well-defined. Although methods for detecting gastrointestinal mucosal blood flow have been proposed, their effectiveness will be difficult to prove due to the relatively low incidence of gastrointestinal complications. Further research on the early diagnosis and treatment of GI bleeding interventions is critical so that interventions can be taken before GI bleeding progresses to more severe conditions.
Recent research has shown that we must be cautious about the effects of vasoactive drug therapies on the splanchnic circulation because the effects of vasoactive agents on pHi (gastric intramucosal pH) are unpredictable (53). For example, the administration of a high dose of vasopressin may reduce rectosigmoidal mucosal perfusion and increase the risk for rectosigmoidal ischemia during and after CPB (54). Animal studies have shown that the supply of oxygen to the gastrointestinal system decreases obviously when the blood is diluted (18). Moreover, clinical outcome studies have shown an inverse relationship between mortality and the lowest hematocrit on bypass, suggesting that excessive hemodilution should be avoided (55, 56). Monitoring indicators that can be considered, such as inflammatory mediators, arterial lactic acid levels, gastrointestinal mucosal pH. In recent years, near infrared spectroscopy has been widely used in continuous monitoring of regional tissue oxygenation during perioperative and postoperative cardiopulmonary bypass in children and some pediatric studies have been conducted to determine near-infrared spectroscopy usage, safety, and efficacy (57–59). Several strategies have been proposed for improvement of CPB, such as maintaining adequate perfusion, avoiding hemodilution and severe anemia, using pulsatile blood flow, and using filters to reduce emboli. The role of pulsatile and non-pulsatile blood flow in cardiopulmonary bypass has been widely debated. Pulsating flow has improved gastrointestinal mucosal oxygenation and perfusion in some previous studies (60–62). Goal-directed therapy has been applied in a variety of perioperative and postoperative areas of medicine with promising results, usually including rigorous oxygen perfusion and monitoring and active management to improve clinical outcomes (63, 64).
The use of proton pump inhibitors to suppress gastric acid is currently recommended to reduce the risk of gastrointestinal bleeding and has been shown to be superior to non-prevention and the use of histamine receptor antagonists to prevent GI bleeding (65). The European Association of Cardiothoracic Surgery has recommended that proton pump inhibitors should be considered in all patients following heart surgery (class IIa recommendation). The improvement in prophylactic use of proton pump inhibitors was statistically significant in reducing GI bleeding after cardiac surgery (66). More cohorts are needed to further evaluate its significance.
Combined with the statistically significant correlation of these risk factors after multivariate logistic regression analysis, we can use the nomograms (Figure 2) to assess the risk of gastrointestinal bleeding after CPB in children with congenital heart disease. In our study, the area under the ROC curve of the nomograms prediction model for GI bleeding was 0.898 (95% CI: 0.884–0.914, P < 0.001), with a sensitivity of 80.9% and specificity of 84.2%. As a result, the nomogram has a good ability to distinguish the risk of GI bleeding after CPB in children. The risk prediction model established in this study has good sensitivity and specificity. The nomograms prediction model we developed can score patients more intuitively and quickly to predict the probability of gastrointestinal bleeding after CPB in children, which can provide certain support for clinical work.
4.1. Limitations
Several limitations should be considered in this study. First, this is a retrospective single-center study. Second, this study is an observational study, which can only demonstrate correlation, and further prospective studies are needed to make causal inference. Third, we noted that there may be a big difference in morbidity and mortality between asymptomatic positive fecal occult blood and a hemodynamically significant GI bleed, but the severity of GI bleeding was not measured in this observational study. The nomograms model we constructed to predict GI bleeding after CPB in children requires further external validation.
5. Conclusion
In this study, we identified several independent risk factors of GI bleeding post-cardiac surgery, including young age, low weight, long operation time, surgical complexity, use of ECMO, LCOS, hepatic function damage, high arterial lactate level, and low postoperative platelet counts. In particular, neonates and patients with low weight have a higher risk of developing GI bleeding. The nomograms prediction model established in this study can evaluate patients more intuitively and quickly, and predict the probability of GI bleeding after CPB operation in children, thus providing support for clinical work.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The research project is in accordance with the ethical principles of “Declaration of Helsinki” and “International Ethical Guidelines for Biomedical Research Involving Human Subjects” promulgated by the Council for International Organization of Medical Sciences. The research was reviewed and approved by the Ethical Committee of Shanghai Children’s Medical Center. The need for informed consent was waived by the Ethical Committee of Shanghai Children’s Medical Center, because of the retrospective nature of the study.
