
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Cardiovasc. Med. , 01 September 2023
Sec. Cardio-Oncology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1222179
 Akshay Mathavan1,†
Akshay Mathavan1,† Akash Mathavan1,†
Akash Mathavan1,† Urszula Krekora2
Urszula Krekora2 Mohit Mathavan3
Mohit Mathavan3 Vanessa Rodriguez4
Vanessa Rodriguez4 Ellery Altshuler1
Ellery Altshuler1 Brianna Nguyen5
Brianna Nguyen5 Mohammed Ruzieh6*
Mohammed Ruzieh6*
Background: Primary cardiac tumors are often benign and commonly present as cardiac myxomas (CMs) or papillary fibroelastomas (CPFEs). There is a paucity of prognostic indicators for tumor burden or potential for embolic cerebrovascular events (CVEs). This study was performed to address these gaps.
Methods: Medical records at the University of Florida Health Shands Hospital between 1996 and 2021 were screened to identify patients with CMs or CPFEs. Clinical features, echocardiographic reports, and CVE outcomes were quantitatively assessed.
Results: A total of 55 patients were included in the study: 28 CM (50.9%) and 27 CPFE (49.1%) patients. Baseline patient characteristics were similar among patients. The neutrophil–lymphocyte ratio was correlated (p < 0.005 in all cases) to three metrics of tumor size in both CM (r = 64–67%) and CPFE (r = 56–59%). CVEs were the presenting symptom in 30 (54.5%) patients. CVE recurrence was high; the 5-year CVE recurrence rate in patients with tumor resection was 24.0% compared to 60.0% without resection. No baseline patient characteristics or tumor features were associated with an initial presentation of CVEs compared to any other indication. Univariate analysis indicated that prolonged duration to surgical resection, left atrial enlargement, male sex, and a neutrophil–lymphocyte ratio >3.0 at the follow-up were significantly associated with 5-year CVE recurrence. Left atrial enlargement and a neutrophil–lymphocyte ratio >3.0 at the follow-up remained significantly associated with 5-year CVE recurrence in multivariate analysis.
Conclusion: The neutrophil–lymphocyte ratio may prognosticate tumor size and recurrence of neurologic events. An increased risk of CVE within 5 years of mass resection is almost exclusive to patients initially presenting with CVEs.
Primary cardiac tumors are rare neoplasms with an annual global incidence of less than 0.2% (1). Approximately 90% of these tumors are benign, the majority of which are cardiac myxomas (CMs) and papillary fibroelastomas (CPFEs). CMs arise from multipotent mesenchymal cells and consist of a myxoid matrix with an acid-mucopolysaccharide-rich stroma (2). Meanwhile, CPFEs are benign lesions derived from valvular endocardium with unknown pathogenesis. There is debate about whether CPFEs represent true neoplasms or reactive tumors; CPFEs are suggested to be hamartomas, neoplasms, or organizing thrombi, in which they originate from aggregates of microthrombi at sites of endothelial damage that evolve into a mass (3). Although generally identified postmortem, increasing utilization and resolution of echocardiographic modalities have shown that CPFEs may have a prevalence equivalent to CMs (4). Clinical presentation for these benign cardiac neoplasms varies and significantly depends on tumor location. Left-sided CMs present with obstructive and constitutional symptoms and systemic embolisms. CPFEs, especially those found on the mitral and aortic valves, are associated with thromboembolic disease (5, 6).
The long-term prognosis of CMs and CPFEs is good, with low recurrence rates often associated with either a relevant family history or an absence of negative margins on surgical excision (1, 7). There is a relative scarcity of understanding of the pathogenesis, clinical profile differences, tumor size markers, and long-term prognosis of CMs and CPFEs. Therefore, this institutional retrospective cohort study aims to provide a comprehensive characterization of the clinical course of patients diagnosed with CMs and CPFEs. In the study group, we emphasize potential prognostic markers, including neutrophil–lymphocyte ratio (NLR), and underline neurovascular manifestations.
A retrospective chart review of adult patients treated at the University of Florida Health Shands Hospital (UFHSH), a large academic tertiary care medical center in Gainesville, FL, USA, was conducted. Patients were identified using the UFHSH integrated data repository. Inclusion criteria for the study included adult patients (age ≥ 18 years) diagnosed with a benign cardiac neoplasm (International Classification of Diseases 9 and 10 codes 212.7 and D15.1, respectively) who were treated at UFHSH between January 1996 and January 2021. The time range was limited by the availability of digitized records prior to 1996. Of this initial pool of patients, only those diagnosed with CMs or CPFEs were included in the final investigation. In all cases in which surgical resection of the tumor was performed, the diagnosis was supported by histopathological confirmation. In the remaining cases in which surgical resection was not performed, the diagnosis was supported by clinical presentation and characteristic features on echocardiographic imaging (8). Baseline demographic data and clinical and pathological information from medical records were also extracted. The study was approved by the University of Florida Institutional Review Board (IRB202102647).
