- 1Graduate School, Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2Department of Cardiology, The Second Affiliated Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
Acute myocardial ischemia is a disease with high morbidity and mortality, and re-perfusion is currently the best intervention. However, re-perfusion may lead to further myocardial injury and increase the area of myocardial infarction. The mechanism of myocardial ischemia-re-perfusion injury is complex, but with more in-depth study, it has been proved that the immune system plays an important role in the process of MIRI. Among them, the γδT cell population has received increasing attention as the main early source of IL-17A in many immune response models. Because γδT cells have the characteristics of linking innate immunity and adaptive immunity,they can rapidly produce IL-17A and produce subsequent immune killing of cardiomyocytes. It can be seen that γδT cells play an important role in MIRI. Therefore, here we review the research progress of immune response in myocardial ischemia-re-perfusion injury, the key characteristics of γδT cells and the role of rapidly produced IL-17 in myocardial ischemia-re-perfusion injury, and propose relevant treatment strategies and prospects for myocardial repair, in order to provide new ideas and methods for clinical treatment of myocardial ischemia-re-perfusion injury.
1. Introduction
Acute myocardial infarction (AMI) is a cardiovascular disease that seriously threatens human health worldwide. Re-perfusion is the preferred treatment strategy for acute myocardial ischemia (1–4). Thrombolysis or percutaneous coronary intervention (PCI) is currently the most effective treatment to reduce ischemic injury and limit infarct size, thereby preventing ventricular remodeling, improving cardiac function, reducing arrhythmia, and effectively reducing mortality (5, 6). However, after the re-perfusion of ischemic myocardium, the abnormal changes of myocardial morphology and function caused by re-perfusion may lead to the necrosis of some ischemic myocardial cells, even more serious than the damage caused by ischemia alone, thus increasing the area of myocardial infarction. Although the best re-perfusion therapy is obtained after AMI, nearly 10% of patients still die, and the incidence of heart failure after AMI is as high as 25%, which seriously affects the effect of ischemic myocardial re-perfusion therapy (7). Therefore, exploring the pathogenesis of myocardial ischemia-re-perfusion injury and taking targeted prevention and control measures have become one of the urgent problems to be solved in cardiovascular clinical treatment, and have important clinical significance for effectively reducing mortality.
At present, it is believed that the mechanism of myocardial ischemia-re-perfusion injury is complex, which involves oxygen free radical injury (8, 9), calcium overload (10, 11), oxidative stress (12, 13), reactive oxygen species production (14), immune cells (15), endothelial cell dysfunction (16), autophagy, ferroptosis and other aspects. With the progress of immunological research, more and more evidence shows that immune response plays a central role in the pathological process of myocardial ischemia-re-perfusion injury. The enhancement of immune response mediated by γδT lymphocytes may aggravate the degree of myocardial ischemia-re-perfusion injury.Therefore, in this review, we discuss the role of immune response in myocardial ischemia-re-perfusion injury and the key characteristics and functions of γδT cells and their potential in the treatment of myocardial ischemia-re-perfusion injury.
2. Overview of the immune system
The immune system is a complex network composed of various immune cells, signaling pathways and effector molecules. It can be divided into two different types: innate immunity and adaptive immunity. Each type can recognize and respond to a variety of antigens. The immune system is activated under conditions such as tissue damage, infection or genotoxic stress, resulting in innate immune responses.
2.1. Innate immunity
The evolutionary innate immune system is much older than the adaptive immune system. It is composed of the complement system and different types of immune cells, including phagocytes (macrophages, neutrophils), antigen presenting cells (dendritic cells) and so on (17). The first line of defense for immune defense is based on the detection of pathogen-associated molecular patterns that cause toxic and inflammatory responses. Pattern recognition receptors in immune cells are activated when they respond to conserved motifs of invading pathogens and non-self elements (pathogen-associated molecular patterns). PPRs may also respond to endogenous molecular patterns released during cell injury or death, namely damage-associated molecular patterns (DAMPs), and subsequently induce aseptic inflammation. Among them, dendritic cells can further activate the adaptive immune response through antigen presentation. In MIRI, dendritic cells are considered to be the source of DAMPs release from cardiomyocytes after myocardial ischemia. The mechanism is that NADPH oxidase-dependent super-oxide production in dendritic cells is enhanced, resulting in the formation of highly active γ-ketoaldehyde. These compounds rapidly form their own protein adducts, which are treated by dendritic cells and presented as DAMPs, leading to vascular dysfunction.
2.2. Adaptive immunity
An adaptive immune system in which pathogenic exposure confers long-term defensive memory to host organisms, including T lymphocytes and B lymphocytes. B lymphocytes mainly detect and process antigens, and further differentiate in plasma cells to produce antibodies (immunoglobulins) to resist the invasion of harmful antigens and participate in humoral immunity (18). T lymphocytes mediate cellular immunity and assist B lymphocytes to produce antibodies (19). In a relatively new immune model, it has been shown that the immune system can respond to “danger signals”, both self and non-self. Exogenous “danger signals”, pathogen-associated molecular patterns (PAMPs), are highly conserved gene sequences in microbial pathogens, such as lipopolysaccharide (LPS), peptidoglycan, bacterial lipid oleic acid and flagellin. Endogenous “danger signals”, namely damage-associated molecular patterns (DAMPs), may come from poor or damaged cells, such as ischemic cardiomyocytes and infarcted cardiomyocytes. Both PAMPs and DAMPs can activate the immune system through PRRs and trigger innate and adaptive immunity. In addition, T lymphocytes also have the characteristics of inhibiting immune response and maintaining self-tolerance. The mechanisms of its inhibitory function include inhibition of cytokine secretion (IL-10, TGF-β and IL-35), direct cytolysis of effector T cells, destruction of metabolism through tryptophan decomposition products, IL-2 deprivation and direct interference with co-stimulation through cytotoxic T lymphocyte-associated protein 4 (CTLA-4) expression (20).
Although these two systems mainly protect organisms from invading pathogens, under disease conditions, their own cells may be the target of destruction, and invading immune cells can cause damage to the host they intend to protect. There are many different links linking the innate and adaptive immune responses, including the complement system, and involving cell types with two systemic functional characteristics, including B1 cells and γδT cells (21, 22).
3. The role of T lymphocyte sub-types and their mediated immune response in MIRI
Recent studies have shown that myocardial ischemia-re-perfusion injury is a complex process involving metabolic and immune factors. Immune response plays a central role in the pathological process of myocardial ischemia-re-perfusion injury. Immune response regulates the whole process of myocardial ischemia-re-perfusion injury by recruiting and activating related immune cells, innate immune system and adaptive immune system. T lymphocyte-mediated immune response plays an important role in myocardial ischemia-re-perfusion injury. Therefore, various T lymphocyte sub-types have been widely studied, including NKT cells, TH17 cells, γδT cells, CD4 + T cells and CD8 + T cells, among which γδT cells play an important role in myocardial ischemia-re-perfusion injury.
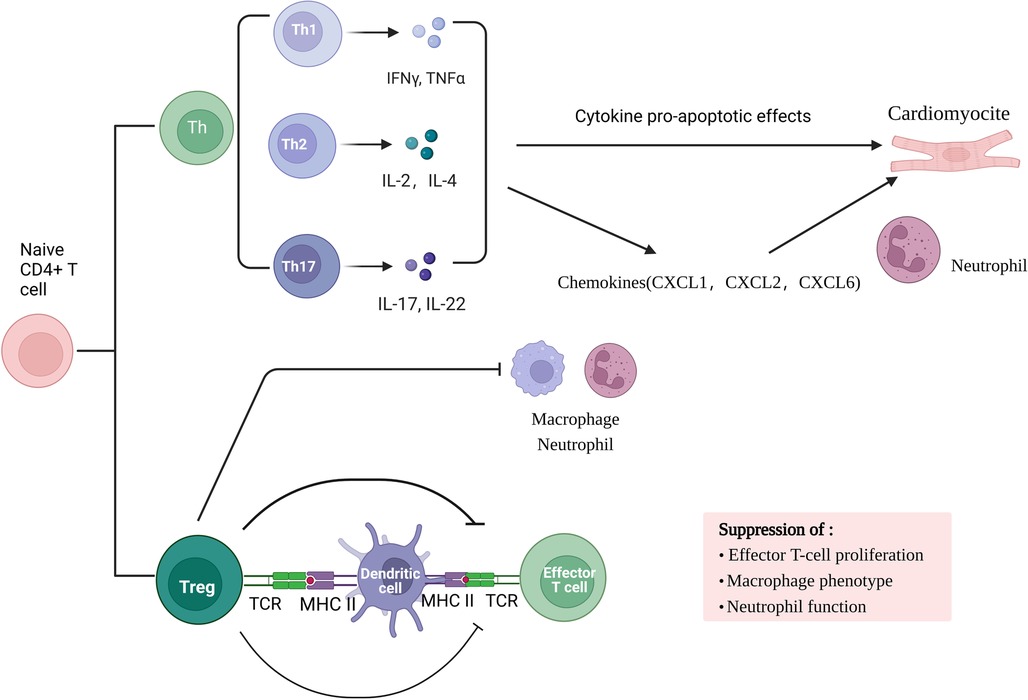
Adaptive immune response involved in myocardial ischemia/re-perfusion is a local inflammatory response based on cellular immunity. Studies have found that the early stage of ischemia-re-perfusion (IR) is dominated by acute inflammatory response. In myocardial ischemia-re-perfusion injury, myocardial tissue in the damaged area is mainly infiltrated with CD4 + T cells as the main effector cells, which can infiltrate into the infarct area 2 min after re-perfusion and participate in sustained and stable myocardial injury. Primitive CD4 + T lymphocytes can differentiate into helper T cells (Th cells) subsets and Foxp3 + regulatory T cells (Treg cells) under the action of various factors. Th cell subsets are mainly Th17 cells. Th17 cells and Treg cell subsets are the main participants in the immune inflammatory response. TH17 cells are a pro-inflammatory subset that promotes autoimmune and tissue damage. Th cells mainly secrete inflammatory cytokines such as interleukin (IL) −2, interferon (IFN) -γ, tumor necrosis factor (TNF) -β.Cytokines have pro-apoptotic effects in mouse myocardial I/R cardiomyocytes, and increase neutrophil infiltration by enhancing the production of chemokines (including CXCL1, CXCL2 and CXCL6), promote inflammatory response, and cause myocardial cell damage. On the contrary, Treg cells have immunosuppressive effects and can prevent autoimmunity. They not only regulate adaptive immunity by inhibiting the proliferation and function of effector T cells, but also regulate innate immunity by inhibiting macrophage inflammatory phenotype and neutrophil function.