Author contributions
Z-QL: responsible for research implementation, data collection and drafting the manuscript. WZ: responsible for supervision of project execution and drafting the article. ZG: responsible for the statistics and analysis of research data. X-WD: responsible for retrieval and screening literature, final approval of the version to be published. WW: responsible for the determination of the research direction, the design of the research program, and the summary of the research questions. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by National Natural Science Fund of China (82070430).
Acknowledgments
Thanks to all the staff of Shanghai Children's Medical Center who have contributed to the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1224872/full#supplementary-material
References
1. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. (2002) 39(12):1890–900. doi: 10.1016/s0735-1097(02)01886-7
2. Liu Y, Chen S, Zühlke L, Black GC, Choy MK, Li N, et al. Global birth prevalence of congenital heart defects 1970-2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. (2019) 48(2):455–63. doi: 10.1093/ije/dyz009
3. Krawiec F, Maitland A, Duan Q, Faris P, Belletrutti PJ, Kent WDT. Duodenal ulcers are a major cause of gastrointestinal bleeding after cardiac surgery. J Thorac Cardiovasc Surg. (2017) 154(1):181–8. doi: 10.1016/j.jtcvs.2017.02.012
4. Bolcal C, Iyem H, Sargin M, Mataraci I, Sahin MA, Temizkan V, et al. Gastrointestinal complications after cardiopulmonary bypass: sixteen years of experience. Can J Gastroenterol. (2005) 19(10):613–7. doi: 10.1155/2005/975612
5. Bhat M, Larocque M, Amorim M, Herba K, Martel M, De Varennes B, et al. Prediction and prevention of upper gastrointestinal bleeding after cardiac surgery: a case control study. Can J Gastroenterol. (2012) 26(6):340–4. doi: 10.1155/2012/121836
6. Guney LH, Araz C, Beyazpinar DS, Arda IS, Arslan EE, Hicsonmez A. Abdominal problems in children with congenital cardiovascular abnormalities. Balkan Med J. (2015) 32(3):285–90. doi: 10.5152/balkanmedj.2015.151045
7. D'Ancona G, Baillot R, Poirier B, Dagenais F, de Ibarra JI, Bauset R, et al. Determinants of gastrointestinal complications in cardiac surgery. Tex Heart Inst J. (2003) 30(4):280–5.
8. Viana FF, Chen Y, Almeida AA, Baxter HD, Cochrane AD, Smith JA. Gastrointestinal complications after cardiac surgery: 10-year experience of a single Australian centre. ANZ J Surg. (2013) 83(9):651–6. doi: 10.1111/ans.12134
9. Chaudhry R, Zaki J, Wegner R, Pednekar G, Tse A, Sheinbaum R, et al. Gastrointestinal complications after cardiac surgery: a nationwide population-based analysis of morbidity and mortality predictors. J Cardiothorac Vasc Anesth. (2017) 31(4):1268–74. doi: 10.1053/j.jvca.2017.04.013
10. Haywood N, Mehaffey JH, Hawkins RB, Zhang A, Kron IL, Kern JA, et al. Gastrointestinal complications after cardiac surgery: highly morbid but improving over time. J Surg Res. (2020) 254:306–13. doi: 10.1016/j.jss.2020.02.019
11. Chor CYT, Mahmood S, Khan IH, Shirke M, Harky A. Gastrointestinal complications following cardiac surgery. Asian Cardiovasc Thorac Ann. (2020) 28(9):621–32. doi: 10.1177/0218492320949084
12. Rodriguez R, Robich MP, Plate JF, Trooskin SZ, Sellke FW. Gastrointestinal complications following cardiac surgery: a comprehensive review. J Card Surg. (2010) 25(2):188–97. doi: 10.1111/j.1540-8191.2009.00985.x