The hematologic laboratory results presented in this study reflect those collected at the time of presentation and at the time of follow-up evaluation. NLR was defined as the ratio between absolute neutrophil and lymphocyte counts. Measurements of tumors in two-dimensional echocardiograms were collected as longer dimension a (mm) and shorter dimension b (mm). Tumor size was estimated using the arithmetic mean (), geometric mean (), and area () of the dimensions.
Nonparametric statistical analysis was performed using SPSS 28.0.1.0 (142) (IBM Corp, Armonk, NY, USA). Continuous variables were compared using the Mann–Whitney U test, while categorical variables were compared using Pearson's chi-squared test. Correlations between continuous variables are presented as the Pearson product-moment correlation coefficient r. Univariate and multivariate survival analyses were performed with Cox proportional hazards regression models; the proportional hazards assumption was not violated in these analyses. All statistical tests were two-sided, and p-values <0.05 were deemed statistically significant.
A total of 62 patients met the inclusion criteria during the study period, including 28 (45.2%) patients with CMs and 27 (43.5%) patients with CPFEs. The remaining seven cases were lipomas (Supplementary Figure S1). The characteristics and presentation of patients are reported in Table 1. Baseline demographics did not vary significantly between CM and CPFE groups. The mean age during tumor identification was 56 years, and patients were predominantly women [n = 17 (60.7%) CM patients and n = 18 (66.7%) CPFE patients]. The most common comorbidities at presentation were hypertension (n = 35, 63.6%), hyperlipidemia (n = 32, 58.2%), and coronary artery disease (n = 24, 43.6%). Compared to patients with CMs, patients with CPFEs were significantly more likely to present with moderate or severe valve stenosis or regurgitation (n = 1, 3.6% vs. n = 6, 22.2%; p = 0.038) or have had prior cardiac surgery (n = 1, 3.6% vs. n = 8, 29.6%; p = 0.009).
The most common presenting symptoms to the hospital were those related to CVE (n = 30, 54.5%), chest pain (n = 11, 20%), and dyspnea (n = 9, 16.4%). In the 30 patients initially presenting with CVEs, 17 (56.7%) patients had CMs, and 13 (43.3%) patients had CPFEs (Supplementary Table S1). Ischemic embolic stroke occurred in 18 patients (10 in the CM and eight in the CPFE group), while 12 patients (seven in the CM and five in the CPFE group) had transient ischemic attacks. No patient presented with primary intracerebral or subarachnoid hemorrhage. In patients with ischemic embolic stroke, the presentation had a wide range of severity with a National Institutes of Health Stroke Scale (NIHSS) score of 5–14. The NIHSS score on presentation was significantly higher in patients with CMs than in patients with CPFEs (8.9 ± 2.3 vs. 5.8 ± 3.8; p = 0.046).
The white blood cell count was elevated but significantly more elevated in patients with CMs than in patients with CPFEs (13.9 ± 6.2 vs. 10.4 ± 4.6 × 109/l; p = 0.021). NLR was similarly elevated in both groups but significantly more elevated in patients with CMs than in patients with CPFEs (8.1 ± 3.9 vs. 4.4 ± 1.7; p < 0.001). Hemoglobin and platelet levels were similar between both groups. The significance of elevations in the white blood cell count and NLR did not vary between the indication for patient presentation (p > 0.05 in all cases).
The results of echocardiograms performed during the initial patient evaluation are presented in Table 2. Of note, 36 (65.5%) benign cardiac neoplasms were initially detected by transthoracic echocardiograms, while the remaining were identified with subsequent transesophageal echocardiograms. The location of CMs was exclusively nonvalvular, with 20 (71.4%) tumors found in the left atrium and four (14.3%) in the right atrium. Multichamber involvement was seen in two patients; one patient had CMs in the left atrium and left ventricle, and another had CMs in the left and right atria. The location of CPFEs was predominantly valvular, with 19 (70.4%) cases on the aortic valve and five (18.5%) on the mitral valve. Multiple tumors were seen in two patients, where both had one mass on the aortic valve and a secondary mass on the left ventricular wall.