At the same time, during myocardial ischemia-re-perfusion injury, endogenous ligands released by tissue damage activate Toll-like receptors, NOD-like receptors, C-type lectin receptors, and RIG-1-like receptors (23), thereby initiating natural immune responses (24), and then transcriptionally regulate the production of pro-inflammatory mediators, including cytokines, chemokines, and adhesion molecules, leading to tissue inflammation, thereby aggravating myocardial ischemia-re-perfusion injury.
In conclusion, in the process of myocardial ischemia-re-perfusion injury, metabolic abnormalities during hypoxia-re-oxygenation release dangerous signals, activate the body's natural immune system, activate TLR, complement system and mast cells, and subsequently recruit a large number of neutrophils and monocytes. Pro-inflammatory factors and oxygen free radicals are involved in the amplification of inflammatory reactions. Any part of the system overreaction will aggravate tissue damage. Most T cell subsets mainly contribute to the antigen-specific effects and memory stages of immunity, but γδT cells combine the characteristics of adaptive immunity with rapid innate immune responses, allowing them to be in the initial stage of immune response Figure 1.
4. Overview of γδT cells
4.1. Characteristics of γδT cells
According to the surface expression of T cell receptor (TCR), T cells are divided into two main groups: αβT cells and γδT cells. Different from αβ T cell population, the surface T cell receptors of γδT cells are composed of γ chain and δ chain. γδT cells are highly heterogeneous cells with various sub-types, variable phenotypes and different biological characteristics among sub-types. γδT cells are T cells that perform innate immune function. Compared with traditional αβT cells, γδT cells have unique properties that link innate immunity and adaptive immunity. They play an important role in the development of infection, tumor and autoimmune diseases. The antigen recognition of mouse or human γδT cells does not require the presentation of major histocompatibility complex (MHC) class I or II antigens (25). Activated γδT cells can enter the activated state within a few minutes after antigen stimulation (26). Activated γδT cells affect other immune cells by producing cytokines and cytotoxic multiple effector functions, regulating antigen presentation functions, thereby enhancing the immune response to dangerous signals formed by invading pathogens or “own” cells.
4.2. The classification and function of γδT cells
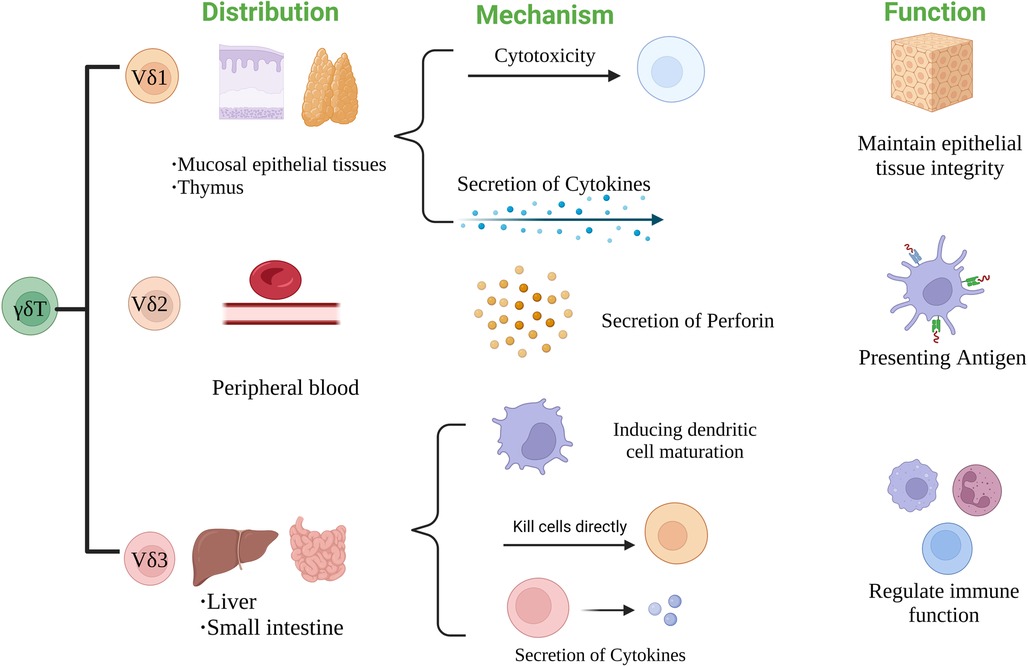
According to the structure of TCR δ chain, γδT cells can be divided into three subgroups: Vδ1, Vδ2 and Vδ3 γδT cells. The distribution and function of each subgroup are also different (27). Vδ1 γδT cells are mainly distributed in mucosal tissues such as skin and small intestine. They can respond to stress antigens of epithelial cells (28) and participate in maintaining epithelial tissue integrity in the face of injury, infection or transformation (29). Vδ2 γδT cells are mainly present in peripheral blood, accounting for 50%–90% of peripheral γδT cells, and are the main γδT cells involved in blood circulation. Activated Vδ2T cells can be used as professional antigen presenting cells (APC) (30), such as antigen presentation, costimulatory and adhesion molecules expression, including MHC-II, CD80 and CD86 (31). Vδ3 γδT cells are mainly present in the liver and intestinal epithelium, accounting for about 0.2% of circulating T cells. They can kill CD1d target cells, release cytokines such as Th1, Th2 and Th17, and induce dendritic cells (DC) to mature into APC+ (32) Figure 2.
They were classified according to the function of γδT cells. The heterogeneity of γδT cell subsets determines the diversity of their functions, so it can also be divided into γδT1 (IFN-γ+ γδT), γδT17, follicular helper γδT (γδTfh), regulatory γδT (γδTreg), memory γδT (memoryγδT) cells, hMSH2-specific γδT cells and recently discovered IL-6-secreting γδT cells. Among them, hMSH2-specific γδT cells and IL-6-secreting γδT cells have not been reported in the literature, and their structural and functional characteristics are still in the preliminary stage. γδT1 (IFN-γ+ γδT) cells can secrete IFN-γ, which can enhance cell-mediated anti-infective immunity. γδT17 cells can secrete IL-17, which plays an important role in initiating inflammatory response, regulating the expansion and recruitment of neutrophils and monocytes, and plays an important role in the initial stage of various inflammatory responses (33, 34). γδTfh cells can promote the maturation of B cells and the ability to produce antibodies; in addition, in some chronic infections and tumors, γδTfh cells can enhance the effector function of CD8 + T cells and the proliferation of CD8 + T cells by secreting IL-21 (35, 36); γδTreg cells can express a specific transcription factor FOXP3 and have immunosuppressive function (37); memory γδT cells have the characteristics of immune memory and can rapidly produce immune response after receiving the same antigen stimulation again (38). In summary, each γδT cell subset plays different roles in the human immune system and has potential clinical value Figure 3.
4.3. The immune regulation of γδT cells is bidirectional
4.3.1. γδt cell-mediated immune response
γδT cells have the characteristics of congenital and adaptive immune cells, and can be activated only through APC cytokine signaling without homologous TCR ligands (39). When activated, γδT cells produce Th1 and/or Th2 inflammatory cytokines and IL-17 (40, 41), and then induce the inflammatory response of adaptive effector cells (42). These characteristics make γδT cells an effective effector T cell-mediated immune cascade in inflammatory tissues (43), and γδT cells can exert cytotoxic mediated killing of multiple target cells through Fas/Fas ligands (44). In addition, γδT cells can also play a variety of roles in the response to infection, including direct antibacterial effects, recruitment of innate immune cells (such as neutrophils, macrophages) and activation of adaptive immunity (45).
In the anti-tumor effect, studies have found that γδT cells are involved in several types of cancer, including breast cancer, stomach, colorectal cancer, hematological malignancies, and glioblastoma (46). As a key participant in anti-tumor immune response, γδT cells have the ability to produce a large number of pro-inflammatory cytokines and directly mediate cell lysis of various tumor types (47). At the same time, studies have confirmed that Vδ1 and Vδ2 subsets play an important role in the tumor immunity of γδT cells, mainly through natural killer cell receptors to identify tumor cells (48), and can play an anti-tumor role through direct contact and secretion of cytokines (49). Vδ1T cells kill cancer cells by up-regulating the expression of CD69, CD107a, perforin, granzyme B, TRAIL and CD57 (50). Vδ2T cells can directly kill or induce tumor cell apoptosis after TCR-dependent activation, and can also induce neutrophil infiltration to tumor sites or affect other immune cells to exert anti-tumor effects by secreting cytokines (51).