13. Marsoner K, Voetsch A, Lierzer C, Sodeck GH, Fruhwald S, Dapunt O, et al. Gastrointestinal complications following on-pump cardiac surgery-a propensity matched analysis. PLoS One. (2019) 14(6):e0217874. doi: 10.1371/journal.pone.0217874
14. Geissler HJ, Fischer UM, Grunert S, Kuhn-Régnier F, Hoelscher A, Schwinger RH, et al. Incidence and outcome of gastrointestinal complications after cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. (2006) 5(3):239–42. doi: 10.1510/icvts.2005.117382
15. Achouh PE, Madsen K, Miller CC 3rd, Estrera AL, Azizzadeh A, Dhareshwar J, et al. Gastrointestinal complications after descending thoracic and thoracoabdominal aortic repairs: a 14-year experience. J Vasc Surg. (2006) 44(3):442–6. doi: 10.1016/j.jvs.2006.05.018
16. Duman ZM, Bayram M, Timur B, Kaplan MC, Aksu T. Predictors and outcomes of gastrointestinal complications after cardiac surgery: a systematic review and meta-analysis. Turk Gogus Kalp Damar Cerrahisi Derg. (2023) 31(1):45–55. doi: 10.5606/tgkdc.dergisi.2023.24003
17. Hess NR, Seese LM, Hong Y, Afflu D, Wang Y, Thoma FW, et al. Gastrointestinal complications after cardiac surgery: incidence, predictors, and impact on outcomes. J Card Surg. (2021) 36(3):894–901. doi: 10.1111/jocs.15321
18. Tao W, Zwischenberger JB, Nguyen TT, Vertrees RA, McDaniel LB, Nutt LK, et al. Gut mucosal ischemia during normothermic cardiopulmonary bypass results from blood flow redistribution and increased oxygen demand. J Thorac Cardiovasc Surg. (1995) 110(3):819–28. doi: 10.1016/s0022-5223(95)70116-8
19. Abboud B, Daher R, Boujaoude J. Acute mesenteric ischemia after cardio-pulmonary bypass surgery. World J Gastroenterol. (2008) 14(35):5361–70. doi: 10.3748/wjg.14.5361
20. Haisjackl M, Birnbaum J, Redlin M, Schmutzler M, Waldenberger F, Lochs H, et al. Splanchnic oxygen transport and lactate metabolism during normothermic cardiopulmonary bypass in humans. Anesth Analg. (1998) 86(1):22–7. doi: 10.1097/00000539-199801000-00005
21. Braun JP, Schroeder T, Buehner S, Dohmen P, Moshirzadeh M, Grosse J, et al. Splanchnic oxygen transport, hepatic function and gastrointestinal barrier after normothermic cardiopulmonary bypass. Acta Anaesthesiol Scand. (2004) 48(6):697–703. doi: 10.1111/j.1399-6576.2004.00392.x
22. Wernovsky G, Wypij D, Jonas RA, Mayer JE Jr, Hanley FL, Hickey PR, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. (1995) 92(8):2226–35. doi: 10.1161/01.cir.92.8.2226
23. Parr GV, Blackstone EH, Kirklin JW. Cardiac performance and mortality early after intracardiac surgery in infants and young children. Circulation. (1975) 51(5):867–74. doi: 10.1161/01.cir.51.5.867
24. Andersson B, Andersson R, Brandt J, Höglund P, Algotsson L, Nilsson J. Gastrointestinal complications after cardiac surgery - improved risk stratification using a new scoring model. Interact Cardiovasc Thorac Surg. (2010) 10(3):366–70. doi: 10.1510/icvts.2009.219113
25. Ohri SK, Becket J, Brannan J, Keogh BE, Taylor KM. Effects of cardiopulmonary bypass on gut blood flow, oxygen utilization, and intramucosal ph. Ann Thorac Surg. (1994) 57(5):1193–9. doi: 10.1016/0003-4975(94)91355-2
26. Adamik B, Kübler A, Gozdzik A, Gozdzik W. Prolonged cardiopulmonary bypass is a risk factor for intestinal ischaemic damage and endotoxaemia. Heart Lung Circ. (2017) 26(7):717–23. doi: 10.1016/j.hlc.2016.10.012