Compared to those in CPFEs, measurements of tumor size in CMs were significantly larger for dimensions a (33.4 ± 9.1 vs. 7.7 ± 2.6; p < 0.001) and b (24.9 ± 7.0 vs. 5.6 ± 2.4; p < 0.001). In the CM group, NLR was significantly correlated with the arithmetic mean [correlation coefficient r(26) = 0.64; p < 0.001], geometric mean [r(26) = 0.65; p < 0.001], and area [r(26) = 0.67; p < 0.001] of the dimensions of the tumor. In the CPFE group, NLR was also significantly correlated with the tumor's size arithmetic mean [correlation coefficient r(25) = 0.59; p = 0.001], geometric mean [r(25) = 0.56; p = 0.002], and area [r(25) = 0.59; p = 0.001]. The NLR per unit size of tumor (cm), defined as the ratio of NLR to the arithmetic mean of tumor dimensions a and b, was significantly higher in patients with CPFEs than in patients with CMs (6.9 ± 2.8 vs. 2.7 ± 1.1; p < 0.001).
A total of 48 (87.3%) patients had surgical resection of the tumor, as reported in Table 3. Surgical resection was more likely to be performed in patients with CMs than in patients with CPFEs (n = 27, 96.4% vs. n = 21, 77.8%; p = 0.038). No patients experienced ischemic or hemorrhagic stroke during or within 30 days of surgical resection, and 30-day survival was 100%.
The mean follow-up period was 69.3 ± 13.1 months, with 54 (98.2%) patients having at least 1 year of follow-up and 50 (90.9%) patients having at least 5 years of follow-up. Compared to the initial presentation, the mean white blood cell count was significantly lower at the follow-up in both the CM group (difference: −6.68 ± 6.73 × 109/l; p < 0.001) and the CPFE group (difference: −2.85 ± 5.17 × 109/l; p = 0.013). Compared to the initial presentation, mean NLR was also significantly lower at the follow-up in both the CM group (difference: −5.12 ± 4.18; p < 0.001) and the CPFE group (difference: −1.44 ± 2.69; p = 0.012). Hematologic values during this follow-up interval did not significantly differ between tumor types (p > 0.05 in all cases).
In the subset of 30 patients with benign cardiac neoplasms who initially presented with CVEs (Table 3 and Supplementary Table S2), four (13.3%) had CVE recurrence within 1 year of diagnosis (n = 2, 11.8% in the CM group, and n = 2, 15.4% in the CPFE group) and nine (30.0%) had recurrence at 5 years (n = 5, 29.4% in the CM group and n = 4, 30.8% in the CPFE group) (Figure 1A). Among the nine patients with 5-year CVE recurrence, three did not have surgical resection at the time of initial presentation. The 5-year CVE recurrence rate in patients with tumor resection was 24.0% (n = 6) compared to 60.0% (n = 3) in those without resection (p = 0.109). Additionally, three patients experienced other embolic events within 5 years of resection/discharge, including embolization to bilateral popliteal arteries, inferior mesenteric arteries, and left renal artery. One patient who did not initially present with CVEs experienced an ischemic stroke within 5 years of resection of his CM.
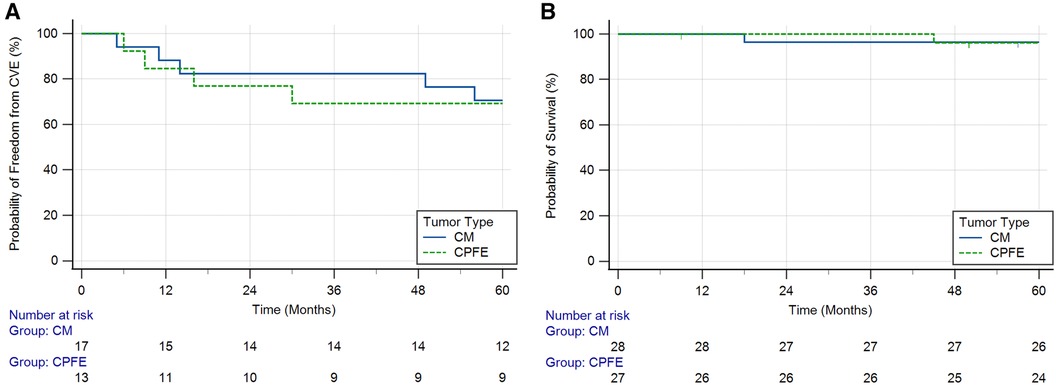
Figure 1. Kaplan–Meier curves showing (A) probability of freedom from cerebrovascular events (CVEs) over time in patients with cardiac myxomas (CMs) and cardiac papillary fibroelastomas (CPFEs) who initially presented with CVEs (log-rank test p = 0.891) and (B) probability of survival over time in patients with CMs and CPFEs (log-rank test p = 0.966).