γδT cells play an important role in re-perfusion injury. In intestinal IRI, γδT cells are involved in the initiation and continuation of the initial inflammatory response as a mediator to promote the acute inflammatory response of intestinal IRI (52, 53); the lack of γδT cells can improve the production of pro-inflammatory cytokines, reduce neutrophil recruitment and distant organ damage (54, 55). In renal IRI, γδT cells mediate innate and adaptive immune responses during the first 72 h of renal IRI, and the absence of γδT cells will delay the inflammatory response in renal IRI (56–58). In brain IRI, studies have shown that IL-23 and IL-17 play a key role in the evolution of cerebral infarction and accompanying neurological dysfunction. IL-23 plays a role in the direct stage of cerebral IRI, while IL-17 plays an important role in the delayed stage of cerebral IRI. IL-23 secreted by activated macrophages can drive γδT cells to produce IL-17, which further increases neuroinflammation and secondary damage after intracerebral hemorrhage (59). Another study has also shown that the expression of IL-23 is mainly derived from infiltrating macrophages, and is an important inducer of IL-17 production by γδT cells in the delayed phase of cerebral ischemia in mice, and γδT cells that produce IL-17 play an important role in late cerebral infarction. Increased expression of IL-17 aggravates secondary brain injury after intracerebral hemorrhage, and γδT cells are the main source of IL-17 in the hemorrhagic hemisphere (60, 61). It has been found in liver IRI that IL-17A produced by liver γδT cells can lead to liver cell damage and enhanced liver inflammation in animals (62).
4.3.2. Immunosuppressive effect of γδT cells
In systemic inflammatory response syndrome (SIRS), studies have found that γδT cells can prevent lung tissue damage by recognizing and eliminating inflammatory PMN (63). It is proved that γδT cells have cytotoxicity to activated macrophages and play an important role in the down-regulation of inflammatory response (64), which indicates that γδT cells are involved in the recovery of infection and can accelerate the recovery of infection. Another study found that γδT cells produce IFN-γ and anti-inflammatory cytokines (such as IL-10), which can inhibit the production of pro-inflammatory mediators (including IL-1, TNF-α and IL-8) in inflammatory cells in affected tissues. In addition to the ability to eliminate inflammatory cells, γδT cells can also play an additional role in protecting the integrity of host tissues and organs.
In the process of liver fibrosis, studies have shown that liver γδT cells, especially γδT1 subsets, play a significant protective role in the development of liver fibrosis. γδT cells can induce the apoptosis of HSC and inactivate activated HSC, so as to delay the process of liver fibrosis. In addition, γδT cells may inhibit the infiltration of inflammatory cells into liver tissue, and the lack of γδT cells will aggravate liver fibrosis, increase serum ALT levels, and accumulate intrahepatic white blood cells (65).
In terms of tumors, γδT cells may promote tumor development. Studies have found that CD39 γδTregs not only have a direct immunosuppressive function on effector T cells, but also secrete a large amount of IL-17A, TNF-α and GM-CSF.These cells may mobilize and recruit PMN-MDSCs into TME, thereby establishing an immunosuppressive network in colorectal cancer. In addition, under the induction of TGF-β1 secreted by tumor cells, cells produce more adenosine, which shows obvious immunosuppressive effect on CD4 + T cells through adenosine-mediated pathway, and promotes tumor progression and metastasis (66–68). Another study found that Th17 γδT cells that produce IL-17 increase the expression of angiogenic factors VEGF-2 and ANG-2 in tumor sites, indicating that they promote tumor development in gallbladder cancer, ovarian cancer and breast cancer by enhancing angiogenesis (69).
It can be seen that γδT cells have a two-way immunomodulatory effect, which can not only mediate the immune response, but also produce immunosuppressive effects under certain conditions.
5. The role of γδT cell-mediated immune response in MIRI
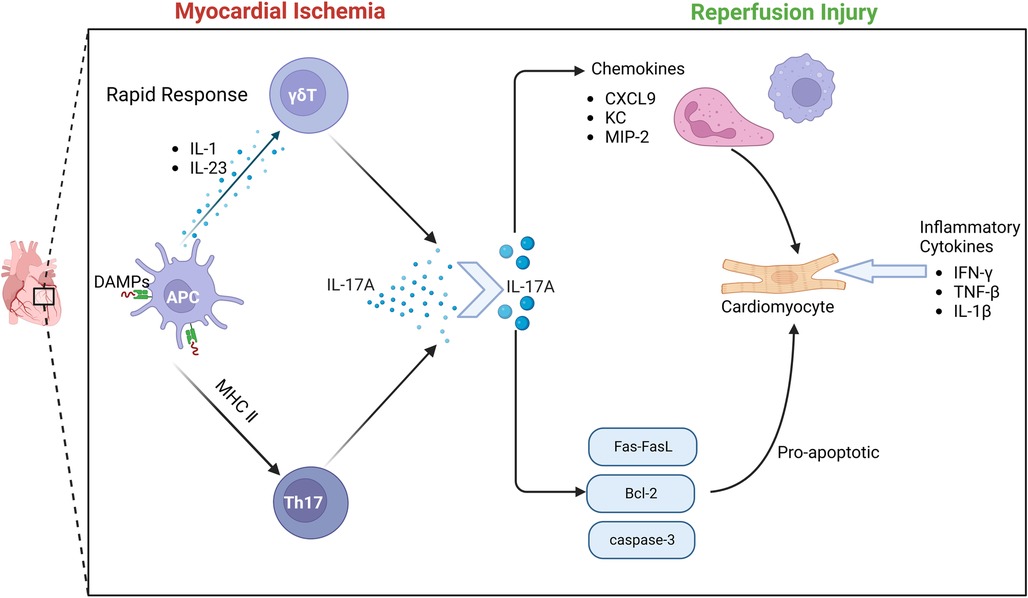
There is a close relationship between γδT cells and IL-17. Through a variety of disease mouse model experiments, γδT cells, NK cells, neutrophils and other innate immune cells can produce IL-17.Early studies have found that γδT cells can also secrete IL-17 after receiving PMA/Ionomycin stimulation in vitro (70). As the most widely studied pro-inflammatory mediator in the IL-17 family, IL-17 A is involved in the occurrence and development of many infectious diseases, tumors and autoimmune diseases (71). γδT cells are the main source of IL-17A in the early immune response. The activation of γδT cells does not depend on the effect of antigen on TCR.IL-1 and IL-23 produced by activated dendritic cells can induce γδT cells to produce IL-17 (72). In the early stage of MIRI, IL-17A produced by γδT cells in the myocardium is the most important inflammatory cytokine. Apoptosis is considered to be an important mechanism of massive cell death in myocardial ischemia-re-perfusion (73). More and more evidence shows that the elements of innate immunity and adaptive immunity are involved in I/R injury (74). γδT cells play an important role in the immune response to myocardial ischemia-re-perfusion injury. γδT cells are T cells that perform innate immune functions. This heterogeneous cell includes multiple cell subsets and has multiple effector functions of producing cytokines and cytotoxicity (75–77). It has been found that the enhancement of immune response mediated by γδT cells aggravates the degree of myocardial ischemia-re-perfusion injury. In the early stage of ischemia-re-perfusion injury, the infiltration of a large number of neutrophils and monocytes/macrophages leads to a strong inflammatory response. At the same time, a large number of neutrophils and monocytes/macrophages produce and secrete various cytokines, chemokines and adhesion molecules, which ultimately aggravate tissue damage (78, 79).
5.1. γδt cells rapidly produce Il-17A
Due to the special pattern of antigen recognition and activation, γδT cells can immediately respond to various pathogens or (IL-1/IL-23), and produce a large amount of IL-17A within a few hours (80). IL-17A produces an immune response by gene-induced recruitment and migration of neutrophils. The IL-17 family is an important cytokine in the human body. In particular, IL-17A has been extensively studied and is mostly secreted by CD4 + T cells. However, the inflammatory cytokine IL-17A during MIRI is mainly produced by γδT cells in the myocardium (81). Studies have shown that IL-17A in the myocardium is almost instantaneously increased after I/R (82), and the process of cytokine expression by γδT cells is a transient process. Although 70% of IL-17A is expressed by Th cells, the differentiation of naive CD4 + T cells into Th17 cells takes a long time and cannot produce a large amount of IL-17A in a process similar to myocardial ischemia. Because the process of IL-17A production by γδT cells does not require pre-induction, γδT cells can quickly produce IL-17A and produce subsequent immune killing of cardiomyocytes. Thus, γδT lymphocytes are the main source of IL-17A.
5.2. IL-17A is the core cytokine of γδT-mediated immune response
The IL-17 cytokine family includes IL-17A, IL-17B, IL-17C, IL-17D, IL-17E and IL-17F. Among them, IL-17A was first discovered in clinical practice (83), and it is also the most widely studied cytokine in the family, and it is one of the most important pro-inflammatory cytokines (84, 85). The genes of IL-17A and IL-17F are located in the same chromosome region and are bound by the same complex IL17RA-IL17RC, so they have the highest structural homology and similar biological functions in the IL-17 cytokine family (86, 87). IL-17A, as a cytokine derived from activated T cells, is now considered to be a key pro-inflammatory cytokine in immune-mediated inflammatory diseases (88).Its mechanism is to recruit neutrophils and monocytes by producing chemokines to cause inflammation. IL-17A also plays an important role in promoting chronic inflammation and autoimmunity in mouse models (89–91). IL-17A and IL-17A-producing cells have become important targets for drug research to treat various forms of autoimmune and inflammatory diseases. Studies have shown that during MIRI, IL-17, as a special pro-inflammatory cytokine, participates in the occurrence of myocardial ischemia-re-perfusion injury by promoting cardiomyocyte apoptosis, recruiting neutrophil infiltration, and leading to myocardial remodeling, and is closely related to the pathogenesis of various cardiovascular diseases (92–94).