27. Altin FH, Yildirim HA, Tanidir IC, Yildiz O, Kahraman MZ, Ozturk E, et al. Alterations in antioxidant and oxidant status of children after on-pump surgery for cyanotic and acyanotic congenital heart diseases. Cardiol Young. (2017) 27(2):325–32. doi: 10.1017/s1047951116000573
28. Rohit M, Shrivastava S. Acyanotic and cyanotic congenital heart diseases. Indian J Pediatr. (2018) 85(6):454–60. doi: 10.1007/s12098-017-2454-6
29. Trojnarska O, Szczepaniak-Chicheł L, Gabriel M, Bartczak-Rutkowska A, Rupa-Matysek J, Tykarski A, et al. Arterial stiffness and arterial function in adult cyanotic patients with congenital heart disease. J Cardiol. (2017) 70(1):62–7. doi: 10.1016/j.jjcc.2016.09.003
30. Aneman A, Pettersson A, Eisenhofer G, Friberg P, Holm M, von Bothmer C, et al. Sympathetic and renin-angiotensin activation during graded hypovolemia in pigs: impact on mesenteric perfusion and duodenal mucosal function. Shock. (1997) 8(5):378–84. doi: 10.1097/00024382-199711000-00011
31. Reilly PM, Wilkins KB, Fuh KC, Haglund U, Bulkley GB. The mesenteric hemodynamic response to circulatory shock: an overview. Shock. (2001) 15(5):329–43. doi: 10.1097/00024382-200115050-00001
32. Smith EE, Naftel DC, Blackstone EH, Kirklin JW. Microvascular permeability after cardiopulmonary bypass. An experimental study. J Thorac Cardiovasc Surg. (1987) 94(2):225–33. doi: 10.1016/S0022-5223(19)36285-3
33. Indrio F, Neu J, Pettoello-Mantovani M, Marchese F, Martini S, Salatto A, et al. Development of the gastrointestinal tract in newborns as a challenge for an appropriate nutrition: a narrative review. Nutrients. (2022) 14(7):1405. doi: 10.3390/nu14071405
34. Rose AT, Patel RM. A critical analysis of risk factors for necrotizing enterocolitis. Semin Fetal Neonatal Med. (2018) 23(6):374–9. doi: 10.1016/j.siny.2018.07.005
35. Hessel EA 2nd. Abdominal organ injury after cardiac surgery. Semin Cardiothorac Vasc Anesth. (2004) 8(3):243–63. doi: 10.1177/108925320400800306
36. Waisman D, Brod V, Wolff R, Sabo E, Chernin M, Weintraub Z, et al. Effects of hyperoxia on local and remote microcirculatory inflammatory response after splanchnic ischemia and reperfusion. Am J Physiol Heart Circ Physiol. (2003) 285(2):H643–52. doi: 10.1152/ajpheart.00900.2002
37. Ohri SK, Bowles CW, Mathie RT, Lawrence DR, Keogh BE, Taylor KM. Effect of cardiopulmonary bypass perfusion protocols on gut tissue oxygenation and blood flow. Ann Thorac Surg. (1997) 64(1):163–70. doi: 10.1016/s0003-4975(97)00290-7
38. Shoor PM, Marzec UM, Griffith LD, Hammill FS, Dilley RB, Bernstein EF. Renin secretion in the chronically perfused pulseless calf. Evidence for failure of stimulation by decreased pulse pressure. Circ Res. (1979) 45(1):100–7. doi: 10.1161/01.res.45.1.100
39. Spotnitz WD, Sanders RP, Hanks JB, Nolan SP, Tribble CG, Bergin JD, et al. General surgical complications can be predicted after cardiopulmonary bypass. Ann Surg. (1995) 221(5):489–96; discussion 96–7. doi: 10.1097/00000658-199505000-00006
40. Sever K, Ozbek C, Goktas B, Bas S, Ugurlucan M, Mansuroglu D. Gastrointestinal complications after open heart surgery: incidence and determinants of risk factors. Angiology. (2014) 65(5):425–9. doi: 10.1177/0003319713482357