Univariate analysis of the subset of patients initially presenting with CVEs indicated that factors significantly associated with CVE recurrence within 5 years included those with a duration between symptom onset and surgical resection >60 days (HR: 4.182; 95% CI: 1.035–16.899; p = 0.046), left atrial enlargement (left atrial diameter >40 mm) on the initial echocardiographic report (HR: 8.648; 95% CI: 1.781–41.992; p = 0.008), male sex (HR: 5.422; 95% CI: 1.343–21.889; p = 0.018), and NLR > 3.0 at the follow-up (HR: 4.173; 95% CI: 1.115–15.618; p = 0.034), as reported in Supplementary Table S3. When controlling for age and risk factors for CVEs (i.e., hypertension, hyperlipidemia, coronary artery disease, diabetes mellitus, or coronary artery bypass grafting) in multivariate analysis, left atrial enlargement (HR: 15.932; 95% CI: 1.940–139.142; p = 0.013) and NLR > 3.0 at the follow-up (HR: 5.017; 95% CI: 1.480–34.647; p = 0.043) remained significantly associated with CVE recurrence within 5 years. The observed trends in these analyses were maintained when stratifying into CM and CPFE tumor types.
Two patients died during the 5-year follow-up period (Figure 1B). One patient with a CM presented 18 months after surgical resection died from severe intraparenchymal hemorrhage, and the second patient with a CPFE presented 45 months after surgical resection died from end-stage heart failure. Tumor recurrence was seen in one patient, who presented with a CM 4 years after the initial resection. This patient had a significant family history of atrial CMs.
In this study, we present clinical, echocardiographic, and laboratory data of 55 patients with benign cardiac tumors—28 patients with CMs and 27 patients with CPFEs (1, 11–13). Similar to prior investigations, most patients in this study presented with embolic phenomena (54.5%) (11, 14–17). Patients were in their fifth decade of life, and about two-thirds were women. We found NLR to correlate with tumor size for both CM and CPFE and predict CVE recurrence. To the best of our knowledge, these are novel findings. Our study showed that patients with CPFEs were more likely to have valvular dysfunction (moderate or severe valve stenosis or regurgitation as assessed in accordance with established guidelines) or a history of cardiac surgery (9, 10). This may lend credence to the theory that endothelial and valvular damage promotes a nidus for microthrombi collection that raises the risk for CPFE. Two reports have also associated CPFEs and β-thalassemia blood disorders, suggesting a similar hemodynamic mechanism (18, 19). However, a recent study investigating the molecular profile of 14 CPFE patients demonstrated mutations of the KRAS oncogene in 11 patients, supporting the neoplastic nature of these lesions (20). Based on echocardiographic reports in this study, CMs were often left-sided (85.7%) and most commonly in the left atrium (71.4%). CPFEs were exclusively left-sided, predominantly valvular (92.6%), and most commonly on the aortic valve (70.4%). These findings correlate with the existing literature (4, 11, 17, 21–23).
No specific biomarkers for benign or malignant cardiac neoplasms are currently validated. Leukocytosis is a nonspecific indicator of inflammation. NLR is a readily accessible adjunct prognostic biomarker of growing contemporary interest. It reflects a shift from adaptive to innate immunity due to a combination of disordered immunity and contributions from disease-specific etiologies. The normal range of NLR is typically between 1 and 2, while values higher than 3.0 and lower than 0.7 are considered pathological. Elevated NLR has been demonstrated as a prognostic marker for morbidity and mortality in various chronic diseases (e.g., atherosclerotic disease, cerebrovascular disease) and cancers (e.g., melanoma, breast cancer) (24). In the case of malignancy, neutrophilia is an observed feature of cancer-elicited chronic inflammation, and it is often accompanied by relative lymphocytopenia due to tumor-driven suppression of the cell-mediated immune response (25, 26). NLR has been associated with tumor size, tumor stage, and metastatic potential and has been shown to predict cancer-specific survival, including progression-free and disease-free survival (27).