5.3. The role of Il-17A produced by γδT cells in MIRI
Innate immunity and adaptive immunity play an important role in the pathological process of MIRI. IL-17A acts as a bridge between innate immunity and adaptive immunity. IL-17A induces a typical inflammatory response through a strong gene-induced innate immunity, presenting a unique positional process in the immune response (95, 96). The recruitment and migration of neutrophils by IL-17A is a key process of myocardial I/R injury. IL-17RA and IL-17RC are key mediators of neutrophil recruitment and migration, which induce neutrophil production and production of neutrophil chemokines, including lipopolysaccharide-induced CXC chemokine(LIX) (97), cytokine-induced neutrophil chemoattractant (KC) and macrophage inflammatory factor protein-2 (MIP-2) -mediated neutrophil migration. IL-17A promotes EC to enhance the expression of neutrophil infiltration E-selectin and ICAM-1 (98), thereby promoting inflammatory response and aggravating myocardial ischemia-re-perfusion injury. In vitro studies have further confirmed that IL-17A has a direct pro-apoptotic effect on cardiomyocytes. When cardiomyocytes are exposed to hypoxia and oxidative stress, the apoptotic signaling pathway is activated, Fas mRNA and Bcl-2 family proteins are up-regulated, and the redox state changes, thereby regulating the Bax/Bcl-2 ratio to induce cardiomyocyte apoptosis. At the same time, caspase-3 apoptotic signaling pathway can also be regulated by IL-17A, thereby inducing cardiomyocyte apoptosis (99, 100).
At the same time, studies have also confirmed that anti-IL-17A monoclonal neutralizing antibody treatment or IL-17A knockout significantly reduced neutrophil infiltration and inhibited cardiomyocyte apoptosis, significantly improving myocardial ischemia-re-perfusion injury. The supplement of exogenous IL-17A aggravated myocardial ischemia-re-perfusion injury. Another study found that IL-17A knockout or γδT cell ablation can improve the survival rate of mice after 7 days, indicating that IL-17A is involved in early myocardial ischemia-re-perfusion injury (101). In summary, the results show that the inflammatory cytokine IL-17 produced by γδT cells causes myocardial pathological damage by inducing cardiomyocyte apoptosis and neutrophil infiltration during myocardial ischemia-re-perfusion injury. Controlling the production of IL-17 may help reduce myocardial injury caused by I/R (102) Figure 4.
6. Therapeutic strategies based on γδT cell-mediated immune response in MIRI
Nowadays, the exact mechanism of MIRI has not been fully revealed, but more and more recent studies have confirmed that immune response plays a central role in various mechanisms of MIRI pathological process. Immune response affects the whole process of MIRI by activating innate immune system and adaptive immune system, as well as related immune cells. Among them, γδT cells play an important role in the MIRI immune response. Therefore, based on the immune response mechanism mediated by γδT cells, it can provide a new strategy for further treatment of MIRI.
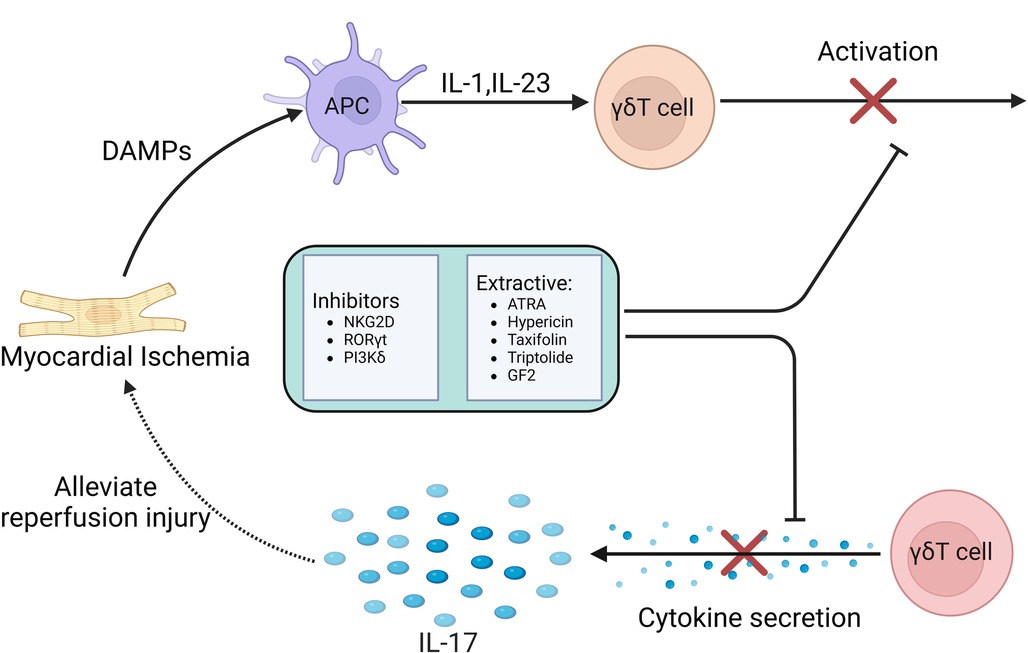
Studies have shown that the adhesion and aggregation of neutrophils in myocardial tissue may be an important factor in mediating MIRI, and the induction of cytokines may play an important role. Under the stimulation of cytokine TNF, cardiomyocytes express higher levels of ICAM-1, which promotes neutrophil infiltration. Treatment with specific anti-ICAM-1 antibody can effectively protect myocardium and coronary vessels (103). Another study found that NKG2D can stimulate CD8 + T cells, γδT cells and NK cells to secrete cytokines (104), and NKG2D blockade can effectively reduce the expression of TnT, MPO, TNF and ICAM. NKG2D can also recognize a variety of ligands to play a variety of functions. NKG2D and its ligands effectively link innate immunity and adaptive immunity (105). Experiments have shown that NKG2D inhibitors can reduce the number of γδT cells that produce IL-17 after myocardial ischemia, and also inhibit the expression of IL-17 (106). NKG2D inhibitors can reduce the production of pro-inflammatory cytokines in myocardial tissue and effectively protect myocardial cells. Therefore, NKG2D inhibitors can be used as an effective means to alleviate MIRI.
In infectious or autoimmune diseases, γδT cells are the early main source of IL-17 production, which is regulated by RORγt expression. Since RORγt is the main transcription factor of IL-17 and is specific to IL-17, RORγt can be used as a therapeutic target for a variety of autoimmune diseases. It was found that RORγt inhibitors had a significant inhibitory effect on γδT cells and their secreted IL-17 in patients with spondyloarthritis and acute pancreatitis, and greatly improved the symptoms of patients. SR1001 is a traditional RORγt inhibitor. Its mechanism is to inhibit the activation and over-expression of γδT cells and inhibit the secretion of IL-17 by inhibiting RORγt transcription (107, 108). Therefore, the development of more types of RORγt inhibitors in the future can expand the therapeutic application in autoimmune diseases and provide new thinking for the prevention and treatment of MIRI.
An experiment found that inhibition of PI3Kδ can inhibit the production of IL-17 by some congenital and adaptive lymphocytes, such as γδT cells and MAITs to produce pro-inflammatory cytokines, thereby inhibiting downstream inflammation and tissue remodeling. Therefore, targeting PI3Kδ may become a new therapeutic approach for the treatment of MIRI mediated by γδT cells (109).
In addition to inhibitors, some extracts may also play an important role. Experimental studies have found that ATRA is an active metabolite of vitamin A, which has multiple effects on cell differentiation and survival by binding to two receptors RARs and RXRs (110, 111). ATRA can reduce antibody production in mice by inhibiting humoral immune responses in vitro and in vivo. In patients with Hashimoto's disease, ATRA attenuated the effect of γδT cells on the production of IgG, TPO-Ab and Tg-Ab by B cells. It can be seen that ATRA has a profound impact on the regulation of γδT cells. The regulation of ATRA can target activated γδT cells, which may promote the further activation and subsequent apoptosis of γδT cells through the mechanism of activation-induced cell death. Therefore, ATRA may be a potential regulator for the treatment of MIRI (112).
In recent years, traditional Chinese medicine is also playing an increasingly important role. It was found that hypericin, the extract of Hypericum perforatum, could inhibit the infiltration of γδT cells in spleen and lymph nodes. In another study, the reduction effect of hypericin on γδT cells was first elucidated, and it was found that hypericin reduced the expression and secretion of IL-17A in γδT cells. Hypericin inhibits the immune response of IL-17A-producing γδT cells and related cytokines by regulating the MAPK/Stat3 pathway (113). Based on the above drug mechanism, hypericin can provide a new treatment for clinical treatment of MIRI.
Psoriasis is a common chronic inflammatory disease. γδT cells accumulate in psoriatic lesions by rapidly secreting IL-17A, inducing and aggravating skin inflammation. It was found that the inhibitory effect of taxifolin on IL-17A may be related to the decrease of γδT cells. Taxifolin can significantly inhibit the activation of immune cells and down-regulate the level of IL-17 A gene in psoriatic skin lesions, and reduce the levels of IL-17 A, IFN-γ, IL-6 and other cytokines in peripheral blood. In addition, low-dose taxifolin also down-regulated the contents of chemokines MIP-1α and MCP-3, indicating that taxifolin can significantly inhibit lymphocyte migration and the effect of mononuclear macrophages on inflammatory lesions (114). Therefore, taxifolin can treat MIRI by inhibiting the activation of γδT cells and down-regulating the level of IL-17A.
Triptolide is a diterpene lactone compound extracted from Tripterygium wilfordii. Its pharmacological effects mainly include anti-tumor, anti-inflammatory and immune regulation. Experimental studies have found that triptolide can regulate the number of γδT cells and the expression level of cytokines. Triptolide can reduce the percentage of γδT cells in peripheral blood of arthritis model rats, and reduce the expression of γδT cells, TNF-α, IL-17 and IL-10 (115). Therefore, triptolide can be used for the treatment of MIRI by regulating the number of γδT cells and the expression level of IL-17A cytokines.