41. Ghadimi K, Quiñones QJ, Karhausen JA. Identifying predictors of gastrointestinal complications after cardiovascular surgery: how do we digest the data? J Cardiothorac Vasc Anesth. (2017) 31(4):1275–7. doi: 10.1053/j.jvca.2017.06.025
42. Mutlu GM, Mutlu EA, Factor P. GI complications in patients receiving mechanical ventilation. Chest. (2001) 119(4):1222–41. doi: 10.1378/chest.119.4.1222
43. Elizalde JI, Hernández C, Llach J, Montón C, Bordas JM, Piqué JM, et al. Gastric intramucosal acidosis in mechanically ventilated patients: role of mucosal blood flow. Crit Care Med. (1998) 26(5):827–32. doi: 10.1097/00003246-199805000-00011
44. Protti A, Iapichino GE, Di Nardo M, Panigada M, Gattinoni L. Anticoagulation management and antithrombin supplementation practice during veno-venous extracorporeal membrane oxygenation: a worldwide survey. Anesthesiology. (2020) 132(3):562–70. doi: 10.1097/aln.0000000000003044
45. Piacente C, Martucci G, Miceli V, Pavone G, Papeo A, Occhipinti G, et al. A narrative review of antithrombin use during veno-venous extracorporeal membrane oxygenation in adults: rationale, current use, effects on anticoagulation, and outcomes. Perfusion. (2020) 35(6):452–64. doi: 10.1177/0267659120913803
46. Panigada M, Cucino A, Spinelli E, Occhipinti G, Panarello G, Novembrino C, et al. A randomized controlled trial of antithrombin supplementation during extracorporeal membrane oxygenation. Crit Care Med. (2020) 48(11):1636–44. doi: 10.1097/ccm.0000000000004590
47. Hogan M, Berger JS. Heparin-induced thrombocytopenia (hit): review of incidence, diagnosis, and management. Vasc Med. (2020) 25(2):160–73. doi: 10.1177/1358863-19898253
48. McKenzie SE, Sachais BS. Advances in the pathophysiology and treatment of heparin-induced thrombocytopenia. Curr Opin Hematol. (2014) 21(5):380–7. doi: 10.1097/moh.0000000000000066
49. Neunert C, Chitlur M, van Ommen CH. The changing landscape of anticoagulation in pediatric extracorporeal membrane oxygenation: use of the direct thrombin inhibitors. Front Med. (2022) 9:887199. doi: 10.3389/fmed.2022.887199
50. Buck ML. Bivalirudin as an alternative to heparin for anticoagulation in infants and children. J Pediatr Pharmacol Ther. (2015) 20(6):408–17. doi: 10.5863/1551-6776-20.6.408
51. Faella KH, Whiting D, Fynn-Thompson F, Matte GS. Bivalirudin anticoagulation for a pediatric patient with heparin-induced thrombocytopenia and thrombosis requiring cardiopulmonary bypass for ventricular assist device placement. J Extra Corpor Technol. (2016) 48(1):39–42. doi: 10.1051/ject/201648039
52. Forbes TJ, Hijazi ZM, Young G, Ringewald JM, Aquino PM, Vincent RN, et al. Pediatric catheterization laboratory anticoagulation with bivalirudin. Catheter Cardiovasc Interv. (2011) 77(5):671–9. doi: 10.1002/ccd.22817
53. Silva E, DeBacker D, Créteur J, Vincent JL. Effects of vasoactive drugs on gastric intramucosal pH. Crit Care Med. (1998) 26(10):1749–58. doi: 10.1097/00003246-199810000-00034
54. Bomberg H, Bierbach B, Flache S, Scheuer C, Novák M, Schäfers HJ, et al. Vasopressin induces rectosigmoidal mucosal ischemia during cardiopulmonary bypass. J Card Surg. (2014) 29(1):108–15. doi: 10.1111/jocs.12242