In this study, the white blood cell count and NLR were elevated in patients presenting with CMs and CPFEs, although values were significantly higher in the former. This is likely due to the substantially larger tumor sizes of CMs than those of CPFEs. It may also naturally reflect the immunologic nature of CMs, which has been described to overproduce interleukin-6 and other various growth factors and cytokines that generate observed constitutional symptoms. Indeed, interleukin-6 has been posited as a marker of recurrence for CMs (28). In CPFEs, the elevated NLR may be a product of endothelial cell activation and chronic valvular inflammation in addition to neoplastic processes (29). Elevated NLR has previously been observed in patients with CMs, and it has been correlated with tumor size (30). NLR values were similarly correlated with various metrics of tumor size in this study (64%–67% in the CM and 56%–59% in the CPFE group), suggesting a role for this biomarker in tumor burden prognostication. Of note, NLR values may also be elevated in ischemic chest pain, CVE, and other stress states present in these patients during hospitalization; however, NLR values were not significantly altered when stratified by the patient presentation (31). Although CMs and CPFEs generally differ in fundamental pathophysiology, there is considerable overlap in the initial clinical manifestation. Moreover, unusual presentations of these tumors (e.g., the deranged echocardiographic appearance of CMs or nonvalvular site of CPFEs) obscure identification (32, 33). Therefore, the patterns of NLR elevation observed in this study may be useful in the setting of a patient in whom a primary cardiac neoplasm is detected, but the exact etiology is unclear.
Importantly, a sizeable portion of patients with either neoplasm (30%–60%) initially present with CVEs, consistent with the results of this investigation. CM-related CVEs have been associated with a friable or villous tumor surface, younger male patients, and tumors with size ≤4.5 cm (14, 15, 34). CPFE-related CVEs have been associated with small tumor size at diagnosis and independent mobility (11, 17). Other echocardiographic characteristics, aortic valve location, or tumor growth rate have not been identified as risk factors (35). In this study, tumors were expectedly left-sided. No baseline patient characteristics, including age, sex, or cardiovascular risk factors such as a history of hypertension, hyperlipidemia, coronary artery disease, diabetes mellitus, smoking, or prior CVE, were significantly associated with an initial cerebrovascular manifestation of the benign cardiac neoplasm compared to any other indication for presentation. The size or independent mobility of the tumor and left atrial enlargement also did not significantly differ between patients initially presenting with CVEs and any other indications.
Elevated recurrence of CVE, compared to baseline age- and sex-matched controls, after excision has also been reported in both CMs and CPFEs. Prolonged duration until surgical excision in patients with CMs and unremoved lesions in patients with CPFEs are associated with CVE recurrence (4, 11, 16). The CVE recurrence rate in our cohort was high: 30.0% at 5 years (24.0% in patients with prior tumor resection and 60.0% in patients without prior resection). The prolonged duration between symptom onset and surgical resection (>60 days), left atrial enlargement, male sex, and NLR > 3.0 at approximately 1-year follow-up were significantly associated with CVE recurrence within 5 years of surgical resection or discharge. When controlling for age and known risk factors for CVE in multivariable analysis, left atrial enlargement and NLR > 3.0 at approximately 1-year follow-up remained significantly associated with 5-year CVE recurrence. Ultimately, these findings may emphasize the need for timely excision of CMs and CPFEs in suitable candidates and the use of NLR as a prognostic biomarker. Importantly, no cases of hemorrhagic transformation intraoperatively or postoperatively have been reported, lessening concern for postischemic stroke patients on bridge therapy.
This investigation has several limitations that must be emphasized. The study design is retrospective and small in sample size, limiting the robustness of the inferences that can be drawn. Our tertiary care center may also be subject to referral bias, possibly explaining the relatively low rates of incidental discovery of these benign cardiac neoplasms compared to other studies. Regarding NLR, the prognostic biomarker is an accessible and cost-effective measure of dysregulated inflammation that has garnered significant contemporary interest in various fields. In numerous large-cohort studies and meta-analyses within the cardiovascular domain, NLR has been significantly associated with the onset of atrial fibrillation, acute coronary syndrome (including correlation with the Global Registry of Acute Coronary Events risk score and SYNTAX score), and acute decompensated heart failure (36–40). However, its use in cardiovascular tumors has not been well characterized, and further large-scale investigations are required to validate the observations within our study. The reference cutoff and the subsequent interpretive potential of NLR may also vary by age, sex, and other factors, although this requires further research (41).