As the main active pharmacological component of ginseng, ginsenosides are often used to treat various diseases. GF2, as a ginseng extract, can play a variety of roles and functions in different tissues with low side effects, and has a variety of pharmacological effects in the treatment of inflammatory skin diseases, tumors, obesity and so on. GF2 has a significant anti-inflammatory effect. Experiments have shown that GF2 can reduce the infiltration of γδT cells, reduce the production of IL-17A, down-regulate the expression of CXCL1 in inflammatory skin tissues, and reduce neutrophil migration. In addition, GF2 also reduced ROS production in neutrophils. The anti-inflammatory effect of GF2 may be mediated by inhibiting the migration of γδT cells and the production of IL-17A and inhibiting the production of ROS and NET in neutrophils (116). Based on this possible potential mechanism, GF2 can be used as a suitable drug for the treatment of MIRI in the future.
In summary, in the process of MIRI, γδT cells combine the characteristics of adaptive immunity with rapid innate immune response, so that they are in the initial stage of immune response. Current studies have confirmed that γδT cells-mediated immune response and the IL-17A produced by γδT cells play a key regulatory role in many infectious or autoimmune diseases. These treatments and drugs have significantly improved these diseases by inhibiting the activation of γδT cells and reducing the secretion of IL-17. Although there is still a lack of relevant clinical experimental studies on the role of MIRI, we believe that the immune response mediated by γδT cells can provide more therapeutic strategies for clinical prevention and treatment of MIRI in the future Figure 5.
7. Prospect of γδT cells in myocardial repair after ischemia-reperfusion injury
In the process of injury/repair of many diseases, the limited regenerative capacity of various tissues and organs has become a challenge in contemporary medicine. When the inflammation is controlled, the damaged tissue heals as the inflammation subsides, and the tissue structure and function recover. However, in some cases, fibrosis and scars are formed at the site of injury after inflammation subsides, which affects healing and leads to organ dysfunction. With continuous research, it has been proved that the immune system has a two-way regulatory role in the process of tissue and organ repair, which can lead to effective tissue regeneration, fibrosis and scar formation. Usually, myocardial injury is irreversible. When various cell types are delivered to the damaged heart, even if the delivered cells cannot survive, transplant or differentiate into functional muscle cells, the improvement of cardiac function is sometimes observed. The reason may be that the inflammatory response caused by activated macrophages temporarily enhances cardiac function.
The results of related studies on the innate immune system show that in the case of injury, activated fibroblasts, cardiomyocytes and various other immune cells release cytokines to polarize existing macrophages and chemokines, thereby recruiting more monocytes, activating and proliferating tissue-resident CCR2−macrophages (117, 118), which may protect and repair the damaged heart (119). Another study demonstrated that enhancing the activity of M2-like macrophages can promote cardiac function recovery after MIRI (120). Thus, CCR2 and CX3CR1 (M2-like) macrophages can be recruited by freezing and thawing/killing cells or local injection of zymosan (an effective stimulator of the innate immune system) to protect and repair damaged hearts.
The results of adaptive immune system showed that T cell infiltration may have pleiotropic effects on damaged myocardium (121). On the one hand, pro-inflammatory CD8 T cells are activated by dendritic cells to produce effective cytokines, including IL-17, IFN-γ and TNF-α, causing myocardial damage. In addition, CD8 T cells are essential for M1-like macrophage infiltration and secretion of pro-inflammatory cytokines and chemokines (122–124); and some CD4 T cells also show destructive effects in the injured heart. On the other hand, some infiltrating T cells have protective effects in damaged myocardium. A major role is to directly activate cardiac fibroblasts and induce fibrosis. The rapid pro-inflammatory response of CD4 T and CD8 T cells can enhance scar formation in the acute phase of injury, but may not be conducive to cardiac function recovery in the long run (125). In addition, dendritic cells can promote myocardial repair by coordinating regulatory T cells to polarize macrophages into M2-like phenotypes; in the experimental model of myocarditis, CD4 non-specific effector T cells have been shown to prevent post-inflammatory fibrosis. In addition, reducing the level of pro-inflammatory cytokines such as IL-17A can also inhibit the formation of fibrosis. CD4 Foxp3 T regulatory cells have been shown to be beneficial to wound healing, scar formation, inflammation regression and skeletal muscle injury repair after myocardial infarction.
Studies on the role of γδT cells in tissue repair have demonstrated that IL-17A produced by γδT cells plays an important role in promoting the proliferation of stem/progenitor cells (126). In muscle fiber injury/repair, it was found that IL-17A can directly promote the proliferation of MuSC, and the key to its repair mechanism is likely to produce IL-17A-mediated neutrophil accumulation through γδT cells to remove necrotic muscle fibers after muscle injury (127). In skin injury/repair, different doses of IL-17A play different roles in wound healing. Low or high doses of IL-17A are not conducive to the repair of skin wounds, while medium doses of IL-17A can effectively promote skin wound healing (128). The γδT cell subset in the skin immune system is usually called dendritic epidermal T cells (DETC). DETC is produced in the thymus during embryonic development and implanted into the epidermis to maintain a steady-state population. DETC has a characteristic dendritic morphology, which can monitor signs of injury or disease, and allow the proliferation and migration of DETC and keratinocytes when keratinocytes are damaged, which is essential for wound healing (129, 130).
γδT cells act as a bridge between innate immunity and adaptive immunity, and have a two-way immune effect. Targeted immunoregulatory γδT cells may be a potential treatment for myocardial ischemia-re-perfusion injury. It can promote the recovery after injury and prevent the secondary injury of myocardial cells after reperfusion. Inhibit fibroblast activation and reduce adverse remodeling. With the continuous deepening of research to further determine the targeted immunomodulator, it can have a specific effect on the components of the immune response, which may be an attractive direction for future clinical treatment of myocardial ischemia-re-perfusion injury.
8. Discussion
In summary, innate immune response and adaptive immune response as the body's defense system play an important role in cardiovascular disease. However, the regulation of the immune system is very complex in different physiological and pathological backgrounds. A large number of studies have shown that the regulation of the immune system in MIRI is a cardiac protection mechanism to protect it from different types of damage, but in some cases, excessive immune response will aggravate the damage to the body. Among them, γδT cells play an important role in MIRI. The activation and release of inflammatory factors is an important cause of myocardial ischemia-re-perfusion injury. Inhibiting the release of inflammatory factors can maintain the stability of cardiomyocytes. Although the proportion of γδT cells in the total T cell population is small, γδT cells have become an important regulator of early immune response and have become a key immune cell type in the prevention and treatment of cardiovascular diseases.As a bridge between innate immune response and adaptive immune response, γδT cells have the characteristics of non-MHC-restricted recognition of receptors, rapid activation, and bidirectional immunity. They have received more and more attention in cardiovascular immunotherapy. Targeted immunoregulatory γδT cells may be a potential treatment method, which is conducive to promoting the recovery after myocardial injury, preventing secondary damage to cardiomyocytes after reperfusion, inhibiting fibroblast activation, and reducing adverse remodeling. Therefore, it can be used as a new idea for the treatment of myocardial ischemia-re-perfusion injury. However, the current research on the treatment of myocardial ischemia-re-perfusion injury based on immune response and γδT cells is still limited, and more research is needed. For example, the combination of optimized immune regulation detection methods and disease animal models can eventually make γδT cells become targeted personalized immunotherapy, which will help us to diagnose and treat diseases more accurately and provide new ideas and methods for clinical prevention and treatment of cardiovascular diseases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