55. DeFoe GR, Ross CS, Olmstead EM, Surgenor SD, Fillinger MP, Groom RC, et al. Lowest hematocrit on bypass and adverse outcomes associated with coronary artery bypass grafting. Northern New England cardiovascular disease study group. Ann Thorac Surg. (2001) 71(3):769–76. doi: 10.1016/s0003-4975(00)02393-6
56. Fang WC, Helm RE, Krieger KH, Rosengart TK, DuBois WJ, Sason C, et al. Impact of minimum hematocrit during cardiopulmonary bypass on mortality in patients undergoing coronary artery surgery. Circulation. (1997) 96(9 Suppl):Ii-194-9.9386097
57. Li J, Van Arsdell GS, Zhang G, Cai S, Humpl T, Caldarone CA, et al. Assessment of the relationship between cerebral and splanchnic oxygen saturations measured by near-infrared spectroscopy and direct measurements of systemic haemodynamic variables and oxygen transport after the Norwood procedure. Heart. (2006) 92(11):1678–85. doi: 10.1136/hrt.2005.087270
58. Hoffman GM, Ghanayem NS, Tweddell JS. Noninvasive assessment of cardiac output. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. (2005) 8(1):12–21. doi: 10.1053/j.pcsu.2005.01.005
59. McQuillen PS, Nishimoto MS, Bottrell CL, Fineman LD, Hamrick SE, Glidden DV, et al. Regional and central venous oxygen saturation monitoring following pediatric cardiac surgery: concordance and association with clinical variables. Pediatr Crit Care Med. (2007) 8(2):154–60. doi: 10.1097/01.Pcc.0000257101.37171.Be
60. Ji B, Undar A. An evaluation of the benefits of pulsatile versus nonpulsatile perfusion during cardiopulmonary bypass procedures in pediatric and adult cardiac patients. ASAIO J. (2006) 52(4):357–61. doi: 10.1097/01.mat.0000225266.80021.9b
61. O'Neil MP, Alie R, Guo LR, Myers ML, Murkin JM, Ellis CG. Microvascular responsiveness to pulsatile and nonpulsatile flow during cardiopulmonary bypass. Ann Thorac Surg. (2018) 105(6):1745–53. doi: 10.1016/j.athoracsur.2018.01.007
62. O'Neil MP, Fleming JC, Badhwar A, Guo LR. Pulsatile versus nonpulsatile flow during cardiopulmonary bypass: microcirculatory and systemic effects. Ann Thorac Surg. (2012) 94(6):2046–53. doi: 10.1016/j.athoracsur.2012.05.065
63. Medikonda R, Ong CS, Wadia R, Goswami D, Schwartz J, Wolff L, et al. A review of goal-directed cardiopulmonary bypass management in pediatric cardiac surgery. World J Pediatr Congenit Heart Surg. (2018) 9(5):565–72. doi: 10.1177/2150135118775964
64. Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. J Am Med Assoc. (2014) 311(21):2181–90. doi: 10.1001/jama.2014.5305
65. Shin JS, Abah U. Is routine stress ulcer prophylaxis of benefit for patients undergoing cardiac surgery? Interact Cardiovasc Thorac Surg. (2012) 14(5):622–8. doi: 10.1093/icvts/ivs019
Keywords: congenital heart disease, cardiopulmonary bypass, children, gastrointestinal bleeding, risk factors
Citation: Li Z-Q, Zhang W, Guo Z, Du X-W and Wang W (2023) Risk factors of gastrointestinal bleeding after cardiopulmonary bypass in children: a retrospective study. Front. Cardiovasc. Med. 10:1224872. doi: 10.3389/fcvm.2023.1224872
Received: 18 May 2023; Accepted: 8 September 2023;
Published: 19 September 2023.
Edited by:
Cristina Barbero, A.O.U. City of Health and Science of Turin, ItalyReviewed by:
Paul Philipp Heinisch, German Heart, GermanyHeidi J. Dalton, Inova Health System, United States
© 2023 Li, Zhang, Guo, Du and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Wang d2FuZ3dlaUBzY21jLmNvbS5jbg==
 Zheng-Qing Li
Zheng-Qing Li Wei Zhang
Wei Zhang Wei Wang
Wei Wang