Furthermore, NLR may be elevated in various acute disease states; therefore, its utility may primarily be as an adjunct prognostic tool (after further validation) and not in a diagnostic capacity. A presentation of ischemic stroke is among the acute etiologies associated with elevated NLR (42). Therefore, it would be of great interest to evaluate NLR in patients presenting with CVEs and underlying benign cardiac neoplasms compared to values in patients with CVEs and no malignancy to further validate this work. Finally, this study included a small number of patients in whom the benign cardiac neoplasm was not excised. In these cases, the diagnosis was formulated via clinical presentation and the presence of characteristic echocardiographic features of the tumor (8). A few contemporary studies have discussed the viability of a conservative approach to asymptomatic benign cardiac neoplasms (especially small, incidentally discovered tumors), one that favors antithrombotic medical therapy as opposed to surgical intervention (16, 17, 43, 44). While our investigation failed to highlight a significant elevation in adverse outcomes in patients in whom surgical excision was not pursued, likely due to the small sample size, we feel it is important to include these cases to reflect these considerations; we bear in mind that without histopathological confirmation of tumors in these cases, the uncertainty and risk of misdiagnosis remains.
In conclusion, our study demonstrated a high CVE recurrence rate in patients with benign cardiac tumors and indicates that NLR may serve as a valid adjunct prognostic tool for tumor burden and recurrence of neurologic events.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the University of Florida Institutional Review Board. The studies were conducted in accordance with local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because it was a cohort study and involved no more than minimal risk to subjects, did not adversely affect their rights, and could not be carried out practicably without the exemption/waiver.
AyM and AM designed the study, performed data collection and analysis, produced tables and figures, and drafted the manuscript. UK and MM performed data collection and analysis and drafted portions of the manuscript. VR, EA, and BN contributed to data analysis and manuscript revision. MR supervised the study, provided subject matter expertise, revised the manuscript, and was responsible for the final content. All authors have agreed on the journal to which the article will be submitted, the final version for publication, and accountability for all aspects of the work. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1222179/full#supplementary-material
1. Bussani R, Castrichini M, Restivo L, Fabris E, Porcari A, Ferro F, et al. Cardiac tumors: diagnosis, prognosis, and treatment. Curr Cardiol Rep. (2020) 22(12):169. doi: 10.1007/s11886-020-01420-z
2. Islam AKMM. Cardiac myxomas: a narrative review. World J Cardiol. (2022) 14(4):206–19. doi: 10.4330/wjc.v14.i4.206
3. Yandrapalli S, Mehta B, Mondal P, Gupta T, Khattar P, Fallon J, et al. Cardiac papillary fibroelastoma: the need for a timely diagnosis. World J Clin Cases. (2017) 5(1):9–13. doi: 10.12998/wjcc.v5.i1.9
4. Sun JP, Asher CR, Yang XS, Cheng GG, Scalia GM, Massed AG, et al. Clinical and echocardiographic characteristics of papillary fibroelastomas. Circulation. (2001) 103(22):2687–93. doi: 10.1161/01.CIR.103.22.2687
5. Kumar V, Soni P, Hashmi A, Moskovits M. Aortic valve fibroelastoma: a rare cause of stroke. BMJ Case Rep. (2016) 2016:bcr2016217631. doi: 10.1136/bcr-2016-217631
6. Valente M, Basso C, Thiene G, Bressan M, Stritoni P, Cocco P, et al. Fibroelastic papilloma: a not-so-benign cardiac tumor. Cardiovasc Pathol. (1992) 1(2):161–6. doi: 10.1016/1054-8807(92)90020-O
7. Mazur P, Kurmann R, Klarich KW, Dearani JA, Arghami A, Daly RC, et al. Operative management of cardiac papillary fibroelastomas. J Thorac Cardiovasc Surg. (2022) S0022-5223(22):00744–9. doi: 10.1016/j.jtcvs.2022.06.022.