LF: contributed to conception and design of the study. WL: wrote the first draft of the manuscript. XB and XL: wrote sections of the manuscript. WZ and QX: checked sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported in by grants from National Natural Science Foundation of China [No. 81774016 and 82274237].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer YL declared a shared parent affiliation with the authors to the handling editor at the time of the review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li L, Wang Y, Guo R, Li S, Ni J, Gao S, et al. Ginsenoside Rg3-loaded, reactive oxygen species-responsive polymeric nanoparticles for alleviating myocardial ischemia-reperfusion injury. J Controlled Release. (2020) 317:259–72. doi: 10.1016/j.jconrel.2019.11.032
2. Guo X, Hu S, Liu J J, Huang L, Zhong P, Fan ZX, et al. Piperine protects against pyroptosis in myocardial ischaemia/reperfusion injury by regulating the miR -383/RP105/AKT signalling pathway. J Cel Mol Med. (2021) 25(1):244–58. doi: 10.1111/jcmm.15953
3. Zhu N, Li J, Li Y L, Zhang Y, Du Q, Hao P, et al. Berberine protects against simulated ischemia/reperfusion injury -induced H9C2 cardiomyocytes apoptosis in vitro and myocardial ischemia/reperfusion -induced apoptosis in vivo by regulating the mitophagy-mediated HIF-1α/BNIP3 pathway. Front Pharmacol. (2020) 11:367. doi: 10.3389/fphar.2020.00367
4. Yuan XX, Juan ZD, Zhang R, Sun X, Yan R, Yue F, et al. Clemastine fumarate protects against myocardial ischemia reperfusion injury by activating the TLR4/PI3K/Akt signaling pathway. Frontiersin Pharmacology. (2020) 11:28. doi: 10.3389/fphar.2020.00028
5. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European society of cardiology (ESC). Eur Heart J. (2018) 39(2):119–77. doi: 10.1093/eurheartj/ehx393
6. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol. (2016) 67(10):1235–50. doi: 10.1016/j.jacc.2015.10.005
7. Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. (2013) 123(1):92–100. doi: 10.1172/JCI62874
8. Yi C, Song M, Sun L, Si L, Yu D, Li B, et al. Asiatic acid alleviates myocardial ischemia-reperfusion injury by inhibiting the ROS-mediated mitochondria-dependent apoptosis pathway. Oxid Med Cell Longev. (2022) 2022:3267450. doi: 10.1155/2022/3267450
9. Cadenas S. ROS And redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med. (2018) 117:76–89. doi: 10.1016/j.freeradbiomed.2018.01.024
10. Wang R, Wang M, Zhou J, Dai Z, Sun G, Sun X. Calenduloside E suppresses calcium overload by promoting the interaction between L-type calcium channels and Bcl2-associated athanogene 3 to alleviate myocardial ischemia/reperfusion injury. J Adv Res. (2020) 34:173–86. doi: 10.1016/j.jare.2020.10.005
11. Pittas K, Vrachatis DA, Angelidis C, Tsoucala S, Giannopoulos G, Deftereos S. The role of calcium handling mechanisms in reperfusion injury. Curr Pharm Des. (2018) 24(34):4077–89. doi: 10.2174/1381612825666181120155953
12. Zhai M, Li B, Duan W, Jing L, Zhang B, Zhang M, et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J Pineal Res. (2017) 63(2):12419. doi: 10.1111/jpi.12419
13. Cheng Y, Cheng L, Gao X, Chen S, Wu P, Wang C, et al. Covalent modification of Keap1 at Cys77 and Cys434 by pubescenoside a suppresses oxidative stress-induced NLRP3 inflammasome activation in myocardial ischemia-reperfusion injury. Theranostics. (2021) 11(2):861–77. doi: 10.7150/thno.48436
14. Jiang L, Yin X, Chen YH, Chen Y, Jiang W, Zheng H, et al. Proteomic analysis reveals ginsenoside Rb1 attenuates myocardial ischemia/reperfusion injury through inhibiting ROS production from mitochondrial complex I. Theranostics. (2021) 11(4):1703–20. doi: 10.7150/thno.43895
15. Boag SE, Andreano E, Spyridopoulos I. Lymphocyte communication in myocardial ischemia/reperfusion injury. Antioxid Redox Signal. (2017) 26(12):660–75. doi: 10.1089/ars.2016.6940
16. Hou M, Wu X, Zhao Z, Deng Q, Chen Y, Yin L. Endothelial cell-targeting, ROS-ultrasensitive drug/siRNA co-delivery nanocomplexes mitigate early-stage neutrophil recruitment for the anti-inflammatory treatment of myocardial ischemia reperfusion injury. Acta Biomater. (2022) 143:344–55. doi: 10.1016/j.actbio.2022.02.018
17. Nègre-Salvayre A, Augé N, Camaré C, Bacchetti T, Ferretti G, Salvayre R. Dual signaling evoked by oxidized LDLs in vascular cells. Free Radic Biol Med. (2017) 106:118–33. doi: 10.1016/j.freeradbiomed.2017.02.006
18. Barrio L, Roman-Garcia S, Diaz-Mora E, Risco A, Jiménez-Saiz R, Carrasco YR, et al. B cell development and T-dependent antibody response are regulated by p38gamma and p38delta. Front Cell Dev Biol. (2020) 8:189. doi: 10.3389/fcell.2020.00189
19. Galloway A, Turner M. Cell cycle RNA regulons coordinating early lymphocyte development. Wiley Interdiscip Rev RNA. (2017) 8(5):e1419. doi: 10.1002/wrna.1419
20. Davidson TS, Shevach EM. Polyclonal treg cells modulate T effector cell trafficking. Eur J Immunol. (2011) 41(10):2862–70. doi: 10.1002/eji.201141503
21. Getz GS. Thematic review series: the immune system and atherogenesis. Bridging the innate and adaptive immune systems. J Lipid Res. (2005) 46(4):619–22. doi: 10.1194/jlr.E500002-JLR200
22. Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. (2010) 20(1):34–50. doi: 10.1038/cr.2009.139
23. Fujiwara M, Matoba T, Koga JI, Okahara A, Funamoto D, Nakano K, et al. Nanoparticle incorporating toll-like receptor 4 inhibitor attenuates myocardial ischaemia reperfusion injury by inhibiting monocyte-mediated inflammation in mice. Cardiovasc Res. (2019) 115(7):1244–55. doi: 10.1093/cvr/cvz066
24. Zuurbier CJ, Abbate A, Cabrera-Fuentes HA, Cohen MV, Collino M, De Kleijn DPV, et al. Innate immunityas a target for acute cardioprotection. Cardiovasc Res. (2019) 115(7):1131–42. doi: 10.1093/cvr/cvy304
25. Born WK, Kemal Aydintug M, O'Brien RL. Diversity of γδ T-cell antigens. Cell Mol Immunol. (2013) 10(1):13–20. doi: 10.1038/cmi.2012.45
26. Morita CT, Lee HK, Leslie DS, Tanaka Y, Bukowski JF, Märker-Hermann E. Recognition of nonpeptide prenyl pyrophosphate antigens by human gammadelta T cells. Microbes Infect. (1999) 1(3):175–86. doi: 10.1016/S1286-4579(99)80032-X
27. Zhao Y, Niu C, Cui J. Gamma-delta(gammadelta)T cells:friend or foe in cancer development?. J Transl Med. (2018) 16(1):3. doi: 10.1186/s12967-017-1378-2
28. Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. (1998) 279:1737–40. doi: 10.1126/science.279.5357.1737
29. Kabelitz D, Glatzel A, Wesch D. Antigen recognition by human γδ T lymphocytes. Int Arch Allergy Appl Immunol. (2000) 122:1–7. doi: 10.1159/000024353
30. Moser B, Eberl M. γδ T-APCs: a novel tool for immunotherapy? Cell Mol Life Sci. (2011) 68(14):2443–52. doi: 10.1007/s00018-011-0706-6
31. Li H, Pauza CD. Rapamycin increases the yield and effector function of human γδ T cells stimulated in vitro. Cancer Immunol Immunother. (2011) 60:361–70. doi: 10.1007/s00262-010-0945-7
32. Mangan BA, Dunne MR, O'Reilly VP, Dunne PJ, Exley MA, O'Shea D, et al. Cutting edge: CD1d restriction and Th1/Th2/Th17 cytokine secretion by human Vδ3T cells. J Immunol. (2013) 191:30–4. doi: 10.4049/jimmunol.1300121
33. Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. (2010) 55(2):500–7. doi: 10.1161/HYPERTENSIONAHA.109.145094
34. Yuan J, Yu M, Lin QW, Cao AL, Yu X, Dong JH, et al. Th17 cells contribute to viral replication in coxsackievirus B3-induced acute viral myocarditis. J Immunol. (2010) 185(7):4004–10. doi: 10.4049/jimmunol.1001718
35. Niogret J, Berger H, Rebe C, Mary R, Ballot E, Truntzer C, et al. Follicular helper-T cells restore CD8+-dependent antitumor immunity and anti-PD-L1/PD-1 efficacy. J Immunother Cancer. (2021) 9(6):e002157. doi: 10.1136/jitc-2020-002157
36. Cui C, Wang J, Fagerberg E, Chen PM, Connolly KA, Damo M, et al. Neoantigen-driven B cell and CD4 T follicular helper cell collaboration promotes anti-tumor CD8 T cell responses. Cell. (2021) 184(25):6101–6118.e13. doi: 10.1016/j.cell.2021.11.007
37. Ye J, Ma C, Hsueh EC, Eickhoff CS, Zhang Y, Varvares MA, et al. Tumor-derivedγδregulatory T cells suppress innate and adaptive immunity through the induc-tion of immunosenescence. J Immunol. (2013) 190(5):2403–14. doi: 10.4049/jimmunol.1202369
38. Romagnoli PA, Sheridan BS, Pham QM, Lefrançois L, Khanna KM. IL-17A-producing resident memory gammadelta T cells orchestrate the innate immune response to secondary oral Listeria monocyto-genes infection. Proc Natl Acad Sci U S A. (2016) 113(30):8502–7. doi: 10.1073/pnas.1600713113
39. Born WK, Jin N, Aydintug MK, Wands JM, French JD, Roark CL, et al. gammadelta T lymphocytes-selectable cells within the innate system? J Clin Immunol. (2007) 27(2):133–44. doi: 10.1007/s10875-007-9077-z
40. O'Brien RL, Roark CL, Born WK. IL-17-producing γδT cells. Eur J Immunol. (2009) 39:662–6. doi: 10.1002/eji.200839120
41. O'Brien RL, Roark CL, Jin N, Aydintug MK, French JD, Chain JL, et al. γδT-cell receptors: functional correlations. Immunol Rev. (2007) 215:77–88. doi: 10.1111/j.1600-065X.2006.00477.x