8. Kurmann R, El-Am E, Ahmad A, Abbasi MA, Mazur P, Akiki E, et al. Cardiac masses discovered by echocardiogram; what to do next? Structural Heart. (2023) 7(4):100154. doi: 10.1016/j.shj.2022.100154
9. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. (2021) 143(5):e72–e184. doi: 10.1161/CIR.0000000000000923
10. Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation. J Am Soc Echocardiogr. (2017) 30(4):303–71. doi: 10.1016/j.echo.2017.01.007
11. Tamin SS, Maleszewski JJ, Scott CG, Khan SK, Edwards WD, Bruce CJ, et al. Prognostic and bioepidemiologic implications of papillary fibroelastomas. J Am Coll Cardiol. (2015) 65(22):2420–9. doi: 10.1016/j.jacc.2015.03.569
12. Steger CM, Hager T, Ruttmann E. Primary cardiac tumours: a single-center 41-year experience. ISRN Cardiol. (2012) 2012:1–7. doi: 10.5402/2012/906109
13. Boyacıoğlu K, Ak A, Dönmez AA, Çayhan B, Aksüt M, Tunçer MA. Outcomes after surgical resection of primary non-myxoma cardiac tumors. Braz J Cardiovasc Surg. (2018) 33(2):162–8. doi: 10.21470/1678-9741-2017-0152
14. Wen X, Chen Y, Yu L, Wang S, Zheng H, Chen Z, et al. Neurological manifestations of atrial myxoma: a retrospective analysis. Oncol Lett. (2018) 16(4):4635–9. doi: 10.3892/ol.2018.9218.30214598
15. Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. Medicine. (2001) 80(3):159–72. doi: 10.1097/00005792-200105000-00002
16. Stefanou MI, Rath D, Stadler V, Richter H, Hennersdorf F, Lausberg HF, et al. Cardiac myxoma and cerebrovascular events: a retrospective cohort study. Front Neurol. (2018) 9:823. doi: 10.3389/fneur.2018.00823
17. Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J. (2003) 146(3):404–10. doi: 10.1016/S0002-8703(03)00249-7
18. Kokotsakis J, Nenekidis I, Anagnostakou V, Paralikas I, Giotopoulou A, Kratimenos T, et al. Papillary fibroelastoma of the aortic valve in a β-thalassemia patient. Gen Thorac Cardiovasc Surg. (2011) 59(10):712–4. doi: 10.1007/s11748-010-0754-0
19. Chin RI, Monda JJ, Sheth M, Ogle W, Merenda G, De D. Papillary fibroelastoma as a cause of cardiogenic embolic stroke in a β-thalassemia patient: case report and literature review. Case Rep Cardiol. (2017) 2017:1–4. doi: 10.1155/2017/8185601
20. Wittersheim M, Heydt C, Hoffmann F, Büttner R. KRAS mutation in papillary fibroelastoma: a true cardiac neoplasm? J Pathol Clin Res. (2017) 3(2):100–4. doi: 10.1002/cjp2.66
21. Jelic J, Milicić D, Alfirević I, Anić D, Baudoin Z, Bulat C, et al. Cardiac myxoma: diagnostic approach, surgical treatment and follow-up. A twenty years experience. J Cardiovasc Surg. (1996) 37(6 Suppl 1):113–7.
22. Val-Bernal JF, Mayorga M, Garijo MF, Val D, Nistal JF. Cardiac papillary fibroelastoma: retrospective clinicopathologic study of 17 tumors with resection at a single institution and literature review. Pathol Res Pract. (2013) 209(4):208–14. doi: 10.1016/j.prp.2013.02.001
23. Karabinis A, Samanidis G, Khoury M, Stavridis G, Perreas K. Clinical presentation and treatment of cardiac myxoma in 153 patients. Medicine. (2018) 97(37):e12397. doi: 10.1097/MD.0000000000012397
24. Buonacera A, Stancanelli B, Colaci M, Malatino L. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int J Mol Sci. (2022) 23(7):3636. doi: 10.3390/ijms23073636
25. Howard R, Kanetsky PA, Egan KM. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci Rep. (2019) 9(1):19673. doi: 10.1038/s41598-019-56218-z
26. Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. JNCI J Natl Cancer Inst. (2014) 106(6):dju124. doi: 10.1093/jnci/dju124
27. Lianos GD, Alexiou GA, Exarchos C, Rausei S, Mitsis M, Voulgaris S. Prognostic significance of neutrophil-to-lymphocyte ratio in several malignancies: where do we stand? Biomark Med. (2020) 14(3):169–72. doi: 10.2217/bmm-2019-0497
28. Mendoza CE, Rosado MF, Bernal L. The role of interleukin-6 in cases of cardiac myxoma. Clinical features, immunologic abnormalities, and a possible role in recurrence. Tex Heart Inst J. (2001) 28(1):3–7.11330738