42. Lalor SJ, Dungan LS, Sutton CE, Basdeo SA, Fletcher JM, Mills KH. Caspase-1-processed cytokines IL-1beta and IL-18 promote IL-17 production by gammadelta and CD4 T cells that mediate autoimmunity. J Immunol. (2011) 186(10):5738–48. doi: 10.4049/jimmunol.1003597
43. Malik S, Want MY, Awasthi A. The emerging roles of gamma-Delta T cells in tissue inflammation in experimental autoimmune encephalomyelitis. Front Immunol. (2016) 7:14. doi: 10.3389/fimmu.2016.00014
44. Ajuebor MN, Jin Y, Gremillion GL, Strieter RM, Chen Q, Adegboyega PA. Gammadeltat cells initiate acute inflammation and injury in adenovirus-infected liver via cytokine-chemokine cross talk. J Virol. (2008) 82(19):9564–76. doi: 10.1128/JVI.00927-08
45. Lawand M, Déchanet-Merville J, Dieu-Nosjean MC. Key features of gamma-Delta T-cell subsets in human diseases and their immunotherapeutic implications. Front Immunol. (2017) 8:761. doi: 10.3389/fimmu.2017.00761
46. Bhat J, Placek K, Faissner S. Contemplating dichotomous nature of gamma delta T cells for immunotherapy. Front Immunol. (2022) 13:894580. doi: 10.3389/fimmu.2022.894580
47. Adams E, Gu S, Luoma A. Human gamma delta T cells:evolution and ligand recognition. Cell Immunol. (2015) 296(1):31–40. doi: 10.1016/j.cellimm.2015.04.008
48. Castella B, Kopecka J, Sciancalepore P, Mandili G, Foglietta M, Mitro N, et al. The ATP-binding cassette transporter A1 regulates phosphoantigen release and Vγ9Vδ2T cell activation by dendritic cells. Nat Commun. (2017) 8:15663. doi: 10.1038/ncomms15663
49. Stolk D, van der Vliet HJ, de Gruijl TD, van Kooyk Y, Exley MA. Positive & negative roles of innate effector cells in controlling cancer progression. Front Immunol. (2018) 9:1990. doi: 10.3389/fimmu.2018.01990
50. Wu D, Wu P, Wu X, Ye J, Wang Z, Zhao S, et al. Ex vivo expanded human circulating Vδ1γδT cells exhibit favorable therapeutic potential for colon cancer. Oncoimmunology. (2015) 4(3):e992749. doi: 10.4161/2162402X.2014.992749
51. Xiang Z, Tu W. Dual face of Vγ9Vδ2-T cells in tumor immunology:anti-versus pro-tumoral activities. Front Immunol. (2017) (8):1041. doi: 10.3389/fimmu.2017.01041
52. Funken D, Yu Y, Feng X, Imvised T, Gueler F, Prinz I, et al. Lack of gamma delta T cells ameliorates inflammatory response after acute intestinal ischemia reperfusion in mice. Sci Rep. (2021) 11(1):18628. doi: 10.1038/s41598-021-96525-y
53. Shigematsu T, Wolf RE, Granger DN. T-lymphocytes modulate the microvascular and inflammatory responses to intestinal ischemia-reperfusion. Microcirculation. (2002) 9:99–109. doi: 10.1038/sj/mn/7800126
54. Watson MJ, Ke B, Shen X-D, Gao F, Busuttil RW, Kupiec-Weglinski JW, et al. Treatment with antithymocyte globulin ameliorates intestinal ischemia and reperfusion injury in mice. Surgery. (2012) 152:843–50. doi: 10.1016/j.surg.2012.03.001
55. Pai M-H, Shih Y-M, Shih J-M, Yeh CL. Glutamine modulates changes in intestinal intraepithelial γδT-lymphocyte expressions in mice with ischemia/reperfusion injury. Shock. (2015) 15:44. doi: 10.1097/SHK.0000000000000375
56. Hochegger K, Schätz T, Eller P, Tagwerker A, Heininger D, Mayer G, et al. Role of alpha/beta and gamma/delta T cells in renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. (2007) 293(3):F741–7. doi: 10.1152/ajprenal.00486.2006
57. Savransky V, Molls RR, Burne-Taney M, Chien CC, Racusen L, Rabb H. Role of the T-cell receptor in kidney ischemia-reperfusion injury. Kidney Int. (2006) 69(2):233–8. doi: 10.1038/sj.ki.5000038
58. Cortvrindt C, Speeckaert R, Moerman A, Delanghe JR, Speeckaert MM. The role of interleukin-17A in the pathogenesis of kidney diseases. Pathology. (2017) 49(3):247–58. doi: 10.1016/j.pathol.2017.01.003
59. Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, et al. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. (2009) 15:946–50. doi: 10.1038/nm.1999
60. Zhong Q, Zhou K, Liang QL, Lin S, Wang YC, Xiong XY, et al. Interleukin-23 secreted by activated macrophages drives γδT cell production of interleukin-17 to aggravate secondary injury after intracerebral hemorrhage. J Am Heart Assoc. (2016) 5(10):e004340. doi: 10.1161/JAHA.116.004340
61. Gelderblom M, Weymar A, Bernreuther C, Velden J, Arunachalam P, Steinbach K, et al. Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood. (2012) 120(18):3793–802. doi: 10.1182/blood-2012-02-412726
62. Loi P, Yuan Q, Torres D, Delbauve S, Laute MA, Lalmand MC, et al. Interferon regulatory factor 3 deficiency leads to interleukin-17-mediated liver ischemia-reperfusion injury. Hepatology. (2013) 57(1):351–61. doi: 10.1002/hep.26022
63. Hirsh MI, Hashiguchi N, Chen Y, Yip L, Junger WG. Surface expression of HSP72 by LPS-stimulated neutrophils facilitates gammadeltaT cell-mediated killing. Eur J Immunol. (2006) 36(3):712–21. doi: 10.1002/eji.200535422
64. Egan PJ, Carding SR. Downmodulation of the inflammatory response to bacterial infection by gammadelta T cells cytotoxic for activated macrophages. J Exp Med. (2000) 191(12):2145–58. doi: 10.1084/jem.191.12.2145
65. Liu M, Hu Y, Yuan Y, Tian Z, Zhang C. γδt cells suppress liver fibrosis via strong cytolysis and enhanced NK cell-mediated cytotoxicity against hepatic stellate cells. Front Immunol. (2019) 10:477. doi: 10.3389/fimmu.2019.00477
66. Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, et al. γδt17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. (2014) 40(5):785–800. doi: 10.1016/j.immuni.2014.03.013
67. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. (2015) 372(26):2509–20. doi: 10.1056/NEJMoa1500596
68. Hu G, Wu P, Cheng P, Zhang Z, Wang Z, Yu X, et al. Tumor-infiltrating CD39+γδTregs are novel immunosuppressive T cells in human colorectal cancer. Oncoimmunology. (2017) 6(2):e1277305. doi: 10.1080/2162402X.2016.1277305
69. Wakita D, Sumida K, Iwakura Y, Nishikawa H, Ohkuri T, Chamoto K, et al. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur J Immunol. (2010) 40(7):1927–37. doi: 10.1002/eji.200940157
70. Girardi M. Immunosurveillance and immunoregulation by gammadelta T cells. J Invest Dermatol. (2006) 126(1):25–31. doi: 10.1038/sj.jid.5700003
71. Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune. Nat Rev Immunol. (2010) 10(7):479–89. doi: 10.1038/nri2800
72. Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. (2009) 31(2):331–41. doi: 10.1016/j.immuni.2009.08.001
73. Hamacher-Brady A, Brady NR, Gottlieb RA. The interplay between pro-death and pro-survival signaling pathways in myocardial ischemia/reperfusion injury: apoptosis meets autophagy. Cardiovasc Drugs Ther. (2006) 20(6):445–62. doi: 10.1007/s10557-006-0583-7
74. Linfert D, Chowdhry T, Rabb H. Lymphocytes and ischemia-reperfusion injury. Transplant Rev. (2009) 23(1):1–10. doi: 10.1016/j.trre.2008.08.003
75. Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. (2002) 2(5):336–45. doi: 10.1038/nri797
76. Chien YH, Meyer C, Bonneville M. γδ T cells: first line of defense and beyond. Annu Rev Immunol. (2014) 32:121–55. doi: 10.1146/annurev-immunol-032713-120216
77. Prinz I, Silva-Santos B, Pennington DJ. Functional development of γδ T cells. Eur J Immunol. (2013) 43(8):1988–94. doi: 10.1002/eji.201343759
78. Toldo S, Mauro AG, Cutter Z, Abbate A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. (2018) 315(6):H1553–68. doi: 10.1152/ajpheart.00158.2018
79. Steffens S, Montecucco F, Mach F. The inflammatory response as a target to reduce myocardial ischaemia and reperfusion injury. Thromb Haemost. (2009) 102(2):240–7. doi: 10.1160/TH08-12-0837
80. Kapsenberg ML. Gammadelta T cell receptors without a job. Immunity. (2009) 31(2):181–3. doi: 10.1016/j.immuni.2009.08.004
81. Liao YH, Xia N, Zhou SF, Tang TT, Yan XX, Lv BJ, et al. Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J Am Coll Cardiol. (2012) 59(4):420–9. doi: 10.1016/j.jacc.2011.10.863
82. Cheng X, Yu X, Ding YJ, Fu QQ, Xie JJ, Tang TT, et al. The Th17/treg imbalance in patients with acute coronary syndrome. Clin Immunol. (2008) 127(1):89–97. doi: 10.1016/j.clim.2008.01.009
83. Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. (1993) 150:5445–56. doi: 10.4049/jimmunol.150.12.5445
84. Hymowitz SG, Filvaroff EH, Yin JP, Lee J, Cai L, Risser P, et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. (2001) 20(19):5332–41. doi: 10.1093/emboj/20.19.5332
85. Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M, et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. (2007) 179(11):7791–9. doi: 10.4049/jimmunol.179.11.7791
86. Akimzhanov AM, Yang XO, Dong C. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J Biol Chem. (2007) 282(9):5969–72. doi: 10.1074/jbc.C600322200
87. Zhang X, Angkasekwinai P, Dong C, Tang H. Structure and function of interleukin-17 family cytokines. Protein Cell. (2011) 2:26–40. doi: 10.1007/s13238-011-1006-5
88. Schett G, Elewaut D, McInnes IB, Dayer JM, Neurath MF. How cytokine networks fuel inflammation: toward a cytokine-based disease taxonomy. Nat Med. (2013) 19:822–4. doi: 10.1038/nm.3260
89. Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. (2006) 177:566–73. doi: 10.4049/jimmunol.177.1.566
90. Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. (2003) 171:6173–7. doi: 10.4049/jimmunol.171.11.6173
91. van Tok MN, van Duivenvoorde LM, Kramer I, Ingold P, Pfister S, Roth L, et al. Interleukin-17A inhibition diminishes inflammation and new bone formation in experimental spondyloarthritis. Arthritis Rheumato l. (2019) 71:612–25. doi: 10.1002/art.40770
92. Cheng X, Taleb S, Wang J, Tang TT, Chen J, Gao XL, et al. Inhibition of IL-17A in atherosclerosis. Atherosclerosis. (2011) 215(2):471–4. doi: 10.1016/j.atherosclerosis.2010.12.034
93. Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, et al. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol. (2009) 183(12):8167–75. doi: 10.4049/jimmunol.0901126
94. Baldeviano GC, Barin JG, Talor MV, Srinivasan S, Bedja D, Zheng D, et al. Interleukin17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ Res. (2010) 106(10):1646–55. doi: 10.1161/CIRCRESAHA.109.213157
95. Raucci F, Mansour AA, Casillo GM, Saviano A, Caso F, Scarpa R, et al. Interleukin-17A (IL-17A), a key molecule of innate and adaptive immunity, and its potential involvement in COVID-19-related thrombotic and vascularmechanisms. Autoimmun Rev. (2020) 19(7):102572. doi: 10.1016/j.autrev.2020.102572
96. Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. (2004) 21:467–76. doi: 10.1016/j.immuni.2004.08.018
97. Frangogiannis NG. Chemokines in ischemia and reperfusion. Thromb Haemost. (2007) 97:738–47. doi: 10.1160/TH07-01-0022
98. Nakamura M, Wang NP, Zhao ZQ, Wilcox JN, Thourani V, Guyton RA, et al. Preconditioning decreases bax expression, PMN accumulation and apoptosis in reperfused rat heart. Cardiovasc Res. (2000) 45:661–70. doi: 10.1016/S0008-6363(99)00393-4
99. Tanaka M, Ito H, Adachi S, Akimoto H, Nishikawa T, Kasajima T, et al. Hypoxia induces apoptosis with enhanced expression of fas antigen messenger RNA in cultured neonatal rat cardiomyocytes. Circ Res. (1994) 75(3):426–33. doi: 10.1161/01.res.75.3.426
100. Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci U S A. (2002) 99(20):12825–30. doi: 10.1073/pnas.202474099
101. Barry SP, Ounzain S, McCormick J, Scarabelli TM, Chen-Scarabelli C, Saravolatz LII, et al. Retraction notice to “enhanced IL-17 signalling following myocardial ischaemia/reperfusion injury”. Int J Cardiol. (2019) 274:404. doi: 10.1016/j.ijcard.2018.10.031
102. Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res. (2004) 61(3):481–97. doi: 10.1016/j.cardiores.2003.10.011
103. Lefer DJ, Flynn DM, Anderson DC, Buda AJ. Combined inhibition of P-selectin and ICAM-1 reduces myocardial injury following ischemia and reperfusion. Am J Physiol. (1996) 271(6 Pt 2):H2421–9. doi: 10.1152/ajpheart.1996.271.6.H2421
104. López-Larrea C, Suárez-Alvarez B, López-Soto A, López-Vázquez A, Gonzalez S. The NKG2D receptor: sensing stressed cells. Trends Mol Med. (2008) 14(4):179–89. doi: 10.1016/j.molmed.2008.02.004
105. Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. NatRev Immunol. (2003) 3(10):781–90. doi: 10.1038/nri1199
106. Shen B, Li J, Yang B. NKG2D Blockade significantly attenuates ischemia-reperfusion injury in a cardiac transplantation model. Transplant Proc. (2013) 45(6):2513–6. doi: 10.1016/j.transproceed.2013.02.126
107. Ding Q, Zhao M, Bai C, Yu B, Huang Z. Inhibition of RORγt activity and Th17 differentiation by a set of novel compounds. BMC Immunol. (2015) 16:32. doi: 10.1186/s12865-015-0097-9
108. Wang J, Xu Y, Jing H, Chang Q, Wu X, Zhang Z. RORγt inhibitor SR1001 alleviates acute pancreatitis by suppressing pancreatic IL-17-producing Th17 and γδ-T cells in mice with ceruletide-induced pancreatitis. Basic Clin Pharmacol Toxicol. (2021) 129(5):357–68. doi: 10.1111/bcpt.13642
109. Chen S, Paveley R, Kraal L, Sritharan L, Stevens E, Dedi N, et al. Selective targeting of PI3Kδ suppresses human IL-17-producing T cells and innate-like lymphocytes and may be therapeutic for IL-17-mediated diseases. J Autoimmun. (2020) 111:102435. doi: 10.1016/j.jaut.2020.102435
110. Saurer L, McCullough KC, Summerfield A. In vitro induction of mucosa-type dendritic cells by all-trans retinoic acid. J Immunol. (2007) 179(6):3504–14. doi: 10.4049/jimmunol.179.6.3504
111. Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. (2007) 129(4):723–33. doi: 10.1016/j.cell.2007.02.050
112. Liu H, Zheng T, Mao Y, Xu C, Wu F, Bu L, et al. γδ Τ cells enhance B cells for antibody production in Hashimoto's Thyroiditis, and retinoic acid induces apoptosis of the γδ Τ cell. Endocrine. (2016) 51(1):113–22. doi: 10.1007/s12020-015-0631-9
113. Zhang S, Zhang J, Yu J, Chen X, Zhang F, Wei W, et al. Hyperforin ameliorates imiquimod-induced psoriasis-like murine skin inflammation by modulating IL-17A-producing γδ T cells. Front Immunol. (2021) 12:635076. doi: 10.3389/fimmu.2021.635076
114. Di T, Zhai C, Zhao J, Wang Y, Chen Z, Li P. Taxifolin inhibits keratinocyte proliferation and ameliorates imiquimod-induced psoriasis-like mouse model via regulating cytoplasmic phospholipase A2 and PPAR-γ pathway. Int Immunopharmacol. (2021) 99:107900. doi: 10.1016/j.intimp.2021.107900
115. Zeng KQ, Hu YH, Zhang MM, Lai XY, Zhang WC. Effect of triptolide on the expression of cytokines in γδT cells of CIA rats. Journal of Beijing University of Chinese Medicine. (2004) 05:39–41.
116. Park SH, Seo W, Eun HS, Kim SY, Jo E, Kim MH, et al. Protective effects of ginsenoside F2 on 12-O-tetradecanoylphorbol-13-acetate-induced skin inflammation in mice. Biochem Biophys Res Commun. (2016) 478(4):1713–9. doi: 10.1016/j.bbrc.2016.09.009
117. Lavine KJ, Pinto AR, Epelman S, Kopecky BJ, Clemente-Casares X, Godwin J, et al. The macrophage in cardiac homeostasis and disease: jACC macrophage in CVD series (part 4). J Am Coll Cardiol. (2018) 72(18):2213–30. doi: 10.1016/j.jacc.2018.08.2149
118. Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci U S A. (2014) 111(45):16029–34. doi: 10.1073/pnas.1406508111
119. Bajpai G, Bredemeyer A, Li W, Zaitsev K, Koenig AL, Lokshina I, et al. Tissue resident CCR2- and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ Res. (2019) 124(2):263–78. doi: 10.1161/CIRCRESAHA.118.314028
120. Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AKZ, Schwanekamp JA, et al. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature. (2020) 577(7790):405–9. doi: 10.1038/s41586-019-1802-2
121. Bansal SS, Ismahil MA, Goel M, Patel B, Hamid T, Rokosh G, et al. Activated T lymphocytes are essential drivers of pathological remodeling in ischemic heart failure. Circ Heart Fail. (2017) 10(3):e003688. doi: 10.1161/CIRCHEARTFAILURE.116.003688
122. Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. (2014) 124(10):4642–56. doi: 10.1172/JCI74084
123. Forte E, Perkins B, Sintou A, Kalkat HS, Papanikolaou A, Jenkins C, et al. Cross-Priming dendritic cells exacerbate immunopathology after ischemic tissue damage in the heart. Circulation. (2021) 143(8):821–36. doi: 10.1161/CIRCULATIONAHA.120.044581
124. Ma F, Feng J, Zhang C, Li Y, Qi G, Li H, et al. The requirement of CD8+ T cells to initiate and augment acute cardiac inflammatory response to high blood pressure. J Immunol. (2014) 192(7):3365–73. doi: 10.4049/jimmunol.1301522
125. Nevers T, Salvador AM, Velazquez F, Ngwenyama N, Carrillo-Salinas FJ, Aronovitz M, et al. Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. J Exp Med. (2017) 214(11):3311–29. doi: 10.1084/jem.20161791
126. Zhao J, Chen X, Herjan T, Li X. The role of interleukin-17 in tumor development and progression. J Exp Med. (2020) 217(1):e20190297. doi: 10.1084/jem.20190297
127. Nishimura D, Sakai H, Sato T, Sato F, Nishimura S, Toyama-Sorimachi N, et al. Roles of ADAM8 in elimination of injured muscle fibers prior to skeletal muscle regeneration. Mech Dev. (2015) 135:58–67. doi: 10.1016/j.mod.2014.12.001
128. Hu W, Shang R, Yang J, Chen C, Liu Z, Liang G, et al. Skin γδ T cells and their function in wound healing. Front Immunol. (2022) 13:875076. doi: 10.3389/fimmu.2022.875076
129. Pitard V, Roumanes D, Lafarge X, Couzi L, Garrigue I, Lafon ME, et al. Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood. (2008) 112(4):1317–24. doi: 10.1182/blood-2008-01-136713
Keywords: myocardial ischemia reperfusion injury, immune response, T lymphocytes, γδT cell, cytokine, IL-17
Citation: Luo W, Bian X, Liu X, Zhang W, Xie Q and Feng L (2023) A new method for the treatment of myocardial ischemia-reperfusion injury based on γδT cell-mediated immune response. Front. Cardiovasc. Med. 10:1219316. doi: 10.3389/fcvm.2023.1219316
Received: 8 May 2023; Accepted: 18 July 2023;
Published: 3 August 2023.
Edited by:
Simon Tual-Chalot, Newcastle University, United KingdomReviewed by:
Yulin Li, Capital Medical University, ChinaYu Liu, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, China
© 2023 Luo, Bian, Liu, Zhang, Xie and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Limin Feng ZmxpbWludGNtQDE2My5jb20=
†These authors have contributed equally to this work
 Wei Luo
Wei Luo Xiaohong Bian
Xiaohong Bian Xiaona Liu1
Xiaona Liu1