29. Matysiak N, Mielanczyk L, Kaczmarek K, Zaba M, Reichman-Warmusz E, Wojnicz R. New insights into the pathogenesis of cardiac papillary fibroelastomas. Folia Histochem Cytobiol. (2021) 59(4):212–25. doi: 10.5603/FHC.a2021.0027
30. Michopanou N, Schizas N, Charitos C, Rontogianni D, Saroglou G, Vatopoulos A, et al. Autopsy of 54 cases of surgically excised cardiac myxomas. Investigation of their impact on immune response. Heliyon. (2020) 6(7):e04535. doi: 10.1016/j.heliyon.2020.e04535
31. Quan K, Wang A, Zhang X, Meng X, Chen P, Li H, et al. Neutrophil to lymphocyte ratio and adverse clinical outcomes in patients with ischemic stroke. Ann Transl Med. (2021) 9(13):1047–1047. doi: 10.21037/atm-21-710
32. Oda T, Yasunaga H, Takaseya T, Amako M, Kawara T, Todo K, et al. Left atrial myxoma mimicking papillary fibroelastoma. J Med Ultrason. (2012) 39(3):173–5. doi: 10.1007/s10396-012-0358-7
33. Ulloa J U, de Vega V M, Gil A F, Cabrera JÁ. A papillary fibroelastoma with myxoma camouflage: a case report. Eur Heart J Case Rep. (2022) 6(8):ytac315. doi: 10.1093/ehjcr/ytac315
34. Wang Z, Chen S, Zhu M, Zhang W, Zhang H, Li H, et al. Risk prediction for emboli and recurrence of primary cardiac myxomas after resection. J Cardiothorac Surg. (2016) 11:22. doi: 10.1186/s13019-016-0420-4
35. Kurmann RD, El-Am EA, Sorour AA, Ahmad A, Lee AT, Scott CG, et al. Papillary fibroelastoma growth. J Am Coll Cardiol. (2021) 77(16):2154–5. doi: 10.1016/j.jacc.2021.02.027
36. Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther. (2016) 14(5):573–7. doi: 10.1586/14779072.2016.1154788
37. Shao Q, Chen K, Rha SW, Lim HE, Li G, Liu T. Usefulness of neutrophil/lymphocyte ratio as a predictor of atrial fibrillation: a meta-analysis. Arch Med Res. (2015) 46(3):199–206. doi: 10.1016/j.arcmed.2015.03.011
38. Guasti L, Dentali F, Castiglioni L, Maroni L, Marino F, Squizzato A, et al. Neutrophils and clinical outcomes in patients with acute coronary syndromes and/or cardiac revascularisation. Thromb Haemost. (2011) 106(10):591–9. doi: 10.1160/TH11-02-0096
39. Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. (2008) 102(6):653–7. doi: 10.1016/j.amjcard.2008.05.006
40. Kurtul S, Sarli B, Baktir AO, Demirbas M, Saglam H, Doğan Y, et al. Neutrophil to lymphocyte ratio predicts SYNTAX score in patients with non-ST segment elevation myocardial infarction. Int Heart J. (2015) 56(1):18–21. doi: 10.1536/ihj.14-175
41. Lee JS, Kim NY, Na SH, Youn YH, Shin CS. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in south Korea. Medicine. (2018) 97(26):e11138. doi: 10.1097/MD.0000000000011138
42. Li W, Hou M, Ding Z, Liu X, Shao Y, Li X. Prognostic value of neutrophil-to-lymphocyte ratio in stroke: a systematic review and meta-analysis. Front Neurol. (2021) 12:686983. doi: 10.3389/fneur.2021.686983
43. Łebek-Szatańska AM, Łebek ZL, Dąbrowski MJ, Kardaszewicz PS. A large tumour of the left atrium—a 10-year follow-up. Hellenic J Cardiol. (2016) 57(6):441–4. doi: 10.1016/j.hjc.2016.11.008
Keywords: cardiac myxomas, cardiac papillary fibroelastoma, neutrophil–lymphocyte (N/L ratio), cerebrovascular events, benign cardiac neoplasms
Citation: Mathavan A, Mathavan A, Krekora U, Mathavan M, Rodriguez V, Altshuler E, Nguyen B and Ruzieh M (2023) Clinical presentation and neurovascular manifestations of cardiac myxomas and papillary fibroelastomas: a retrospective single-institution cohort study. Front. Cardiovasc. Med. 10:1222179. doi: 10.3389/fcvm.2023.1222179
Received: 13 May 2023; Accepted: 11 August 2023;
Published: 1 September 2023.
Edited by:
Reto Asmis, Wake Forest University, United StatesReviewed by:
Orlando Parise, Maastricht University, Netherlands© 2023 Mathavan, Mathavan, Krekora, Mathavan, Rodriguez, Altshuler, Nguyen and Ruzieh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammed Ruzieh bW9oYW1tZWQucnV6aWVoQG1lZGljaW5lLnVmbC5lZHU=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.